Abstract
Ignicoccus hospitalis forms many cell surface appendages, the Iho670 fibers (width, 14 nm; length, up to 20 μm), which constitute up to 5% of cellular protein. They are composed mainly of protein Iho670, possessing no homology to archaeal flagellins or fimbrins. Their existence as structures different from archaeal flagella or fimbriae have gone unnoticed up to now because they are very brittle.
The existence of surface appendages on archaeal cells has been known for a long time (6; for a recent review, see reference 15); functional studies defined archaeal flagella as motility organelles (e.g., see references 1 and 2) and archaeal fimbriae as adhesins (3, 7, 22). Two other types of very special archaeal cell surface appendages are the hami formed by the SM1 euryarchaeum (13) and the cannulae produced by Pyrodictium occultum (16, 21). In the case of hami, their function is obvious: they are 1- to 3-μm-long filamentous structures with regular spikes, ending in a hook to resemble in ultrastructure a barbed wire, which ends in a grappling hook (13). Thereby, SM1 cells adhere to each other (and structures in their biotope); SM1, indeed can be harvested from polyethylene nets placed into its natural habitat (cold sulfidic springs) (8). The function of the cannulae is still not known: they are extracellular tubes 25 nm wide with an inner diameter of ca. 20 nm and are composed of five related proteins (12). Cannulae enter the periplasmic space but do not reach into the cytoplasm; they have different lengths and connect the highly irregular Pyrodictium cells to form nets easily visible by naked eye. Here, we describe a new type of archaeal cell surface appendage, the fibers which we have detected on cells of the crenarchaeum Ignicoccus hospitalis KIN4/IT.
I. hospitalis originally was described to possess up to nine flagellum-like appendages, anchored at one pole into the cell (17). In repeated experiments designed to examine possible motility of I. hospitalis, however, we never observed such behavior. For those experiments, we used our thermomicroscope, allowing analyses of cells under anaerobic conditions at 90°C (9). To study these cell surface appendages in more detail, cells were grown at 90°C (in medium described in reference 17) either in 120-ml serum bottles filled with 20 ml medium, an 80:20 H2-CO2 gas phase, and with shaking at 100 rpm or in a 300-liter fermentor filled with 250 liters as described previously (10). In initial experiments, the cell surface appendages were removed from cells by shearing, differential centrifugation, and a final CsCl gradient centrifugation (similar to the protocol established for the preparation of flagella from Pyrococcus furiosus [14]). Yields, using this method, however, were very low (data not shown). A breakthrough was our finding that the cell surface appendages are very brittle and that the majority of fibers were removed from the cell surface by normal lab handling (compare Fig. 1A and B). Such manipulations include especially sampling of cells using the syringe-needle method (to transfer the strictly anaerobic archaea) and centrifugations to concentrate cells. Therefore, another strategy was used to isolate the cell surface appendages. To aliquots of the supernatant of the harvest (after overnight centrifugation at 16,000 × g) of cells grown to stationary phase in a 300-liter fermentor—containing the majority of fibers, broken off during the harvest centrifugation—NaCl was added to a 5.8% final concentration and polyethylene glycol 6000 (PEG 6000; Fluka, Sigma-Aldrich, Steinheim, Germany) was added to a final concentration of 10.5%. Precipitation was carried out overnight at 6°C, followed by centrifugation (30 min at 10,000 × g). The pelleted material obtained from a 10-liter aliquot of centrifugation supernatant was dissolved in 8 ml of Millipore-purified water (aqua bidest). After addition of 3.6 g of CsCl, the sample was centrifuged for 48 h (SW60 Ti rotor, 250,000 × g, 4°C in Beckman Optima LE-80K centrifuge) and the resulting band (Fig. 1C) was dialyzed extensively against 5 mM MES buffer (1 mM MgSO4·7H2O with 1 mM dithiothreitol; pH 6.0). Yields did increase dramatically: the PEG 6000 precipitation method resulted in at least 100-fold more fiber material than the method used initially. (Shearing from 10 liters of cells which had been concentrated by centrifugation resulted in 10 to 50 μg of fiber protein.) In repeated experiments, the total yield of fiber protein, precipitated from 10 liters of centrifugation supernatant by PEG-NaCl addition, varied from 35 to 45 mg. Since the cell yield of 10 liters of culture was 0.75 g, we estimate that the fiber protein constitutes at least 5% of the cellular protein.
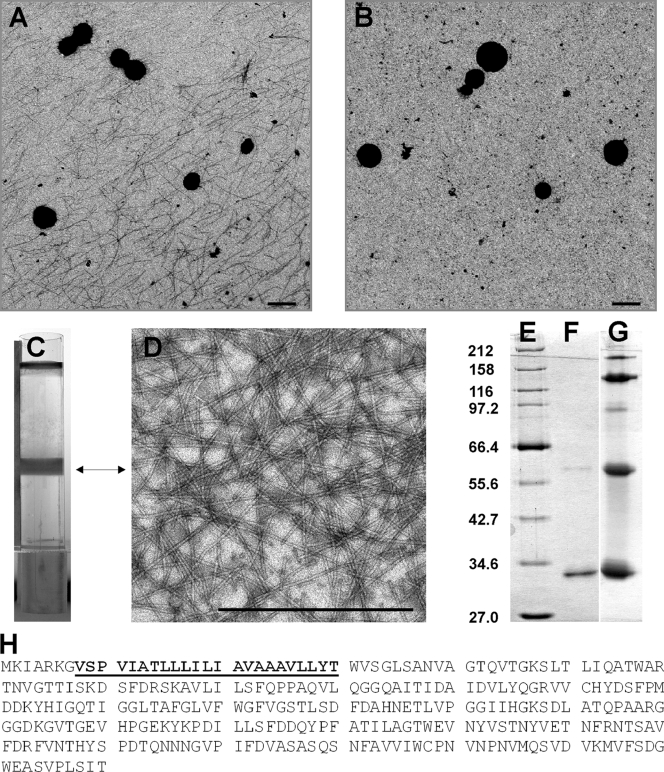
FIG. 1.
Ultrastructural and biochemical analyses of Ignicoccus hospitalis fibers. (A) A 5-μl aliquot of a fermentor culture, grown to stationary phase, was applied onto a carbon-coated grid, negatively stained with 2% uranyl acetate, and analyzed by TEM. The sample was collected using a wide-bore pipette. (B) An aliquot of the same culture as that in panel A was analyzed by the TEM procedure as described for panel A. The sample was collected with a syringe and needle. (C) CsCl gradient (volume, 10 ml) of I. hospitalis fibers concentrated by PEG precipitation from 10 liters of supernatant of a 16,000 × g centrifugation (used to harvest cells from the fully grown fermentor culture). (D) TEM analysis of the CsCl gradient-purified fibers, prepared and negatively stained as for panel A. (E to G) SDS-PAGE analyses of CsCl gradient-purified fibers (12.5% gel, silver stained). (E) Broad-range protein marker (NEB). Sizes in kDa are indicated to the left. (F) Heat-denatured fibers (0.5 μg treated for 15 min at 100°C). (G) Partially heat-denatured fibers (5 μg treated for 3 min at 100°C). (H) Amino acid sequence of the fiber protein. The sequence given in boldface and underlined was obtained by N-terminal protein sequencing and used to identify Igni_0670 as the structural gene coding for the I. hospitalis fibers (NCBI reference sequence NC_009776.1). The size bars in panels A, B, and D are 1 μm each.
Biochemical and bioinformatic analyses of the fiber protein indicated the following. The material obtained after PEG 6000 precipitation and CsCl gradient centrifugation consisted of mainly one protein with a mass of ca. 33 kDa, as indicated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 1F). Protein samples were resolved by SDS-PAGE with 12.5% polyacrylamide (11); proteins were stained with Coomassie brilliant blue G250 or via silver staining (4). The same preparation, if not completely denatured by heat treatment, resulted in protein bands of ca. 33, 60, and 120 kDa (with weaker bands of ca. 90 and 180 kDa) (Fig. 1G). We take this to indicate that these bands represent fiber protein oligomers. The fiber protein seems not to be glycosylated, as indicated by the lack of a positive reaction (data not shown) with periodic acid-Schiff staining (24), although we note that there is no absolute correlation between the presence or absence of glycosylation with a positive or negative periodic acid-Schiff staining. A lack of glycosylation would differentiate the fiber protein from most other archaeal cell surface appendages described as flagellins, for which such a modification is the rule rather than an exception (23). N-terminal sequencing by Edman degradation (performed by the central protein analytic facility of the Biology Department of the University of Regensburg) determined the N terminus over a length of 23 amino acids (Fig. 1H). Since the genome sequence of I. hospitalis is known (18), identification of the fiber protein was possible: it is encoded by I. hospitalis gene Igni_0670. In order to differentiate fiber proteins potentially occurring in other Ignicoccus species (and to comply with the generally accepted rule to name proteins with a three-letter code), we propose to name the fiber protein of I. hospitalis Iho670. The Iho670 protein is processed since the first 7 amino acids are not found in the mature fiber protein. The two programs Flafind and SOSUI indeed predict such a short signal peptide, while other programs (like signalP) indicate a much longer signal peptide of 38 amino acids: similar difficulties with prediction of signal peptides had been observed earlier for archaeal cell surface proteins, e.g., for the Mth60 fimbrin and the I. hospitalis outer membrane protein Ihomp1 (5, 22; see also reference 3 for a detailed discussion of archaeal signal peptides). A signal peptidase processing the Iho670 protein at the correct site (i.e., after amino acid 7) very recently was identified in I. hospitalis (S.-V. Albers, personal communication). The fiber protein Iho670 shows no homologies to other proteins in its amino acid sequence, especially to the archaeal cell surface appendage proteins identified up to now: archaeal flagellins, the archaeal fimbrin Mth60, the hamus protein, and the three cannula proteins. The Igni_0670 gene might be argued to be part of an operon, because Igni_0668 to Igni_0677 are predicted to be transcribed counterclockwise from the I. hospitalis genome, with a maximum intergenic space of 73 nucleotides (18). Predictions of the proteins encoded by these genes, however, do not favor this possibility, because Igni_0668, Igni_0669, Igni_0670, and Igni_0672 code for hypothetical proteins; Igni_0671 and Igni_0673 code for flavin adenine dinucleotide-dependent pyridine nucleotide disulfide oxidoreductases; Igni_0674 codes for an NiFe hydrogenase maturation protein; Igni_0675 encodes a nonspecific serine/threonine protein kinase; Igni_0676 encodes a protein homologous to eukaryotic initiation factor 1A; and Igni_0677 encodes a 30S ribosomal protein, S6e. Obviously, there is no functional context between the encoded proteins.
The results of our ultrastructural analyses of the fibers can be summarized as follows. Transmission electron microscopic (TEM) analyses of the purified Iho670 fibers indicate that they can be up to 20 μm long, with a diameter of 14 nm. TEM analyses of I. hospitalis cells growing on carbon-coated grids (see reference 14 for technical details) confirmed these data. Obviously, these cell surface appendages are very long and brittle; therefore, we were not able to decide how many fibers are synthesized per cell: we estimate this number to be at least 20. For TEM, a drop of cell-suspension was placed on a carbon-coated 200-mesh copper grid (Plano, Wetzlar, Germany). These samples were either unidirectionally shadowed with platinum and carbon at 15° (CFE 50; Cressington Ltd., Watford, United Kingdom) or negatively stained for 1 min with 2% uranyl acetate. All TEM micrographs were recorded using a slow-scan charge-coupled device camera (TEM 1000; TVIPS-Tietz, Gauting, Germany) attached to a CM 12 transmission electron microsope (FEI, Eindhoven, The Netherlands).
It turned out that scanning electron microscopic (SEM) analyses of the coccoid cells with emanating fibers is extremely difficult. This is due to the fact that the outer membrane of I. hospitalis is a very delicate structure, being destroyed on nearly every cell during standard fixation and processing steps for TEM and SEM. We have proven that under the same conditions, other archaeal cells and their appendages are well preserved and can be nicely visualized (13, 14, 17, 19, 20). TEM analyses of I. hospitalis cells need special precautions to conserve the labile membranes, like growth in cellulose capillaries and high-pressure freezing and freeze-substitution (19); a simple glutaraldehyde fixation and dehydration at room-temperature will destroy, especially, the outer membrane. For SEM analyses, a protocol preserving the outer membrane is not yet available.
Conclusions.
We have shown here that I. hospitalis forms a multitude of cell surface appendages which we name “fibers” to differentiate them from fimbriae and flagella. Archaeal flagella are defined as motility organelles with a diameter from 10 to ca. 15 nm, and archaeal fimbriae are defined as adhesion structures with a diameter of ca. 5 nm. The fibers described here are distinct from flagella and fimbriae, since they have a diameter of ca. 15 nm, but are not related to flagella, concerning the primary structure of the constituting protein and the fact that they are not motility organelles. These fibers have remained undetected in their multitude and extensions up to now, because they are very brittle. The normal sampling of cells via syringes and needles removes the fibers nearly quantitatively from cells, as does centrifugation to collect cells (Fig. 1B). These surface appendages could be observed only rarely using routine TEM preparation techniques and were proposed to represent flagella, due to their diameter, which is similar to that of other archaeal flagella (17): the data reported here identify those structures as a new cell surface appendage. The Iho670 fibers are observed on cells in planktonic stage (grown in liquid medium) and on cells adhering to surfaces; therefore, a definite answer to whether they are used for adhesion is not possible at the moment. Obviously, they are of major importance for I. hospitalis, since they constitute—to the best of our estimates—at least 5% of all cellular proteins.
Further analyses will address the question of whether similar fibers are present on the two other known species of the genus Ignicoccus, namely Ignicoccus islandicus and Ignicoccus pacificus. Furthermore, it will be very interesting to define the function of these cell surface appendages: bioinformatics seems to be not helpful in this connection, because no homologies to proteins in the databases were found. Another question is how these structures are anchored in the cell. Do fibers extend from the cytoplasmic membrane (and thereby cross the periplasm, which can vary in width between 20 to 500 nm [17]), or are they anchored only in the outer membrane?
Acknowledgments
We thank E. Papst, S. Dobner, Y. Bilek, T. Hader and K. Eichinger for expert technical help and two reviewers for very thoughtful comments.
This work was supported by DFG grant WI 731/10-1 to R.R. and R.W.
Footnotes
Published ahead of print on 14 August 2009.
REFERENCES
- 1.Alam, M., M. Claviez, D. Oesterhelt, and M. Kessel. 1984. Flagella and motility behaviour of square bacteria. EMBO J. 3:2899-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam, M., and D. Oesterhelt. 1984. Morphology, function and isolation of halobacterial flagella. J. Mol. Biol. 176:459-475. [DOI] [PubMed] [Google Scholar]
- 3.Albers, S.-V., Z. Szabó, and A. J. M. Driessen. 2003. Archaeal homolog of bacterial type IV prepilin signal peptidases with broad substrate specificity. J. Bacteriol. 185:3918-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blum, H., H. Beier, and H. J. Gross. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93-99. [Google Scholar]
- 5.Burghardt, T., M. Saller, S. Gürster, D. Müller, C. Meyer, U. Jahn, E. Hochmuth, R. Deutzmann, F. Siedler, P. Babinger, R. Wirth, H. Huber, and R. Rachel. 2008. Insight into the proteome of the hyperthermophilic crenarchaeon Ignicoccus hospitalis: the major cytosolic and membrane proteins. Arch. Microbiol. 190:379-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodemma, H. J., J. W. M. Derksen, and G. D. Vogels. 1979. Fimbriae and flagella of methanogenic bacteria. FEMS Microbiol. Lett. 5:135-138. [Google Scholar]
- 7.Fröls, S., A. Malgorzata, M. Wagner, D. Teichmann, B. Zolghadr, M. Folea, E. J. Boekema, A. J. M. Driessen, C. Schleper, and S.-V. Albers. 2008. UV-inducible cellular aggregation of the hyperthermophilic archaeon Sulfolobus solfataricus is mediated by pili formation. Mol. Microbiol. 70:938-952. [DOI] [PubMed] [Google Scholar]
- 8.Henneberger, R., C. Moissl, T. Amann, C. Rudolph, and R. Huber. 2006. New insights into the lifestyle of the cold-loving SM1 euryarchaeon: natural growth as a monospecies biofilm in the subsurface. Appl. Environ. Microbiol. 72:192-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horn, C., B. Paulmann, G. Kerlen, N. Junker, and H. Huber. 1999. In vivo observation of cell division of anaerobic hyperthermophiles by using a high-intensity dark-field microscope. J. Bacteriol. 181:5114-5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jahn, U., H. Huber, W. Eisenreich, M. Hügler, and G. Fuchs. 2007. Insights into the autotrophic CO2 fixation pathway of the archaeon Ignicoccus hospitalis: comprehensive analysis of the central carbon metabolism. J. Bacteriol. 189:4108-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 12.Mai, B. 1998. In vitro Untersuchungen zum extrazellulären Netzwerk von Pyrodictium abyssi TAG11. Ph.D. thesis. University of Regensburg, Regensburg, Germany.
- 13.Moissl, C., R. Rachel, A. Briegel, H. Engelhardt, and R. Huber. 2005. The unique structure of archaeal hami, highly complex cell appendages with nano-grappling hooks. Mol. Microbiol. 56:361-370. [DOI] [PubMed] [Google Scholar]
- 14.Näther, D. J., R. Rachel, G. Wanner, and R. Wirth. 2006. Flagella of Pyrococcus furiosus: multifunctional organelles, made for swimming, adhesion to various surfaces, and cell-cell contacts. J. Bacteriol. 188:6915-6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng, S. Y. M., B. Zolghadr, A. J. M. Driessen, S.-V. Albers, and K. F. Jarrell. 2008. Cell surface structures of archaea. J. Bacteriol. 190:6039-6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nickell, S., R. Hegerl, W. Baumeister, and R. Rachel. 2003. Pyrodictium cannulae enter the periplasmic space but do not enter the cytoplasm, as revealed by cryo-electron tomography. J. Struct. Biol. 141:32-42. [DOI] [PubMed] [Google Scholar]
- 17.Paper, W., U. Jahn, M. J. Hohn, M. Kronner, D. J. Näther, T. Burghardt, R. Rachel, K. O. Stetter, and H. Huber. 2007. Ignicoccus hospitalis sp. nov., the host of “Nanoarchaeum equitans.” Int. J. Syst. Evol. Bacteriol. 57:803-808. [DOI] [PubMed] [Google Scholar]
- 18.Podar, M., I. Anderson, K. S. Makarova, J. G. Elkins, N. Ivanova, M. A. Wall, A. Lykidis, K. Mavromatis, H. Sun, M. E. Hudson, W. Chen, C. Deciu, D. Hutchison, J. R. Eads, A. Anderson, F. Fernandes, E. Szeto, A. Lapidus, N. C. Kyrpides, M. H. Saier, Jr., P. M. Richardson, R. Rachel, H. Huber, J. A. Eisen, E. V. Koonin, M. Keller, and K. O. Stetter. 10 November 2008, posting date. A genomic analysis of the archaeal system Ignicoccus hospitalis-Nanoarchaeum equitans. Genome Biol. 9:R158. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rachel, R., I. Wyschkony, S. Riehl, and H. Huber. 2002. The ultrastructure of Ignicoccus: evidence for a novel outer membrane and for intracellular vesicle budding in an archaeon. Archaea 1:9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schopf, S., G. Wanner, R. Rachel, and R. Wirth. 2008. An archaeal bi-species biofilm formed by Pyrococcus furiosus and Methanopyrus kandleri. Arch. Microbiol. 190:371-377. [DOI] [PubMed] [Google Scholar]
- 21.Stetter, K. O., R. Huber, E. Blöchl, M. Kurr, R. D. Eden, M. Fiedler, H. Cash, and I. Vance. 1983. Hyperthermophilic archaea are thriving in deep North Sea and Alaskan oil reservoirs. Nature 365:743-745. [Google Scholar]
- 22.Thoma, C., M. Frank, R. Rachel, S. Schmid, D. Näther, G. Wanner, and R. Wirth. 2008. The Mth60 fimbriae of Methanothermobacter thermoautotrophicus are functional adhesins. Environ. Microbiol. 10:2785-2795. [DOI] [PubMed] [Google Scholar]
- 23.Thomas, N. A., S. L. Bardy, and K. F. Jarrell. 2001. The archaeal flagellum: a different kind of prokaryotic motility structure. FEMS Microbiol. Rev. 25:147-174. [DOI] [PubMed] [Google Scholar]
- 24.Zacharius, R. M., T. Zell, J. H. Morrison, and J. J. Woodlock. 1969. Glycoprotein staining following electrophoresis on acrylamide gels. Anal. Biochem. 172:320-329. [DOI] [PubMed] [Google Scholar]