Abstract
Recently, a novel type of secretory pathway, type VII secretion systems (T7SSs), has been characterized in mycobacteria. The chromosomes of Mycobacterium tuberculosis and Mycobacterium bovis encode five T7SSs (ESX-1 to ESX-5). The best characterized of them, ESX-1, is involved in host-pathogen interactions, and its deletion is one of the main causes of M. bovis BCG attenuation. Another T7SS, ESX-3, has been previously shown to be transcriptionally controlled by the zinc uptake repressor (Zur) and by the iron-dependent transcriptional repressor (IdeR), suggesting that it might be involved in zinc and iron homeostasis. In this study, we characterized an M. tuberculosis conditional mutant in which transcription of the ESX-3 gene cluster can be downregulated by anhydrotetracycline. We showed that this T7SS is essential for growth and that this phenotype can be complemented by zinc, iron, or supernatant from a wild-type parental strain culture, demonstrating that the ESX-3 secretion system is responsible for the secretion of some soluble factor(s) required for growth that is probably involved in optimal iron and zinc uptake.
The Mycobacterium tuberculosis chromosome encodes several protein secretion pathways. The Sec and Sec2 systems are responsible for the secretion of proteins containing typical N-terminal signal sequences, while the two-arginine translocation (TAT) system is responsible for the secretion of a subset of proteins containing a peculiar N-terminal signal sequence characterized by a couple of arginine residues (23). Recently, a novel type of secretory pathway, type VII secretion systems (T7SSs), has been characterized in mycobacteria (1). The chromosome of M. tuberculosis encodes five T7SSs (ESX-1 to ESX-5) (15). The best-characterized T7SS is ESX-1; its secretion machinery includes at least seven proteins and is responsible for the secretion of a heterodimer formed from two small proteins of the WXG-100 family (ESAT-6 and CFP-10) (6). The initial observation suggesting ESX-1 involvement in host-pathogen interactions was that both Mycobacterium bovis BCG and Mycobacterium microti were natural mutants lacking a functional ESX-1 (20). Moreover, complementation of M. bovis BCG with a functional ESX-1 partially restored virulence (21). Since then, several studies have focused on the role of this secretion system in host-pathogen interactions in M. tuberculosis and Mycobacterium marinum, characterizing its involvement in suppression of the proinflammatory response, phagosome evasion, and cell-to cell migration (5, 9, 14, 16, 28). In the nonpathogenic bacterium Mycobacterium smegmatis, however, ESX-1 was shown to be involved in conjugation (8).
Even if the mechanism at the base of these phenotypes remains elusive, it is worth mentioning that the ESAT-6/CFP-10 dimers are able to dissociate under acidic conditions and that once dissociated from CFP-10, ESAT-6 exhibits membrane-lysing activity (10).
The only other T7SS for which information are available is ESX-5, which is present only in slow-growing mycobacteria (1). This was characterized in M. marinum, where it was shown to be essential for hemolysis and virulence (2). Interestingly, ESX-5 was also shown to be essential for PPE and PE_PGRS secretion (3, 4). PPE and PE represent two large families of exported proteins of unknown function that distinguish mycobacteria and lack a typical signal sequence (7, 15).
No information is available on ESX-2, -3, or -4 function, even though ESX-3 was predicted to be essential for growth (27). Interestingly, the latter is conserved in all of the mycobacterial genomes whose complete sequences are available (data not shown), suggesting that it has an important physiological role. It was recently shown that in M. tuberculosis the expression of the ESX-3 gene cluster is regulated by the zinc uptake repressor (Zur) (18) and by the iron-dependent transcriptional repressor (IdeR) (26), while in M. smegmatis its transcription is only regulated by IdeR (19), suggesting that this T7SS might be involved in zinc and/or iron homeostasis.
Almost nothing is known regarding zinc uptake in M. tuberculosis; however, iron uptake is well characterized (25). M. tuberculosis produces two siderophores: the water-soluble carboxymycobactin and the water-insoluble mycobactin. While their biosynthetic pathways are well known, the mechanism of their translocation through the mycobacterial envelope remains partially uncharacterized (22).
In this work, we constructed an M. tuberculosis conditional mutant in which expression of the ESX-3 gene cluster was placed under the control of a repressible promoter system recently developed in our laboratory, showing that its expression is required for growth in Middlebrook 7H9 and 7H10 media and that this phenotype can be complemented by the addition of iron, zinc, or conditioned supernatant.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
All experiments were performed with M. tuberculosis H37Rv. Bacteria were grown in either liquid Middlebrook 7H9 medium or solid Middlebrook 7H10 medium (Difco) supplemented with 10% albumin-dextrose-sodium chloride complex (17), 0.2% glycerol, and 0.05% Tween 80 (Sigma) at 37°C. ZnSO4 and ferric ammonium citrate were added at concentrations of 30 and 300 μM, respectively. Plates were incubated at 37°C in sealed plastic bags. Culture supernatants were filtered with low-protein-binding polyvinylidene difluoride filters (0.22 μm, Millex-GV; Millipore) and, when required, heated in a heat block at 100°C for 10 min.
Escherichia coli strains DH5α and HB101 were grown in Luria broth (Difco) at 37°C. When required, antibiotics were added at the following concentrations: kanamycin, 50 μg ml−1; hygromycin, 150 μg ml−1 (E. coli); streptomycin, 20 μg ml−1; hygromycin, 100 μg ml−1 (M. tuberculosis).
DNA manipulations.
Recombinant DNA techniques were performed according to standard procedures with E. coli HB101 as the initial host. DNA restriction and modifying enzymes were obtained from New England BioLabs and used according to the manufacturer's suggestion.
Electroporation of M. tuberculosis.
Preparation of electrocompetent cells and electroporation was performed as previously described (18).
Construction of M. tuberculosis ESX-3 conditional mutant.
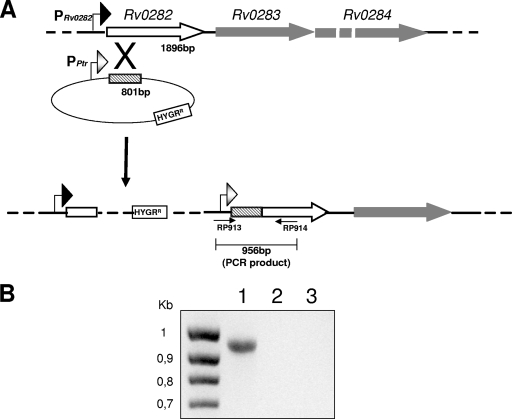
The 807-bp NsiI/PvuII fragment containing the first 801 bp of the Rv0282 open reading frame was amplified by PCR from H37Rv DNA and cloned in frame with Pptr (a Streptomyces pristinaespiralis promoter regulated by the transcriptional repressor Pip) into the NsiI/PvuII sites of suicide vector pFRA50 (F. Boldrin et al., unpublished data) to obtain pAGN8. The sequences of the primers used for amplification are the following: upper (RP860), 5′ ATG CAT GCG GGC GTA GGT GAA GGA 3′; lower (RP877), 5′ CAG CTG CGA CTC CTC ATC CAC ATG 3′. pAGN8 was electroporated into M. tuberculosis TB38, an H37Rv derivative carrying the genes encoding the tetracycline repressor TetR (12) and the S. pristinaespiralis Pip repressor integrated at the L5 attB site (Boldrin et al., unpublished data). Since in this strain the gene encoding Pip is under TetR transcriptional control, by adding anhydrotetracycline (ATc; a tetracycline derivative less toxic for M. tuberculosis than tetracycline), the gene encoding Pip is induced, leading to stringent transcriptional repression of the gene downstream of Pptr (Boldrin et al., unpublished data). Transformants were selected on hygromycin and analyzed by PCR. The amplification of a 956-bp fragment by utilizing a primer inside Pptr (5′ CAG CGT ATG GGA ATC TCT TG 3′, RP913 upper) and a second primer external to the homology region used for recombination (5′ GAC GAT CCC GAA GCT GGT AT 3′, RP914 lower) confirmed the exact integration of the suicide vector in the Rv0282 gene (Fig. 1).
FIG. 1.
Construction of the ESX-3 conditional mutant. (A) Schematic representation of the recombination event used to achieve the conditional mutant TB79 (see the text for details). (B) PCR analysis of total DNA isolated from conditional mutant strain TB79 and parental strain TB38. The primers used to verify the exact gene structure are indicated; RP913 is located within the Pptr promoter, and RP914 is located within the coding region and external to the homology region used for recombination. Unnumbered lane, molecular size standard; lane 1, TB79; lane 2, TB38; lane 3, no-DNA control.
RESULTS AND DISCUSSION
Construction of an ESX-3 conditional mutant.
The M. tuberculosis ESX-3 gene cluster includes 11 genes and is principally transcribed from two promoters (under Zur and IdeR transcriptional control, respectively) placed upstream of Rv0282 (18, 26). To construct a conditional mutant, we replaced these two promoters with Pptr, an S. pristinaespiralis repressible promoter regulated by the transcriptional regulator Pip (13). For this purpose, the 801-bp 5′ portion of Rv0282 was cloned in frame with Pptr into a suicide plasmid and electroporated into TB38, an M. tuberculosis H37Rv-derived strain with the genes encoding the tetracycline repressor TetR and Pip integrated at the L5 attB site (Boldrin et al. unpublished data). Since in this strain pip was placed under TetR transcriptional repression, addition of ATc to the medium induces Pip expression, repressing the activity of Pptr (Boldrin et al. unpublished data). Recombinant strains were selected and analyzed by PCR to demonstrate the correct integration of the plasmid (Fig. 1). One strain with the correct chromosomal structure (TB79) was isolated and conserved for further studies.
ESX-3 is essential for growth.
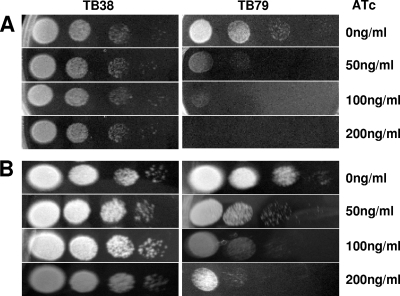
To determine if ESX-3 is essential for growth, different dilutions of TB38 and ESX-3 conditional mutant derivative TB79 were spotted onto Middlebrook 7H10 medium plates with or without different concentrations of ATc. As shown in Fig. 2, while the presence of ATc did not affect TB38 growth, the conditional mutant TB79 grew at a rate inversely proportional to the ATc concentration. After 15 days of incubation, no growth was observed at an ATc concentration of 200 ng/ml, while some growth was detectable in plates with lower ATc concentrations; however, after 21 days of incubation, some growth was detectable also on the plates with the highest ATc concentration. Taken together, these data demonstrate that ESX-3 expression is required for optimal growth of M. tuberculosis in Middlebrook 7H10 medium.
FIG. 2.
Effect of Rv0282 repression on growth. Cells were grown to exponential phase, and 5-μl volumes of 10-fold serial dilutions were spotted onto Middlebrook 7H10 medium supplemented with ATc at the indicated concentrations. Plates were incubated at 37°C. Pictures were taken after 15 (A) and 21 (B) days.
The growth of the conditional mutant can be rescued by iron and zinc.
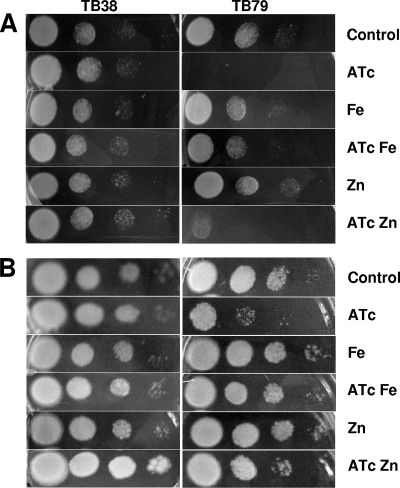
The previous finding that the M. tuberculosis ESX-3 cluster is regulated by IdeR and Zur (18, 26) suggests its involvement in the uptake of these two essential elements. To verify this hypothesis, different dilutions of the two strains were spotted onto Middlebrook 7H10 medium plates (with or without ATc at 200 ng/ml) supplemented with different iron (ferric ammonium citrate) or zinc (ZnSO4) concentrations. As shown in Fig. 3, plates with a final iron concentration of 450 μM (versus the 150 μM present in normal Middlebrook 7H10 medium) or a final zinc concentration 36 μM (versus the 6 μM present in normal Middlebrook 7H10 medium) supported the growth of the conditional mutant even when the presence of ATc turned off ESX-3 expression. The effect of iron supplementation was clearly visible after 15 days of incubation, while that of zinc addition was clear after 21 days of incubation.
FIG. 3.
Rescue of strain TB79 growth by iron and zinc on solid medium. Cells were grown to exponential phase, and 5-μl volumes of 10-fold serial dilutions were spotted onto Middlebrook 7H10 medium supplemented with ATc, Zn, or Fe. Plates were incubated at 37°C. Pictures were taken after 15 (A) and 21 (B) days.
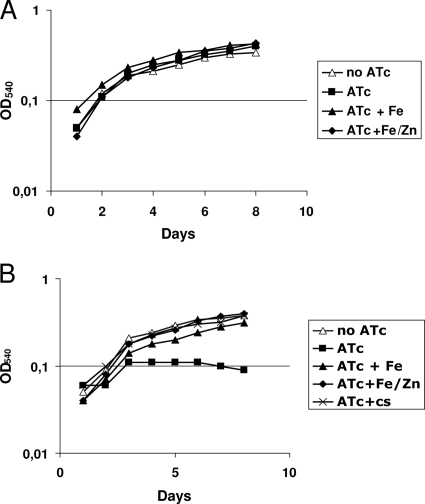
A similar experiment was performed with liquid medium, and also in this case, the addition of iron alone was able to complement the growth phenotype, but when both iron and zinc were added, complementation was more efficient (Fig. 4). In order to detect the TB79 growth phenotype, liquid cultures needed to be pregrown for about three generations in the presence of ATc before being diluted 1:10 in fresh medium (with ATc), suggesting that ESX-3 components needed to be titrated down and/or that some time was required to use the iron and Zn stored in the cells.
FIG. 4.
Rescue of strain TB79 growth by iron, zinc, or conditioned supernatant (cs). Cells were grown standing in 3 ml of Middlebrook 7H9 medium; optical density (OD) at 540 nm was measured for 6 days. When indicated, ATc (200 ng/ml), Fe (300 μM), Zn (20 μM), or cs was added to the cultures. Panels: A, TB38 (parental strain); B, TB79 (conditional mutant strain).
Taken together, these data suggest that ESX-3 could be involved in the optimal uptake of both of these essential metals. Interestingly, the amounts of iron and zinc present in Middlebrook 7H9 or 7H10 medium are not considered limiting. Indeed, M. tuberculosis mutants that do not produce siderophores or are unable to transport them inside the cytoplasm are still able to grow in 7H9 medium (11, 24). Moreover, during growth in this medium, the iron and zinc uptake regulons (regulated by IdeR and Zur) are expressed at their basal level but are not induced (18, 26). The fact that ESX-3, but not the mycobactin/carboxymycobactin system, is essential for growth in Middlebrook 7H9 or 7H10 medium suggests that this T7SS encodes (or is essential for the function of) a novel iron/zinc uptake system or has a strong effect on mycobacterial cell surface permeability to these metals.
Effect of conditioned medium.
The ESX-3 secretion system could participate in iron and zinc uptake either by allowing the secretion of required molecules or by modifying the M. tuberculosis cell surface composition, increasing its permeability to these metals. To discriminate between these two hypotheses, we designed an experiment to determine if conditioned medium could complement the growth defect of TB79 in the presence of ATc. For this purpose, the pellet of a 3-ml culture of the TB79 mutant was resuspended in the same volume of filtered supernatant from a culture of parental strain TB38 containing ATc at 200 ng/ml. As shown in Fig. 4, the conditioned supernatant was able to complement the growth defect of TB79. Preliminary experiments with boiled supernatants suggested that the soluble factor(s) involved in the complementation of the phenotype is not heat sensitive (data not shown).
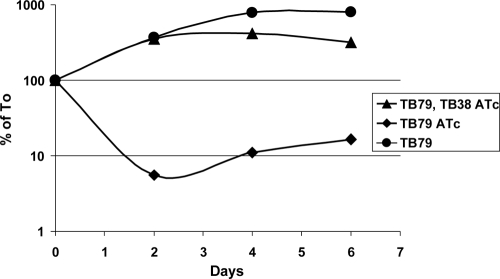
In a similar experiment, we inoculated equal amounts of TB79 and parental strain TB38 into the same tube containing Middlebrook 7H9 medium with added ATc at 200 ng/ml. TB79 viable counts were monitored for 1 week by plating triplicate serial dilutions on plates containing hygromycin (in order to counterselect against TB38) and compared to those obtained from two pure TB79 cultures incubated with or without ATc. As shown in Fig. 5, the TB79 viable counts in the presence of ATc decreased drastically (more than 10-fold) during the first 2 days and then stabilized. However, this effect was totally eliminated in the presence of parental strain TB38 in the same culture. These data confirm that the ESX-3 cluster is essential and suggest that the residual growth shown in Fig. 2 and 3 at 21 days was due either to an inoculum effect (some division cycles are needed to titrate down ESX-3 components and/or to use the iron and zinc stored in the cell) or to the growth of a few survivors after the effect of ATc is reduced by natural decay.
FIG. 5.
Conditional mutant strain TB79 viable counts after incubation in Middlebrook 7H9 medium without ATc, with 200 ng/ml ATc, or with 200 ng/ml ATc and an equal amount of parental strain TB38. Data were obtained from a representative experiment by calculating the weighted average of triplicate serial dilutions and shown as a percentage of the time zero (T0) value.
Taken together, these experiments strongly suggest that the ESX-3 secretion system is responsible for the secretion of some soluble factor(s) that is required for growth and that is probably involved in optimal iron and zinc uptake.
Since ATc can be administrated to mice or cell cultures (12), it will be interesting in the future to analyze the role of the ESX-3 gene cluster during experimental infection.
Conclusions.
We constructed a conditional mutant of M. tuberculosis in which the expression of the ESX-3 gene cluster was repressed upon the addition of ATc. We showed that ESX-3 is required for optimal growth of M. tuberculosis in Middlebrook 7H9 and 7H10 media and that this phenotype can be complemented by zinc, iron, or supernatant from the parental strain. Taken together, our data suggest that ESX-3 is responsible for the secretion of some still unrecognized factor(s) required for the optimal uptake of iron and zinc.
The finding that ESX-3 is conserved in Mycobacterium leprae, which is unable to produce any siderophore and thus must rely on alternative iron uptake mechanisms (22), suggests that ESX-3 could be the main factor responsible for iron uptake by this microorganism.
We can conclude that ESX-3 encodes (or is essential for the correct functioning of) a still unrecognized novel metal uptake (or surface permeabilization) system which can either complement both the mycobactin/carboxymycobactin system and the zinc transporters (as in M. tuberculosis) or even be the main iron uptake system used by the microorganism (as in M. leprae).
Acknowledgments
We thank G. Marcela Rodriguez, Giovanni Delogu, Roberta Provvedi, Paolo Visca, and Roland Brosch for useful discussion.
This research received funding from the European Community Seventh Framework Program (FP7/2007-2013) under grant agreement 201762.
Footnotes
Published ahead of print on 14 August 2009.
REFERENCES
- 1.Abdallah, A. M., N. C. Gey van Pittius, P. A. Champion, J. Cox, J. Luirink, C. M. Vandenbroucke-Grauls, B. J. Appelmelk, and W. Bitter. 2007. Type VII secretion—mycobacteria show the way. Nat. Rev. Microbiol. 5:883-891. [DOI] [PubMed] [Google Scholar]
- 2.Abdallah, A. M., N. D. Savage, M. van Zon, L. Wilson, C. M. Vandenbroucke-Grauls, N. N. van der Wel, T. H. Ottenhoff, and W. Bitter. 2008. The ESX-5 secretion system of Mycobacterium marinum modulates the macrophage response. J. Immunol. 181:7166-7175. [DOI] [PubMed] [Google Scholar]
- 3.Abdallah, A. M., T. Verboom, F. Hannes, M. Safi, M. Strong, D. Eisenberg, R. J. Musters, C. M. Vandenbroucke-Grauls, B. J. Appelmelk, J. Luirink, and W. Bitter. 2006. A specific secretion system mediates PPE41 transport in pathogenic mycobacteria. Mol. Microbiol. 62:667-679. [DOI] [PubMed] [Google Scholar]
- 4.Abdallah, A. M., T. Verboom, E. M. Weerdenburg, N. C. van Pittius, P. W. Mahasha, C. Jimenez, M. Parra, N. Cadieux, M. J. Brennan, B. J. Appelmelk, and W. Bitter. 2009. PPE and PE_PGRS proteins of Mycobacterium marinum are transported via the type VII secretion system ESX-5. Mol. Microbiol. 73:329-340. [DOI] [PubMed] [Google Scholar]
- 5.Bottai, D., and R. Brosch. 2009. Mycobacterial PE, PPE and ESX clusters: novel insights into the secretion of these most unusual protein families. Mol. Microbiol. 73:325-328. [DOI] [PubMed] [Google Scholar]
- 6.Brodin, P., L. Majlessi, L. Marsollier, M. I. de Jonge, D. Bottai, C. Demangel, J. Hinds, O. Neyrolles, P. D. Butcher, C. Leclerc, S. T. Cole, and R. Brosch. 2006. Dissection of ESAT-6 system 1 of Mycobacterium tuberculosis and impact on immunogenicity and virulence. Infect. Immun. 74:88-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cascioferro, A., G. Delogu, M. Colone, M. Sali, A. Stringaro, G. Arancia, G. Fadda, G. Palu, and R. Manganelli. 2007. PE is a functional domain responsible for protein translocation and localization on mycobacterial cell wall. Mol. Microbiol. 66:1536-1547. [DOI] [PubMed] [Google Scholar]
- 8.Coros, A., B. Callahan, E. Battaglioli, and K. M. Derbyshire. 2008. The specialized secretory apparatus ESX-1 is essential for DNA transfer in Mycobacterium smegmatis. Mol. Microbiol. 69:794-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis, J. M., and L. Ramakrishnan. 2009. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136:37-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Jonge, M. I., G. Pehau-Arnaudet, M. M. Fretz, F. Romain, D. Bottai, P. Brodin, N. Honore, G. Marchal, W. Jiskoot, P. England, S. T. Cole, and R. Brosch. 2007. ESAT-6 from Mycobacterium tuberculosis dissociates from its putative chaperone CFP-10 under acidic conditions and exhibits membrane-lysing activity. J. Bacteriol. 189:6028-6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Voss, J. J., K. Rutter, B. G. Schroeder, H. Su, Y. Zhu, and C. E. Barry III. 2000. The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. Proc. Natl. Acad. Sci. USA 97:1252-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrt, S., X. V. Guo, C. M. Hickey, M. Ryou, M. Monteleone, L. W. Riley, and D. Schnappinger. 2005. Controlling gene expression in mycobacteria with anhydrotetracycline and Tet repressor. Nucleic Acids Res. 33:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folcher, M., R. P. Morris, G. Dale, K. Salah-Bey-Hocini, P. H. Viollier, and C. J. Thompson. 2001. A transcriptional regulator of a pristinamycin resistance gene in Streptomyces coelicolor. J. Biol. Chem. 276:1479-1485. [DOI] [PubMed] [Google Scholar]
- 14.Gao, L. Y., S. Guo, B. McLaughlin, H. Morisaki, J. N. Engel, and E. J. Brown. 2004. A mycobacterial virulence gene cluster extending RD1 is required for cytolysis, bacterial spreading and ESAT-6 secretion. Mol. Microbiol. 53:1677-1693. [DOI] [PubMed] [Google Scholar]
- 15.Gey van Pittius, N. C., S. L. Sampson, H. Lee, Y. Kim, P. D. van Helden, and R. M. Warren. 2006. Evolution and expansion of the Mycobacterium tuberculosis PE and PPE multigene families and their association with the duplication of the ESAT-6 (esx) gene cluster regions. BMC Evol. Biol. 6:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagedorn, M., K. H. Rohde, D. G. Russell, and T. Soldati. 2009. Infection by tubercular mycobacteria is spread by nonlytic ejection from their amoeba hosts. Science 323:1729-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs, W. R., Jr., G. V. Kalpana, J. D. Cirillo, L. Pascopella, S. B. Snapper, R. A. Udani, W. Jones, R. G. Barletta, and B. R. Bloom. 1991. Genetic systems for mycobacteria. Methods Enzymol. 204:537-555. [DOI] [PubMed] [Google Scholar]
- 18.Maciag, A., E. Dainese, G. M. Rodriguez, A. Milano, R. Provvedi, M. R. Pasca, I. Smith, G. Palu, G. Riccardi, and R. Manganelli. 2007. Global analysis of the Mycobacterium tuberculosis Zur (FurB) regulon. J. Bacteriol. 189:730-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maciag, A., A. Piazza, G. Riccardi, and A. Milano. 2009. Transcriptional analysis of ESAT-6 cluster 3 in Mycobacterium smegmatis. BMC Microbiol. 9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pym, A. S., P. Brodin, R. Brosch, M. Huerre, and S. T. Cole. 2002. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol. Microbiol. 46:709-717. [DOI] [PubMed] [Google Scholar]
- 21.Pym, A. S., P. Brodin, L. Majlessi, R. Brosch, C. Demangel, A. Williams, K. E. Griffiths, G. Marchal, C. Leclerc, and S. T. Cole. 2003. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat. Med. 9:533-539. [DOI] [PubMed] [Google Scholar]
- 22.Quadri, L. E. 2008. Iron uptake in mycobacteria, p. 167-184. In M. Daffe and J. M. Reyrat (ed.), The mycobacterial cell envelope. ASM Press, Washington, DC.
- 23.Rigel, N. W., and M. Braunstein. 2008. A new twist on an old pathway—accessory secretion systems. Mol. Microbiol. 69:291-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez, G. M., and I. Smith. 2006. Identification of an ABC transporter required for iron acquisition and virulence in Mycobacterium tuberculosis. J. Bacteriol. 188:424-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez, G. M., and I. Smith. 2003. Mechanisms of iron regulation in mycobacteria: role in physiology and virulence. Mol. Microbiol. 47:1485-1494. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez, G. M., M. I. Voskuil, B. Gold, G. K. Schoolnik, and I. Smith. 2002. ideR, an essential gene in Mycobacterium tuberculosis: role of IdeR in iron-dependent gene expression, iron metabolism, and oxidative stress response. Infect. Immun. 70:3371-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sassetti, C. M., D. H. Boyd, and E. J. Rubin. 2003. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48:77-84. [DOI] [PubMed] [Google Scholar]
- 28.Tan, T., W. L. Lee, D. C. Alexander, S. Grinstein, and J. Liu. 2006. The ESAT-6/CFP-10 secretion system of Mycobacterium marinum modulates phagosome maturation. Cell. Microbiol. 8:1417-1429. [DOI] [PubMed] [Google Scholar]