Abstract
The pyrene-degrading Mycobacterium sp. strain AP1 grew in nutrient-supplemented artificial seawater with a heavy fuel oil as the sole carbon source, causing the complete removal of all linear (C12 to C40) and branched alkanes from the aliphatic fraction, as well as an extensive degradation of the three- and four-ring polycyclic aromatic hydrocarbons (PAHs) phenanthrene (95%), anthracene (80%), fluoranthene (80%), pyrene (75%), and benzo(a)anthracene (30%). Alkylated PAHs, which are more abundant in crude oils than the nonsubstituted compounds, were selectively attacked at extents that varied from more than 90% for dimethylnaphthalenes, methylphenanthrenes, methylfluorenes, and methyldibenzothiophenes to about 30% for monomethylated fluoranthenes/pyrenes and trimethylated phenanthrenes and dibenzothiophenes. Identification of key metabolites indicated the utilization of phenanthrene, pyrene, and fluoranthene by known assimilatory metabolic routes, while other components were cooxidized. Detection of mono- and dimethylated phthalic acids demonstrated ring cleavage and further oxidation of alkyl PAHs. The extensive degradation of the alkanes, the two-, three-, and four-ring PAHs, and their 1-, 2-, and 3-methyl derivatives from a complex mixture of hydrocarbons by Mycobacterium sp. strain AP1 illustrates the great substrate versatility of alkane- and PAH-degrading mycobacteria.
Accidental oil spills cause extensive ecological damage to marine shorelines and also have an enormous impact on related economic activities due to the potential risk to public health. One of the most recent examples is the heavy fuel oil spill from the tanker Prestige in 2002, which affected 1,900 km of coast in northwestern Spain. While the light fractions of the oil evaporate in the early stages of a spill, microbial degradation plays a major role in the removal of the heavier fractions. Stimulation of natural biodegradation processes by nutrient and fertilizer addition has proven to enhance oil degradation in a variety of coastal environments (3, 42, 44).
Oil is a complex mixture of hundreds of components that can be separated into saturates, aromatics, resins, and asphaltenes. The saturated hydrocarbons are usually the most abundant, while polycyclic aromatic hydrocarbons (PAHs) cause the greatest concern because of their toxic and genotoxic potentials.
Most of the available knowledge on the microbial processes involved in PAH biodegradation has been obtained from studies involving bacterial isolates acting on single substrates that serve as the sole source of carbon and energy for growth (7, 20, 22). The pathways for the complete degradation of hydrocarbons containing two and three aromatic rings by gram-negative bacteria are well characterized for such conditions (7, 22). Conversely, degradation of hydrocarbons containing four or more fused aromatic rings, such as pyrene, has been reported only for soil actinomycetes (20, 25, 29, 30, 36, 45), which use multibranched pathways involving both classical dioxygenation and meta-cleavage reactions and novel ortho-cleavage mechanisms uncommon in gram-negative organisms (23). Due to the relaxed specificity of some degradative enzymes, mainly dioxygenases (15, 37), PAH-degrading strains have a wide range of substrates, being able to act simultaneously on a number of structural analogs and to oxidize them to different extents (18, 37). However, the individual processes involved in the degradation of naturally occurring complex mixtures of PAHs (crude oils and coal derivatives) have rarely been addressed (18, 31).
Early studies on biodegradation of crude oil were carried out with bacterial strains able to use this mixture for growth. Since PAHs and other components are contained within a predominantly aliphatic matrix in crude oil, most of these studies reported actions of alkane degraders on individual oil components (2, 34, 38, 41, 50). In addition to alkanes, these alkane degraders selectively depleted some alkylated PAHs (2, 41), a process that has been attributed to partial oxidation due to a monooxygenase attack on the methyl groups to produce the corresponding carboxylic acids (35). Recent studies reported the isolation of a number of two- and three-ring-PAH-degrading bacterial strains from coastal sediments affected by crude oil spills. These strains include members of genera commonly isolated from PAH-contaminated soils, such as Pseudomonas (39, 43) and Sphingomonas (49), as well as less common genera, such as Marinobacter (13), Moraxella (43), Vibrio (51), and Cycloclasticus (12). The last genus seems to play a major role in the fate of low-molecular-weight PAHs in the marine environment, as members of this genus have been isolated from several crude oil-contaminated locations (6, 14, 21). When incubated with crude oil, Cycloclasticus strains degraded most of the two- and three-ring PAHs and some of their alkyl derivatives (C0-4 naphthalene, C0-2 dibenzothiophene, C0-2 phenanthrene, and C0-2 fluorene [numerals indicate the number of methyl groups]). However, neither alkanes, trimethyl derivatives of three-ring PAHs, or higher-molecular-weight PAHs were significantly depleted (21). On the other hand, no attempts were made to identify metabolic intermediates indicative of specific degradation or cometabolic pathways.
Alkyl-PAH degradation is isomer specific, a feature that has been used in geochemistry to define source recognition and oil weathering ratios (47). For example, given the resistance of 9-methyl phenanthrene to microbial oxidation in relation to the other isomers, the ratio of 3-methylphenanthrene plus 2-methylphenanthrene to 9-methylphenanthrene plus 1-methylphenanthrene has been utilized as a diagnostic ratio (47). These ratios have been defined on the basis of analysis of environmental samples (47) and results of crude oil biodegradation assays with mixed cultures (10, 48) or single strains (2, 41), mainly alkane-degrading pseudomonads. The actions of high-molecular-weight-PAH-degrading mycobacteria on the alkylated families of PAHs present in crude oil and derivatives have not been addressed.
Mycobacterium strains isolated by their ability to grow on pyrene have often been shown to also utilize phenanthrene, fluoranthene, and high-molecular-weight alkanes as single carbon sources (8, 45). In a recent study, we showed that when Mycobacterium strain AP1, isolated from an oil-polluted marine beach, was incubated with a mixture of PAHs from creosote, this strain caused a significant depletion of the three-aromatic-ring PAHs but had a limited action on the higher-molecular-weight PAHs fluoranthene and pyrene (31). Given the wide substrate versatility of pyrene-degrading mycobacteria, especially for alkane degradation, their presence in marine environments (16), and their distinctive reactions during PAH degradation (22, 25, 30), in this study we used strain AP1 to investigate the catabolic potential of mycobacteria in the removal of the most abundant hydrocarbon families and their derivatives from crude oil in a marine medium under laboratory conditions. The identification of key metabolites indicative of previously proposed reactions gave insight into the metabolic and cometabolic processes involved. As a model mixture, we used the heavy fuel oil spilled from the Prestige, a Russian M100 fuel oil especially rich in aromatic hydrocarbons (52%) (27).
MATERIALS AND METHODS
Chemicals.
Pyrene and oxygenated PAHs used for the identification of metabolites were obtained from Sigma-Aldrich, Milwaukee, WI. The 17α(H),21β(H)-hopane used was purchased from Chiron AS (Trondheim, Norway), while the 16-PAH standard solution used for gas chromatographic (GC) quantification (PAH-mix 9) was purchased from Dr. Ehrenstorfer GmbH (Ausburg, Germany). Diazomethane was generated by alkaline decomposition of Diazald (N-methyl-N-nitroso-p-toluenesulfonamide), using a Diazald kit with Clear-Seal joints from Sigma-Aldrich. Solvents were obtained from J. T. Baker, Deventer, The Netherlands.
Culture medium and supply of hydrocarbons.
The composition of the artificial seawater used as a basal medium was as follows (per liter): NaCl, 24.53 g; Na2SO4, 4.1 g; MgCl2, 11.2 g; CaCl2, 1.16 g; SrCl2, 0.042 g; KCl, 0.7 g; NaHCO3, 0.2 g; KBr, 0.1 g; H3BO3, 0.0029 g; and NaF, 0.003 g. Prior to inoculation, the sterile seawater was supplemented with 1% of the following sterile solutions: nitrogen (1 M NH4NO3), phosphorus (0.06 M K2HPO4), and metals (with the following amounts per liter in the concentrated solution: MgSO4·7H2O, 20 g; FeSO4·7H2O, 1.2 g; MnSO4·H2O, 0.3 g; ZnSO4·7H2O, 0.3 g; CoCl2·6H2O, 0.1 g; and nitrilotriacetic acid disodium salt [Sigma-Aldrich Chemie, Steinheim, Germany], 12.3 g).
The fuel used in this work was obtained from a water-in-oil emulsion (40% water content [wt/wt]) collected directly from the sea after the Prestige incident. The chemical composition of this fuel oil was 22.9% saturated hydrocarbons, 52.7% aromatic hydrocarbons, 12% resins, and 12.4% asphaltenes (27). Prior to use as a substrate for biodegradation studies, the emulsion was sterilized by autoclave, the water phase decanted, and the fuel dissolved in methylene chloride (1:2 [wt/vol]). This solution (750 μl) was added to empty sterile flasks, the solvent was allowed to evaporate at room temperature for 1 day, and the sterile supplemented seawater medium (50 ml) was then added. The final concentration of fuel oil in the culture medium was 5 g per liter.
Utilization of pyrene as sole carbon source in marine medium.
Growth of Mycobacterium sp. strain AP1 (CECT 7328) at the expense of pyrene in marine medium was verified by demonstrating an increase in bacterial protein concentration concomitant with a decrease in pyrene concentration in cultures established in supplemented artificial seawater with pyrene (0.2 g/liter), as described elsewhere (45). The efficiency of cellular assimilation of pyrene carbon was determined by assuming that in bacterial cells proteins account for about 50% of cell dry weight and 50% of cell dry weight is carbon (17).
Time course of biodegradation of fuel oil.
Several colonies of strain AP1 grown on LB plates for 5 days were suspended in artificial seawater (A650, 0.7), and the suspension was used to inoculate (1 ml) triplicate 250-ml Erlenmeyer flasks containing 50 ml of supplemented artificial seawater and fuel oil (5 g/liter). Sterile, noninoculated flasks were used as controls. Cultures were incubated at 26°C under fully aerobic conditions (rotary shaking at 200 rpm). At 0, 15, 30, and 60 days, the entire flask contents of triplicate cultures and controls were extracted five times with 20 ml of dichloromethane, acidified to pH 2, and extracted in the same manner, using ethyl acetate as a solvent. Neutral and acidic extracts were dried using Na2SO4 and concentrated under vacuum to a final volume of 1 ml for further analysis. Growth of strain AP1 throughout the incubation time was determined in separate replicate cultures by plate counting on LB agar.
Chemical analysis of residual fuel oil.
Total petroleum hydrocarbons (TPHs) present in the residual fuel oil recovered from cultures were obtained by column chromatography of the neutral organic extracts, using USEPA method 3611b. In brief, one-half of the neutral extract (0.5 ml) was concentrated to dryness under vacuum, weighed, redissolved in n-hexane, and applied to an SPE glass column (J. T. Baker, Deventer, The Netherlands) packed with 2.5 g of alumina (Merck, Darmstad, Germany) which was previously activated at 120°C. The saturated hydrocarbons were eluted with n-hexane (4 ml), the aromatics with dichloromethane (25 ml), and the resins with methanol (25 ml). All of the fractions were concentrated to dryness under vacuum. Prior to GC-flame ionization detection (GC-FID) and GC-mass spectrometry (GC-MS) analyses, the saturated and aromatic fractions were redissolved in dichloromethane (1 ml), and 0.1 ml of each fraction was combined to obtain the TPHs. Prior to analysis, o-terphenyl (0.01 g/liter) (Sigma-Aldrich) was added as an internal standard. GC-FID analyses were performed using a Trace GC2000 (Thermo) gas chromatograph equipped with a flame ionization detector. GC-MS analyses were performed in a Hewlett Packard HP5890 series II gas chromatograph coupled to an HP5989 mass spectrometer operated at 70 eV. For both GC-FID and GC-MS analyses, the gas chromatographs were equipped with a DB5 (J&W Scientific, Folsom, CA) capillary column (30 m × 0.25-mm internal diameter) with a 0.25-μm film thickness. The column temperature was held at 50°C for 1 min and then programmed to increase to 320°C at 5°C/min. This final temperature was held for 10 min. Injector, transfer line, and detector temperatures were set at 290, 290, and 320°C, respectively. The samples (1 μl) were injected in the splitless mode, using helium as the carrier gas, at a flow rate of 1.1 ml/min.
To detect the 16 PAHs included in the USEPA list of priority pollutants, the MS detector was operated in the selected ion monitoring mode. In addition to the 16 PAHs, the internal standard o-terphenyl and the conservative internal biomarker 17α(H),21β(H)-hopane (33) were detected. The ions selected to detect these products were those reported elsewhere (26). The concentrations of PAHs and hopane were quantified using standard calibration curves. To estimate the percentage of recovery for each PAH at each different sampling point, the concentration was normalized with respect to that of the internal biomarker and compared to that obtained for the abiotic controls at time zero, as shown in the following equation: % remaining = [(Ct/Ht)/(C0/H0)] × 100, where Ct and Ht are the concentrations of the target analyte (PAH) and hopane, respectively, at time t and C0 and H0 are the concentrations of the same compounds at the beginning of the experiment (t = 0).
The alkyl-PAHs were quantified from the reconstructed ion chromatograms obtained by GC-MS by using the molecular ions for these compounds and m/z 191 for 17α(H),21β(H)-hopane. The concentrations of alkyl-PAHs were estimated using calibration curves obtained with the corresponding nonsubstituted PAHs (26).
Identification of neutral and acidic bacterial metabolites.
To detect the accumulation of possible neutral metabolites, the dichloromethane extracts (pH 7) from cultures and controls were fractionated by column chromatography (19). One-half of each neutral extract (0.5 ml) was concentrated to dryness under a nitrogen stream, redissolved in 1 ml of n-hexane, and applied to a glass column (20 x 1 cm) packed with 8 g of aluminum oxide (top) and 8 g of silica gel (bottom), previously activated at 320°C and 125°C, respectively, and then inactivated with water (5%). The stationary phase was preequilibrated with n-hexane. The elution of the different fractions was performed with the following solvent system: n-hexane (20 ml; FI), n-hexane-methylene chloride (9:1) (20 ml; FII), n-hexane-methylene chloride (8:2) (40 ml; FIII), n-hexane-methylene chloride (1:1) (20 ml; FIV), methylene chloride (20 ml; FV), methylene chloride-methanol (8:2) (20 ml; FVI), methanol (40 ml; FVII), and acidified methanol (0.05 N HCl) (40 ml; FVIII). All fractions were concentrated to a final volume of 1 ml under vacuum and analyzed by GC-FID and GC-MS. Fractions VI to VIII were derivatized with ethereal diazomethane prior to analysis. To detect possible acidic metabolites accumulated in the cultures, the ethyl acetate extracts (pH 2) from cultures and controls were derivatized with ethereal diazomethane and analyzed by GC and GC-MS. The new chromatographic peaks appearing in cultures and not present in abiotic controls were considered metabolites.
The GC-MS analytical conditions used for detection of metabolites were those described previously (45). When possible, oxidation products were identified by comparison of their MS spectra and GC retention times with those obtained for authentic commercial standards or for metabolites isolated and identified in previous biodegradation studies with single PAHs (29, 30, 45). When commercial products were not available, identification was performed by comparison of the MS spectra with those in databases (National Institute of Standards and Technology). In this case, identification was accepted when the similarity was above 90%. Metabolites were quantified by using standard calibration curves obtained for the methyl ester derivatives of the commercial products.
RESULTS
Biodegradation of TPHs of fuel oil by Mycobacterium sp. strain AP1 in marine medium.
Strain AP1 grew in nutrient-supplemented artificial seawater with pyrene as the sole carbon source with an efficiency in the cellular assimilation of the pyrene carbon of approximately 16%, which was higher than that observed in nonsaline medium (10%) (45). Metabolite accumulation profiles (high-performance liquid chromatography) showed a similar pattern to those observed in nonsaline medium.
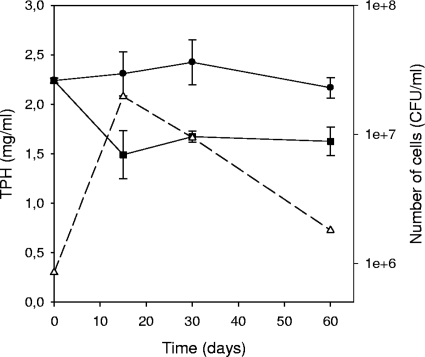
The action of strain AP1 on different components of the Prestige fuel oil was determined for cultures grown in nutrient-supplemented artificial seawater with this fuel oil (5 g/liter) as the sole carbon source. During the first 15 days of incubation, strain AP1 increased its population from 8.5 × 105 CFU/ml to 2 × 107 CFU/ml and caused a 35% reduction in the fuel TPH content (GC-FID). From this point to the end of incubation (60 days), the TPH content remained almost constant, while the bacterial population decreased to initial values (Fig. 1).
FIG. 1.
Utilization of fuel oil TPHs by strain AP1. The TPH contents in cultures (▪) and abiotic controls (•) were determined by GC-FID. The number of cells of Mycobacterium sp. strain AP1 in nutrient-supplemented artificial seawater containing Prestige fuel oil was determined by plate counting (▵).
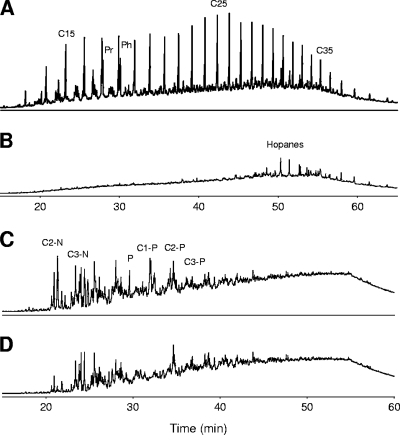
The GC profiles of both the aliphatic and aromatic fractions of the residual fuel oil recovered from 15-day cultures showed important changes with respect to controls (Fig. 2). The aliphatic fraction from abiotic controls (Fig. 2A), which was similar to that of the initial fuel added to the cultures, exhibited a bimodal distribution of the n-alkanes, centered at n-C16 and n-C26 and extending up to n-C40, which is typical of a heavy petroleum residue diluted with a middle-distillate product (10). This profile revealed a slight degree of weathering with respect to fuel recovered directly from the tanker, for example, the pristane/phytane ratio was 0.62 instead of the 0.98 of the original Prestige fuel oil. In the biodegraded fuel recovered from cultures (Fig. 2B), all of the chromatographic peaks corresponding to linear alkanes were completely depleted, including those corresponding to long-chain alkanes (C20 to C36). Branched alkanes, such as pristane and phytane, were also extensively attacked, leaving the hopanes as the major resolved peaks.
FIG. 2.
GC-FID chromatograms of aliphatic (A and B) and aromatic (C and D) fractions recovered from abiotic controls (A and C) and from cultures of strain AP1 (B and D) after 15 days of incubation. Pr, pristane; Ph, phytane; C15, C25, and C35, n-alkanes (the number indicates the number of carbon atoms).
The aromatic fraction from abiotic controls (Fig. 2C) showed a predominance of alkyl-naphthalenes and alkyl-phenanthrenes. Naphthalene and monomethylnaphthalenes (C1-N) were detected only in small traces as a result of the abiotic weathering processes. The GC analysis of the aromatic fraction from the fuel recovered from cultures (Fig. 2D) revealed an important reduction of alkyl-PAHs (especially dimethylnaphthalenes [C2-N] and monomethylphenanthrenes [C1-P]), which suggested further analysis by selective detection techniques (GC-MS).
Biodegradation of fuel oil PAHs.
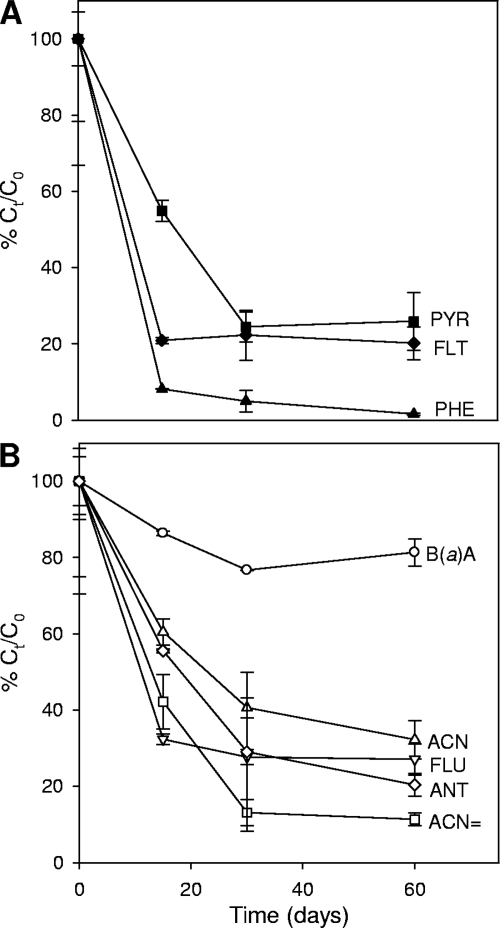
Mycobacterium sp. strain AP1 caused a significant (P < 0.05) depletion of all of the three- and four-ring PAHs, except for chrysene. Anthracene and phenanthrene, for example, were degraded to high extents (80 and 99%, respectively, at 60 days), while the higher-molecular-weight compounds fluoranthene, pyrene, and benzo(a)anthracene were reduced 80, 75, and 30%, respectively (Fig. 3). The three PAHs known to be degraded through growth-linked reactions (phenanthrene, fluoranthene, and pyrene) were attacked simultaneously, showing maximum depletion rates during the first 2 weeks of incubation (Fig. 3A). Thereafter, the degradation of phenanthrene and fluoranthene was strongly attenuated, while that of pyrene was still significant at day 30. Strain AP1 caused considerable reductions of a number of PAHs that do not support its growth, including fluorene, acenaphthene, acenaphthylene, anthracene, and benzo(a)anthracene (Fig. 3B).
FIG. 3.
Disappearance of significantly depleted PAHs (P ≤ 0.05) in liquid cultures of Mycobacterium sp. strain AP1 growing at the expense of Prestige fuel oil. (A) Evolution of substrates that support the growth of the strain. PHE, phenanthrene; FLT, fluoranthene; PYR, pyrene. (B) Evolution of non-growth-supporting substrates. ACN=, acenaphthylene; ACN, acenaphthene; FLU, fluorene; ANT, anthracene; B(a)A, benzo(a)anthracene. The relative recovery (Ct/C0) was calculated for the hopane-normalized concentration of each analyte with respect to that found in the time zero cultures. Each data point corresponds to the average of the values obtained for three replicate cultures. Error bars represent 1 standard deviation.
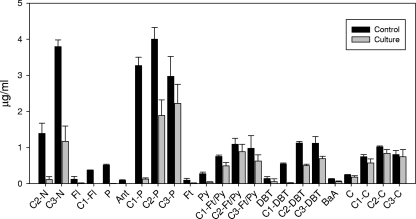
Strain AP1 effected an extensive attack on alkyl-PAHs (Fig. 4), significantly (P < 0.05) reducing the dimethylnaphthalenes (C2-N) (92%), C3-N (70%), C1-fluorenes (96%), C1-phenanthrenes (C1-P) (97%), C2-P (53%), C3-P (28%), C1-dibenzothiophenes (C1-DBT) (96%), C2-DBT (55%), C3-DBT (36%), and C1-fluoranthenes/pyrenes (35%). The order of preference of the strain in attacking the different alkyl-PAH families was C1-P ≈ C1-DBT ≈ C1-fluorenes ≈ C2-N > C3-N > C2-DBT ≈ C2-P > C3-DBT > C1-pyrenes > C3-P, and within each family this attack was isomer selective (see Fig. S1 in the supplemental material). The isomer discrimination patterns observed for C2-naphthalenes, C1-phenanthrenes, and C1-pyrenes were similar to those reported for gram-negative bacteria (2). All of the C1-dibenzothiophenes were almost completely removed, and therefore the previously described preference for 2-/3-methyldibenzothiophenes was not observed (2, 5, 47).
FIG. 4.
Concentrations of PAHs in abiotic controls and cultures of strain AP1 after 60 days of incubation in nutrient-supplemented artificial seawater and fuel. N, naphthalene; Fl, fluorene; P, phenanthrene; Ant, anthracene; Ft, fluoranthene; Py, pyrene; DBT, dibenzothiophene; BaA, benzo(a)anthracene; C, chrysene. C1, C2, and C3 indicate the numbers of methyl groups.
The analysis of the C3-naphthalenes (m/z 170) indicated that the 1,3,6-, 1,3,5-, 1,4,6-, 1,2,4-, and 1,2,5-isomers were the most resistant to biodegradative attack by strain AP1, while the rest of the isomers were extensively depleted. These results differ from data reported for mixed cultures and environmental samples indicating that 1,3,6- and 1,2,5-isomers were the most easily oxidized isomers (47, 48), while 1,2,7-, 1,6,7-, and 1,2,6-isomers were refractory to biodegradation. The profiles obtained for the C2-phenanthrenes (m/z 206) showed 1,7-, 1,8-, 1,6- and 2,9-isomers as the most refractory to biodegradation by strain AP1. In contrast, 2,6- and 2,7-isomers were almost absent in cultures. This also differs from results obtained by other authors after the incubation of two microbial consortia with the Prestige fuel, which indicated the 1,7- and 2,7-isomers as the most refractory and the 3,6-, 2,6-, and 2,3-isomers as the more easily degraded isomers (10).
Within the C2-dibenzothiophene family, the 1,4-, 1,6-, 1,8-, 1,3-, and 2,6-isomers showed the most resistance to attack by strain AP1. The group of 1,4-, 1,6-, and 1,8-isomers was also found to be recalcitrant to biodegradation in the mentioned study with the two microbial consortia (10). However, in that work, the 1,3- and 2,6-isomers were significantly depleted, and the 4,6- and 3,6-isomers, considerably reduced by strain AP1, were attacked to a limited extent. Alterations in the isomer distribution of C3-phenanthrenes and C3-dibenzothiophenes were less evident, as found for the cited consortia (10).
Identification of accumulated metabolites.
Two PAH metabolites were identified in the polar fractions (FIV to FVIII) of the neutral extracts from cultures. The first one, eluted in FV, exhibited a GC retention time (Rt) and mass spectrum [m/z at 194(100), 165(72), 150(1), 139(8), 126(1), 115(2), 97(4), 89(1), 82(11), 69(5), and 63(3)] indistinguishable from those of authentic anthrone. The second metabolite eluted in FVII and FVIII and exhibited a mass spectrum [m/z at 236(100), 221(14), 205(79), 177(65), 163(4), 151(10), 139(3), 126(2), 118(3), 111(2), 102(6), 88(32), 75(6), and 63(2)] and Rt identical to those of authentic 4-phenanthrene carboxylic acid. Other chromatographic peaks corresponding to possible neutral metabolites could not be identified. However, their mass spectra showed fragment ions consistent with ketones, quinones, and phenolic products resulting from the partial oxidation of alkyl-fluorenes, phenanthrenes, and naphthalenes.
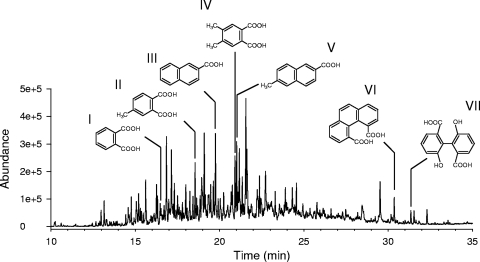
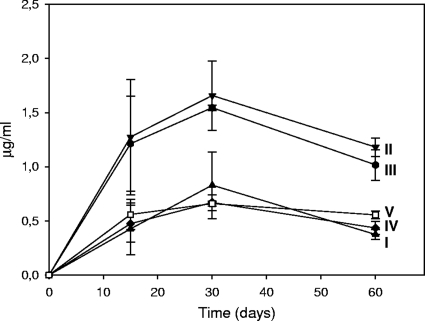
Seven metabolites were identified in the acidic extracts from cultures (Fig. 5). Three of them were identified as methyl ester derivatives of phthalic acid (I), 4-methylphthalic acid (II) [m/z at 208(10), 177(100), 149(7), 134(2), 118(4), 106(5), 91(15), 77(4), and 63(5)], and 2-naphthalene carboxylic acid (III) [m/z at 186(55), 155(100), 127(88), 115(4), 101(9), 77(15), 63(14), and 51(10)] by comparison with derivatized authentic standards. Two metabolites exhibited GC and spectral characteristics indistinguishable from those found for the methyl derivatives of products identified previously (45) as phenanthrene 4,5-dicarboxylic acid (VI) and 6,6′-dihydroxy-2,2′-biphenyl dicarboxylic acid (VII). Compound IV showed a mass spectrum [m/z at 222(17), 191(100), 179(2), 177(2), 163(43), 148(3), 133(7), 120(10), 105(14), 95(14), and 77(16)] consistent with that of the dimethyl ester derivative of a dimethylphthalic acid. Lastly, compound V exhibited a mass spectrum [m/z at 200(29), 169(6), 141(100), 115(25), 102(2), 89(4), 77(2), 70(6), and 63(6)] consistent with the methyl ester derivative of a methylnaphthalene carboxylic acid. Quantitative results for metabolites for which standard products were available are shown in Fig. 6. Maximum accumulation values were observed at 30 days. The subsequent decreases in the levels of methylphthalic acid (II), naphthalene carboxylic acid (III), and methylnaphthalene carboxylic acid (V) could indicate further utilization of these oxidation products by strain AP1 once the PAHs became limiting.
FIG. 5.
GC-FID profile of acidic extract (treated with diazomethane) from a 60-day culture of Mycobacterium sp. strain AP1 in nutrient-supplemented artificial seawater containing Prestige fuel oil, showing the identities of metabolites determined by GC-MS. Metabolites: I, phthalic acid; II, 4-methylphthalic acid; III, 2-naphthoic acid; IV, dimethylphthalic acid; V, methylnaphthoic acid; VI, phenanthrene-4,5-dicarboxylic acid; and VII, 6,6′-dihydroxy-2,2′-biphenyl dicarboxylic acid. All metabolites were identified as methyl ester derivatives.
FIG. 6.
Concentrations of phthalic (I), 4-methylphthalic (II), 2-naphthoic (III), dimethylphthalic (IV), and methylnaphthoic (V) acids in cultures of Mycobacterium sp. strain AP1 in artificial seawater and Prestige fuel oil. Each data point corresponds to the average of the values obtained for three replicate independent cultures.
DISCUSSION
In this study, we used the heavy fuel oil spilled by the Prestige as a model to investigate the potential oxidative actions of high-molecular-weight-PAH-degrading mycobacteria on petroleum and derivative complex mixtures. This Russian M-100 fuel oil is rich in long-chain alkanes and has a predominant aromatic fraction (52%) (27), which makes it more resistant to biodegradation than most commercial petroleum products. The pyrene-degrading Mycobacterium sp. strain AP1 grew at the expense of this heavy fuel oil, acting on a variety of alkanes and aromatic hydrocarbons. During the incubation, strain AP1 completely removed all of the linear (C12 to C40) and branched alkanes present in the aliphatic fraction and caused an extensive depletion of most of the three- and four-ring PAHs [including phenanthrene, anthracene, fluoranthene, pyrene, and benzo(a)anthracene] and their alkyl derivatives.
As expected, the bulk of the alkanes and the growth-supporting PAHs were degraded during the period when bacterial growth was observed (15 days). The subsequent decrease in cells, even when some PAHs continued to be oxidized, has also been observed in cultures of this strain with a single PAH (45) and could be attributed to the fact that the reduced availability of a carbon source could not support the reached cell concentrations, as demonstrated elsewhere (30).
The ability of strain AP1 and other pyrene-degrading mycobacteria to utilize single alkanes for growth has been reported previously (8, 45), but the high capacity of strain AP1 to remove all of the resolved compounds in the saturated fraction of an oil derivative was unexpected. Alkane-degrading actinobacterial strains, for which the ability to degrade PAHs has not been reported, have been shown to degrade the saturated fraction of crude oils, but the reported degradation extents were always lower. For example, a Rhodococcus strain removed 50% of the aliphatic fraction of Assam crude oil, completely depleting all linear alkanes (up to C20) and pristane but showing limited action on higher-molecular-weight alkanes and phytane (38).
The three growth-supporting PAHs, pyrene, fluoranthene, and phenanthrene, were also extensively removed (75 to 95%). Identification of key metabolites, such as phenanthrene 4,5-dicarboxylic acid (VI) and 6,6′-dihydroxy-2,2′-biphenyl dicarboxylic acid (VII), indicates the utilization of pyrene by the metabolic pathway previously described for strain AP1 (9, 36, 45), while phthalate (I) is a common intermediate in the degradation of all three PAHs. Simultaneous to the utilization of these substrates, strain AP1 cooxidized the non-growth-supporting PAHs acenaphthene, acenaphthylene, anthracene, fluorene, and benzo(a)anthracene (90 to 30%).
The percentages of degradation observed here for those PAHs contrasted with results obtained for incubations of the strain with single substrates (30, 45) or with a mixture of creosote PAHs (31). In the latter study, for example, only fluorene and anthracene were significantly degraded. For growth with PAH crystals or with drops of creosote PAHs, the low levels of biodegradation were attributed to a progressive reduction in the bioavailability of these compounds due to the formation of a biofilm that limited the transfer of the PAHs to the water phase (30, 31, 45). During growth of strain AP1 on fuel, biofilm formation was not observed. Instead, a stable emulsion of the fuel oil was observed after the first week of incubation. It has been demonstrated that the presence of fatty acids in the culture medium favors emulsion of the crude oil (28, 40). Other authors suggested that fatty acids excreted into the medium during growth of an alkane-degrading Rhodococcus strain on crude oil, together with the increase of cellular lipophobicity due to changes in cell fatty acid composition, may favor the uptake of hydrocarbons by bacterial cells (38). In fact, in this study, GC-MS analyses of the acidic extracts from the cultures of strain AP1 with the Prestige fuel oil revealed the formation of hexadecanoic and octadecanoic acids and their accumulation throughout the incubation period, with maximum concentrations after 15 days (results not shown). Therefore, this fatty acid production could play an important role in increasing the bioavailability of the PAHs and thus enhancing their degradation.
Although alkyl-PAHs have received much less attention than the nonsubstituted PAHs in biodegradation studies and our knowledge on the biochemical routes for their removal from the environment is limited, this class of compounds is far more abundant than the nonsubstituted PAHs in crude oils and derivatives. Mycobacterium sp. strain AP1 carried out a substantial attack on mono- and dimethylated PAHs (degradation of 53 to 97%) as well as on trimethylated phenanthrenes, trimethylated dibenzothiophenes, and monomethylated fluoranthenes/pyrenes (28 to 35%). As found in laboratory and field studies (44, 46, 47), the biodegradation percentages within each of these series of alkyl-PAHs for strain AP1 decreased with increasing alkyl substitution. However, the preference in degradation of the different families found for Mycobacterium sp. strain AP1 showed some differences with respect to that reported for single gram-negative strains or environmental samples (21, 44, 48), where C1-phenanthrenes and C1-dibenzothiophenes were more resistant to biodegradation than C2- and C3-naphthalenes. Conversely, the pattern found for strain AP1 in the sequence of degradation of those families was more similar to that reported by other authors analyzing the degradation of the Prestige fuel oil by specialized PAH-degrading consortia (10).
In general, the isomer selectivity shown by strain AP1 within each of the C1-PAH families and for the C2-naphthalenes was similar to that previously described for pure and mixed cultures. There are no data in the literature on the selective degradation of C3-naphthalenes, C2-phenanthrenes, and dibenzothiophenes by pure bacterial cultures, and the results found for strain AP1 clearly differ from those obtained for mixed cultures and environmental samples (10, 47, 48).
These singularities of strain AP1 relating the preference in degradation of the different alkyl-PAH families and the isomer discrimination within some of these families could be due to the types of reactions involved in the degradative attack of those compounds. The depletion of methyl derivatives of PAHs from crude oil by alkane-degrading strains (2, 11, 21, 41) has been attributed to the mere oxidation of the alkylic carbons to form carboxylic aromatic acids (35). In this case, the accumulation of naphthalene carboxylic (III) and methylnaphthalene carboxylic (V) acids would indicate similar reactions by strain AP1, but the presence of mono (II)- and dimethylphthalates (IV) demonstrates that aromatic ring opening and further degradation of alkylated compounds also occur.
In addition to the identified acidic metabolites, strain AP1 accumulated aromatic ketones, quinones, and phenolic products in the medium. Ketones and quinones are often formed either as a result of fortuitous oxidations on nongrowth substrates or from lateral reactions alternative to assimilative routes during the degradation of PAHs by mycobacteria. For example, anthraquinone is produced by various PAH-degrading mycobacterial strains, apparently as a result of the nonproductive oxidation of anthracene, which is degraded by the same strains by alternative dioxygenation routes (32). The further degradation of quinones has not been demonstrated. The formation of ketones and quinones by gram-negative PAH degraders has been attributed to the action of dioxygenases (37). Since some mycobacterial strains have been shown to possess cytochrome P450 monooxygenases that act on PAHs (4), here the production of ketones and quinones could also result from the action of this type of enzymes.
It is interesting that the acidic metabolites accumulated during the biodegradation of fuel reached maximum concentrations (0.5 to 1.3 μg/ml) that were within the same range as the concentrations of the parent PAHs and alkyl-PAHs (0.1 to 4 μg/ml). Given that some of those metabolites (i.e., phenanthrene-4,5-dicarboxylic acid and 6,6′-dihydroxy-2,2′-biphenyl dicarboxylic acid) are characteristic of specific metabolic pathways for the degradation of PAHs by mycobacteria (8, 25, 36, 45) and that these compounds have also been reported to accumulate during the degradation of creosote PAHs by strain AP1, these compounds could be used as indicators of mycobacterial activity in PAH-polluted sites. In general, the identification of metabolites indicative of these and other PAH degradation pathways could be used as evidence of active degradation in monitored natural attenuation processes and to determine the progress of other bioremediation strategies.
The results obtained here demonstrate the high catabolic potential of alkane- and pyrene-degrading mycobacteria in the degradation of petrogenic complex mixtures if the appropriate culture conditions are given. The detection of key metabolites indicative of specific oxidative actions permitted us to identify the general processes by which individual components of the mixture are degraded or transformed and were decisive in establishing the degradation of the aromatic rings of alkyl-PAHs. The versatility of Mycobacterium sp. strain AP1 in acting on all the alkanes and PAHs in the oil, the extension of the degradation observed, especially for high-molecular-weight compounds, and the fact that the combination of these capabilities has not been observed in other microbial strains point to the possible important role of mycobacteria in removing these compounds from polluted environments. More research is needed to confirm the significance of the observed catabolic activities in polluted environments and the potential of Mycobacterium sp. strain AP1 and similar strains in the bioremediation of oil spills, soils, and contaminated waters.
Supplementary Material
Acknowledgments
This research was funded by grants from the Spanish Ministry of Education and Science (VEM2004-08-556 and CGL2007-64199/BOS). We are members of the Xarxa de Referència d'R+D+I en Biotecnologia (XRB), which receives funding from the Generalitat de Catalunya.
We are grateful to Asunción Marín (Serveis Científico-Tècnics, Universitat de Barcelona) for the acquisition of GC-MS data.
Footnotes
Published ahead of print on 7 August 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Reference deleted.
- 2.Bayona, J. M., J. Albaigés, A. M. Solanas, R. Parés, P. Garrigues, and M. Ewalt. 1986. Selective aerobic degradation of methyl-substituted polycyclic aromatic hydrocarbons in petroleum by pure microbial cultures. Int. J. Environ. Anal. Chem. 23:289-303. [Google Scholar]
- 3.Bragg, J. R., R. C. Prince, E. J. Harner, and R. M. Atlas. 1994. Effectiveness of bioremediation for the Exxon Valdez oil spill. Nature 368:413-418. [Google Scholar]
- 4.Brezna, B., O. Kweon, R. L. Stingley, J. P. Freeman, A. A. Khan, B. Polek, R. C. Jones, and C. E. Cerniglia. 2006. Molecular characterization of cytochrome P450 genes in the polycyclic aromatic hydrocarbon degrading Mycobacterium vanbaalenii PYR-1. Appl. Microbiol. Biotechnol. 71:522-532. [DOI] [PubMed] [Google Scholar]
- 5.Budzinsky, H., N. Raymond, T. Nadalig, M. Gilewicz, P. Garrigues, J. C. Bertrand, and P. Caumette. 1998. Aerobic biodegradation of alkylated aromatic hydrocarbons by a bacterial community. Org. Geochem. 28:337-348. [Google Scholar]
- 6.Button, D. K., F. Schut, P. Quang, R. Martin, and B. R. Robertson. 1993. Viability and isolation of marine bacteria by dilution culture: theory, procedures, and initial results. Appl. Environ. Microbiol. 59:881-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerniglia, C. E. 1992. Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation 3:351-368. [Google Scholar]
- 8.Churchill, S. A., J. P. Harper, and P. F. Churchill. 1999. Isolation and characterization of a Mycobacterium species capable of degrading three- and four-ring aromatic and aliphatic hydrocarbons. Appl. Environ. Microbiol. 65:549-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean-Ross, D., and C. E. Cerniglia. 1996. Degradation of pyrene by Mycobacterium flavescens. Appl. Microbiol. Biotechnol. 46:307-312. [DOI] [PubMed] [Google Scholar]
- 10.Díez, S., J. Sabaté, M. Viñas, J. M. Bayona, A. M. Solanas, and J. Albaigés. 2005. The Prestige oil spill. I. Biodegradation of a heavy fuel oil under simulated conditions. Environ. Toxicol. Chem. 24:2203-2217. [DOI] [PubMed] [Google Scholar]
- 11.Dutta, T. K., S. A. Selifonov, and I. C. Gunsalus. 1998. Oxidation of methyl-substituted naphthalenes: pathways in a versatile Sphingomonas paucimobilis strain. Appl. Environ. Microbiol. 64:1884-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyksterhouse, S. E., J. P. Gray, R. P. Herwig, J. C. Lara, and J. T. Staley. 1995. Cycloclasticus pugetii gen. nov., sp. nov., an aromatic hydrocarbon-degrading bacterium from marine sediments. Int. J. Syst. Bacteriol. 45:116-123. [DOI] [PubMed] [Google Scholar]
- 13.Gauthier, M. J., B. Lafay, R. Christen, L. Fernandez, M. Acquaviva, P. Bonin, and J.-C. Bertrand. 1992. Marinobacter hydrocarbonoclasticus gen. nov., sp. nov., a new, extremely halotolerant, hydrocarbon-degrading marine bacterium. Int. J. Syst. Bacteriol. 42:568-576. [DOI] [PubMed] [Google Scholar]
- 14.Geiselbrecht, A. D., B. P. Hedlund, M. A. Tichi, and J. T. Staley. 1998. Isolation of marine polycyclic aromatic hydrocarbon (PAH)-degrading Cycloclasticus strains from the Gulf of Mexico and comparison of their PAH degradation ability with that of Puget Sound Cycloclasticus strains. Appl. Environ. Microbiol. 64:4703-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson, D. T., S. M. Resnick, K. Le, J. M. Brand, D. S. Torok, L. P. Wackett, M. J. Schoken, and B. E. Haigler. 1995. Desaturation, dioxygenation, and monooxygenation reactions catalyzed by naphthalene dioxygenase from Pseudomonas sp. strain 9816-4. J. Bacteriol. 177:2615-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gontang, E. A., W. Fenical, and P. R. Jensen. 2007. Phylogenetic diversity of gram-positive bacteria cultured from marine sediments. Appl. Environ. Microbiol. 73:3272-3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grifoll, M., S. A. Selifonov, and P. J. Chapman. 1994. Evidence for a novel pathway in the degradation of fluorene by Pseudomonas sp. strain F274. Appl. Environ. Microbiol. 60:2438-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grifoll, M., S. A. Selifonov, C. V. Gatlin, and P. J. Chapman. 1995. Actions of a versatile fluorene-degrading bacterial isolate on polycyclic aromatic hydrocarbons. Appl. Environ. Microbiol. 61:3711-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimalt, J. O., M. Grifoll, A. M. Solanas, and J. Albaigés. 1991. Microbial degradation of marine evaporitic crude oils. Geochim. Cosmochim. Acta 55:1903-1913. [Google Scholar]
- 20.Kanaly, R. A., and S. Harayama. 2000. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J. Bacteriol. 182:2059-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasai, Y., H. Kishira, and S. Harayama. 2002. Bacteria belonging to the genus Cycloclasticus play a primary role in the degradation of aromatic hydrocarbons released in a marine environment. Appl. Environ. Microbiol. 68:5625-5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kästner, M. 2000. Degradation of aromatic and polyaromatic compounds, p. 212-239. In H. J. Rehm, G. Reed, A. Pühler, and P. Stadler (ed.), Environmental processes II: soil decontamination, biotechnology, vol. 11b. Wiley, New York, NY. [Google Scholar]
- 23.Keum, Y.-S., J.-S. Seo, Y. Hu, and Q. X. Li. 2006. Degradation pathways of phenanthrene by Sinorhizobium sp. C4. Appl. Microbiol. Biotechnol. 71:935-941. [DOI] [PubMed] [Google Scholar]
- 24.Reference deleted.
- 25.Kim, Y. H., J. P. Freeman, J. D. Moody, K. H. Engesser, and C. E. Cerniglia. 2005. Effects of pH on the degradation of phenanthrene and pyrene by Mycobacterium vanbaalenii PYR-1. Appl. Microbiol. Biotechnol. 67:275-285. [DOI] [PubMed] [Google Scholar]
- 26.Kostecki, P. T., and E. J. Calabrese. 1992. Contaminated soils. Diesel fuel contamination. Lewis Publishers Inc., Chelsea, MI.
- 27.Le Cedre. 2003. Study of the Prestige fuel cargo. Cedre, Brest, France. http://www.le-cedre.fr/index.gb.html.
- 28.Lee, R. F. 1999. Agents which promote and stabilize water-in-oil emulsions. Spill Sci. Technol. Bull. 5:117-126. [Google Scholar]
- 29.López, Z., J. Vila, and M. Grifoll. 2005. Metabolism of fluoranthene by mycobacterial strains isolated by their capacity of growing in fluoranthene and pyrene. J. Ind. Microbiol. Biotechnol. 32:455-464. [DOI] [PubMed] [Google Scholar]
- 30.López, Z., J. Vila, C. Minguillón, and M. Grifoll. 2006. Metabolism of fluoranthene by Mycobacterium sp. strain AP1. Appl. Microbiol. Biotechnol. 70:747-756. [DOI] [PubMed] [Google Scholar]
- 31.López, Z., J. Vila, J. J. Ortega-Calvo, and M. Grifoll. 2008. Simultaneous biodegradation of creosote-polycyclic aromatic hydrocarbons by a pyrene-degrading Mycobacterium. Appl. Microbiol. Biotechnol. 78:165-172. [DOI] [PubMed] [Google Scholar]
- 32.Moody, J. D., J. P. Freeman, D. R. Doerge, and C. E. Cerniglia. 2001. Degradation of phenanthrene and anthracene by cell suspension of Mycobacterium sp. strain PYR-1. Appl. Environ. Microbiol. 67:1476-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prince, R. C., D. L. Elmendorf, J. R. Lute, C. S. Hsu, C. E. Haith, J. D. Senius, G. J. Dechert, G. S. Douglas, and E. L. Butler. 1994. 17α(H)-21β(H)-hopane as a conserved internal marker for estimating the biodegradation of crude oil. Environ. Sci. Technol. 28:142-145. [DOI] [PubMed] [Google Scholar]
- 34.Quek, E., Y. P. Ting, and H. M. Tan. 2006. Rhodococcus sp. F92 immobilized on polyurethane foam shows ability to degrade various petroleum products. Biores. Technol. 97:32-38. [DOI] [PubMed] [Google Scholar]
- 35.Raymond, R. L., V. W. Jamison, and J. O. Hudson. 1967. Microbial hydrocarbon co-oxidation. Appl. Microbiol. 15:857-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rehmann, K., H. P. Noll, C. E. W. Steinberg, and A. A. Kettrup. 1998. Pyrene degradation by Mycobacterium sp. strain KR2. Chemosphere 36:2977-2992. [DOI] [PubMed] [Google Scholar]
- 37.Selifonov, S. A., M. Grifoll, R. W. Eaton, and P. J. Chapman. 1996. Oxidation of naphthenoaromatic and methyl-substituted aromatic compounds by naphthalene 1,2-dioxygenase. Appl. Environ. Microbiol. 62:507-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma, S. L., and A. Pant. 2000. Biodegradation and conversion of alkanes and crude oil by a marine Rhodococcus sp. Biodegradation 11:289-294. [DOI] [PubMed] [Google Scholar]
- 39.Shiraris, M. P., and J. J. Cooney. 1983. Replica plating method for estimating phenanthrene-utilizing and phenanthrene-cometabolizing microorganisms. Appl. Environ. Microbiol. 45:706-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sjöblom, J., H. Sönderlund, S. Lindblad, E. J. Johansen, and I. M. Skjärvö. 1990. Water-in-crude oil emulsions from the Norwegian continental shelf. II. Chemical destabilization and interfacial tensions. Colloid Polym. Sci. 268:389-398. [Google Scholar]
- 41.Solanas, A. M., R. Parés, J. M. Bayona, and J. Albaigés. 1984. Degradation of aromatic petroleum hydrocarbons by pure microbial cultures. Chemosphere 13:593-601. [Google Scholar]
- 42.Swannell, R. P. J., D. Mitchell, G. Lethbridge, D. Jones, D. Heath, M. Hagley, M. Jones, S. Petch, R. Milne, R. Croxford, and K. Lee. 1999. A field demonstration of the efficacy of bioremediation to treat oiled shorelines following the Sea Empress incident. Environ. Technol. 20:863-873. [Google Scholar]
- 43.Tagger, S., N. Truffaunt, and J. Le Petit. 1990. Preliminary study of relationships among strains forming a bacterial community selected on naphthalene from a marine sediment. Can. J. Microbiol. 36:676-681. [DOI] [PubMed] [Google Scholar]
- 44.Venosa, A. D., M. T. Suidan, B. A. Wrenn, K. L. Strohmeier, J. R. Haines, B. L. Eberhart, D. King, and E. Holder. 1996. Bioremediation of an experimental oil spill on the shoreline of Delaware Bay. Environ. Sci. Technol. 30:1764-1775. [Google Scholar]
- 45.Vila, J., Z. López, J. Sabaté, C. Minguillón, A. M. Solanas, and M. Grifoll. 2001. Identification of a novel metabolite in the degradation of pyrene by Mycobacterium sp. strain AP1. Actions of the isolate on two- and three-ring polycyclic aromatic hydrocarbons. Appl. Environ. Microbiol. 67:5497-5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, Z., M. Fingas, and G. Sergy. 1995. Chemical characterization of crude oil residues from an arctic beach by GC/MS and GC/FID. Environ. Sci. Technol. 29:2622-2631. [DOI] [PubMed] [Google Scholar]
- 47.Wang, Z., M. Fingas, S. Blenkinsopp, G. Sergy, M. Landriault, L. Sigouin, J. Foght, K. Semple, and D. W. S. Westlake. 1998. Comparison of oil composition changes due to biodegradation and physical weathering in different oils. J. Chromatogr. A 809:89-107. [DOI] [PubMed] [Google Scholar]
- 48.Watson, J. S., D. M. Jones, R. P. J. Swanell, and A. C. T. van Duin. 2002. Formation of carboxylic acids during aerobic biodegradation of crude oil and evidence of microbial oxidation of hopanes. Org. Geochem. 33:1153-1169. [Google Scholar]
- 49.West, P. A., G. C. Okpokwasili, and P. R. Brayton, D. J. Grimes, and R. R. Colwell. 1984. Numerical taxonomy of phenanthrene-degrading bacteria isolated from Chesapeake Bay. Appl. Environ. Microbiol. 48:988-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuste, L., M. E. Corbella, M. J. Turiégano, U. Karlson, A. Puyet, and F. Rojo. 2000. Characterization of bacterial strains able to grow on high molecular mass residues from crude oil processing. FEMS Microbiol. Ecol. 32:69-75. [DOI] [PubMed] [Google Scholar]
- 51.Zylstra, G. J., E. Kim, and A. K. Goyal. 1997. Comparative molecular analysis of genes for polycyclic aromatic hydrocarbon degradation. Genet. Eng. 19:257-269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.