Abstract
Industrial production of biodegradable polyesters such as polyhydroxyalkanoates is hampered by high production costs, among which the costs for substrates and for downstream processing represent the main obstacles. Inexpensive fermentable raw materials such as crude glycerol, an abundant by-product of the biodiesel industry, have emerged to be promising carbon sources for industrial fermentations. In this study, Zobellella denitrificans MW1, a recently isolated bacterium, was used for the production of poly(3-hydroxybutyrate) (PHB) from glycerol as the sole carbon source. Pilot-scale fermentations (42-liter scale) were conducted to scale up the high PHB accumulation capability of this strain. By fed-batch cultivation, at first a relatively high cell density (29.9 ± 1.3 g/liter) was obtained during only a short fermentation period (24 h). However, the PHB content was relatively low (31.0% ± 4.2% [wt/wt]). Afterwards, much higher concentrations of PHB (up to 54.3 ± 7.9 g/liter) and higher cell densities (up to 81.2 ± 2.5 g/liter) were obtained by further fed-batch optimization in the presence of 20 g/liter NaCl, with optimized feeding of glycerol and ammonia to support both cell growth and polymer accumulation over a period of 50 h. A high specific growth rate (0.422/h) and a short doubling time (1.64 h) were attained. The maximum PHB content obtained was 66.9% ± 7.6% of cell dry weight, and the maximum polymer productivity and substrate yield coefficient were 1.09 ± 0.16 g/liter/h and 0.25 ± 0.04 g PHB/g glycerol, respectively. Furthermore, a simple organic solvent extraction process was employed for PHB recovery during downstream processing: self-flotation of cell debris after extraction of PHB with chloroform allowed a convenient separation of a clear PHB-solvent solution from the cells. Maximum PHB recovery (85.0% ± 0.10% [wt/wt]) was reached after 72 h of extraction with chloroform at 30°C, with a polymer purity of 98.3% ± 1.3%.
Polyhydroxybutyrate (PHB) is the best-studied example of biodegradable polyesters belonging to the group of polyhydroxyalkanoates (PHAs), which are synthesized by many bacteria and archaea as intracellular carbon and energy reserves (1, 40, 43). In the last decades, these biopolymers have received great attention due to their properties which resemble those of conventional petrochemical-based polymers (49). For instance, PHB is very similar to thermoplastic polypropylene (17). Their production from renewable resources and their complete biodegradability give PHAs promising advantages from an environmental point of view (6). In addition to their special physical traits, such as the elasticity of medium-chain-length PHAs and the high crystallization rate of PHB, PHAs are biocompatible, water resistant, oxygen impermeable, and enantiomerically pure; all of these characteristics broaden the scope of their applications in industry and medicine.
So far, higher production costs than those of petrochemical plastics have hindered the successful commercialization of PHB (9). Many efforts have been devoted to reducing the production costs by developing superior microbial strains capable of utilizing cheap substrates and also by applying more efficient fermentation strategies and economical recovery processes (10).
Fed-batch fermentation regimens are usually applied to achieve a high cell density, which is necessary for a high productivity and yield, in particular in cases of intracellular products, by frequent or continuous feeding of nutrients when growth proceeds (46). Several fed-batch fermentation processes have been reported for PHA production (21, 28). There are two prevalent cultivation modes for PHB production that are imposed on the microorganisms being used. The more frequently used mode is realized by a complex two-stage cultivation process. In this mode, all nutrients needed for growth to a high cell density are provided during the first phase of the process. In the second phase, imbalanced growth conditions are enforced by providing growth-limiting amounts of nutrients such as nitrogen, phosphate, or oxygen to trigger PHA biosynthesis and accumulation. The model organism for this mode is Ralstonia eutropha (formerly known as Alcaligenes eutrophus and recently reclassified as Cupriavidus necator) (26, 27). In the other cultivation mode, PHB is accumulated concurrently with growth, and therefore a single-stage process is applicable. A well-known example of this mode is PHB production by Alcaligenes latus (18, 47).
Although several new downstream processes for the extraction of PHA have been reported as being economically effective, such as the application of surfactants and hypochlorite (9, 38), dispersions of hypochlorite solution and chloroform (14, 15), and the selective dissolution of cell mass by proteolytic enzymes (25) or by sulfuric acid and hypochlorite (48), solvent extraction methods are still regarded as an adequate way to gain intact polymers with a high purity and recovery yield (39). However, there is still a need to develop and improve these extraction methods further to make the entire process much simpler and cheaper (22).
In addition to increased prices for crude oil, the abundance of inexpensive raw materials from agriculture and industry as cheap substrates for microbial fermentations, such as crude glycerol from the biodiesel industry, could make the production of PHA from renewable resources more competitive with common plastics (32). Due to increased glycerol production by the growing biodiesel industry, the prices for glycerol became low enough to make this residual compound a cheap carbon source for several industrial fermentation processes, especially for the production of microbial polyesters (11, 34). However, the various amounts of actual fermentable substrates and the presence of other nonfermentable components in feedstock, such as the various concentrations of glycerol and salts in biodiesel coproducts, hinder their use (42). Therefore, tolerant bioprocesses and/or strains tolerant to such variable factors are required.
The production of PHA from glycerol has been investigated in only a few studies (4, 12, 24, 33, 42). In a recent study (32), crude glycerol from different biodiesel manufacturers was examined for suitability as a substrate for PHB production. However, significant decreases in PHB productivity and product yields were recorded when NaCl-contaminated crude glycerol was used.
Recently, a newly isolated bacterium, Zobellella denitrificans MW1, was characterized as producing large amounts of PHB from glycerol, with enhanced growth and polymer productivity in the presence of NaCl (20). The present study aimed at developing a strategy to improve the volumetric production of PHB by Z. denitrificans MW1, using glycerol as the sole carbon source. For this purpose, several fed-batch cultivations were set up to steadily improve the nutrient supply to attain a high cell density and high PHB productivity. Moreover, the conventional organic solvent extraction method was modified with regard to an economically more feasible large-scale PHB extraction, achieving a high purity and recovery of the polymer.
MATERIALS AND METHODS
Microorganism and culture conditions.
Z. denitrificans strain MW1, a recently isolated and characterized bacterium accumulating PHB from glycerol (20), was used in this study. Cells were grown in mineral salt medium (MSM) (41) containing the following (g/liter): Na2HPO4·12H2O, 9.0; KH2PO4, 1.5; MgSO4·7H2O, 0.2; NH4Cl, 1.0; CaCl2·2H2O, 0.02; and Fe(III)NH4+-citrate, 0.0012. The MSM also included 1 ml of trace element solution containing the following (g/liter): EDTA, 50.0; FeCl3, 8.3; ZnCl2, 0.84; CuCl2·2H2O, 0.13; CoCl2·6H2O, 0.1; MnCl2·6H2O, 0.016; and H3BO3, 0.1. Glycerol (15 g/liter) was used as the sole carbon source. The pH was adjusted to 7.3 before sterilization. MSM with 15 g/liter agar was used as a solid medium for maintenance of the bacterium at 4°C. Precultures were grown in 250-ml and 2-liter Erlenmeyer flasks containing 50 and 400 ml MSM, respectively. The flasks were incubated at 41°C and 200 rpm for 24 h.
Cultivation at 42-liter scale.
A Biostat UD-30 stainless steel reactor (B. Braun Biotech International, Melsungen, Germany) with a total volume of 42 liters (28-cm inner diameter and 71-cm height) and a d/D value relation (relation of stirrer diameter to vessel diameter) of 0.375 was used for cultivations at the 30-liter scale. This bioreactor was equipped with three stirrers, each containing six paddles and a Funda-Foam mechanical foam destroyer (B. Braun Biotech International, Melsungen, Germany). In addition, sterilized probes were inserted into ports to measure dissolved oxygen (pO2) (model 25; Mettler Toledo GmbH, Steinbach, Switzerland), pH (model Pa/25; Mettler Toledo GmbH), foam (model L300/Rd. 28; B. Braun Biotech International), temperature (pt 100 electrode; M. K. Juchheim GmbH, Fulda, Germany), and optical density at 850 nm (OD850) (model CT6; Sentex/Monitek Technology Inc.). The operations were controlled and recorded by a digital control unit in combination with the MFCS/win software package (B. Braun Biotech International).
Cultivations were done at 41°C and at a pO2 between 0 and 100% saturation in the medium, which was controlled by agitation rates between 100 and 800 rpm and aeration rates between 0.4 and 1.67 volume per volume per minute. Unless stated otherwise, the pH in the medium was held between 7.0 and 7.3 by controlled addition of 4 N HCl or NaOH. Foam was removed by a mechanical foam destroyer; if this was not sufficient, the antifoam agent Silikon Antischaum Emulsion SLE (Wacker, Darwin Vertriebs GmbH, Ottobrunn, Germany) was added. Small samples were withdrawn from the culture fluid for analytical purposes.
Cell harvest from 42-liter cultivations.
Cells were harvested by centrifugation in a CEPA type Z41 or type Z61 continuous centrifuge (Carl Padberg Zentrifugenbau GmbH, Lahr, Germany). Harvested cells were frozen at −30°C and then lyophilized (Beta 1-16; Christ, Osterode, Germany).
Determination of cell growth.
Growth of Z. denitrificans MW1 was monitored by measuring the increase of the OD600. Moreover, defined volumes of cultures were harvested by centrifugation for 20 min at 1,200 × g and 4°C; afterwards, the cells were washed with distilled water, frozen, and lyophilized. Cell density, defined as the cell dry weight (CDW) per liter of culture broth, was determined by weighing aliquots of lyophilized cells.
Fluorescence microscopy.
The presence of cytoplasmic PHA inclusions was evidenced by staining the biopolymer with Nile red and by observing the cells under a fluorescence microscope (30).
PHB extraction from Z. denitrificans MW1 whole cells.
Small-scale solvent extraction experiments were done to investigate the extraction efficiencies of different organic solvents for optimum PHB recovery from Z. denitrificans MW1 cells. One hundred milligrams of lyophilized cells was mixed overnight at room temperature with 20 volumes of different solvents (chloroform, methylene chloride, carbon tetrachloride, diethyl ester, ethyl acetate, and mixtures of chloroform and acetone [1:1, 1:2, 1:3, 1:4, 2:1, 3:1, and 4:1] [vol/vol]) in 2-ml sealed glass tubes. After extraction, cells were separated by flotation or precipitation, depending on their behavior in the solvent being used. PHB was recovered after solvent evaporation. Larger-scale solvent extraction experiments were done by stirring 100 g of lyophilized cells in 1,000 ml chloroform or methylene chloride (10 volumes) at a temperature of 24, 30, or 41°C. Samples of 50 ml were withdrawn after different incubation periods lasting up to 228 h. Before filtration, samples were left overnight in separation funnels until complete flotation of cells had occurred, thereby yielding a clear PHB-solvent solution for easy and simple filtration. After filtration, the PHB-solvent solutions were concentrated by distillation (solvent recovery), and 2 to 4 volumes of cold methanol was used to precipitate the PHB. Afterwards, the precipitated PHB was filtered and dried under vacuum.
Analyses of ammonium and glycerol.
The concentrations of ammonium in cell-free supernatants were determined by employing a gas-sensitive type 152303000 ammonium electrode (Mettler Toledo GmbH, Greifensee, Switzerland). Analysis of residual carbon sources was carried out with a LaChrom Elite high-performance liquid chromatography apparatus (VWR-Hitachi International GmbH, Darmstadt, Germany) consisting of a Metacarb 67H advanced C column (Bio-Rad Aminex equivalent; Varian, Palo Alto, CA) and a VWR-Hitachi model 22350 column oven. The column (300 mm by 6.5 mm) consisted of a sulfonated polystyrene resin in the protonated form. The primary separation mechanism included ligand exchange, ion exclusion, and adsorption. A VWR-Hitachi refractive index detector (type 2490) with an active-flow-cell temperature control and automated reference flushing eliminating temperature effects on the refractive index baseline was used for detection. Aliquots of 20 μl were injected and eluted with 0.005 N sulfuric acid in double-distilled water at a flow rate of 0.8 ml/min. Online integration and analysis of the data were done with EZ Chrome Elite software (VWR International GmbH, Darmstadt, Germany).
Quantitative analyses.
To determine the PHB contents of the cells, samples were subjected to methanolysis in the presence of 15% (vol/vol) sulfuric acid. The resulting 3-hydroxybutyric acid methyl esters were analyzed by gas chromatography (GC) (19). PHB content (% [wt/wt]) was defined as a percentage of CDW. The purity of extracted PHB was also determined by GC from a known mass of solvent-extracted PHB. The percentage of PHB recovered was calculated from a known amount of PHB in the biomass, based on the purity of the total mass of samples recovered. The substrate conversion factor (g PHB/g glycerol) was calculated as grams of PHB produced per grams of glycerol utilized. All results are from duplicate or triplicate measurements, and mean values and standard deviations are presented.
RESULTS
Production of PHB by fed-batch cultivation of Z. denitrificans MW1.
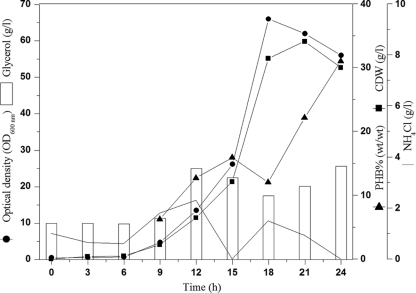
Four fed-batch fermentations at the 42-liter scale were carried out to obtain high cell densities and high polymer contents of cells of the newly isolated bacterium Z. denitrificans MW1, using glycerol as the sole carbon source. In accordance with the results of a previous study done with small Erlenmeyer flasks (20), cultivation temperature and pH were controlled at 41°C and 7.3, respectively. In the first fermentation (Fig. 1), an overnight seed culture was used to inoculate 24 liters of MSM (4% [vol/vol] inoculum size). Feeding was started after 6 h of batch culture, using a 50% (vol/vol) solution of glycerol. The dissolved oxygen (pO2) level in the medium was controlled automatically at 30% by increasing the agitation and aeration rates to 800 rpm and 1.67 volume per volume per minute, respectively.
FIG. 1.
Fed-batch cultivation of Z. denitrificans for PHB production from glycerol in the absence of NaCl (fed-batch 1). Z. denitrificans MW1 was cultivated in a Biostat UD-30 stirred-tank reactor containing 24 liters of MSM with glycerol as the sole carbon source. The bioreactor was inoculated with 4% (vol/vol) 18-h preculture. The pO2 was controlled automatically at 30% saturation by adjusting aeration and agitation rates. Cells were grown for 24 h at 41°C and pH 7.3. During the time course of cultivation, samples were withdrawn, and the concentrations of glycerol and ammonium, as well as the cell density and the PHB content of the cells, were determined as described in Materials and Methods.
The feeding regimen was designed to provide sufficient amounts of glycerol (10 to 20 g/liter) to support both cell growth and polymer accumulation during growth of Z. denitrificans MW1. Ammonium chloride solution (25% [wt/vol]) was added if the ammonium concentration in the culture fell below 1.0 g/liter. The specific growth rate (μ) during the batch phase was 0.09/h, and the maximum specific growth rate recorded was 0.351/h, with a doubling time of 1.97 h. At the end of this fermentation (24 h), a high cell density and also high volumetric polymer productivity were obtained (29.9 ± 1.3 g CDW/liter and 9.28 ± 1.7 g PHB/liter, respectively). However, the PHB content was relatively low (31.0% ± 4.2% [wt/wt]). The two main problems which prevented the extension of this fermentation to reach higher cell densities and polymer contents were the excessively added NaOH solution to control the sharp decrease in pH during the last 6 hours of this fermentation, which should cause significant cell lysis, and the increased medium volume because of nutrient feeding and base addition. The total amount of 4 kg glycerol used yielded a total of 0.33 kg PHB, thereby indicating a low substrate conversion factor of 0.10 ± 0.02 g PHB/g glycerol.
Fed-batch fermentation in the presence of NaCl.
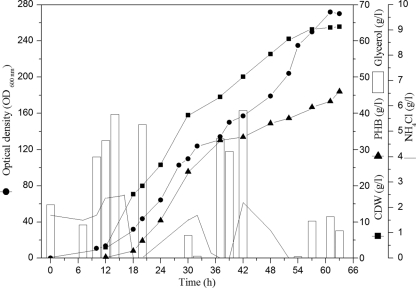
In the next fed-batch fermentations (batches 2, 3, and 4) (Table 1), the reported enhancing effect of sodium chloride on growth and polymer accumulation (20) was investigated on a large scale (42 liters). In addition, some modifications in the feeding strategy were conducted. A 24-h-old preculture (800 ml) with approximately 3 g CDW/liter was used to inoculate 20 liter MSM containing 15 g/liter glycerol. The total amount of sodium chloride for 30 liters (600 g) was added at the beginning of the fermentation. The feeding solution used contained 1% (wt/vol) MgSO4 and 50% (vol/vol) glycerol. The pO2 was kept at 20% saturation. Culture pH was controlled at 7.3 during the first 20 h of growth and then at 6.8 until the end of fermentation, using sodium hydroxide (4 N) and ammonia water (25% [wt/vol]); the latter was also used as a nitrogen source, as well as NH4Cl (25% [wt/vol]). In fed-batch 2, a similar cell density (32.8 ± 0.6 g CDW/liter) to that of fed-batch 1 was reached, in spite of using only half the amount of glycerol (Table 2). The relatively optimized cultivation conditions resulted in a higher polymer content of 52% ± 1.4% (wt/wt), producing 17.1 ± 0.8 g PHB/liter after 36 h of cultivation. The product yield was improved to 0.26 ± 0.01 g PHB/g glycerol.
TABLE 1.
PHB production by Zobellella denitrificans MW1 in fed-batch fermentationsa
| Fed-batch fermentation no. | Initial vol (liters) | Final vol (liters) | Cultivation time (h) | Amt of glycerol (g) | Residual amt of glycerol (g) | CDW (g/liter) | % PHA (wt/wt) | PHA concn (g/liter) | PHB productivity (g/liter/h) | Yield (g PHB/g glycerol) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 24 | 36 | 24 | 4,059 | 768 | 29.9 ± 1.3 | 31.0 ± 4.2 | 9.3 ± 1.7 | 0.39 ± 0.07 | 0.10 ± 0.02 |
| 2 (NaCl) | 20 | 26 | 36 | 2,100 | 360 | 32.8 ± 0.6 | 52.0 ± 1.4 | 17.1 ± 0.8 | 0.47 ± 0.02 | 0.26 ± 0.01 |
| 3 (NaCl) | 20 | 33 | 63 | 5,725 | 250 | 63.9 ± 2.0 | 56.0 ± 0.7 | 35.8 ± 1.6 | 0.57 ± 0.02 | 0.22 ± 0.01 |
| 4 (NaCl) | 20 | 30 | 50 | 8,400 | 1,884 | 81.2 ± 2.5 | 66.9 ± 7.6 | 54.3 ± 7.9 | 1.09 ± 0.16 | 0.25 ± 0.04 |
Fed-batch fermentations were done by cultivating Z. denitrificans MW1 in a 42-liter Biostat UD-30 stainless steel reactor. Medium components, feeding solutions, and cultivation conditions were as described in the text. All fermentations were operated at 41°C. Fed-batch fermentations 2, 3, and 4 were supplied with 20 g/liter NaCl. Analyses were done in triplicate, and averages and standard deviations are presented.
TABLE 2.
Overview of literature describing production of PHAs from glycerol by bacteria
| Bacterium | Cultivation vessel, fermentation strategy | Mediuma | Cultivation temp (°C) | Cultivation time (h) | Cell density (g CDW/liter) | PHA content (% [wt/wt]) | Type of PHA | PHA concn (g/liter) | PHA productivity (g/liter/h) | Yield (g PHA/g substrate) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Methylobacterium rhodesianum MB-126 | 2.5-liter bioreactor (batch) | 5.0-7.5% Gly, 3% Cas-Pep or Cas A | 30 | 45 | 21-22 | 50 | PHB | 13.50/8.40 | 0.223 | 0.17 | 4 |
| Ralstonia eutropha DSM 11348 | 2.5-liter bioreactor (batch) | 5.0-7.5% Gly, 3% Cas-Pep or Cas A | 30 | 67/45 | 12-32 | 47/65 | PHB | 17.00 | 0.254 | 0.17 | 4 |
| Recombinant E. coli JM109 harboring genomic DNA fragment from Streptomyces aureofaciens NRRL 2209 | Flask culture | BM, 0.5% YE, 0.5% Pep, 1% Gly | 37 | 40 | 60 | PHB | 37 | ||||
| Recombinant E. coli (ATCC PTA-1579) harboring Streptomyces aureofaciens PHB biosynthesis genes | 250-ml flask | BM, 0.5% YE, 0.5% Pep, 1% Gly | 37 | 48 | 8 | 60 | PHB | 4.80 | 0.100 | 31 | |
| Pseudomonas oleovorans NRRL B-14682 | 500-ml flask | Medium E,b 1-5% CSBP | 30 | 72 | 1.3 | 13-27 | PHB | 0.17-0.35 | 0.002-0.005 | 2 | |
| Pseudomonas corrugate 388 | 500-ml flask | Medium E,b 1% CSBP | 30 | 72 | 2.1 | 42 | mcl-PHA | 0.88 | 0.012 | 2 | |
| Mixed culture of Pseudomonas oleovorans NRRL B-14682 and Pseudomonas corrugate 388 | 500-ml flask (P. oleovorans incubated for 24 h before addition of P. corrugate and then 48 or 72 h of mixed incubation) | Medium E,b 1-5% Gly | 30 | 72/96 | 1.9, 3.4 | 40, 20 | s/mcl-PHA blends | 0.97, 0.67 | 3 | ||
| Unidentified osmophilic organism | 42-liter fed-batch culture | 1% GLP, 2.5% YE, 2.5% Pep | 37 | 182 | 21.3 | 76 | PHBV (8-10 mol% HV) | 16.20 | 0.090 | 0.23 | 24 |
| Vibrio sp. isolates M11, M14, M20, and M31 | 1,000-ml flask | MBM, 0.25% Try, 0.1% YE, 3% Gly | 30 | 34 | 0.31-0.45 | 31.4-40.7 | PHB | 0.10-0.17 | 0.003-0.005 | 7 | |
| Recombinant E. coli strain with PhaPc | 5.6-liter bioreactor (batch) | MYA, 3% Gly | 37 | 48 | 15.3 | 51.9 | PHB | 7.90 | 0.200 | 0.08 | 12 |
| Recombinant E. coliarcA mutant | 5.6-liter fed-batch culture | SGA, >0.5% Gly | 37 | 60 | 21.17 | 51 | PHB | 10.81 | 0.180 | 33 | |
| Cupriavidus necator JMP 134 | 2-liter fed-batch culture | MSM, 1% Gly, 1% crude Gly (ADM [5.5% NaCl]) | 35 | 30-50 after nitrogen limitation | 22-25 | 70/48 | PHB | 12.00 | 0.160-0.240 | 0.37/0.14 | 32 |
| Zobellella denitrificans MW1 | 250-ml flask | MSM, 1.5% Gly, 2% NaCl | 41 | 96 | 5 | 87 | PHB | 4.27 | 0.044 | 0.29 | 20 |
| 42-liter fed-batch culture | MSM, 1.5% Gly, 2% NaCl | 41 | 50 | 81.2 | 66.9 | PHB | 54.32 | 1.090 | 0.25 | This study |
CSBP, coproduct steam from soy-based biodiesel production (1% CSBP has 2.3 g available glycerol); GLP, glycerol liquid phase from biodiesel production (70% [wt/wt] glycerol); YE, yeast extract; Try, tryptone; Cas, casein; Cas A, Casamino Acids; Gly, glycerol; Pep, peptone; SGA, Sabouraud glucose agar medium; MBM, marine basal medium; BM, basal medium; ADM, ADM Hamburg AG (biodiesel manufacturer).
See reference 5.
Granule-associated protein from Azotobacter sp. strain FA8.
Using these improved results, further optimizations were done using the same fed-batch technique, but with increased amounts of glycerol and ammonia. Aiming to reach a higher cell density despite the lack of information available for the recently isolated Z. denitrificans strain MW1, nutrient requirements for cell growth were estimated according to the general calculation method for bacteria (13). In the third fed-batch fermentation (Fig. 2), the same feeding solution used in fed-batch 2 was supplemented with trace elements, iron, and calcium, providing two times their original concentrations in MSM during the entire time course of the fermentation. The total estimated amounts of glycerol and ammonia were fed to keep concentrations of 10 to 20 g/liter of glycerol and 1 to 2 g/liter of ammonium. In this fed-batch fermentation, significant increases in cell density (195%) and volumetric PHB productivity (201%) compared to those in fed-batch 2 were obtained using a semicontinuous feeding regimen, which provided adequate carbon and nitrogen for growth and polymer accumulation over a period of 63 h. In addition, the polymer content increased slightly, to 56% ± 0.7% (wt/wt), and the PHB productivity increased substantially, to 0.57 ± 0.02 g/ liter/h. The substrate conversion factor was kept higher than 0.2 g PHB/g glycerol.
FIG. 2.
Fed-batch cultivation of Z. denitrificans for PHB production from glycerol in the presence of NaCl (fed-batch 3). Z. denitrificans MW1 was cultivated in the presence of NaCl in a Biostat UD-30 stirred-tank reactor containing 20 liters of MSM with glycerol as the sole carbon source. A total of 2% NaCl was added at the beginning of the fermentation. The fermenter was inoculated with 4% (vol/vol) 24-h preculture. The pH of the medium was controlled at 7.3 during the first 20 h of growth and then at 6.8 until the end of fermentation, using NaOH (4 N) or NH4OH (25% [wt/vol]). The glycerol feeding solution (50% [vol/vol]) was supplemented with 1% MgSO4 and two times the normal concentrations of trace elements, iron, and calcium in MSM. The pO2 was controlled automatically at 20% saturation by adjusting aeration and agitation rates. Cells were grown for 63 h at 41°C. During the time course of cultivation, samples were withdrawn, and the concentrations of glycerol and ammonium, as well as the CDW and PHB content of the cells, were determined as described in Materials and Methods.
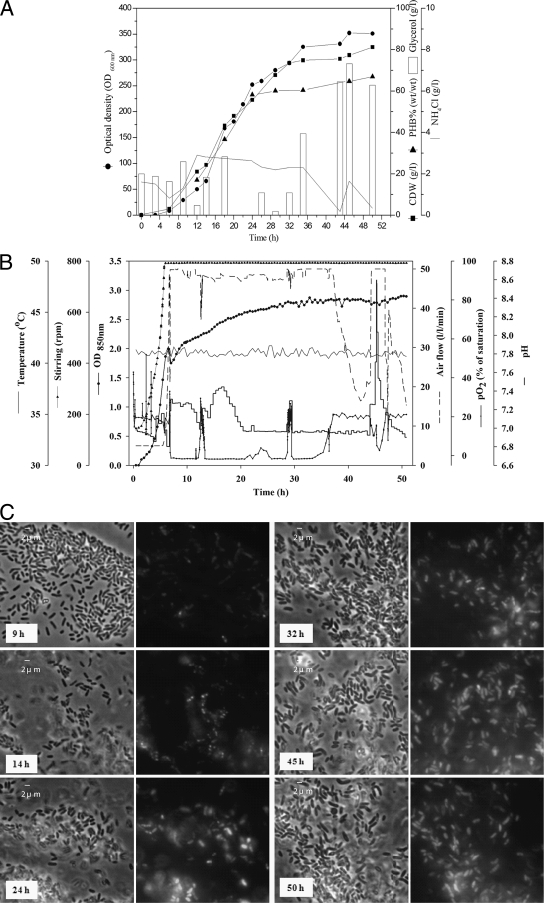
In the last fed-batch fermentation (fed-batch 4) (Fig. 3), a higher cell density and polymer content were targeted while keeping the satisfying product yield obtained during the previous fed-batch cultivations. The concentration of glycerol in the feeding solution was increased to 80% (vol/vol). NH4OH (25% [wt/vol]) was used in parallel with NaOH (4 N), instead of ammonium chloride solution, for improved control of the pH of the medium and also to provide enough nitrogen for growth. On the basis of offline analytical data (high-performance liquid chromatography analysis of the glycerol concentration in samples withdrawn from the bioreactor), the feeding rate was increased or decreased if necessary during continuous feeding to keep the glycerol concentration between 10 and 20 g/liter. The maximum growth rate and doubling time were 0.422/h and 1.64 h, respectively. The highest cell density achieved was 81.2 ± 2.5 g CDW/liter, and the maximum OD600 obtained was 351 ± 5.3. In addition, a high PHB content (66.9% ± 7.6% [wt/wt]) was reached after a relatively short fermentation time (50 h), thereby enhancing the polymer volumetric productivity up to 54.2 ± 7.9 g PHB/liter, with an improved product yield (0.25 ± 0.04 g PHB/g glycerol). In summary, during this fed-batch fermentation (30-liter final volume), 2.44 kg CDW containing 1.63 kg PHB was produced from 6.52 kg glycerol. Growth-associated PHB accumulation was monitored microscopically. Figure 3C shows the increased prevalence of PHB granules inside Z. denitrificans MW1 cells during fed-batch fermentation 4.
FIG. 3.
PHB production by Z. denitrificans MW1 during continuous feeding of glycerol (fed-batch 4). Cultivation was done in a Biostat UD-30 stirred-tank reactor containing 20 liters of MSM with glycerol as the sole carbon source. A total of 2% NaCl was added at the beginning of the fermentation. The bioreactor was inoculated with 4% (vol/vol) 24-h preculture. (A) Glycerol was fed continuously to keep its concentration higher than 10 g/liter. The glycerol feeding solution (80% [vol/vol]) was supplemented with MgSO4 (1% [wt/vol]) and two times the normal concentrations of trace elements, iron, and calcium in MSM. During the time course of cultivation, samples were withdrawn, and the concentrations of glycerol and ammonium, as well as the CDW and PHB content of cells, were determined as described in Materials and Methods. (B) The following parameters were obtained by online monitoring: OD850, pO2 (controlled automatically at 20% saturation by adjusting aeration and agitation rates), and the pH of the medium (controlled at 7.3 during the first 20 h of growth and then at 6.8 to the end of fermentation, using NaOH [4 N] and NH4OH [25% {wt/vol}] in parallel). Fermentation was operated for 50 h at 41°C. (C) Microscopic photographs showing the accumulation of PHB granules in cells during the entire time course of fermentation (9, 14, 24, 32, 45, and 50 h). The left panels (with size bars) show pictures obtained by phase-contrast light microscopy of cells possessing bright cytoplasmic inclusions. The right panels (without size bars) show fluorescence micrographs of PHB inclusions stained with Nile red.
Extraction of PHB from Z. denitrificans MW1 cells.
In this study, extraction of PHB from cells of Z. denitrificans MW1 was investigated by different methods, such as the use of detergents or decolorizing agents, dispersion of cells in hypochlorite solution and chloroform, and the selective dissolution of cell mass by sulfuric acid and hypochlorite. However, the degree of purity obtained by these methods was very low in comparison to the purity of PHB prepared by solvent extraction (data not shown). Many problems were faced during separation of non-PHB cell materials or during residual hypochlorite wash-off steps, in addition to an excessive need for high-speed centrifugation. These problems would be magnified in the large-scale extraction process for PHB and would require special precautions, especially if highly viscous PHB-solvent solutions were employed.
During the search for a simple but perfect recovery of PHB from Z. denitrificans MW1 cells, different organic solvents were investigated in small-scale experiments (100 mg cells in 2 ml organic solvent) to determine their efficiency in recovering PHB and how easy the separation of PHB from cell debris after extraction could be. Only chloroform and methylene chloride showed remarkable efficiencies in PHB recovery. No detectable PHB was extracted from the cells with carbon tetrachloride, diethyl ester, ethyl acetate, or mixtures of chloroform and acetone under the conditions studied (data not shown). Self-flocculation and flotation of Z. denitrificans MW1 cells were recorded for carbon tetrachloride, chloroform, and methylene chloride, whereas in diethyl ester and ethyl acetate the cells settled down. On the other hand, floating/settling priority in chloroform-acetone mixtures depended on the prevalence of chloroform, i.e., if the percentage of chloroform was less than 75% (vol/vol), then the cells would sediment.
Because of their PHB extraction efficiencies, chloroform and methylene chloride were selected for large-scale experiments (100 g cells in 10 volumes solvent) to optimize PHB extraction from Z. denitrificans MW1 cells with a high recovery and efficient floating. The data in Table 3 show the effects of incubation temperature and time on the efficiency of solvent recovery of PHB. At room temperature (24°C), chloroform recovered 54.5% ± 0.06% (wt/wt) of the PHB from the cells after 24 h of extraction. Extending the extraction time to up to 228 h was not correlated with an increase in the amount of recovered polymer. However, the chloroform extraction efficiency increased significantly when incubation was done at a higher temperature. At 30°C, 74.2% ± 0.09% (wt/wt) of the PHB was recovered from the cells after 24 h. The maximum recovery rate, 85.0% ± 0.10% (wt/wt) of PHB, with a purity of 98.3% ± 1.3%, was achieved after 72 h by use of 10 volumes of CHCl3 at 30°C. Figure 4 shows the complete flotation of cells in a separation funnel after 16 h of incubation at room temperature. A slightly lower recovery rate (81.7% ± 0.09% [wt/wt]) and purity (97.9% ± 0.8%) were detected after 120 h. The latter may be due to excessive cell lysis during a prolonged incubation time, which may hinder flotation of cell debris.
TABLE 3.
Solvent extraction of PHB from Z. denitrificans MW1 cellsa
| Solvent (temp) | Time (h) | Amt of extracted PHB (g/50 ml solvent) | Recovery (%) | Purity (%) |
|---|---|---|---|---|
| Chloroform (24°C) | 24 | 1.82 ± 0.04 | 54.5 ± 0.06 | ND |
| 48 | 1.84 ± 0.04 | 55.4 ± 0.06 | ND | |
| 72 | 1.85 ± 0.06 | 55.6 ± 0.06 | 97.6 ± 2.1 | |
| 228 | 1.81 ± 0.03 | 54.4 ± 0.06 | 96.8 ± 1.8 | |
| Chloroform (30°C) | 24 | 2.47 ± 0.10 | 74.2 ± 0.09 | ND |
| 48 | 2.81 ± 0.03 | 84.3 ± 0.10 | ND | |
| 72 | 2.83 ± 0.13 | 85.0 ± 0.10 | 98.3 ± 1.3 | |
| 96 | 2.82 ± 0.12 | 84.7 ± 0.10 | ND | |
| 120 | 2.72 ± 0.21 | 81.7 ± 0.09 | 97.9 ± 0.8 | |
| Chloroform (41°C) | 24 | 2.19 ± 0.05 | 65.9 ± 0.08 | 94.2 ± 2.5 |
| Methylene chloride (24°C) | 24 | 1.09 ± 0.03 | 32.6 ± 0.04 | ND |
| 48 | 1.13 ± 0.03 | 34.1 ± 0.04 | ND | |
| 72 | 1.21 ± 0.10 | 36.2 ± 0.04 | 98.8 ± 1.0 | |
| 228 | 1.05 ± 0.03 | 31.5 ± 0.04 | 96.4 ± 2.7 |
Lyophilized cells of Z. denitrificans MW1 (100-g portion) from fed-batch fermentation 4 (66.9% ± 7.6% PHB [wt/wt]) were stirred into 10 volumes of solvent at different extraction temperatures. Fifty-milliliter sample portions were withdrawn at the indicated times. Samples were separated in separation funnels overnight at room temperature. Clear PHB-solvent solutions were withdrawn and concentrated. Afterwards, 4 volumes of methanol was added to precipitate PHB, which was then filtered and dried under vacuum. Recovery and purity of PHB were analyzed by GC. Analyses were done in triplicate, and averages and standard deviations for three different analyses are presented. ND, not determined.
FIG. 4.
Flocculation and flotation of Z. denitrificans MW1 cells in separation funnels after extraction of PHB with chloroform. This experiment was done with 200 g lyophilized cells in 2 liters of CHCl3. (A) Cell-PHB-chloroform mixture directly after extraction. (B) Floating cell layer on top of clear PHB-chloroform solution after overnight incubation at room temperature.
Increasing the temperature during chloroform extraction to 41°C did not enhance the recovery efficiency, since low PHB recovery (65.9% ± 0.08%) and low purity (94.2% ± 2.5%) were observed in comparison to those reached at 30°C. Also, at high temperature, increased cell lysis resulted in a very low floating velocity of cell debris, and after 48 h of extraction at 41°C, cells could not be separated by flotation.
Extraction with methylene chloride yielded a lower recovery rate than that attained with chloroform. For instance, after 24 h, only 32.6% ± 0.04% (wt/wt) of the PHB could be recovered from the cells at 24°C; this is only about 60% of the PHB recovered by chloroform for the same temperature and incubation period. PHB recovery by methylene chloride was only marginally enhanced by increasing the extraction time to 72 h (Table 3).
DISCUSSION
Production of PHB from glycerol by the recently isolated bacterium Z. denitrificans MW1 was scaled up by using the fed-batch fermentation mode at the 42-liter scale. In the first fed-batch fermentation, a relatively high cell density and high polymer productivity were achieved after a short incubation time (24 h). Both were higher, by factors of 8.5 and 13.4, respectively, than those recently recorded in flask-scale experiments after 96 h (20). In spite of the low PHB content and the low product yield reached in this fed-batch cultivation experiment, the cell density and also the PHB content and product yield were comparable to the data published for the production of PHB from glycerol (Table 2). A slightly lower product yield (0.080 g PHB/g glycerol) was recorded for recombinant E. coli strains in 5.6-liter batch fermentations (12). A similar cell density was also reported for a recombinant arcA mutant of E. coli in a 5.6-liter fed-batch culture (33). A higher PHB content (76% [wt/wt]) was achieved by an unidentified osmophilic organism in a 42-liter fed-batch fermentation using glycerol liquor phase with yeast extract and peptone. However, the productivity was very low (0.09 g/liter/h) because of the long cultivation time (182 h) (24). Several flask-scale cultivations were carried out with a variety of bacterial strains, such as a recombinant E. coli strain harboring PHB synthesis genes from Streptomyces aureofaciens (31, 37), different Pseudomonas strains (2, 3), and several Vibrio sp. isolates (7), but only comparably low PHA productivities were attained. Batch culture of R. eutropha DSM 11348 in a 2.5-liter fermenter with glycerol and casein peptone or Casamino Acids resulted in the maximum productivity (0.254 g/liter/h) reported for PHB production from glycerol (4).
Significant decreases in PHB content (from 70 to 48% [wt/wt]) and also in product yield (from 0.37 to 0.14 g/g) were recorded for PHB production by Cupriavidus necator JMP 134 using NaCl-contaminated crude glycerol (5.5% [wt/vol] NaCl) (32), which might be related to the increased osmotic pressure caused by NaCl accumulation during glycerol feeding. This problem can be ignored if halophilic bacteria or moderate halophiles are used under such conditions. On the other hand, some of these bacteria have been studied for PHB productivity (16, 36), but the need for additional salts for strain requirements elevated the production costs (29, 40) and could further accelerate the corrosion of commonly used stainless steel fermenters (8, 35).
In this respect, further optimizations of fed-batch fermentations by Z. denitrificans MW1 were made by utilizing the reported enhanced growth and polymer content in the presence of sodium chloride (20). First, the modified feeding and pH control improved the product yield to 260%, since half the amount of glycerol (1.74 kg) was utilized, producing 445.4 g PHB with a higher polymer productivity (0.47 ± 0.02 g/liter/h). Further optimizations of fed-batch fermentation with increased feeding rates of glycerol and ammonia, together with the precise control of pH, maximized PHB productivity, to 1.09 ± 0.16 g/liter/h, with a higher polymer content (66.9% ± 7.6% [wt/wt]) and a high product yield (0.25 ± 0.04 g PHB/g glycerol), which is the highest yield reported so far for the production of PHA from glycerol (Table 2).
Although Z. denitrificans MW1 accumulates PHB during growth, slightly increased PHB contents were detected at a low concentration of ammonium chloride (20). Therefore, a short finale of limited nitrogen conditions was provided in all fed-batch fermentations (batches 1, 2, 3, and 4) for enhanced polymer accumulation. An increased PHB content after applying the nitrogen limitation condition was also reported for growth-associated PHB accumulation by A. latus during batch and fed-batch cultures (45).
As shown in Fig. 3A, the offline direct control feeding regimen used has some critical points, such as the drop in glycerol concentration to <5 g/liter at 12 and 29 h, and also the ammonia concentration decreased to 0.17 g/liter at 43 h. At the same time that glycerol was depleted (12 and 29 h), an increase in pO2 was detected (Fig. 3B), and this could be a basis for the application of indirect control of glycerol concentration in further optimization. However, precise direct control of glycerol is also anticipated to maximize cell density and polymer productivity. High PHB productivity from glucose (2.42 g/liter/h) was achieved by a glucose-utilizing mutant of A. eutrophus H16 when a direct online glucose control was applied compared to the productivity reached with pH state for a fed-batch (0.25 g/liter/h) of the same strain (23). Also, for higher-cell-density cultivation, a pure oxygen supply is a significant concern, since the maximum stirring and airflow used in fed-batch 4 could not provide enough dissolved oxygen in the exponential growth phase (up to 36 h) (Fig. 3B). Due to its physicochemical characteristics, glycerol as a substrate will pose a challenge in oxygen transfer rates as well.
Eventually, ease of recovery of PHA is a very important parameter for economically feasible production of the polyester (22). In this respect, flotation of Z. denitrificans MW1 cells after solvent extraction is considered the easiest way to achieve a cell-free PHB-solvent solution by an in situ separation step, avoiding additional costs for centrifugation and waste of polymer during recovery steps. The highest recovery rate was reached with chloroform at 30°C after 72 h. Recently, another method of selective dissolved-air flotation for PHA recovery from cell debris of Pseudomonas putida was also investigated (44). A slightly lower recovery and purity were reported for the extraction of PHB from A. eutrophus by chlorinated solvent reflux (39). In spite of the short extraction time recorded (15 min), the high temperatures (61°C for CHCl3 and 40°C for CH2Cl2) at which reflux was done and the need for 24 h of cell pretreatment with acetone should hinder the application of this recovery method at the industrial scale.
Compared to all other strains used for PHB production from glycerol (Table 2), this study recommends Z. denitrificans MW1 as a new and attractive option for large-scale production of PHB by use of residual crude glycerol from the biodiesel industry.
Acknowledgments
Financial support of this study by BASF AG (Ludwigshafen, Germany) is gratefully acknowledged.
We thank Yasser Elbahloul, Ahmad Sallam, Kaichien Lin, Kay Frey, and Herbert Ahlers for their assistance during fermentation.
Footnotes
Published ahead of print on 7 August 2009.
REFERENCES
- 1.Anderson, A. J., and E. A. Dawes. 1990. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol. Rev. 54:450-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashby, R. D., D. K. Y. Solaiman, and T. A. Foglia. 2004. Bacterial poly(hydroxyalkanoate) polymer production from the biodiesel co-product stream. J. Polym. Environ. 12:105-112. [Google Scholar]
- 3.Ashby, R. D., D. K. Y. Solaiman, and T. A. Foglia. 2005. Synthesis of short-/medium-chain-length poly(hydroxyalkanoate) blends by mixed culture fermentation of glycerol. Biomacromolecules 6:2106-2112. [DOI] [PubMed] [Google Scholar]
- 4.Bormann, E. J., and M. Roth. 1999. The production of polyhydroxybutyrate by Methylobacterium rhodesianum and Ralstonia eutropha in media containing glycerol and casein hydrolysates. Biotechnol. Lett. 21:1059-1063. [Google Scholar]
- 5.Brandl, H., R. A. Gross, R. W. Lenz, and R. C. Fuller. 1988. Pseudomonas oleovorans as a source of poly(β-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl. Environ. Microbiol. 54:1977-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braunegg, G., G. Lefebvre, and K. F. Genser. 1998. Polyhydroxyalkanoates, biopolyesters from renewable resources: physiological and engineering aspects. J. Biotechnol. 65:127-161. [DOI] [PubMed] [Google Scholar]
- 7.Chien, C. C., C. C. Chen, M. H. Choi, S. S. Kung, and Y. H. Wei. 2007. Production of poly-beta-hydroxybutyrate (PHB) by Vibrio spp. isolated from marine environment. J. Biotechnol. 132:259-263. [DOI] [PubMed] [Google Scholar]
- 8.Chisti, Y. 1992. Build better industrial bioreactors. Chem. Eng. Prog. 88:55-58. [Google Scholar]
- 9.Choi, J., and S. Y. Lee. 1997. Process analysis and economic evaluation for poly(3-hydroxybutyrate) production by fermentation. Bioproc. Eng. 17:335-342. [Google Scholar]
- 10.Choi, J., and S. Y. Lee. 1999. Factors affecting the economics of polyhydroxyalkanoates production by bacterial fermentation. Appl. Microbiol. Biotechnol. 51:13-21. [Google Scholar]
- 11.da Silva, G. P., M. Mack, and J. Contiero. 2009. Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol. Adv. 27:30-39. [DOI] [PubMed] [Google Scholar]
- 12.de Almeida, A., P. I. Nikel, A. M. Giordano, and M. J. Pettinari. 2007. Effects of granule-associated protein PhaP on glycerol-dependent growth and polymer production in poly(3-hydroxybutyrate)-producing Escherichia coli. Appl. Environ. Microbiol. 73:7912-7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greasham, R. L. 1993. Media for microbial fermentations, p. 127-139. In H.-J. Rehm and G. Reed (ed.), Biotechnology, 2nd ed., vol. 3. Bioprocessing. VCH Verlagsgesellschaft GmbH, Weinheim, Germany. [Google Scholar]
- 14.Hahn, S. K., Y. K. Chang, B. S. Kim, K. M. Lee, and H. N. Chang. 1993. The recovery of poly(3-hydroxybutyrate) by using dispersions of sodium hypochlorite solution and chloroform. Biotechnol. Tech. 7:209-212. [DOI] [PubMed] [Google Scholar]
- 15.Hahn, S. K., Y. K. Chang, B. S. Kim, and H. N. Chang. 1994. Optimization of microbial poly(3-hydroxybutyrate) recovery using dispersions of sodium hypochlorite solution and chloroform. Biotechnol. Bioeng. 44:256-261. [DOI] [PubMed] [Google Scholar]
- 16.Hezayen, F. F., B. H. A. Rehm, R. Eberhardt, and A. Steinbüchel. 2000. Polymer production by two newly isolated extremely halophilic archaea: application of a novel corrosion-resistant bioreactor. Appl. Microbiol. Biotechnol. 54:319-325. [DOI] [PubMed] [Google Scholar]
- 17.Holmes, P. A. 1985. Applications of PHB—a microbially produced biodegradable thermoplastic. Phys. Technol. 16:32-36. [Google Scholar]
- 18.Hrabak, O. 1992. Industrial production of poly-β-hydroxybutyrate. FEMS Microbiol. Rev. 103:251-256. [Google Scholar]
- 19.Huijberts, G. N. M., H. van der Wal, C. Wilkinson, and G. Eggink. 1994. Gas-chromatographic analysis of poly(3-hydroxyalkanoates) in bacteria. Biotechnol. Tech. 8:187-192. [Google Scholar]
- 20.Ibrahim, M. H. A., and A. Steinbüchel. Zobellella denitrificans strain MW1, a newly isolated bacterium suitable for poly(3-hydroxybutyrate) production from glycerol. J. Appl. Microbiol. doi: 10.1111/j.1365-2672.2009.04413.x. [DOI] [PubMed]
- 21.Kessler, B., R. Weusthuis, B. Witholt, and G. Eggink. 2001. Production of microbial polyesters: fermentation and downstream processes. Adv. Biochem. Eng. Biotechnol. 71:159-182. [DOI] [PubMed] [Google Scholar]
- 22.Khanna, S., and A. K. Srivastava. 2005. Recent advances in microbial polyhydroxyalkanoates. Processes Biochem. 40:607-619. [Google Scholar]
- 23.Kim, B. S., S. C. Lee, S. Y. Lee, H. N. Chang, Y. K. Chang, and S. I. Woo. 1994. Production of poly(3-hydroxybutyric acid) by fed-batch culture of Alcaligenes eutrophus with glucose concentration control. Biotechnol. Bioeng. 43:892-898. [DOI] [PubMed] [Google Scholar]
- 24.Koller, M., R. Bona, G. Braunegg, C. Hermann, P. Horvat, M. Kroutil, J. Martinz, J. Neto, L. Pereira, and P. Varila. 2005. Production of polyhydroxyalkanoates from agricultural waste and surplus materials. Biomacromolecules 6:561-565. [DOI] [PubMed] [Google Scholar]
- 25.Lakshman, K., and T. R. Shamala. 2006. Extraction of polyhydroxyalkanoate from Sinorhizobium meliloti cells using Microbispora sp. culture and its enzymes. Enzyme Microb. Technol. 39:1471-1475. [Google Scholar]
- 26.Lee, S. Y. 1996. Bacterial polyhydroxyalkanoates. Biotechnol. Bioeng. 49: 1-14. [DOI] [PubMed] [Google Scholar]
- 27.Lee, S. Y. 1996. Plastic bacteria? Progress and prospects for polyhydroxyalkanoate production in bacteria. Trends Biotechnol. 14:431-438. [Google Scholar]
- 28.Lee, S. Y., J. I. Choi, and S. H. Lee. 2000. Production of polyhydroxyalkanoates by fermentation of bacteria. Macromol. Symp. 159:259-266. [Google Scholar]
- 29.Lillo, J. G., and F. Rodriguez-Valera. 1990. Effects of culture conditions on poly(3-hydroxybutyric acid) production by Haloferax mediterranei. Appl. Environ. Microbiol. 56:2517-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.López-Cortés, A., A. Lanz-Landázuri, and J. Q. Garacía-Maldonado. 2008. Screening and isolation of PHB-producing bacteria in a polluted marine microbial mat. Microb. Ecol. 56:112-120. [DOI] [PubMed] [Google Scholar]
- 31.Mahishi, L. H., G. Tripathi, and S. K. Rawal. 2003. Poly(3-hydroxybutyrate) (PHB) synthesis by recombinant Escherichia coli harbouring Streptomyces aureofaciens PHB biosynthesis genes: effect of various carbon and nitrogen sources. Microbiol. Res. 158:19-27. [DOI] [PubMed] [Google Scholar]
- 32.Mothes, G., C. Schnorpfeil, and J. U. Ackermann. 2007. Production of PHB from crude glycerol. Eng. Life Sci. 7:475-479. [Google Scholar]
- 33.Nikel, P. I., M. J. Pettinari, M. A. Galvagno, and B. S. Méndez. 2008. Poly(3-hydroxybutyrate) synthesis from glycerol by a recombinant Escherichia coli arcA mutant in fed-batch microaerobic cultures. Appl. Microbiol. Biotechnol. 77:1337-1343. [DOI] [PubMed] [Google Scholar]
- 34.Pachauri, N., and B. He. 2006. Value-added utilization of crude glycerol from biodiesel production: a survey of current research activities. Paper no. 066223. American Society of Agricultural and Biological Engineers, St. Joseph, MI.
- 35.Park, J. O., S. Matsch, and H. Böhni. 2002. Effects of temperature and chloride concentration on pit initiation and early pit growth of stainless steel. J. Electrochem. Soc. 149:B34-B39. [Google Scholar]
- 36.Quillaguamán, J., S. Hashim, F. Bento, B. Mattiasson, and R. Hatti-Kaul. 2005. Poly(β-hydroxybutyrate) production by a moderate halophile, Halomonas boliviensis LC1 using starch hydrolysate as substrate. J. Appl. Microbiol. 99:151-157. [DOI] [PubMed] [Google Scholar]
- 37.Ramachander, T. V. N., D. Rohini, A. Belhekar, and S. K. Rawal. 2002. Synthesis of PHB by recombinant E. coli harboring an approximately 5 kb genomic DNA fragment from Streptomyces aureofaciens NRRL2209. Int. J. Biol. Macromol. 31:63-69. [DOI] [PubMed] [Google Scholar]
- 38.Ramsay, J. A., E. Berger, B. A. Ramsay, and C. Chavarie. 1990. Recovery of poly-3-hydroxyalkanoic acid granules by a surfactant-hypochlorite treatment. Biotechnol. Tech. 4:221-226. [Google Scholar]
- 39.Ramsay, J. A., E. Berger, R. Voyer, C. Chavarie, and B. A. Ramsay. 1994. Extraction of poly-3-hydroxybutyrate using chlorinated solvents. Biotechnol. Tech. 8:589-594. [Google Scholar]
- 40.Rodriguez-Valera, F., and J. A. G. Lillo. 1992. Halobacteria as producers of polyhydroxyalkanoates. FEMS Microbiol. Rev. 103:181-186. [Google Scholar]
- 41.Schlegel, H. G., H. Kaltwasser, and G. Gottschalk. 1961. Ein Submersverfahren zur Kultur Wasserstoffoxydierender Bakterien: Wachstumsphysiologische Untersuchungen. Arch. Mikrobiol. 38:209-222.13747777 [Google Scholar]
- 42.Solaiman, D. K. Y., R. D. Ashby, T. A. Foglia, and W. N. Marmer. 2006. Conversion of agricultural feedstock and coproducts into poly(hydroxyalkanoates). Appl. Microbiol. Biotechnol. 71:783-789. [DOI] [PubMed] [Google Scholar]
- 43.Steinbüchel, A., and B. Füchtenbusch. 1998. Bacterial and other biological systems for polyester production. Trends Biotechnol. 16:419-427. [DOI] [PubMed] [Google Scholar]
- 44.Van Hee, P., A. C. M. R. Elumbaring, R. G. J. M. van der Lans, and L. A. M. van der Wielen. 2006. Selective recovery of polyhydroxyalkanoates inclusion bodies from fermentation broth by dissolved air flotation. J. Colloid Interface Sci. 297:595-606. [DOI] [PubMed] [Google Scholar]
- 45.Wang, F., and S. Y. Lee. 1997. Poly(3-hydroxybutyrate) production with high productivity and high polymer content by a fed-batch culture of Alcaligenes latus under nitrogen limitation. Appl. Environ. Microbiol. 63:3703-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamane, T., and S. Shimizu. 1984. Fed-batch techniques in microbial processes. Adv. Biochem. Eng. Biotechnol. 30:147-194. [Google Scholar]
- 47.Yamane, T., M. Fukunaga, and Y. W. Lee. 1996. Increased PHB productivity by high-cell-density fed-batch culture of Alcaligenes latus, a growth-associated PHB producer. Biotechnol. Bioeng. 50:197-202. [DOI] [PubMed] [Google Scholar]
- 48.Yu, J., and L. X. L. Chen. 2006. Cost-effective recovery and purification of polyhydroxyalkanoates by selective dissolution of cell mass. Biotechnol. Prog. 22:547-553. [DOI] [PubMed] [Google Scholar]
- 49.Zinn, M., and R. Hany. 2005. Tailored material properties of polyhydroxyalkanoates through biosynthesis and chemical modification. Adv. Eng. Mat. 7:408-411. [Google Scholar]