Abstract
Human infection with Campylobacter jejuni is often associated with the consumption of foods that have been exposed to both chilling and high temperatures. Despite the public health importance of this pathogen, little is known about the effects of cold exposure on its ability to survive a subsequent heat challenge. This work examined the effect of rapid exposure to chilling, as would occur in poultry processing, on the heat resistance at 56°C of two C. jejuni strains, 11168 and 2097e48, and of Escherichia coli K-12. Unlike E. coli K-12, whose cold-exposed cells showed increased sensitivity to 56°C, such exposure had only a marginal effect on subsequent heat resistance in C. jejuni. This may be explained by the finding that during rapid chilling, unlike E. coli cells, C. jejuni cells are unable to alter their fatty acid composition and do not adapt to cold exposure. However, their unaltered fatty acid composition is more suited to survival when cells are exposed to high temperatures. This hypothesis is supported by the fact that in C. jejuni, the ratio of unsaturated to saturated fatty acids was not significantly different after cold exposure, but it was in E. coli. The low-temperature response of C. jejuni is very different from that of other food-borne pathogens, and this may contribute to its tolerance to further heat stresses.
Campylobacter jejuni is the leading cause of bacterial diarrheal disease worldwide and is the most common antecedent to the peripheral neuropathies Guillain-Barré syndrome and Miller Fisher syndrome (32, 35). In England and Wales, there were ca. 47,000 Campylobacter cases reported in 2006, a probable 10-fold underestimate of the true incidence, as the World Health Organization calculates that ca. 1% of the population of Europe will be infected each year.
Most Campylobacter infections are food borne, although contaminated water and environmental exposure have also been implicated (5). Currently, identified high-risk factors include the consumption of chicken, especially when undercooked (15), barbecued meat (2), and raw or improperly pasteurized milk (10, 22). In all of these cases, the infecting Campylobacter population would have been exposed to both low and high temperatures before being consumed.
Despite their importance as human pathogens, little is known about how campylobacters cope with hostile conditions in the transmission chain from animals to humans. Campylobacter presents an interesting conundrum. It is generally considered to be fragile compared to other food-borne pathogens (7) but is recognized as the leading cause of food-borne disease, and vehicles are frequently foods that have received a degree of heat exposure.
Although Campylobacter has been shown to be able to mount an acid tolerance response (28), it lacks a σ38 (RpoS) homologue (23) and cold shock proteins (17), which is thought to limit its ability to respond to hostile conditions common in the food chain. Despite lacking many of the classical bacterial stress responses, Campylobacter can survive for extended periods at low temperatures on key foods such as raw chicken (12). It is important to determine how the responses of Campylobacter to cold could affect its survival in other parts of the food chain. Does preexposure to cold make it more heat sensitive, as is the case for Escherichia coli and Salmonella spp. (20), or are its responses different, as work on one strain (27) suggests?
The ability to deal with cold is inherently important to a wide range of mesophilic bacteria (29), and it is likely that some cold exposure tolerance mechanisms are highly conserved. One of the most important of these is a change in fatty acid composition, particularly in the outer membrane (36). Maintaining membrane homoviscosity is critical for continuing growth, and cellular membrane fatty acid composition is altered as the growth temperature changes (30). For example, during exposure to reduced temperatures, Salmonella spp. increase levels of unsaturated fatty acids (UFA), whereas Listeria spp. raise those of branched fatty acids in cellular membranes (33). Both responses increase membrane fluidity. Increased levels of cyclic fatty acids have been shown to have a role in raising the heat resistance of Pediococcus spp. (3) and to increase the stability of the membrane structure (9). Ulmer et al. (37) showed that as the growth temperature for Lactobacillus plantarum decreased, the proportion of UFA in cell membranes rose, leading to increased membrane fluidity and allowing the membrane to be maintained in a liquid gel state. A temperature-dependent shift in fatty acid production has been seen in E. coli. When the growth temperature is reduced, the enzyme activity of FabF increases, leading to an increase in the conversion of 16:1 fatty acids to 18:1 fatty acids (8, 11). Desaturation enhances the effects of the branched fatty acids produced during acclimation of bacterial cells to low temperatures (34).
Although changes in fatty acid synthesis have been seen in Campylobacter coli upon a temperature downshift from 42 to 20°C (19), there is currently very little information on the effects of exposure to refrigeration conditions on the whole-cell fatty acid composition of C. jejuni. A study by Hazeleger et al. (17) compared changes in fatty acid composition in coccoid cells held at 4 or 12°C for 14 days with those in control spiral cells grown at 37°C for 24 h. They found that few significant changes in composition occurred at the lower temperatures, whereas at 25°C there were more marked changes in many fatty acids.
The work reported here examines the effects of exposure to 6°C for 24 h on whole-cell fatty acid composition and heat resistance of C. jejuni to determine whether Campylobacter has a similar response to chill to the prototypic response of E. coli.
MATERIALS AND METHODS
Cultivation of bacteria.
All strains were recovered from stocks kept at −80°C by plating beads (Cryobank; Pro-Lab Diagnostics, Neston, United Kingdom) onto Columbia base agar containing 5% defibrinated horse blood (BA; Oxoid, Basingstoke, United Kingdom).
Campylobacter jejuni on BA plates was incubated under microaerobic conditions (5.3% O2) at 37°C for 48 h. For each experiment, triplicate broth cultures were prepared by inoculating a single colony from the above plates into 6.5 ml of nutrient broth number 2 (NB2; Oxoid) containing Campylobacter growth supplement (Oxoid) in a 7-ml container. Broths were incubated for 24 h at 37°C, aliquots required for assays were removed, and the remaining culture was stored at 6°C for 24 h.
Escherichia coli K-12 was incubated on BA plates at 37°C for 24 h. For each experiment, triplicate broth cultures were prepared by inoculating a single colony from the above plates into 5 ml of NB2, which was incubated for 24 h at 37°C. Aliquots required for assays were removed, and the remaining culture was stored at 6°C for 24 h.
Effect of prechill on survival of C. jejuni and E. coli during heat shock.
Cultures were prepared as described above, in triplicate. A 10-μl volume of each was inoculated into 1 ml of preheated NB2. For all Campylobacter strains, Campylobacter growth supplement (Oxoid) was added to the medium, which was maintained at 56°C throughout the assay. Samples were taken after 5, 10, 15, 20, 25, and 30 min of exposure to 56°C and decimally diluted in maximum recovery diluent (Oxoid). The dilutions were plated onto BA plates and incubated under microaerobic conditions at 37°C for 48 h for C. jejuni. Plates for E. coli were incubated aerobically at 37°C for 24 h. Colonies were counted and the log10 reduction in CFU/ml−1 determined.
Exposure to 6°C and whole-cell fatty acid compositions of C. jejuni and E. coli.
Six 50-ml aliquots of NB2 containing Campylobacter supplement in 55-ml centrifuge tubes were inoculated with 5 μl of C. jejuni culture prepared as described above and then were incubated at 37°C for 22 h. The resulting culture was centrifuged at 8,000 × g for 5 min. The supernatants were discarded, and the pellets were resuspended in a fresh tube in 5 ml of prewarmed (37°C) broth for the control cultures and prechilled (6°C) broth for the treated cultures. Medium at the appropriate temperature was added to give a final volume of 50 ml. After incubation at 37 or 6°C for a total of 24 h, cells were centrifuged at either room temperature or 6°C at 8,000 × g for 5 min and the supernatant removed. Cells were resuspended in phosphate-buffered saline and transferred to a Pyrex tube, and then 0.1% hydroquinone in methanol and a concentrated solution of KOH were added to give a final concentration of 2 M KOH. An internal standard of C21:0 was added, and the sample was hydrolyzed at 60°C for 2 h. Samples were acidified and fatty acids extracted into light petroleum (bp, 40 to 60°C), followed by methylation using a solution of diazomethane in diethyl ether. Resulting fatty acid methyl esters (FAME) were analyzed by gas-liquid chromatography using a Finnigan Focus gas chromatograph (Thermoelectron Corporation, Cheshire, United Kingdom) equipped with a flame ionization detector (FID). Samples were injected in the split mode (50:1), using helium at a constant flow as the carrier gas. The injector temperature was 275°C, and the FID temperature was 250°C. Separation was carried out on a 50-m by 0.25-mm-internal-diameter CP Sil 88 FAME capillary column (Varian, Oxford, United Kingdom). The initial oven temperature was 150°C held for 40 min and then was increased by 1°C/min to 180°C, held for 1 min, increased by 5°C/min to 220°C, and held for 10 min. The output from the FID was integrated using a Chrom-Card data system 2.3 (Thermoelectron, Cheshire, United Kingdom). The linearity of the system was tested using a reference monoenoic FAME mix (Thames Restek UK Ltd., Saunderton, United Kingdom). Fatty acids were identified using commercial standards (Supelco [Matreya, PA] or Sigma-Aldrich [St. Louis, MO]).
Analysis of data.
All experiments were carried out with three biological replicates per treatment. The observed bacterial death curves proved to be concave from below. Thus, the Weibull model (log S = −βtα, where S and t represent the survivor fraction and time, respectively, and α and β define curvature and scaling parameters, respectively) (31) was fitted to every death curve generated in these experiments, and these derived curves were used for further analysis.
For curve shape (α values), a value of <1 indicates that the curve is concave from below, whereas an α value of 1 indicates a linear relationship between log S and t (i.e., exponential decay of cell concentration) and α values of >1 would generate a convex curve from below. The β value is related to how fast the instantaneous specific rate of death [d(log S)/dt] changes along the death curve. Statistical analysis of Weibull death curves for each strain under different conditions was carried out using two-way analysis of variance (ANOVA) in GraphPad Prism (GraphPad Software, CA). Differences in fatty acid composition were analyzed using Student's t test. P values of <0.05 were considered significant. The ratio of UFA to saturated fatty acids (SFA) was used as a measure of membrane fluidity (6).
RESULTS
Exposure to 6°C for 24 h and subsequent heat tolerance of C. jejuni and E. coli.
All bacterial strains used in this study showed susceptibility to 56°C in NB2, regardless of the preexposure temperature. All death curves analyzed using the Weibull method had α values (Table 1) of <1, indicating the presence of tailing and a resistant subpopulation in the C. jejuni and E. coli cultures tested.
TABLE 1.
Weibull analysis of death curves for C. jejuni 2097E48, C. jejuni 11168, and E. coil K-12 at 56°C in nutrient broth, before and after exposure to 6°Ca
| Strain | α (mean ± SE)
|
P value | β (mean ± SE)
|
P value | ||
|---|---|---|---|---|---|---|
| Control | Chill group | Control | Chill group | |||
| C. jejuni 2097E48/1 | 0.410 ± 0.04 | 0.652 ± 0.23 | 0.185 | 0.789 ± 0.13 | 0.466 ± 0.32 | 0.11 |
| C. jejuni 11168 | 0.611 ± 0.09 | 0.475 ± 0.06 | 0.0006 | 0.546 ± 0.04 | 0.697 ± 0.11 | 0.073 |
| E. coli | 0.479 ± 0.05 | 0.50 ± 0.014 | 0.21 | 0.519 ± 0.07 | 0.674 ± 0.72 | 2.6 × 10−5 |
Weibull analysis was performed on resulting death curves for triplicate biological replicates of each bacterial strain at 56°C, recovered on BA plates before and after exposure to 6°C. P values of <0.05 were considered significant and are shown in bold.
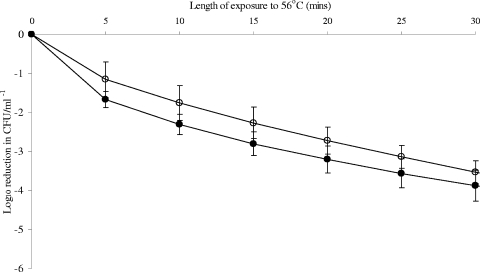
Preexposure to 6°C for 24 h did not have a significant effect on the heat tolerance of C. jejuni 2097e48 (Fig. 1), with no significant difference between treatments (P value of 0.1614) determined using ANOVA. Weibull analysis also showed no significant difference between α (0.410 ± 0.04 and 0.652 ± 0.23, respectively) and β (0.789 ± 0.13 and 0.466 ± 0.32, respectively) values (Table 1) for control and chill-exposed cultures. There were also no significant differences over time between treatments for this strain (P value = 0.3532).
FIG. 1.
Effect of exposure to 6°C on the shape of the death curve for C. jejuni 2097e48 at 56°C. Weibull analysis was performed on triplicate biological replicates for the resulting death curves of C. jejuni 2097e48 at 56°C, before and after exposure to 6°C. Open circles represent controls (37°C), and closed circles represent cells exposed to 6°C before heat treatment. Death values were analyzed using ANOVA at each time point. P values of <0.05 were considered significantly different; no significant differences were seen at any time points.
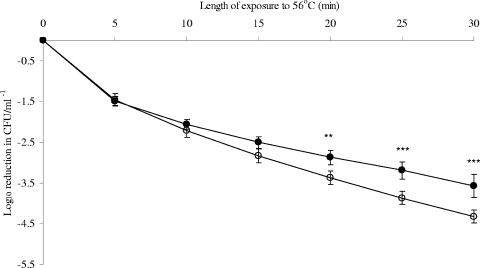
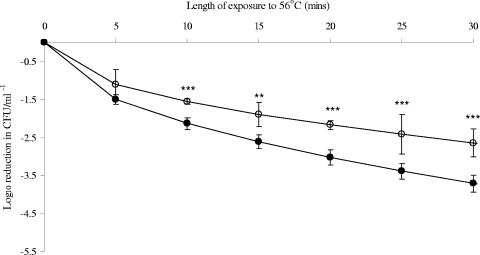
However, preexposure to 6°C significantly increased the heat tolerance of C. jejuni 11168 at later time points (Fig. 2) and significantly reduced that of E. coli K-12 at all time points beyond 5 min of heating (Fig. 3). P values were <0.0001 for both bacteria. Weibull analysis (Table 1) also showed an increase in the heat tolerance of C. jejuni 11168 at 56°C, with α values of 0.611 ± 0.09 and 0.475 ± 0.06 for control cultures and cultures after exposure to 6°C, respectively. No significant change in β values was seen. Weibull analysis of E. coli K-12 death curves confirmed that exposure to 6°C for 24 h significantly reduced the heat tolerance, with β values increasing from 0.519 ± 0.07 to 0.673 ± 0.72. No significant difference in α values (curve shape) was detected.
FIG. 2.
Effect of exposure to 6°C on the shape of the death curve for C. jejuni 11168 at 56°C. Weibull analysis was performed on triplicate biological replicates for the resulting death curves of C. jejuni 11168 at 56°C, before and after exposure to 6°C. Open circles represent controls (37°C), and closed circles represent cells exposed to 6°C before heat treatment. Mean values resulting from Weibull analysis of death curves are shown, with standard deviations given as error bars. Death values were analyzed using ANOVA at each time point. P values of <0.05 were considered significantly different and are shown on the figure as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
FIG. 3.
Effect of exposure to 6°C on the shape of the death curve for E. coli at 56°C. Weibull analysis was performed on triplicate biological replicates for the resulting death curves of E. coli K-12 at 56°C, before and after exposure to 6°C. Open circles represent controls (37°C), and closed circles represent cells exposed to 6°C before heat treatment. Mean values resulting from Weibull analysis of death curves are shown, with standard deviations given as error bars. Death values were analyzed using ANOVA at each time point. P values of <0.05 were considered significantly different and are shown on the figure as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
Exposure to 6°C and whole-cell fatty acid compositions of C. jejuni and E. coli K-12.
The method used in this study allowed the identification of 22 fatty acids, although the column used excluded the running of hydroxy fatty acids. The fatty acid compositions of each of the two strains of C. jejuni were changed little by cold exposure. However, significant changes were seen in the fatty acid composition of E. coli after exposure to 6°C, which affected the UFA-to-SFA ratio.
Campylobacter jejuni 2097e48 had reduced hexadecanoic acid (16:0) and increased heptadecanoic acid (17:0) present after exposure to 6°C (Table 2). However, these changes do not have a significant effect on the ratio of UFA to SFA and thus are unlikely to affect membrane fluidity (Table 3). The ratio of UFA to SFA under control conditions was 0.55 ± 0.02, and that after exposure to 6°C for 24 h was 0.57 ± 0.01. A similar response was seen with C. jejuni 11168, where there was a decrease in dodecanoic acid (12:0) and an increase in trans-18:1, dihydrosterculic (cis-9,10-cyc19) and alpha-linolenic (18:3n-3) acids (Table 2). There was no significant change in the ratio of UFA to SFA under control conditions (0.69 ± 0.02) compared to exposure to 6°C for 24 h (0.73 ± 0.07) (Table 3).
TABLE 2.
Whole-cell fatty acid compositions of C. jejuni 2097e48 and 11168 and E. coli K-12 before and after exposure to 6°C for 24 ha
| Strain (group) | % Fatty acid (mean ± SD)
|
% Fatty acid (mean ± SD)
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12:0 | 13:0 | 14:0 | 15:0 | 16:0 | 16:1 | 17:0 | Δ17:0 | 18:0 | t18:1 | 18:1n-9 | 18:1n-7 | c9, 10 Δ19:0 | c11, 12 Δ19:0 | 18:3n-3 | 20:3n-6 | 20:4n-6 | |
| C. jejuni 2097e48 (control) | 0.91 ± 0.08 | 0.16 ± 0.20 | 17.80 ± 0.40 | 0.17 ± 0.01 | 36.63 ± 0.17 | 7.97 ± 0.17 | 0.07 ± 0.01 | 0.17 ± 0.03 | 1.20 ± 0.18 | 0.08 ± 0.03 | 0.30 ± 0.06 | 22.91 ± 0.80 | 0.16 ± 0.02 | 8.97 ± 0.10 | 0.03 ± 0.02 | ND | ND |
| C. jejuni 2097e48 (chill) | 0.93 ± 0.05 | 0.03 ± 0.00 | 17.41 ± 0.40 | 0.19 ± 0.02 | 35.96 ± 0.19*** | 8.05 ± 0.07 | 0.09 ± 0.01* | 0.20 ± 0.02 | 1.46 ± 0.30 | 0.10 ± 0.01 | 0.36 ± 0.10 | 23.25 ± 0.51 | 0.17 ± 0.05 | 8.96 ± 0.52 | 0.02 ± 0.02 | ND | ND |
| C. jejuni 11168 (control) | 0.81 ± 0.09 | 0.05 ± 0.01 | 13.76 ± 0.34 | 0.20 ± 0.02 | 33.13 ± 0.47 | 8.09 ± 0.05 | 0.10 ± 0.00 | 0.33 ± 0.03 | 2.55 ± 0.02 | 0.10 ± 0.02 | 0.55 ± 0.16 | 26.22 ± 1.46 | 0.17 ± 0.02 | 10.21 ± 0.65 | 0.01 ± 0.01 | ND | ND |
| C. jejuni 11168 (chill) | 0.74 ± 0.00** | 0.02 ± 0.03 | 13.09 ± 0.16 | 0.21 ± 0.02 | 32.96 ± 0.14 | 8.08 ± 0.35 | 0.10 ± 0.00 | 0.27 ± 0.09 | 2.92 ± 0.27 | 0.20 ± 0.01*** | 0.66 ± 0.04 | 27.75 ± 3.05 | 0.21 ± 0.01* | 8.86 ± 2.38 | 0.05 ± 0.00*** | ND | ND |
| E. coli K-12 (control) | 3.87 ± 0.12 | 0.59 ± 0.03 | 8.62 ± 0.15 | 4.40 ± 0.16 | 31.05 ± 0.69 | 15.42 ± 0.16 | 1.21 ± 0.04 | 14.90 ± 0.22 | 0.57 ± 0.07 | 0.246 ± 0.00 | 0.20 ± 0.03 | 10.37 ± 0.28 | 0.01 ± 0.01 | 1.84 ± 0.03 | 0.05 ± 0.02 | 0.02 ± 0.00 | 0.04 ± 0.03 |
| E. coli K-12 (chill) | 3.62 ± 0.06** | 0.48 ± 0.01*** | 8.09 ± 0.25** | 3.75 ± 0.06*** | 28.81 ± 0.51*** | 16.62 ± 0.21*** | 1.06 ± 0.03*** | 14.58 ± 0.41 | 0.55 ± 0.02 | 0.24 ± 0.02 | 0.23 ± 0.06 | 13.16 ± 0.53*** | 0.01 ± 0.01 | 1.72 ± 0.12 | 0.05 ± 0.01 | 0.02 ± 0.00 | 0.04 ± 0.00 |
Fatty acids making up less than 1% of the total and which showed no significant difference between conditions were 18:2n-6, anteiso15:0, iso15:0, iso17:0, and CLA9c11t. Percentages are averages for three biological replicates. P values of <0.05 were considered significant. *, P values of 0.01 to 0.05; **, P values of 0.001 to 0.01; ***, P < 0.001. ND, not determined.
TABLE 3.
Sums of SFA, UFA, cyclic fatty acids, and branched fatty acids for C. jejuni 2097e48 and 11168 and E. coli K-12 before and after exposure to 6°C for 24 ha
| Strain (group) | % Fatty acid (mean ± SD)
|
||||
|---|---|---|---|---|---|
| UFA | SFA | UFA/SFA | BFA | CYC | |
| C. jejuni 2097e48 (control) | 31.29 ± 0.62 | 57.00 ± 0.66 | 0.55 ± 0.02 | 0.66 ± 0.09 | 9.31 ± 0.08 |
| C. jejuni 2097e48 (chill) | 31.79 ± 0.5 | 56.13 ± 0.26 | 0.57 ± 0.01 | 0.71 ± 0.07 | 9.33 ± 0.48 |
| C. jejuni 11168 (control) | 35.02 ± 1.24 | 50.63 ± 0 | 0.69 ± 0.02 | 0.75 ± 0.08 | 10.71 ± 0.70 |
| C. jejuni 11168 (chill) | 36.77 ± 3.33 | 50.17 ± 0.41 | 0.73 ± 0.07 | 0.80 ± 0.05 | 9.34 ± 2.48 |
| E. coli K-12 (control) | 26.46 ± 0.39 | 50.34 ± 0.71 | 0.53 ± 0.01 | 0.50 ± 0.02 | 16.74 ± 0.24 |
| E. coli K-12 (chill) | 30.47 ± 0.72 | 46.38 ± 0.64 | 0.66 ± 0.02*** | 0.52 ± 0.06 | 16.31 ± 0.54 |
Data are means for three biological replicates. BFA, branched fatty acids; CYC, cyclic fatty acids. P values of <0.05 were considered significant. ***, P < 0.001.
A different response was seen with E. coli K-12 (Table 2), where decreases were seen in dodecanoic (12:0), tridecanoic (13:0), tetradecanoic (14:0), pentadecanoic (15:0), hexadecanoic (16:0), and heptadecanoic (17:0) acids and increases were seen in palmitoleic acid (16:1) and cis-vaccenic acid (18:1n-7). These changes resulted in a significant alteration in the ratio of UFA to SFA, from 0.53 ± 0.01 to 0.66 ± 0.02, with a P value of <0.0001. Such changes would result in increased membrane fluidity after 24 h at 6°C (Table 3) as the percentage of UFA increased and that of SFA decreased.
We found two isomers of cyclic 19 in C. jejuni, with the major one (9 to 10.2%) being lactobacillic acid (cis-11,12-methylene-octadecanoic acid [cis-11,12-cyc19]) and the minor one (0.16 to 0.2%) being dihydrosterculic acid (cis-9,10-methylene-octadecanoic acid [cis-9,10-cyc19]). The substrates for cyclic 19 fatty acid biosynthesis were also present in similar proportions, at 23 to 28% for 18:1n-7 and 0.3 to 0.7% for 18:1n-9. Similarly, cyclic 17 (cis-9,10-methylene-hexadecanoic acid) and its substrate, 16:1, were present, at levels of 0.07 to 0.1% and 7.9 to 8.1%, respectively.
The opposite was seen with E. coli K-12, where lower levels of 9c18:1 (0.2%) and 11c18:1 (10.37%) were seen alongside cis-9,10-cyc19 (0.01%) and cis-11,12-cyc19 (1.84%). However, higher levels of cyclic 17 (14.9%) and its substrate, 16:1 (15.42%), were seen in E. coli than in C. jejuni.
DISCUSSION
Chilled storage is a key control measure in the food chain, and in many foods, C. jejuni and E. coli will be exposed to low temperatures prior to heating. In this work, we investigated whether the response of C. jejuni to chilling altered its sensitivity to heat, as is the case with other food-borne bacteria, such as E. coli. All strains grown at 37°C were heat sensitive at 56°C, but death rates were variable. Fatty acid profiles determined in this study for C. jejuni under control conditions were similar to those seen in other studies (13, 18). Similar fatty acid profiles were also seen for E. coli in this study and in a previous study at 37°C (25).
Exposure to 6°C for 24 h prior to heat treatment at 56°C caused a significant decrease in the heat tolerance of E. coli K-12. Previous studies have also shown that prior exposure of E. coli to refrigeration temperatures leads to an increased sensitivity to subsequent heat exposure (21). Cold exposure had subtly different effects on the heat tolerance of the two C. jejuni strains. Thus, with strain 2097e48, heat tolerance was not significantly altered by chilling, but with strain 11168, a small increase was seen. However, it should be noted that there is a degree of variation seen in these data sets due to the natural variation present in Campylobacter cultures. These data indicate that refrigeration, a classic step in food processing to minimize bacterial contamination, does not act on C. jejuni in the same way as it does on E. coli.
A common bacterial response to a reduction in temperature is to change the composition of membrane fatty acids. Different bacteria alter their fatty acid composition in different ways to increase the fluidity of the cell membrane. Campylobacter has been seen to change fatty acid profiles in response to a temperature downshift from 42°C to 20 to 25°C, especially altering cyclic fatty acids (16, 19). Data on other bacteria suggest that exposure to refrigeration temperatures should cause an increase in the amounts of UFA and cyclic fatty acids (1). Neither response occurred with C. jejuni strains 11168 and 2097e48 in this study, but they did occur in E. coli K-12 upon exposure to 6°C. Our data are in line with previous work on Campylobacter coli exposed to a temperature downshift from 37°C to 4°C, where minimal changes in fatty acid composition occurred (19). The changes in fatty acid composition seen in E. coli in this study were also reported in earlier studies where the percentage of UFA increased as growth temperature decreased (25). The fatty acid compositions of both control and cold-treated Campylobacter cells are similar to published results for normal spiral forms (16), with 14:0, 16:0, 16:1, 18:1n-7, and cyclic 19 (cis-11,12-cyc19) being the predominant fatty acids. Where there were significant changes due to temperature in the fatty acid profiles of the Campylobacter strains used in this study, taking into account all of the fatty acids measured, those showing a change accounted for less than a maximum of a 2% change in fatty acid composition for any one strain. This is likely to have had a very limited effect on the overall membrane fluidity. This lack of adaptation means that under chilling conditions, the C. jejuni cellular membrane will become more solid, possibly preventing membrane leakage upon a temperature upshift. In contrast, the adaptation to cold exposure seen in E. coli would indicate that the membrane would be more unstable during a rapid temperature upshift, therefore leading to the reduction in subsequent tolerance to heat exposure, as seen in this study.
It has been reported that species such as Salmonella, Listeria, and Bacillus are metabolically active at cold temperatures (4, 33). Previous work (R. A. Hughes, T. Cogan, and T. Humphrey, unpublished data) has shown a marked reduction in electron transport activity after 24 h at 6°C for 19 C. jejuni strains. This very low level of metabolic activity may account for the lack of major change in cellular fatty acids, as the cell is essentially shutting down. It may be that the ability to mount such a response is lost when the temperature drops below 20 to 25°C, where strains are still metabolically active and significant changes in fatty acids have been reported (16, 19). Significant changes in whole-cell fatty acids have also been reported for the transition from exponential growth phase to stationary phase, indicating that C. jejuni is able to alter its fatty acid composition under stressful conditions in which the cells are metabolically active, but these changes did not result in increased resistance to heat treatment (26). The rapid chilling of the cultures in this work, analogous to poultry carcass chilling or milk cooling, would result in a rapid and large-scale shutdown of cellular processes. This would severely limit the ability of Campylobacter to synthesize the desaturase required to alter the fatty acid composition of its membrane, even if its expression was induced. However, this may not be required due to the existing presence of cyclic fatty acids (Tables 2 and 3), which have been shown to increase fluidity at low temperatures and to increase membrane stability at higher temperatures (9). The presence of these fatty acids within the C. jejuni membrane may allow fluidity to be maintained without requiring changes in composition. By not altering its fatty acid composition, C. jejuni may also be able to adapt rapidly when environmental conditions become more favorable, as reorganization of the membrane to adapt to higher or more optimal temperatures would not be necessary. This lack of change to the membrane also explains the lack of alteration in heat sensitivity seen in chilled C. jejuni.
This appears to be the first report of the presence of two isomers of cyclic 19 in C. jejuni, namely, cis-11,12-cyc19 and cis-9,10-cyc19, since previous studies have shown only cis-11,12-cyc19 (18). The substrates for cyclic 19 fatty acid biosynthesis are also present in similar proportions to those expected from previous studies (14). The synthesis of cyclic 19 appears to be more efficient than that of cyclic 17 in C. jejuni in our work. However, higher levels of cyclic 17 (14.9%) and its substrate, 16:1 (15.42%), were also seen in E. coli than in C. jejuni, suggesting that synthesis of cyclic 17 is more efficient in E. coli than in C. jejuni. Law (24) showed that the formation of cyclic fatty acids is environmentally dependent, as the production of cyclic fatty acids is driven by environmental conditions and substrate availability.
This paper helps to explain a critical process by which C. jejuni can survive in the food chain in sufficient numbers to cause disease. Unlike many other pathogens, such as E. coli, C. jejuni is not sensitized to heat by being chilled, and its essentially quiescent state at low temperature would make it better able to adapt rapidly to higher temperatures. This may help to explain the success of Campylobacter as a zoonotic food-borne pathogen and shows that the bacterium responds to exposure to 6°C in a different way from other food-borne pathogens, such as E. coli.
Acknowledgments
R.-A.H. was funded during this work by Bristol University.
Footnotes
Published ahead of print on 31 July 2009.
REFERENCES
- 1.Aguilar, P. S., J. E. Cronan, Jr., and D. de Mendoza. 1998. A Bacillus subtilis gene induced by cold shock encodes a membrane phospholipid desaturase. J. Bacteriol. 180:2194-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allerberger, F., N. Al-Jazrawi, P. Kreidl, M. P. Dierich, G. Feierl, I. Hein, and M. Wagner. 2003. Barbecued chicken causing a multi-state outbreak of Campylobacter jejuni enteritis. Infection 31:19-23. [DOI] [PubMed] [Google Scholar]
- 3.Annous, B. A., M. F. Kozempel, and M. J. Kurantz. 1999. Changes in membrane fatty acid composition of Pediococcus sp. strain NRRL B-2354 in response to growth conditions and its effect on thermal resistance. Appl. Environ. Microbiol. 65:2857-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckering, C. L., L. Steil, M. H. Weber, U. Volker, and M. A. Marahiel. 2002. Genomewide transcriptional analysis of the cold shock response in Bacillus subtilis. J. Bacteriol. 184:6395-6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, P. E., O. F. Christensen, H. E. Clough, P. J. Diggle, C. A. Hart, S. Hazel, R. Kemp, A. J. Leatherbarrow, A. Moore, J. Sutherst, J. Turner, N. J. Williams, E. J. Wright, and N. P. French. 2004. Frequency and spatial distribution of environmental Campylobacter spp. Appl. Environ. Microbiol. 70:6501-6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadei, M. A., P. Mañas, G. Niven, E. Needs, and B. M. Mackey. 2002. Role of membrane fluidity in pressure resistance of Escherichia coli NCTC 8164. Appl. Environ. Microbiol. 68:5965-5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cason, J. A., and M. E. Berrang. 2002. Variation in numbers of bacteria on paired chicken carcass halves. Poult. Sci. 81:126-133. [DOI] [PubMed] [Google Scholar]
- 8.de Mendoza, D., A. K. Ulrich, and J. E. Cronan, Jr. 1983. Thermal regulation of membrane fluidity in Escherichia coli. Effects of overproduction of beta-ketoacyl-acyl carrier protein synthase I. J. Biol. Chem. 258:2098-2101. [PubMed] [Google Scholar]
- 9.Dufourc, E. J., I. C. P. Smith, and H. C. Jarrell. 1984. Role of cyclopropane moieties in the lipid properties of biological membranes: a 2H NMR structural and dynamical approach. Biochemistry 23:2300-2309. [Google Scholar]
- 10.Fahey, T., D. Morgan, C. Gunneburg, G. K. Adak, F. Majid, and E. Kaczmarski. 1995. An outbreak of Campylobacter jejuni enteritis associated with failed milk pasteurisation. J. Infect. 31:137-143. [DOI] [PubMed] [Google Scholar]
- 11.Garwin, J. L., A. L. Klages, and J. E. Cronan, Jr. 1980. Beta-ketoacyl-acyl carrier protein synthase II of Escherichia coli. Evidence for function in the thermal regulation of fatty acid synthesis. J. Biol. Chem. 255:3263-3265. [PubMed] [Google Scholar]
- 12.Georgsson, F., A. E. Thornorkelsson, M. Geirsdottir, J. Reiersen, and N. J. Stern. 2006. The influence of freezing and duration of storage on Campylobacter and indicator bacteria in broiler carcasses. Food Microbiol. 23:677-683. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin, C. S., W. McConnell, R. K. McCulloch, C. McCullough, R. Hill, M. A. Bronsdon, and G. Kasper. 1989. Cellular fatty acid composition of Campylobacter pylori from primates and ferrets compared with those of other campylobacters. J. Clin. Microbiol. 27:938-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grogan, D. W., and J. E. Cronan, Jr. 1997. Cyclopropane ring formation in membrane lipids of bacteria. Microbiol. Mol. Biol. Rev. 61:429-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris, N. V., N. S. Weiss, and C. M. Nolan. 1986. The role of poultry and meats in the etiology of Campylobacter jejuni/coli enteritis. Am. J. Public Health 76:407-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hazeleger, W. C., J. D. Janse, P. M. Koenraad, R. R. Beumer, F. M. Rombouts, and T. Abee. 1995. Temperature-dependent membrane fatty acid and cell physiology changes in coccoid forms of Campylobacter jejuni. Appl. Environ. Microbiol. 61:2713-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazeleger, W. C., J. A. Wouters, F. M. Rombouts, and T. Abee. 1998. Physiological activity of Campylobacter jejuni far below the minimal growth temperature. Appl. Environ. Microbiol. 64:3917-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hébert, G. A., D. G. Hollis, R. E. Weaver, M. A. Lambert, M. J. Blaser, and C. W. Moss. 1982. Thirty years of campylobacters: biochemical characteristics and biotyping proposal for Campylobacter jejuni. J. Clin. Microbiol. 15:1065-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Höller, C., D. Witthuhn, and B. Janzen-Blunck. 1998. Effect of low temperature on growth, structure and metabolism of Campylobacter coli SP10. Appl. Environ. Microbiol. 64:581-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphrey, T. J. 1990. Heat resistance in Salmonella enteritidis phage type 4: the influence of storage temperatures before heating. J. Appl. Bacteriol. 69:463-497. [DOI] [PubMed] [Google Scholar]
- 21.Jackson, T. C., M. D. Hardin, and G. R. Acuff. 1996. Heat resistance of Escherichia coli O157:H7 in a nutrient medium and in ground beef as influenced by storage and holding temperatures. J. Food Prot. 59:230-237. [DOI] [PubMed] [Google Scholar]
- 22.Jayarao, B. M., S. C. Donaldson, B. A. Straley, A. A. Sawant, N. V. Hegde, and J. L. Brown. 2006. A survey of foodborne pathogens in bulk tank milk and raw milk consumption among farm families in Pennsylvania. J. Dairy Sci. 89:2451-2458. [DOI] [PubMed] [Google Scholar]
- 23.Kelly, A. F., S. F. Park, R. Bovill, and B. M. Mackey. 2001. Survival of Campylobacter jejuni during stationary phase: evidence for the absence of a phenotypic stationary-phase response. Appl. Environ. Microbiol. 67:2248-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Law, J. H. 1971. Biosynthesis of cyclopropane rings. Acc. Chem. Res. 4:199-203. [Google Scholar]
- 25.Marr, A. G., and J. L. Ingraham. 1962. Effect of temperature on the composition of fatty acids in Escherichia coli. J. Bacteriol. 84:1260-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martínez-Rodriguez, A., and B. M. Mackey. 2005. Physiological changes in Campylobacter jejuni on entry into stationary phase. Int. J. Food Microbiol. 101:1-8. [DOI] [PubMed] [Google Scholar]
- 27.Mattick, K., K. Durham, G. Domingue, F. Jørgensen, M. Sen, D. W. Schaffner, and T. Humphrey. 2003. The survival of foodborne pathogens during domestic washing-up and subsequent transfer onto washing-up sponges, kitchen surfaces and food. Int. J. Food Microbiol. 85:213-226. [DOI] [PubMed] [Google Scholar]
- 28.Murphy, C., C. Carroll, and K. N. Jordan. 2003. Induction of an adaptive tolerance response in the foodborne pathogen, Campylobacter jejuni. FEMS Microbiol. Lett. 223:89-93. [DOI] [PubMed] [Google Scholar]
- 29.Panoff, J. M., B. Thammavongs, M. Gueguen, and P. Boutibonnes. 1998. Cold stress responses in mesophilic bacteria. Cryobiology 36:75-83. [DOI] [PubMed] [Google Scholar]
- 30.Pearson, L. K., and H. S. Raper. 1927. The influence of temperature on the nature of the fat formed by living organisms. Biochem. J. 21:875-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peleg, M., and M. B. Cole. 2000. Estimating the survival of Clostridium botulinum spores during heat treatments. J. Food Prot. 63:190-195. [DOI] [PubMed] [Google Scholar]
- 32.Rhodes, K. M., and A. E. Tattersfield. 1982. Guillain-Barre syndrome associated with Campylobacter infection. Br. Med. J. 285:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russle, N. J. 2002. Bacterial membranes: the effect of chill and storage and food processing. An overview. Int. J. Food Microbiol. 79:27-34. [DOI] [PubMed] [Google Scholar]
- 34.Sakamoto, T., and N. Murata. 2002. Regulation of the desaturase of fatty acids and its role in tolerance to cold and salt stress. Curr. Opin. Microbiol. 5:206-210. [DOI] [PubMed] [Google Scholar]
- 35.Servan, J., D. Elghozi, P. Waisbord, and H. Duclos. 1995. Miller-Fisher syndrome: role of Campylobacter jejuni infection. Presse Med. 24:651. [PubMed] [Google Scholar]
- 36.Sinensky, M. 1974. Homeoviscous adaptation—a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc. Natl. Acad. Sci. USA 71:522-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ulmer, H. M., H. Herberhold, S. Fahsel, G. Gänzle, R. Winter, and R. F. Vogel. 2002. Effects of pressure-induced membrane phase transitions on inactivation of HorA, an ATP-dependent multidrug-resistant transporter, in Lactobacillus plantarum. Appl. Environ. Microbiol. 68:1088-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]