Abstract
Outbreaks of salmonellosis related to consumption of fresh produce have raised interest in Salmonella-plant interactions leading to plant colonization. Incubation of gfp-tagged Salmonella enterica with iceberg lettuce leaves in the light resulted in aggregation of bacteria near open stomata and invasion into the inner leaf tissue. In contrast, incubation in the dark resulted in a scattered attachment pattern and very poor stomatal internalization. Forcing stomatal opening in the dark by fusicoccin had no significant effect on Salmonella internalization. These results imply that the pathogen is attracted to nutrients produced de novo by photosynthetically active cells. Indeed, mutations affecting Salmonella motility and chemotaxis significantly inhibited bacterial internalization. These findings suggest a mechanistic account for entry of Salmonella into the plant's apoplast and imply that either Salmonella antigens are not well recognized by the stoma-based innate immunity or that this pathogen has evolved means to evade it. Internalization of leaves may provide a partial explanation for the failure of sanitizers to efficiently eradicate food-borne pathogens in leafy greens.
Salmonella enterica is a common cause of food-borne gastroenteritis, with an estimated number of 1 to 3 million human cases per year in the United States (15). Outbreaks related to consumption of fresh produce have been increasingly reported (28) and result in morbidity and high economic losses. For example, the recent produce-associated salmonellosis outbreak (5), the largest yet reported, has resulted in more than 1,400 persons infected with S. enterica serovar Saintpaul in 43 U.S. states and in Canada. Needless to say, such outbreaks are economically destructive to farmers and the fresh produce industry and damage consumer confidence in the safety of the food supply.
Plants might become contaminated in the field through the use of contaminated irrigation water, such as raw sewage or partially treated recycled water, as well as through the use of animal manure for fertilization (2, 4, 16). Fresh produce can also become contaminated during harvest and at postharvest stages due to poor worker hygiene and low sanitation in the processing plant (2, 4). Enteropathogens can adapt to the phyllosphere environment, where they might interact with epiphytic bacteria and gain a foothold (3, 4, 14). It was suggested that transient occupants of the leaf, such as enteropathogens, may become incorporated into phylloplane biofilms and consequently gain protection from environmental stress (11). Studies of the interactions between Escherichia coli O157:H7 and cut lettuce leaves demonstrated attachment of bacteria to the surface, trichomes, stomata, and cut edges. Bacteria were also seen entrapped 20 to 100 μm below the surface in stomata and cut edges (27). Potential localization of human pathogens in the phyllosphere at sites inaccessible to sanitizers may lead to contamination of the food supply.
The produce industry currently lacks an efficient control method to ensure complete removal or killing of food-borne pathogens in fresh or minimally processed fruits and vegetables. Therefore, understanding the contamination routes and the interplay between food-borne pathogens and plant tissues is essential in order to design new intervention strategies for ensuring the safety of fresh produce. Lettuce was associated with several outbreaks related to contamination with Salmonella (12, 16, 23, 32); therefore, interactions between this pathogen and lettuce leaves were investigated in this study.
MATERIALS AND METHODS
Lettuce preparation.
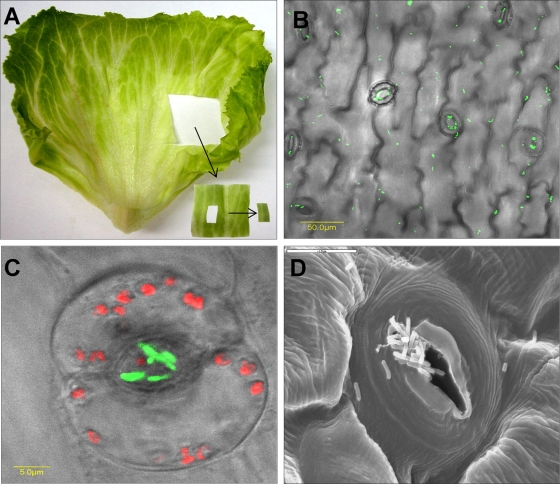
Fresh iceberg lettuce (Lactuca sativa) was purchased at a local retail store or collected in the field and kept at 8°C for a maximum of 3 days before the onset of the experiments. The outermost leaves of the lettuce head were aseptically removed, and two or three inner leaves were taken for the experiments. The leaves were aseptically cut into ca. 3- by 3-cm pieces using a sterile scalpel (Fig. 1A).
FIG. 1.
Interactions of S. enterica serovar Typhimurium with lettuce leaves. (A) S. enterica serovar Typhimurium was incubated for 2 h with a 3- by 3-cm piece of lettuce leaf as described in Materials and Methods and shown. A small (ca. 1- by 0.5-cm) centrally located piece of leaf (not containing large veins) was mounted on a microscopic slide and examined by confocal microscopy. (B and C) Microscopic images of GFP-tagged Salmonella (green) showing both diffuse and stomatal-associated attachment (B) and a higher magnification of a single stoma harboring Salmonella cells are presented (C). Red fluorescence indicates autofluorescence of the chlorophyll of guard cells. The fluorescent images were overlaid with the transmitted light image obtained using Nomarski differential interference contrast. (D) SEM image showing the complex topography of a single stomatal region and multiple bacteria (potentially Salmonella) residing within the stomatal space. Bars, 50 μm (B), 5 μm (C), and 10 μm (D).
In some cases, crude leaf extracts were prepared from 1-h-light-adapted (100 μE s−1 m−2) lettuce heads as follow: 100 g of leaves was mixed (1:1) with 100 ml saline, and the mixture was pureed by a blender (model 32BL79; Waring Products Inc., Torrington, CT) at maximum speed for 40 s. The extract was filtered and immediately used in the internalization assay.
Bacterial strains and inoculum preparation.
S. enterica serovar Typhimurium SL1344 strain expressing a green fluorescent protein (GFP) was used in this study. GFP labeling of S. enterica strains was performed by conjugal transfer of pUC18T-mini-Tn7T-Gm-gfpmut3 into Salmonella and incorporation of the gfp gene into the chromosomal attB site as described before (7). S. enterica serovar Typhimurium SL1344 M913 (fliGHI::Tn10) and M935 (cheY::Tn10) mutants, deficient in motility and chemotaxis, respectively (30), were obtained from W.-D. Hardt (Institute for Microbiology, ETH, Zurich, Switzerland). Pseudomonas syringae pv. tomato was obtained from S. Manulis (The Volcani Center). Bacterial cultures were kept in Luria-Bertani (LB) broth (10 g Bacto peptone, 5 g yeast extract, 10 g NaCl) containing 25% glycerol at −20°C. For each experiment, a fresh culture was made, and bacteria were grown in LB broth for 18 to 20 h at 37°C (S. enterica serovar Typhimurium) and 30°C (P. syringae pv. tomato) with shaking (150 rpm) to obtain stationary-phase cultures. Bacteria were washed with 0.85% NaCl (saline) by centrifugation at 2,700 × g for 10 min, and the pellet was finally resuspended in saline.
Salmonella-leaf interactions.
Lettuce pieces were submerged in 30 ml saline in a 50-ml sterile polypropylene tube (Labcon, Petaluma, CA) (one piece per tube). The leaves were preconditioned for 20 min at the following illumination conditions: dark (0 μE m−2 s−1; tubes covered with aluminum foil), laboratory neon light (3 μE m−2 s−1), or high-intensity bulb (100 μE m−2 s−1). The saline was removed and replaced with 10 ml of GFP-tagged Salmonella suspension (in saline) containing 108 CFU per ml. The tubes were incubated for 2 h under the same illumination conditions at 30°C. In some experiments, the whole lettuce head was preadapted to light by exposure for 30 min. In other experiments, incubation temperatures of 4, 25, and 37°C were also used. Following incubation of Salmonella with lettuce, the leaf pieces were rinsed twice for 1 min each time in saline to remove unattached bacteria and an internal 10- by 5-mm piece (to the edge) was excised (Fig. 1A) and taken immediately for confocal laser scanning microscopy. Bacterial localization was determined on the leaf surface (attachment) and in deeper layers (internalization) in 30 randomly chosen microscopic fields (magnification of ×40). Bacterial numbers were scored as follows: 0, 1 to 10, 10 to 50, 50 to 100, and >100 Salmonella cells per one microscopic field. In order to facilitate the quantification of surface-associated and internalized bacteria, the data are presented in most cases as incidence of Salmonella, i.e., percentage of fields (magnification of ×40) containing one or more surface-attached or internal, GFP-tagged bacteria in 30 microscopic fields of the same leaf. Scoring the incidence of Salmonella in microscopic fields rather than in individual stoma is preferable since it allows comparison of attachment and internalization under conditions where Salmonella is not confined to stomata. Each experiment included three lettuce pieces from different leaves (a total of 90 microscopic fields at a magnification of ×40 examined per experiment) and was repeated at least twice on different days with different plants.
In some experiments, the bacteria and lettuce were incubated in the presence of fusicoccin (10 μM), sucrose (100 mM), glucose (100 mM), fructose (100 mM), and leaf extract derived from a light-adapted lettuce head (1 h at 100 μE m−2 s−1). In other experiments, Salmonella cells were killed by incubation in 4% (vol/vol) formalin for 30 min at room temperature. The cells were washed twice and resuspended in saline. Formalin-killed Salmonella exhibited green fluorescence comparable to that of live bacteria.
Confocal microscopy.
GFP-labeled bacteria were visualized by using a confocal laser scanning microscope (Olympus IX81; Olympus, Tokyo, Japan) and an objective with a magnification of 40× and a numerical aperture of 0.7. Fluorescent bacteria were visualized using an excitation wavelength of 488 nm and a BA505-525 emission filter. For chlorophyll autofluorescence, BA660 emission filter was used. Transmitted light images were obtained using Nomarski differential interference contrast (DIC). To visualize real-time movement of bacteria on the phylloplane, lettuce was incubated with GFP-tagged bacteria in the light for 2 h at 30°C. The leaf pieces were washed twice to remove unattached bacteria and observed by confocal microscopy. Time-lapse microscopy of a single field was employed. Frames were captured every 0.44 s.
SEM.
Lettuce pieces infected with S. enterica serovar Typhimurium for 2 h were washed twice in phosphate-buffered saline, pH 7.2, and fixed in 5% glutaraldehyde in 0.1 M phosphate buffer for 2 h. The pieces were washed five times (10 min each) in phosphate-buffered saline, and internal-leaf 2- by 2-mm squares were excised and processed for scanning electron microscopy (SEM) by washing with increasing concentrations of ethanol. The pieces were dried with CO2 in a critical-point drier (CPD 030; Bal-Tec AG, Balzers, Liechtenstein), coated with gold (Polaron Equipment Ltd., Watford, United Kingdom), and taken for observation with a JEOL JSM 35C scanning electron microscope (Tokyo, Japan). Clusters of rod-shaped bacteria on the leaf surface were observed only in Salmonella-infected leaf samples and not in uninfected leaves (data not shown).
Light intensity measurements.
Illumination intensity was measured by a photometer (model LI-185B; Li-COR, Inc., Lincoln, NE).
Determination of sucrose concentration.
Lettuce leaf pieces prepared as described above were immersed in saline (1 piece per 30 ml) and exposed to light (100 μE m−2 s−1) or kept in the dark for 2 h without bacteria. Soluble sugars were extracted three times, 1 h each time, with 5 ml of 80% (vol/vol) hot (70°C) ethanol, from 0.5 g of fresh lettuce pieces. The pooled alcoholic extracts were concentrated, resuspended in 1 ml water, and deproteinized by 30% KOH. The sucrose concentration in the extract was assayed with anthrone by the method of Van Handel (36).
Statistical methods.
Statistical calculations were performed by using the Instat program, version 3.0.6 (GraphPad Software, Inc., La Jolla, CA).
RESULTS
Salmonella enterica serovar Typhimurium penetrates lettuce leaves via open stomata.
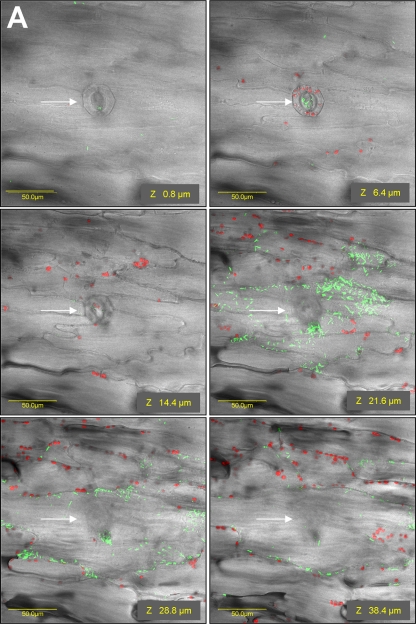
Iceberg lettuce leaf pieces were incubated in a suspension of GFP-tagged S. enterica serovar Typhimurium for 2 h at room temperature. Following extensive washing steps, numerous bacteria were observed attached to the leaf surface with a distinct clustering pattern near and within stomata (Fig. 1B, C, and D). Confocal microscopy images taken at various depths below the surface of the leaf demonstrated the presence of Salmonella particularly underneath stomata in the substomatal space and in the intercellular region (apoplast) of the spongy parenchyma (Fig. 2A) (see movie S1 in the supplemental material). The internal localization of Salmonella is supported by the presence of GFP-tagged S. enterica serovar Typhimurium in the vicinity of parenchymal cells, characterized by morphological structures different from those of the epidermis (see z sections 28.8 and 38.4 μm versus 6.4 μm in Fig. 2A). Furthermore, the red fluorescence observed in images of deeper tissue is characteristic of the chloroplasts, which are present at the parenchyma but not in the epidermis. The only cells that do possess chloroplasts in the epidermis are the guard cells. A three-dimensional reconstruction of fluorescent images taken at the same leaf region is also presented (Fig. 2C). Salmonella cells (green) are evidently observed in the stoma (rectangle outlined by the white broken line) and below within the parenchymal tissue, characterized by red fluorescence of the chloroplasts. The yellow fluorescence corresponds to the presence of bacteria (green) in proximity to the chloroplast (red), apparently in the intercellular space.
FIG. 2.
Photomicrographs showing the distribution (depth) of Salmonella cells in lettuce leaf tissues following exposure to light (A) and dark (B) for 2 h at room temperature. Representative photomicrographs showing fluorescent images along a z section overlaid with DIC images are shown. (A) Under illumination, numerous green fluorescent bacteria were observed beneath stomata (indicated by white arrows) and in the intercellular space in the underlying parenchyma cells. (B) Following incubation in the dark, GFP-labeled bacteria were localized on the leaf surface and no bacterium was observed in inner tissues. Red fluorescence indicates autofluorescence of chlorophyll within chloroplasts. Since the epidermis is devoid of chloroplasts (besides the guard cells), the presence of chloroplast (red) and nearby Salmonella (green) in the same focal plane confirms the localization of Salmonella cells within the parenchymal tissue. (C) A three-dimensional reconstruction of confocal microscopy images taken of the same leaf section shown in panel A further demonstrate the existence of S. enterica cells (green) inside a stoma (rectangle outlined by a white broken line) as well as deeper within the parenchymal tissue, characterized by the presence of chloroplasts (red autofluorescence). The yellow color corresponds to the localization of bacteria (green) and chloroplast (red) close together. The distance in microns (0 to 200 μm) for the images is indicated.
The localization of Salmonella underneath stomata implies that this pathogen exploits open stomata as a portal of entry into deeper tissues of the plant host. Control of stomatal opening and closure is a key factor enabling the regulation of gas exchange at the leaf level and balancing water loss with CO2 uptake. Since light is known to modulate stomatal opening, we have repeated the experiment in the dark, a condition known to favor stomatal closure (25). Indeed, almost no Salmonella penetration was observed following incubation in the dark and bacteria were confined to the surface only (Fig. 2B), suggesting that S. enterica serovar Typhimurium penetration requires open stomata.
Light induces Salmonella internalization.
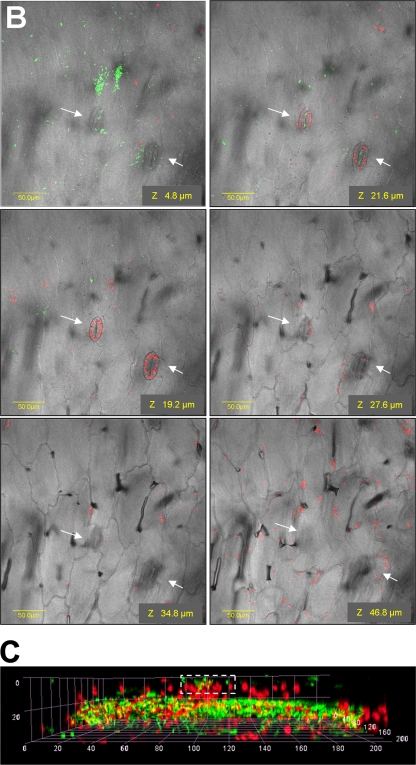
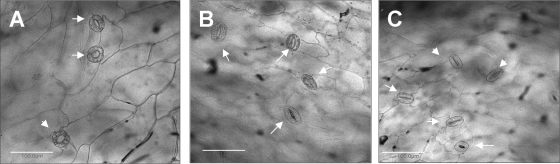
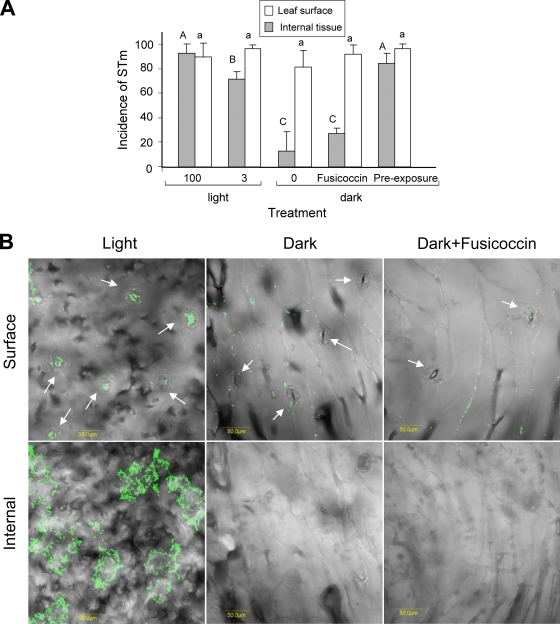
Stomatal opening is dependent on multiple biotic and abiotic factors, including light (35). We reasoned that under high-intensity illumination, maximal stomatal opening will occur, resulting in a higher internalization rate. Therefore, S. enterica serovar Typhimurium entry was assessed under three illumination intensities (0, 3, and 100 μE m−2 s−1). Salmonella internalization was also assessed in the dark in the presence of the stomatal opening reagent fusicoccin (33). To ensure that differential stomatal opening was achieved under the various illumination conditions, stomatal opening was traced following the light or dark conditioning period (20 min) just before bacteria were added (Fig. 3). In the dark, nearly all stomata were tightly closed and only 1.0% ± 1.4% were open, while 65% ± 4% and 88% ± 4% were open under illumination intensities of 3 and 100 μE m−2 s−1, respectively. Following 2 h of incubation of Salmonella with lettuce, internalization was evaluated (Fig. 4A and B; Table 1). Table 1 shows the incidence of Salmonella cells at the surface or underneath the epidermis at two light intensities (0 and 100 μE m−2 s−1). A simplified presentation showing the incidence of Salmonella at the various sites is presented in Fig. 4A. In the light, Salmonella cells were observed both on the surface and within the leaf tissue (Fig. 4B). The highest internalization rate was evident under intense illumination and was significantly inhibited in the dark. It should be noted that the images shown in Fig. 4B in the light represent a high internalization rate, although variable internalization rates were also observed. It seems that more GFP-tagged bacteria are found within the tissue rather than on the leaf surface, suggesting that S. enterica serovar Typhimurium has entered through multiple stomata and spread underneath to nearby tissues.
FIG. 3.
Microscopic photomicrographs showing stomatal guard cells (white arrows) following 20 min of preconditioning at the following light intensities: 100 (A), 3 (B), and 0 (C) μE m−2 s−1. Bars, 100 μm.
FIG. 4.
Effect of light on the localization of Salmonella in leaf tissue. (A) Incidence of S. enterica serovar Typhimurium (STm) on leaf surface and in internal tissue. Preexposure refers to experiments in which the whole lettuce head was preexposed to light (100 μE m−2 s−1) for 30 min before the leaves were cut and taken for internalization assay performed in the dark. Each experiment was performed in triplicate and repeated at least twice at different days. Different letters indicate significant difference (P < 0.05) between the means of surface (capital letters) and internal (lowercase letters) fields harboring bacteria by analysis of variance by the Tukey-Kramer multiple-comparison test. (B) Confocal microscopy images showing GFP-tagged bacteria residing on the surface of the leaf and in internal leaf tissues following 2-h internalization assay. Internal leaf tissue images are composed of a stack of fluorescent images taken every 1.2 μm to a depth of 100 μm along a z section of the same field. All images were overlaid with DIC images. Note Salmonella aggregation near and within stomata (indicated by white arrows) under illumination, but not in the dark. Bars, 50 μm.
TABLE 1.
Effect of light on the localization of Salmonella enterica serovar Typhimurium in leaf tissue
| Light intensity (μE m−2 s−1) | Location in the leaf | % (Mean ± SD) of microscopic fields harboring the following no. of S. enterica serovar Typhimurium cellsa:
|
||||
|---|---|---|---|---|---|---|
| 0 | 1-10 | 10-50 | 50-100 | ≥100 | ||
| 100 | Surface | 10 ± 11 a | 47 ± 23 a | 33 ± 21 a | 11 ± 11 a | 0 a |
| Internal | 8 ± 8 A | 34 ± 25 A | 23 ± 16 A | 24 ± 18 A | 10 ± 11 A | |
| 0 | Surface | 19 ± 13 a | 59 ± 17 a | 22 ± 20 a | 1 ± 1 b | 0 a |
| Internal | 88 ± 16 B | 8 ± 9 B | 3 ± 6 B | 1 ± 3 B | 0 B | |
Thirty microscopic fields (magnification of ×40) were examined per treatment in triplicate (30 × 3). Each experiment was repeated three times on different days with different pieces of lettuce (n = 270). Different capital and lowercase letters indicate significant differences (P < 0.05) between the mean percentages of surface and internal fields, respectively, in the same column by analysis of variance by the Tukey-Kramer multiple-comparison test.
Intermediate internalization was observed in medium lighting conditions (3 μE m−2 s−1). Interestingly, no significant entry of Salmonella into the leaf was observed in the dark when fusicoccin was added, although the majority (92% ± 6%) of stomata were open (Fig. 4A and B). These results imply that light is the main factor that affects S. enterica serovar Typhimurium internalization. To confirm these unexpected results, another experiment was performed; in this experiment, the lettuce was initially adapted to light for 30 min, following by internalization assay in dark. The Salmonella penetration rate was comparable to that obtained under high illumination for 2 h (Fig. 4A), corroborating the major role of light in the internalization phenomenon.
Light induces Salmonella taxis toward stomata.
To determine whether S. enterica serovar Typhimurium can move on the leaf surface toward stomata, bacterial motility was examined in lettuce incubated with GFP-tagged bacteria by time-lapse fluorescence microscopy. Salmonella cells were observed moving toward and vanishing within the substomatal cavity when the experiments were performed in the light but not in the dark (see movies S2 and S3 in the supplemental material).
Effect of S. enterica serovar Typhimurium on stomata.
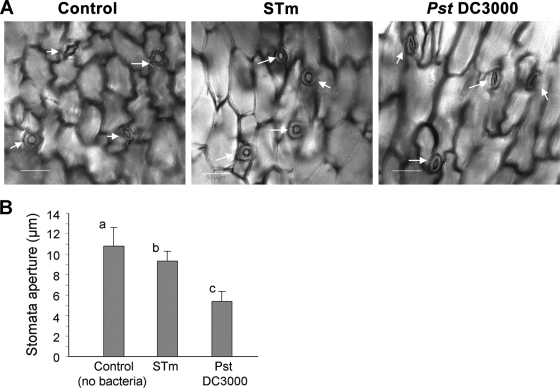
Stomata were recently shown to participate in the innate immunity response against microbial pathogens (24, 25, 37). In a recent study, Arabidopsis thaliana leaves demonstrated rapid stomatal closure (within 2 h) in the presence of E. coli O157:H7 and Pseudomonas syringae pv. tomato (24). P. syringae pv. tomato, but not E. coli O157:H7, was able to force stomatal opening 4 h after expression of coronatine, a virulence factor (24). The apparent efficient entry of S. enterica serovar Typhimurium into stomata within 2 h implies that this human pathogen does not induce stomatal closure. To test this hypothesis, the stomatal aperture was determined in lettuce leaves exposed to saline (control), S. enterica serovar Typhimurium, and P. syringae pv. tomato for 2 h in light (Fig. 5). While both Salmonella and P. syringae pv. tomato seem to induce stomatal closure, the stomatal response to P. syringae pv. tomato infection is significantly more prominent. This suggests that either S. enterica serovar Typhimurium antigens are not well recognized by the stoma-based innate immunity or that this pathogen can somehow inhibit stomatal closure.
FIG. 5.
S. enterica serovar Typhimurium does not trigger stomatal closure. (A) Confocal microscopic images of lettuce leaf exposed to saline (control), S. enterica serovar Typhimurium (STm), and P. syringae pv. tomato (Pst) DC3000 for 2 h in light. White arrows indicate stomata. (B) Stomatal aperture in lettuce leaf exposed to saline (control), S. enterica serovar Typhimurium, and P. syringae pv. tomato DC3000. Results are shown as means plus standard deviations (error bars) (n = 60). Different letters indicate significant differences (P < 0.05) between the means by analysis of variance by the Tukey-Kramer multiple-comparison test.
Effect of Salmonella viability on internalization.
A previous investigation showed that both live and dead E. coli O157:H7 attach equally to iceberg lettuce leaves (29), suggesting that bacterial processes and cell surface moieties were not required for initial attachment of the pathogen to the plant tissue. This report is in agreement with the notion that infiltration of food-borne pathogens through stomata was considered a passive event (9, 14, 35). It was recently reported that vacuum cooling results in increased infiltration of E. coli O157:H7 into romaine lettuce tissue. E. coli clusters were scattered around the locations of many guard cells, the vacuoles of stomata, and other random subsurface locations. It was suggested that vacuuming forcibly changed the structure of lettuce tissue such as the stomata, resulting in bacterial infiltration via a passive mechanism (21). To determine whether entry of Salmonella into substomatal regions observed in our studies is merely a physical process unrelated to Salmonella viability, we have repeated the internalization assay employing formalin-killed bacteria. Neither attachment nor internalization was observed with dead bacteria (data not shown), inferring that Salmonella internalization in iceberg lettuce entails viable bacteria capable of moving toward stomata.
Effect of storage temperature on Salmonella attachment and internalization.
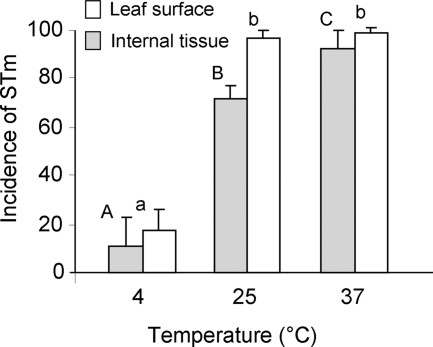
Environmental temperature affects bacterial metabolism and energy production, which consequently influences bacterial motility. To examine the effect of temperature on the interactions between Salmonella and lettuce, attachment and internalization experiments were conducted under the following temperatures: 4, 25, and 37°C (Fig. 6). Elevated internalization and attachment rates were evident at the higher temperatures (25 and 37°C), supporting the involvement of active metabolism in the two processes.
FIG. 6.
Effect of temperature on the incidence of Salmonella enterica serovar Typhimurium (STm) in leaf tissue. Internalization experiments were performed in light (3.0 μE m−2 s−1). The data represent the mean plus standard deviations (error bars) for two independent experiments, each performed in triplicate. Different letters indicate significant differences (P < 0.05) between the means of surface (capital letters) and internal (lowercase letters) fields containing bacteria by analysis of variance by the Tukey-Kramer multiple-comparison test.
Salmonella motility and chemotaxis are required for stomatal penetration.
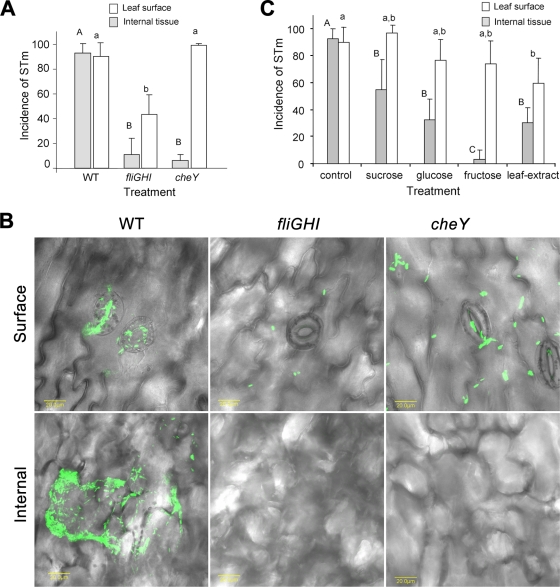
We surmised that S. enterica serovar Typhimurium internalization occurs by chemotaxis toward nutrients produced by photosynthetic guard cells and mesophyll cells. To investigate this hypothesis, the penetration of fliGHI and cheY mutants defective in motility and chemotaxis, respectively, was examined. The entry of the two mutants was significantly inhibited, suggesting that the two processes are important for Salmonella internalization. However, the fliGHI mutant, but not the cheY mutant, also exhibited reduced attachment, about 50% that of the wild type, implying that flagella are also required for efficient attachment (Fig. 7A and B).
FIG. 7.
Effects of fliGHI and cheY mutations (A and B) and exogenous sugars (C) on Salmonella localization in leaf tissue. Internalization experiments were conducted in light (100 μE m−2 s−1) for 2 h. (A and C) Incidence of S. enterica serovar Typhimurium (STm) on the leaf surface and in internal tissues. (B) Confocal microscopy image stacks show bacterial distribution in the various leaf locations. Sucrose, glucose, and fructose were added to the bacterial suspension at a concentration of 100 mM. Leaf extract was prepared from lettuce leaves adapted to light for 1 h. Control denotes bacterial suspension in saline only. The data represent the means plus standard deviations (error bars) from at least two independent experiments, each performed in triplicate. Different letters indicate significant differences (P < 0.05) between the means of surface (capital letters) and internal (lowercase letters) fields harboring bacteria by analysis of variance by the Tukey-Kramer multiple-comparison test. WT, wild type.
Photosynthesis products seem to be required for Salmonella internalization.
The induction of Salmonella movement and internalization in light suggests that photosynthesis plays a major role in this phenomenon. Sucrose is a major translocatable product of photosynthesis and the main soluble component of the phloem sap whose concentration near guard cells may reach 150 mM (18, 20). Sucrose levels measured in extracts of lettuce leaves exposed to light and dark conditions were 1.25 ± 0.07 and 0.88 ± 0.06 mg g−1 (fresh leaves), respectively. To further investigate the potential role of chemotaxis toward substances synthesized during photosynthesis, Salmonella internalization assays were conducted in the presence of exogenous sucrose and its monosaccharide metabolites glucose and fructose. Indeed, all three sugars each at a concentration of 100 mM significantly inhibited Salmonella internalization, with fructose having the maximal inhibition effect. Furthermore, leaf extract derived from light-exposed lettuce also inhibited Salmonella penetration (Fig. 7C).
DISCUSSION
Stomata are involved in exchange of gases between cells in internal leaf tissues, underneath the cuticle, and the atmospheric environment. They are essential for photosynthesis since they allow carbon dioxide to diffuse into the leaf. Plants have evolved complex regulation pathways that include, among others, plant hormones to modulate stomatal opening in response to environmental conditions, such as light intensity, humidity, and carbon dioxide concentration. Stomatal opening is tightly controlled by guard cells in order to maximize photosynthesis efficiency and minimize water loss (25).
Most pathogenic microbes must access the plant interior, either by penetrating the leaf or root surface directly or by entering through wounds or natural openings, such as stomata. However, plants have evolved mechanisms to detect and mount a defense response to potential pathogenic microorganisms. Pathogen-associated molecular pattern-triggered immunity is considered the plant's first active response to microbial perception. It is initiated upon recognition of conserved microbial features, such as lipopolysaccharide and flagella by plant cell surface receptors (6).
Upon introduction to the leaf surface, both plant- and food-borne pathogens encounter multiple stresses, such as limited nutrients, UV irradiation, and desiccation (4, 9, 14, 22, 35). Although penetration of plant pathogens into leaf tissue was considered a passive mechanism (9, 14, 35), recent studies suggest that stomata play an active role in controlling internalization of both human and plant pathogens as part of the plant's innate immune system (24, 35). Several plant pathogens were shown to exploit stomatal openings to gain entry into internal leaf compartments, which provide them with a more favorable environment (24, 35). Although stoma-based innate immunity response initially triggered stomatal closure, some pathogens can overcome this defense mechanism by secreting a virulence factor that forces stomatal reopening (13, 24). Interestingly, it seems that exposure of lettuce leaves to S. enterica serovar Typhimurium does not trigger extensive stomatal response, while P. syringae pv. tomato induces substantial stomatal closure. Since stoma-based innate immunity was reported only for A. thaliana (24), our findings are the first to demonstrate active stoma-based innate immunity in postharvest lettuce infected with P. syringae pv. tomato. The weak response observed with Salmonella may suggest that the plant's innate immunity cannot fully recognize S. enterica serovar Typhimurium pathogen-associated molecular patterns. Alternatively, the pathogen may have evolved means to evade stomatal response. In support of the latter notion is the recent finding that S. enterica serovar Typhimurium can invade Arabidopsis plants via intact shoot or root tissues, overcoming the plant's innate defense mechanisms and cause disease symptoms (26).
Recent studies have shown that enteric bacteria can colonize the interiors of plants (10, 34). Endophytic colonization was shown to result from root infection or contamination of seeds (8, 10, 34). The extent of endophytic colonization is determined by the genetic background of both the microbe and the host plant (34). The occurrence of Salmonella and E. coli O157:H7 near stomata or within the substomatal cavity in lettuce was previously reported (27, 31). E. coli O157:H7 was also shown to colonize the inner tissues and stomata of cotyledons of radish sprouts (17); however, stomatal penetration was not investigated. In the present study, we have shown that a human pathogen (S. enterica serovar Typhimurium) is capable of penetrating lettuce leaf epidermis through open stomata. Our results suggest the involvement of chemotaxis and motility in this phenomenon. The reduced attachment to the surface of lettuce observed with the fliGHI mutant is in agreement with recent findings showing that flagella are involved in the attachment of S. enterica serovar Senftenberg to basil leaves (1). Nevertheless, no difference in the attachment of S. enterica serovar Typhimurium (wild type) and fliC mutant was observed (1). This discrepancy might be related to the different plant models utilized in the two studies, as well as to variation in the physiological conditions of the plants.
Our data suggest that sucrose (and perhaps glucose and fructose) might be a potential candidate for leaf chemoattractant, as its concentration was found to be higher in leaves exposed to light compared to leaves kept in the dark, and exogenous sucrose was capable of inhibiting Salmonella internalization. Salmonella chemotaxis toward the roots of lettuce seedlings was previously reported (19). The researchers observed active movement of S. enterica serovar Dublin toward root exudates and suggested a tentative route of plant contamination that involves chemotaxis toward roots, followed by internalization and endophytic spread comparable to that of plant pathogens (19). Our findings imply that chemotaxis might be a common strategy utilized by Salmonella colonizing two different ecological niches, the rhizosphere and phyllosphere. Whether similar chemoattractant was involved in the two processes remains to be elucidated.
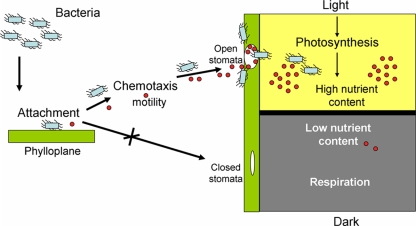
To the best of our knowledge, a correlation between photosynthesis and stomatal internalization, while seemingly predictable, has not been documented before either with plant pathogens or with human pathogens. A number of findings suggest that photosynthesis and not merely stomatal opening is the major motive force behind Salmonella internalization in harvested iceberg lettuce. (i) Efficient internalization occurs in the light but not in the dark. (ii) Preexposure to light is sufficient to allow internalization in the dark. (iii) Force opening stomata in the dark is not sufficient to promote internalization. (iv) Exogenous sugars produced during photosynthesis significantly inhibit Salmonella penetration. A diagram summarizing a proposed model of stomatal penetration by Salmonella is depicted in Fig. 8.
FIG. 8.
A model summarizing our current understanding regarding Salmonella internalization through stomata. Red circles denote putative chemoattractant nutrients produced by stomatal guard cells and by parenchyma cells during photosynthesis.
The elucidation of the mechanism by which Salmonella invades intact leaves has important implications for both pre- and postharvest handling of lettuce and probably other leafy vegetables. The capacity to inhibit internalization should limit bacterial colonization to the phylloplane and consequently might enhance the effectiveness of surface sanitizers (31). Moreover, inhibition of bacterial taxis and/or controlling light exposure throughout postharvest handling and during shelf-life might be considered part of a multiple hurdle approach to minimize internal contamination by Salmonella and perhaps by other human pathogens. Additional studies are required to assess internal leaf contamination in growing plants.
Supplementary Material
Acknowledgments
We thank A. Lichter for initial discussions, M. Bar-Joseph and S. Lurie for reviewing an early version of the manuscript, and I. Ofek for continuous encouragement. We are grateful to Wolf-Dietrich Hardt for providing M913 (fliGHI) and M935 (cheY) mutants, Herbert P. Schweizer for supplying plasmid pUC18T-mini-Tn7T-Gm-gfpmut3, and S. Manulis for providing Pseudomonas syringae pv. tomato (DC3000).
This study was partially supported by The United States-Israel Binational Agricultural Research and Development (BARD) Fund (grant US-3949-06).
Footnotes
Published ahead of print on 31 July 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Berger, C. N., R. K. Shaw, D. J. Brown, H. Mather, S. Clare, G. Dougan, M. J. Pallen, and G. Frankel. 2009. Interaction of Salmonella enterica with basil and other salad leaves. ISME J. 3:261-265. [DOI] [PubMed] [Google Scholar]
- 2.Beuchat, L. R., and J. H. Ryu. 1997. Produce handling and processing practices. Emerg. Infect. Dis. 3:459-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beuchat, L. R. 2002. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 4:413-423. [DOI] [PubMed] [Google Scholar]
- 4.Brandl, M. T. 2006. Fitness of human enteric pathogens on plants and implications for food safety. Annu. Rev. Phytopathol. 44:367-392. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2008. Investigation of outbreak of infections caused by Salmonella Saintpaul. Update for August 28, 2008. Centers for Disease Control and Prevention, Atlanta, GA. http://www.cdc.gov/Salmonella/saintpaul/.
- 6.Chisholm, S. T., G. Coaker, B. Day, and B. J. Staskawicz. 2006. Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124:803-814. [DOI] [PubMed] [Google Scholar]
- 7.Choi, K. H., and H. P. Schweizer. 2006. Mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa. Nat. Protoc. 1:153-161. [DOI] [PubMed] [Google Scholar]
- 8.Cooley, M. B., W. G. Miller, and R. E. Mandrell. 2003. Colonization of Arabidopsis thaliana with Salmonella enterica and enterohemorrhagic Escherichia coli O157:H7 and competition by Enterobacter asburiae. Appl. Environ. Microbiol. 69:4915-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delaquis, P., S. Bach, and L. D. Dinu. 2007. Behavior of Escherichia coli O157:H7 in leafy vegetables. J. Food Prot. 70:1966-1974. [DOI] [PubMed] [Google Scholar]
- 10.Dong, Y. M., A. L. Iniguez, B. M. M. Ahmer, and E. W. Triplett. 2003. Kinetics and strain specificity of rhizosphere and endophytic colonization by enteric bacteria on seedlings of Medicago sativa and Medicago truncatula. Appl. Environ. Microbiol. 69:1783-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fett, W. F. 2000. Naturally occurring biofilms on alfalfa and other types of sprouts. J. Food Prot. 63:625-632. [DOI] [PubMed] [Google Scholar]
- 12.Gillespie, I. A. 2004. Outbreak of Salmonella Newport infection associated with lettuce in the UK. Euro. Surveill. 8:2562. [Google Scholar]
- 13.Gudesblat, G. E., P. S. Torres, and A. A. Vojnov. 2009. Xanthomonas campestris overcomes Arabidopsis stomatal innate immunity through a DSF cell-to-cell signal-regulated virulence factor. Plant Physiol. 149:1017-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heaton, J. C., and K. Jones. 2008. Microbial contamination of fruit and vegetables and the behaviour of enteropathogens in the phyllosphere: a review. J. Appl. Microbiol. 104:613-626. [DOI] [PubMed] [Google Scholar]
- 15.Hohmann, E. L. 2001. Nontyphoidal salmonellosis. Clin. Infect. Dis. 32:263-269. [DOI] [PubMed] [Google Scholar]
- 16.Horby, P. W., S. J. O'Brien, G. K. Adak, C. Graham, J. I. Hawker, P. Hunter, C. Lane, A. J. Lawson, R. T. Mitchell, M. H. Reacher, E. J. Threlfall, and L. R. Ward. 2003. A national outbreak of multi-resistant Salmonella enterica serovar Typhimurium definitive phage type (DT) 104 associated with consumption of lettuce. Epidemiol. Infect. 130:169-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itoh, Y., Y. Sugita-Konishi, F. Kasuga, M. Iwaki, Y. Hara-Kudo, N. Saito, Y. Noguchi, H. Konuma, and S. Kumagai. 1998. Enterohemorrhagic Escherichia coli O157:H7 present in radish sprouts. Appl. Environ. Microbiol. 64:1532-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang, Y., W. H. Outlaw, Jr., P. C. Andersen, and G. B. Fiore. 2007. Guard-cell apoplastic sucrose concentration—a link between leaf photosynthesis and stomatal aperture size in the apoplastic phloem loader Vicia faba L. Plant Cell Environ. 30:551-558. [DOI] [PubMed] [Google Scholar]
- 19.Klerks, M. M., E. Franz, M. van Gent-Pelzer, C. Zijlstra, and A. H. van Bruggen. 2007. Differential interaction of Salmonella enterica serovars with lettuce cultivars and plant-microbe factors influencing the colonization efficiency. ISME J. 1:620-631. [DOI] [PubMed] [Google Scholar]
- 20.Lemoine, R. 2000. Sucrose transporters in plants: update on function and structure: a review. Biochim. Biophys. Acta 1465:246-262. [DOI] [PubMed] [Google Scholar]
- 21.Li, H., M. B. Tajkarimi, and I. Osburn. 2008. Impact of vacuum cooling on Escherichia coli O157:H7 infiltration into lettuce tissue. Appl. Environ. Microbiol. 74:3138-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindow, S. E., and M. T. Brandl. 2003. Microbiology of the phyllosphere. Appl. Environ. Microbiol. 69:1875-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long, S. M., G. K. Adak, S. J. O'Brien, and I. A. Gillespie. 2002. General outbreaks of infectious intestinal disease linked with salad vegetables and fruit, England and Wales, 1992-2000. Commun. Dis. Public Health 5:101-105. [PubMed] [Google Scholar]
- 24.Melotto, M., W. Underwood, J. Koczan, K. Nomura, and S. Y. He. 2006. Plant stomata function in innate immunity against bacterial invasion. Cell 126:969-980. [DOI] [PubMed] [Google Scholar]
- 25.Melotto, M., W. Underwood, and S. Y. He. 2008. Role of stomata in plant innate immunity and foliar bacterial diseases. Annu. Rev. Phytopathol. 46:101-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schikora, A., A. Carreri, E. Charpentier, and H. Hirt. 2008. The dark side of the salad: Salmonella typhimurium overcomes the innate immune response of Arabidopsis thaliana and shows an endopathogenic lifestyle. PLoS ONE 3:e2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seo, K. H., and J. F. Frank. 1999. Attachment of Escherichia coli O157:H7 to lettuce leaf surface and bacterial viability in response to chlorine treatment as demonstrated by using confocal scanning laser microscopy. J. Food Prot. 62:3-9. [DOI] [PubMed] [Google Scholar]
- 28.Sivapalasingam, S., C. R. Friedman, L. Cohen, and R. V. Tauxe. 2004. Fresh produce: a growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J. Food Prot. 67:2342-2353. [DOI] [PubMed] [Google Scholar]
- 29.Solomon, E., and K. R. Matthews. 2006. Interaction of live and dead Escherichia coli O157:H7 and fluorescent microspheres with lettuce tissue suggests bacterial processes do not mediate adherence. Lett. Appl. Microbiol. 42:88-93. [DOI] [PubMed] [Google Scholar]
- 30.Stecher, B., S. Hapfelmeier, C. Müller, M. Kremer, T. Stallmach, and W. D. Hardt. 2004. Flagella and chemotaxis are required for efficient induction of Salmonella enterica serovar Typhimurium colitis in streptomycin-pretreated mice. Infect. Immun. 72:4138-4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeuchi, K., and J. F. Frank. 2001. Quantitative determination of the role of lettuce leaf structures in protecting Escherichia coli O157:H7 from chlorine disinfection. J. Food Prot. 64:147-151. [DOI] [PubMed] [Google Scholar]
- 32.Takkinen, J., U. M. Nakari, T. Johansson, T. Niskanen, A. Siitonen, and M. Kuusi. 2005. A nationwide outbreak of multiresistant Salmonella Typhimurium in Finland due to contaminated lettuce from Spain. Euro. Surveill. 10:E050630.1. [DOI] [PubMed] [Google Scholar]
- 33.Turner, N. C., and A. Graniti. 1969. Fusicoccin: a fungal toxin that opens stomata. Nature 223:1070-1071. [Google Scholar]
- 34.Tyler, H. L., and E. W. Triplett. 2008. Plants as a habitat for beneficial and/or human pathogenic bacteria. Annu. Rev. Phytopathol. 46:53-73. [DOI] [PubMed] [Google Scholar]
- 35.Underwood, W., M. Melotto, and S. Y. He. 2007. Role of plant stomata in bacterial invasion. Cell. Microbiol. 9:1621-1629. [DOI] [PubMed] [Google Scholar]
- 36.Van Handel, E. 1968. Direct microdetermination of sucrose. Anal. Biochem. 22:280-283. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, W., S. Y. He, and S. M. Assmann. 2008. The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. Plant J. 56:984-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.