Abstract
Genital herpes, caused by herpes simplex virus type 2 (HSV-2), is one of the most prevalent sexually transmitted diseases worldwide and a risk factor for acquiring human immunodeficiency virus. Although many vaccine candidates have shown promising results in animal models, they have failed to be effective in human trials. In this study, a humanized mouse strain was evaluated as a potential preclinical model for studying human immune responses to HSV-2 infection and vaccination. Immunodeficient mouse strains were examined for their abilities to develop human innate and adaptive immune cells after transplantation of human umbilical cord stem cells. A RAG2−/− γc−/− mouse strain with a BALB/c background was chosen as the most appropriate model and was then examined for its ability to mount innate and adaptive immune responses to intravaginal HSV-2 infection and immunization. After primary infection, human cells in the lymph nodes were able to generate a protective innate immune response and produce gamma interferon (IFN-γ). After intravaginal immunization and infection, human T cells and NK cells were found in the genital tract and iliac lymph nodes. In addition, human T cells in the spleen, lymph nodes, and vaginal tract were able to respond to stimulation with HSV-2 antigens by replicating and producing IFN-γ. Human B cells were also able to produce HSV-2-specific immunoglobulin G. These adaptive responses were also shown to be protective and reduce local viral replication in the genital tract. This approach provides a means for studying human immune responses in vivo using a small-animal model and may become an important preclinical tool.
Genital herpes, caused primarily by herpes simplex virus type 2 (HSV-2), is one of the most prevalent sexually transmitted diseases in the world and is associated with substantial morbidity (13). After initial infection of the genital tract, the virus establishes latency within the nervous system and thus maintains lifelong infection in humans. Latent virus can reactivate and cause recurrent symptoms, including genital lesions; however, subclinical infection and asymptomatic viral shedding also occur (11, 35, 40, 53). HSV-2 has gained increasing interest in the light of evidence that it is a major risk factor for human immunodeficiency virus type 1 (HIV-1) acquisition and transmission and for the progression of HIV-1 infection (8, 9, 17, 25, 37, 55, 56). In addition, there is evidence that anti-HSV therapy can reduce the amount of infectious HIV-1 in the genital tracts of women (9, 45). Although antiviral treatment is available and can reduce the severity of the infection, compliance problems, as well as difficulty in diagnosing infection in patients, have hampered efforts to control the disease. A vaccine would provide a more effective way of preventing or limiting infection and would therefore greatly reduce the social and economic burdens caused by HSV-2 infection.
Several vaccine candidates exist; however, they have proven to be less successful in clinical trials than anticipated, and new strategies may need to be developed (24, 61). A key concern is that preclinical vaccine strategies have been evaluated largely by using studies performed with mouse models of HSV-2 infection and, thus, the immune responses observed were mediated by murine cells. As a consequence, the results of these studies may not accurately represent the human immune response to infection. In order to develop an effective vaccine and/or treatment, it is necessary to understand which immune mechanisms provide protection against infection at the site of viral entry, the vaginal tract, and how these immune responses can be induced in humans.
Innate and adaptive immune responses are both important for controlling HSV-2 infection. Innate immune cells such as NK and NKT cells are required for protection against genital HSV-2 infection in mice (1) and in humans; NK cells accumulate at sites of HSV-2 infection and can lyse HSV-infected cells (30, 67). Adaptive immune responses to HSV-2 include the cellular response mediated by CD4+ and CD8+ T cells and the humoral response mediated by B cells and antibodies. There is much evidence that T cells play a crucial role in protection against HSV-2 in mice and humans (28). T cells are present in herpes lesions, and depletion of T cells in mice greatly reduces protection (16, 27, 29, 30, 44, 51, 70). Gamma interferon (IFN-γ), which is produced early after infection by NK cells and later by CD4+ T cells, has been shown to be a crucial cytokine for the control of HSV (43, 52, 58, 63). Although HSV-2-specific antibodies are produced in response to infection and vaccination, a correlation with protection in humans has not been established (2, 3, 7, 10, 11, 48). In mice, a role for antibodies early after infection has been shown; however, if B cells are knocked out, mice are still able to eventually clear the virus (16, 50). Although we do not have a complete understanding of the components that are necessary for protection, it appears that both innate and adaptive immune responses will be required and that it will be important to elicit these responses at the site of infection in the genital tract.
The lack of an effective vaccine and accurate translation of results obtained with mice to humans indicates a need for a more relevant preclinical model to study human immune responses and disease. Substantial improvements in the development of humanized mice have made them a novel tool for the study of human diseases (69). Human CD34+ stem cells have been injected into several immunodeficient mouse strains, such as NOD/SCID/γc−/− and RAG2−/− γc−/− mice, in which superior engraftment has resulted in multilineage differentiation of the human cells (23, 64). These novel humanized mice have been shown to develop human immune responses to pathogens such as Epstein-Barr virus, dengue virus, and influenza virus and to immunization with cholera toxin (33, 64, 66, 68). In addition, humanized mice can support infection with HIV after systemic or mucosal challenge in the vaginal tract and rectum (4-6, 62, 65). HSV-2 infection in humanized mice has not been examined, and mucosal immunization that can provide protection from infection with wild-type virus has also not been demonstrated. In addition, although it is clear that adaptive immune responses can be generated in humanized mice, innate responses to viral infection have not been extensively examined.
In this study, we evaluated three immunodeficient mouse strains for their abilities to engraft human umbilical cord-derived stem cells and support the differentiation of these cells into important innate and adaptive immune cells. The most appropriate model was then used to examine mucosal immune responses following primary HSV-2 infection, immunization, and secondary HSV-2 challenge. We show for the first time that the humanized mice can mount protective human NK cell-mediated innate immune responses to primary mucosal infection with HSV-2. In addition, mucosal immunization and infection can induce HSV-2-specific antibody production and, to a greater extent, T-cell-mediated responses both systemically and locally in the genital tracts of humanized mice. We further show that mucosal immunization can provide protection against a lethal intravaginal (IVAG) challenge with HSV-2.
MATERIALS AND METHODS
Mice.
NOD/SCID mice were purchased from the Jackson Lab (Bar Harbor, ME). C57BL/6 RAG2−/− γc−/− mice were purchased from Taconic (Germantown, NY). Breeding pairs of BALB/c RAG2−/− γc−/− mice were generously provided by M. Ito (Central Institute for Experimental Animals, Kawasaki, Japan). All mice were bred and maintained under specific-pathogen-free conditions at the McMaster University Health Sciences Center. Mouse colonies were maintained on a 12-h light/12-h dark cycle.
Isolation of human stem cells from umbilical cord blood and transplantation into mice.
Human cord blood samples (Department of Labor and Delivery, McMaster University Health Sciences Center, Hamilton, Ontario, Canada) were obtained with written informed parental consent. Mononuclear cells were isolated from cord blood using HetaSep (Stem Cell Technologies, Vancouver, British Columbia, Canada). Samples were enriched for CD34+ cells through the removal of lineage-committed cells by a cocktail of antibodies with the RosetteSep system according to the instructions of the manufacturer (Stem Cell Technologies, Vancouver, BC, Canada). Samples were frozen immediately until use.
Mice were inoculated with human stem cells on the day of birth. NOD/SCID and RAG2−/− γc−/− mice were irradiated twice over a 3- to 4-h interval. NOD/SCID mice received two doses of 1.5 Gy, and RAG2−/− γc−/− mice received two doses of 3 Gy. After the second dose of irradiation, mice were inoculated intrahepatically with approximately 1 × 106 to 2 × 106 CD34-enriched cells in 30 μl of phosphate-buffered saline (PBS) by using a 30-gauge needle. Control mice were irradiated using the same protocol but did not receive any human cells. Mice were weaned at 3 weeks of age.
Analysis of human cell engraftment.
Mice were euthanized 12 to 16 weeks posttransplantation and examined for the presence of human lymphocytes. Single-cell suspensions were prepared from bone marrow, spleen, lymph node, thymus, blood, and vaginal tract samples. Bone marrow cells were removed from the leg bones by flushing with PBS and then passed through a 40-μm filter. Spleens, lymph nodes, and thymuses were pressed through a 40-μm filter, and red blood cells were removed with ACK lysis buffer. Vaginal tracts were digested using collagenase A (Life Technologies, Rockville, MD) for 2 h and then passed through a 40-μm filter.
Cells were stained for fluorescence-activated cell sorter (FACS) analysis with antibodies against human CD45, CD19, CD3, CD4, CD8, CD56, and CD16 (BD Pharmingen, San Diego, California). Flow cytometric data were collected using a FACScan or LSR II instrument (Becton Dickinson) and were analyzed using FlowJo software.
Vaccination of BALB/c RAG2−/− γc−/− mice.
Mice with and without reconstituted human cell populations were injected subcutaneously with 2 mg of medroxyprogesterone (Depo-Provera; Upjohn, Don Mills, Ontario, Canada) 5 days before immunization. Mice were vaccinated IVAG with 103 PFU/ml thymidine kinase-negative HSV-2 (HSV-2 Tk−) in PBS at a total volume of 10 μl/mouse. To deliver vaccine, mice were anesthetized with ketamine-xylazine (0.75:0.25 ml) given intraperitoneally; their tails were lifted, and vaccine was administered into the vagina. Mice were kept on their backs under the influence of anesthesia for 45 min to 1 h to allow the inoculum to be taken up.
Infection of BALB/c RAG2−/− γc−/− mice with HSV-2.
Three weeks after immunization or 5 days after medroxyprogesterone treatment only, mice with and without reconstituted human cell populations were challenged with wild-type HSV-2 strain 333. Immunized mice were challenged with 3 × 102 PFU, and unimmunized mice were challenged with 2 × 102 PFU (low dose). Both doses were lethal to mice without reconstituted human cell populations. Mice were anesthetized and placed on their backs, and virus in PBS at a total volume of 10 μl/mouse was administered IVAG. Mice remained anesthetized for 45 min to 1 h to allow infection.
Viral titration.
Vaginal wash fluids were collected from mice for 5 days following challenge. Wash samples were obtained by pipetting 2× 30 μl of PBS into and out of the vagina several times. Viral titers in vaginal wash fluids were determined by a plaque assay. Vero cells were grown in α-minimal essential medium (Gibco Laboratories, Burlington, Canada) supplemented with 5% fetal calf serum (Gibco, Burlington, Canada), 1% penicillin-streptomycin (Invitrogen, Burlington, Ontario), and l-glutamine (Gibco). Vero cells were grown to confluence in 12-well plates. Vaginal wash samples were diluted and added to Vero cell monolayers. Infected monolayers were incubated at 37°C for 1 h and were rocked every 15 min for viral absorption. Infected monolayers were overlaid with α-minimal essential medium supplemented with 0.05% globulin from human immune serum (Canadian Blood Services). Infection was allowed to occur for 48 h at 37°C. Monolayers were then fixed and stained with crystal violet, and viral plaques were counted under a light microscope.
T-cell proliferation assay using CFSE (carboxyfluorescein diacetate succinimidyl ester).
Spleens, lymph nodes, and vaginal tracts were removed from immunized mice with reconstituted human cell populations 4 to 6 days after challenge with HSV-2. Unimmunized mice with reconstituted human cell populations were used as controls. Single-cell suspensions were made from samples of each tissue. Spleens and lymph nodes were passed through a 40-μm mesh, and vaginal tracts were cut into small pieces by using a scalpel and then digested with collagenase A (Life Technologies, Rockville, MD). Red blood cells were removed using ACK lysis buffer.
Cells (5 × 105) were labeled with CFSE and cultured with either medium alone, HSV lysate (10 μg/ml), or concanavalin A (ConA; 5 μg/ml) in a 96-well plate for 4 days at 37°C. The HSV lysate was prepared from a large virus stock that was UV inactivated and lysed by freeze-thawing, with the protein measured after lysis. The lysate was tested on Vero cells to ensure that no live virus was detected. After 4 days, cells were harvested and stained with CD45 and CD3. Flow cytometric data were collected using an LSR II machine (BD Biosciences). Data were analyzed using FlowJo software. Antigen-specific proliferation was calculated by taking the percentage of CD3+ cells that divided in response to stimulation with HSV lysale and subtracting the percentage of CD3+ cells that divided with no stimulation in medium alone.
IFN-γ ELISA.
Human IFN-γ in cell culture supernatants was measured using a human IFN-γ enzyme-linked immunosorbent assay (ELISA) kit (BD Biosciences). Single-cell suspensions of spleen, lymph node, and vaginal tract cells were prepared as described above.
To evaluate adaptive immune responses, tissues were taken from immunized mice with reconstituted human cell populations 4 to 6 days after infection with wild-type HSV-2. Cells (5 × 105/well) were cultured in a 96-well plate with either medium alone, HSV-2 lysate (10 μg/ml), or ConA (5 μg/ml), and supernatants were removed after 72 h. Cells from unimmunized mice with reconstituted human cell populations were used as controls.
To evaluate innate production of IFN-γ, mesenteric lymph nodes were taken from mice with reconstituted human cell populations 48 h after challenge with wild-type HSV-2. Cells (5 × 105/well) were cultured in 96-well plates and stimulated with either medium alone or 200 ng/ml interleukin-15 (IL-15; R&D Systems) for 48 h. Cells from uninfected mice with reconstituted human cell populations were used as controls.
Antibody measurement.
Blood was taken from mice by retro-orbital bleeding. Sera were separated by centrifugation. Total human antibody levels in sera were measured with an ELISA kit (Bethyl Laboratories, Montgomery, TX). HSV-specific immunoglobulin G (IgG) levels were measured in sera taken from mice with reconstituted human cell populations before immunization, 3 weeks after immunization with HSV-2 Tk−, or 1 week after challenge with wild-type HSV-2 (using previously immunized mice). HSV-specific human IgG was measured using an ELISA kit (Alpco Diagnostics, Salem, NH).
Statistics.
The statistical significance of the survival rates was determined by the χ2 test. Viral titer data were analyzed using analysis of variance. Differences in percentages of cells divided, IFN-γ production, and antibody production were evaluated using Student's t test. A P value of <0.05 was considered statistically significant.
RESULTS
Reconstitution of human lymphocyte populations in immunodeficient mouse strains.
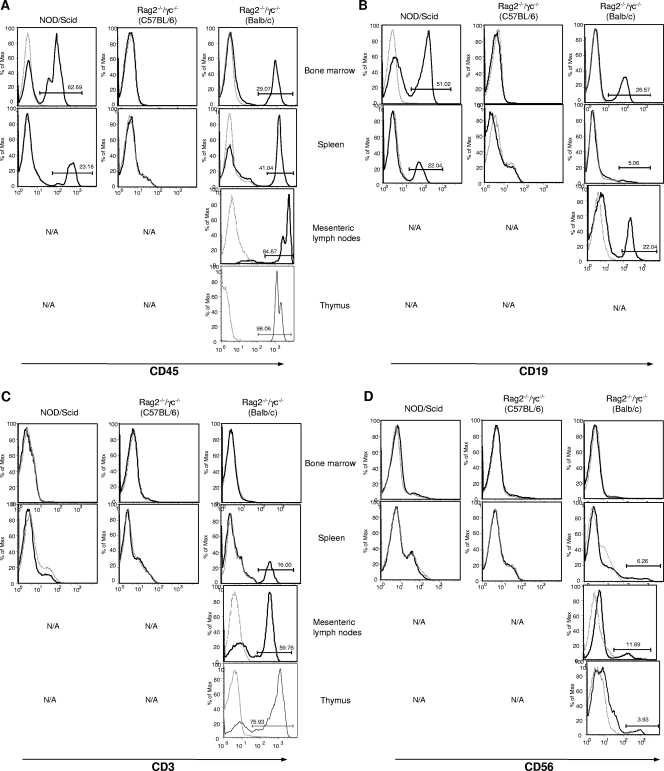
Newborn NOD/SCID, C57BL/6 RAG2−/− γc−/−, and BALB/c RAG2−/− γc−/− mice were irradiated and injected with human CD34-enriched cord blood cells. Twelve to fourteen weeks later, reconstitution in various tissues was evaluated by FACS analysis. Reconstitution of human CD45+ cell populations was observed only in NOD/SCID and BALB/c RAG2−/− γc−/− mice and not in C56BL/6 RAG2−/− γc−/− mice (Fig. 1A). The reconstitution was observed in all tissues examined; however, only the BALB/c RAG2−/− γc−/− mice developed lymph nodes and a thymus.
FIG. 1.
Reconstitution of human lymphocyte populations in immunodeficient mice. Mice of three different strains received human umbilical cord blood-derived stem cells at birth. Cells from the bone marrow, spleen, mesenteric lymph node, and thymus tissues were removed after 12 weeks and examined for the presence of human lymphocytes. Cells were labeled with the markers CD45, CD19, CD3, and CD56 and detected using FACS analyses. Solid histogram lines represent results for mice with reconstituted human cell populations, and dotted lines represent results for control mice without reconstituted human cell populations. N/A, not applicable (analysis was not performed due to the absence of tissue from the mouse strain).
In order for a humanized mouse model to be useful for immunization and infection studies, the primary innate and adaptive immune cells must be present. For this study, the development of human B cells, T cells, and NK cells was important. Differences in the types of human cells that developed in the two mouse strains with reconstituted cell populations were observed. B cells (CD19+) developed in the bone marrow and spleens of NOD/SCID mice and were the predominant human cell type in these tissues (Fig. 1B). The BALB/c RAG2−/− γc−/− mice developed B cells in the bone marrow, lymph nodes, and spleen (Fig. 1B). As shown in Fig. 1C, T cells (CD3+) were not detected in the NOD/SCID mice; however, the BALB/c RAG2−/− γc−/− mice developed T cells in the thymus, lymph nodes, and spleen, but no CD3+ cells were detected in the bone marrow of these mice. Figure 1D shows that NK/NKT cells (CD56+) developed in the spleens, lymph nodes, and thymuses of the BALB/c RAG2−/− γc−/− mice; however, no CD56+ cells were detected in the NOD/SCID mice. In light of the above-described observations (Table 1), it was concluded that the BALB/c RAG2−/− γc−/− mice were the most appropriate model to use in this study. Therefore, all further experiments were carried out with the BALB/c RAG2−/− γc−/− mice only.
TABLE 1.
Distribution of human cells in immunodeficient mice
| Tissue type and marker | % of marker-positive cells in:
|
||
|---|---|---|---|
| NOD/SCID micea | C56BL/6 RAG2−/− γc−/− mice | BALB/c RAG2−/− γc−/− miceb | |
| Bone marrow | |||
| CD45 | 50 | 0 | 14.6 |
| CD19 | 39 | 0 | 12.1 |
| CD3 | 0 | 0 | 0 |
| CD56 | 0 | 0 | 0 |
| Spleen | |||
| CD45 | 27 | 0 | 15.3 |
| CD19 | 27 | 0 | 2.8 |
| CD3 | 0 | 0 | 5 |
| CD56 | 0 | 0 | 2.5 |
| Mesenteric lymph node | |||
| CD45 | NAc | NA | 58.5 |
| CD19 | NA | NA | 24.5 |
| CD3 | NA | NA | 53.2 |
| CD56 | NA | NA | 23.3 |
| Thymus | |||
| CD45 | NA | NA | 53.6 |
| CD19 | NA | NA | 0 |
| CD3 | NA | NA | 63.2 |
| CD56 | NA | NA | 7.1 |
Results are averages for 18 mice.
Results are averages for 11 mice.
NA, not applicable.
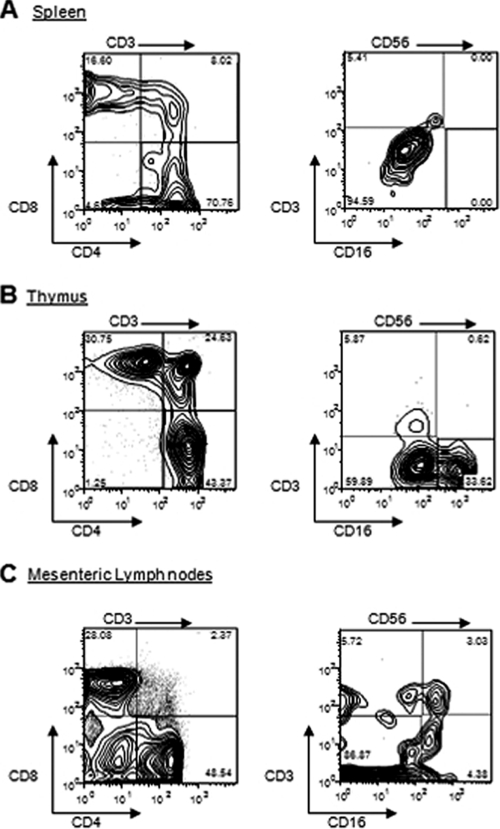
In order to further characterize the T and NK cells, cells from the spleen, thymus, and lymph node samples were examined for the presence of CD4/CD8 on T cells and CD16/CD3 on NK cells. Figure 2 shows representative distribution patterns of cells in the spleen (Fig. 2A), thymus (Fig. 2B), and mesenteric lymph node (Fig. 2C) tissues, although it is important that the proportions of CD3+ and CD56+ cells were variable among mice and that the proportions of subsets of these cells were similarly variable.
FIG. 2.
Human T and NK cells were detected in BALB/c RAG2−/− γc−/− mice. Cells from spleens, thymuses, and mesenteric lymph nodes were removed from mice with reconstituted human cell populations. Cells were gated using either CD3 (CD3→) or CD56 (CD56→). CD3+ cells were stained with T-cell markers CD4 and CD8. CD56+ cells were stained with NK/NKT-cell markers CD3 and CD16. The presence of these markers was detected by FACS analyses. Isotype controls for all antibodies were negative (data not shown).
Mice with reconstituted human cell populations develop innate immune responses after HSV-2 challenge.
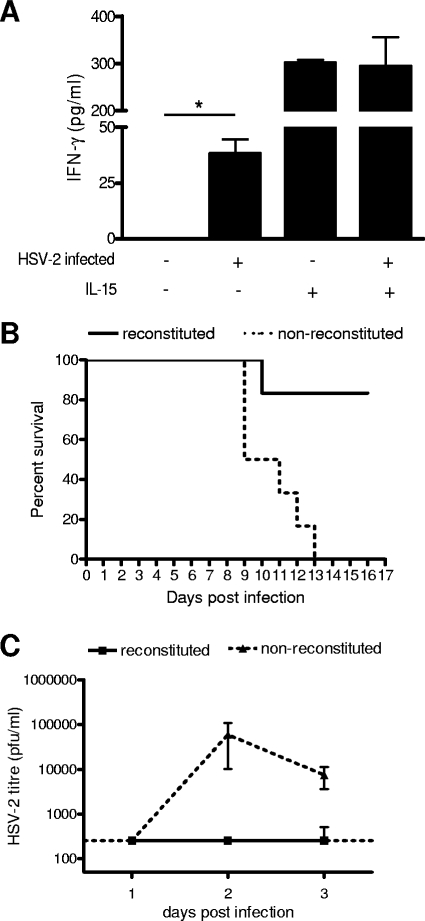
We have previously shown that NK and NKT cells in BALB/c RAG2−/− γc−/− mice were functional against a K562 tumor challenge (A. Kwant-Mitchell, E. A. Pek, K. L. Rosenthal, and A. A. Ashkar, submitted for publication). In order to determine whether the innate cells were functional against a mucosal viral challenge, mice were infected IVAG with 2 × 102 PFU of wild-type HSV-2. Forty-eight hours after infection, cells from the mesenteric lymph nodes were examined for the production of IFN-γ. As a positive control, some of the cells from each mouse were stimulated with human IL-15, which is a potent stimulator of NK cells and causes them to produce IFN-γ (59). As a control, IFN-γ production by cells from uninfected mice with reconstituted human cell populations was also measured. In the absence of IL-15 stimulation, the cells from infected mice produced significantly more IFN-γ than the cells from uninfected mice (P < 0.05) (Fig. 3A). When stimulated with IL-15, cells from both infected and uninfected mice produced IFN-γ (Fig. 3A).
FIG. 3.
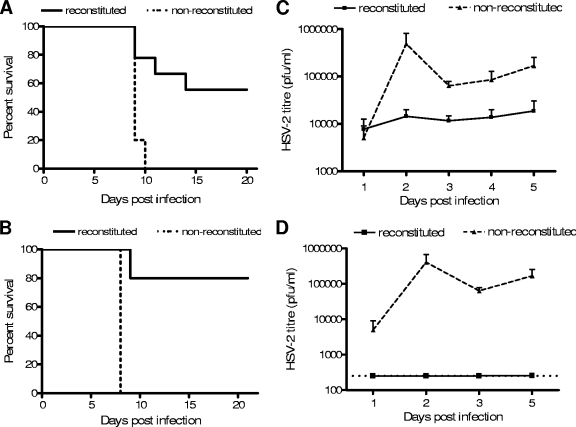
Innate immune response to HSV-2 infection in BALB/c RAG2−/− γc−/− mice. (A) Mice with reconstituted human cell populations were infected IVAG with HSV-2 (+) or not infected (−). Cells from the mesenteric lymph nodes were removed 48 h after infection and cultured with (+) or without (−) IL-15 for a further 48 h. The graph shows the average amounts (± standard errors of the means [SEM]) of human IFN-γ detected in culture supernatants by ELISA. In the absence of IL-15, cells from infected mice produced significantly more human IFN-γ than cells from uninfected mice (*, P < 0.05). Cells from both infected (n = 6) and uninfected (n = 4) mice produced human IFN-γ when stimulated with IL-15. (B) Mice with (n = 6) and without (n = 6) reconstituted cell populations were infected IVAG with wild-type HSV-2. The survival rate of mice with reconstituted cell populations was significantly higher than that of mice without reconstituted cell populations after primary infection (P < 0.05). (C) Vaginal washes were taken from mice with (n = 6) and without (n = 6) reconstituted cell populations on days 1, 2, and 3 after HSV-2 infection, and viral titers were measured using a plaque assay on Vero cells. The graph shows that the average HSV-2 titers (± SEM) were below the detectable level in mice with reconstituted cell populations and were much higher in mice without reconstituted cell populations.
The innate immunity conferred by the human cells was further examined by measuring survival rates of and viral titers in mice with reconstituted human cell populations after a mucosal IVAG HSV-2 challenge. As shown in Fig. 3B, over 80% of the mice with reconstituted human cell populations survived the viral challenge, and all of the mice without reconstituted human cell populations succumbed to infection. In addition, the mice with reconstituted human cell populations had significantly (P < 0.05) lower viral titers than the mice without reconstituted human cell populations (Fig. 3C), supporting a role for innate immune responses in controlling viral replication early after infection.
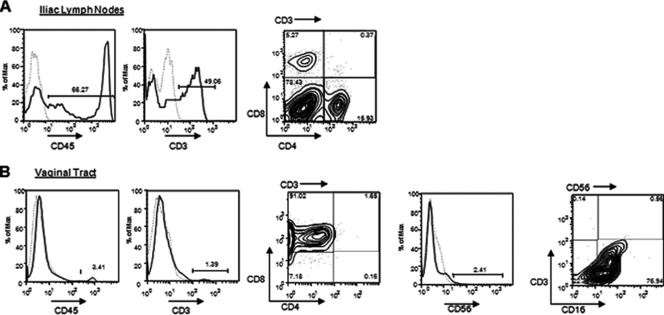
Human lymphocytes home to vaginal tissues after mucosal immunization and challenge.
Mice with reconstituted human cell populations were immunized IVAG with attenuated HSV-2 Tk− and challenged IVAG 3 weeks later with wild-type HSV-2. Five to seven days after challenge, mice were euthanized and tissues were removed. Among mice with reconstituted cell populations, several that had been immunized and/or infected IVAG developed iliac lymph nodes; however, none of the unimmunized or uninfected mice developed iliac lymph nodes. In addition, human cells were detected in the vaginal tract only after immunization and/or infection. The cells in the iliac lymph nodes were primarily T cells, including both CD4+ and CD8+ cells (Fig. 4A). In the vaginal tract, the human cells that were detected were CD8+ T cells and CD3− CD16+ NK cells (Fig. 4B). Thus, cross-species interactions facilitating homing appear to be successful in this humanized mouse model.
FIG. 4.
Human cells home to the vaginal tract and iliac lymph nodes after immunization and infection. Mice with reconstituted human cell populations were immunized IVAG with HSV-2 Tk− and infected 3 weeks later with wild-type HSV-2. Mice were euthanized 4 to 6 days after infection. (A) Iliac lymph nodes formed in immunized/infected mice. The lymph nodes were removed, and cells were stained with human CD45 (lymphocytes) and CD3 (T cells). CD3+ (CD3→) cells were further stained with CD4 and CD8. (B) The vaginal tract was removed, and human lymphocytes were identified by staining with CD45. T cells were identified by staining with CD3, and the CD3+ cells (CD3→) were stained for CD4 and CD8. NK/NKT cells were identified by staining with CD56 and were further stained with CD3 and CD16.
Mice with reconstituted human cell populations mount HSV-2-specific human adaptive immune responses.
After the demonstration that human adaptive immune cells are present in the humanized mice and exist within the systemic and mucosal compartments after IVAG immunization, the human T and B cells were examined for their antigen-specific functions.
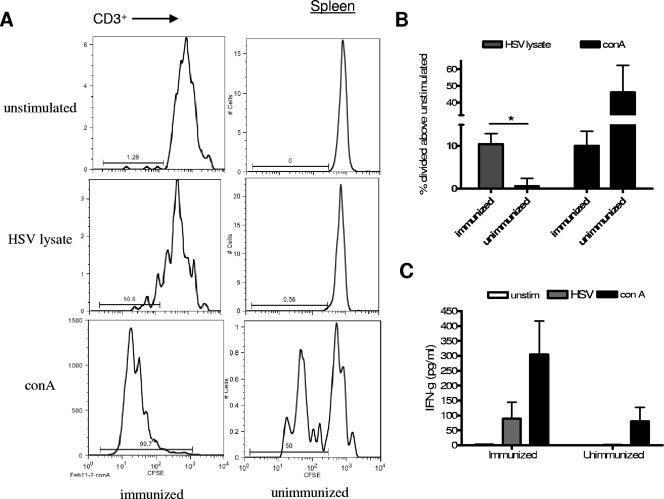
Cells were taken from the spleens, lymph nodes, and vaginal tracts of immunized and unimmunized mice, and T-cell activities in response to HSV-2 antigens were compared. Human T cells from the spleens of immunized mice divided in response to HSV antigen and ConA, while cells from unimmunized mice divided only when stimulated with ConA (Fig. 5A). As shown in Fig. 5B, a significantly higher percentage of T cells from immunized mice than from unimmunized mice divided (P < 0.05). In addition, spleen cells from immunized mice produced human IFN-γ when stimulated with HSV lysate, whereas cells from unimmunized mice produced IFN-γ only in response to the positive control, ConA (Fig. 5C).
FIG. 5.
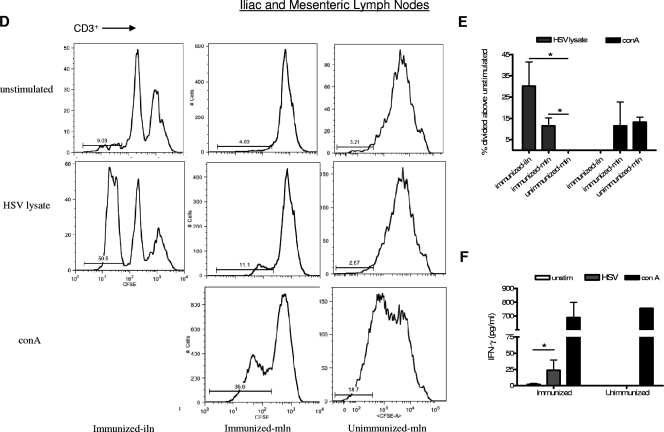
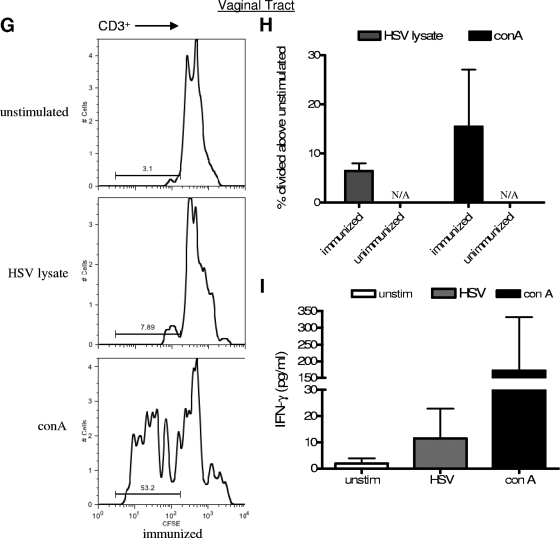
Human HSV-2-specific T-cell responses are observed in mice with reconstituted human cell populations after IVAG immunization and infection. Mice with reconstituted human cell populations were immunized IVAG with HSV-2 Tk− or left unimmunized and infected 3 weeks later with wild-type HSV-2. Spleens (A to C), lymph nodes (D to F), and vaginal tracts (G to I) were removed 5 days after infection, and cells were labeled with CFSE. The labeled cells were then stimulated with medium alone (unstimulated), HSV-2 lysate, or ConA for 4 days. (A) Representative FACS data demonstrating CFSE expression by CD3+ spleen cells from immunized and unimmunized mice. (B) Summary of the increase (± SEM) in the percentage of CD3+ spleen cells that divided above the percentage in the unstimulated control sample. The percentage of CD3+ cells that divided in response to stimulation with HSV-2 lysate was significantly higher in samples from immunized mice than in samples from unimmunized mice (*, P < 0.05; n = 10 immunized and 5 unimmunized mice). (C) Human IFN-γ in supernatants from the stimulated spleen cell cultures was measured by ELISA (n = 5 cultures from immunized mice and 7 from unimmunized mice). unstim, unstimulated. (D) Representative FACS data showing CFSE expression by CD3+ iliac lymph node (iln) and mesenteric lymph node (mln) cells. Iliac lymph nodes were not found in unimmunized mice, and therefore, only mesenteric lymph nodes were used as control samples from unimmunized mice. (E) Summary of the increase (± SEM) in the percentage of lymph node cells that divided above the percentage in the unstimulated control sample. The percentages of CD3+ cells that divided in response to HSV-2 lysate were significantly higher in both iliac and mesenteric lymph nodes from immunized mice than in those from unimmunized mice (*, P < 0.05; n = 7 mesenteric lymph node samples from immunized mice, 3 iliac lymph node samples from immunized mice, and 3 control samples from unimmunized mice). (F) Human IFN-γ levels in mesenteric lymph node cell culture supernatants as measured by ELISA (n = 6 samples from immunized and 3 from unimmunized mice). Cells from immunized mice produced significantly more IFN-γ in response to stimulation with HSV-2 lysate than cells from unimmunized mice (*, P < 0.05). (G) Representative FACS data showing that the percentage of human CD3+ cells from the vaginal tract expressing low levels of CFSE is increased after stimulation with HSV-2 lysate and further increased after stimulation with ConA. Human cells were found in the vaginal tract only after immunization; therefore, no control sample from unimmunized mice was included. (H) Summary of the increase (± SEM) in the percentage of cells that divided above the percentage in the unstimulated control sample (n = 6 immunized mice). N/A, not applicable. (I) Human IFN-γ levels in vaginal cell culture supernatants as measured by ELISA (n = 3).
Lymph node cells were also examined; however, unimmunized mice did not develop iliac lymph nodes, and therefore only mesenteric lymph nodes from unimmunized mice were used as controls. As shown in Fig. 5D and E, T cells from the mesenteric and, to a greater extent, the iliac lymph nodes from immunized mice divided in response to HSV lysate. T cells from mesenteric lymph nodes from unimmunized mice divided significantly less in response to HSV-2 lysate (P < 0.05) but did divide when stimulated with ConA. Lymph node cells from immunized mice also produced significantly more human IFN-γ when stimulated with HSV-2 lysate than cells from unimmunized mice (P < 0.05) (Fig. 5F).
Human cells were detected in the vaginal tract only after immunization, and therefore, no unimmunized mice could be used as controls. Figure 5G and H show that T cells from the vaginal tracts of immunized mice divided in response to HSV lysate and ConA much more than cells in the absence of stimulation. In addition, vaginal cells produced human IFN-γ in response to HSV-2 lysate but made very little when left unstimulated (Fig. 5I).
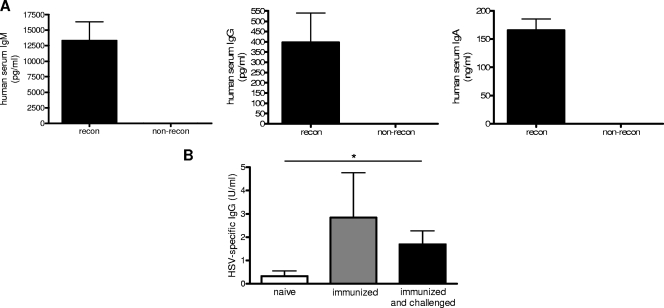
Human B-cell responses were also examined by measuring antibody production in sera from immunized and unimmunized mice. To ensure that isotype switching could occur in the mice, total IgM, IgG, and IgA levels were first measured. The humanized mice were able to produce all three antibody isotypes (Fig. 6A). In order to examine antigen-specific responses, sera from mice were taken before immunization, 2 weeks after immunization, or after challenge. Immunized and immunized/challenged mice produced detectable and significantly higher levels of HSV-2-specific IgG than naïve mice (P < 0.05) (Fig. 6B).
FIG. 6.
Human antibodies are produced by mice with reconstituted cell populations. (A) Total serum IgM, IgG, and IgA levels in naïve mice (eight with [recon] and four without [non-recon] reconstituted cell populations) were measured by ELISA. (B) Mice with reconstituted cell populations were immunized IVAG with HSV-2 Tk− and infected 3 weeks later with wild-type HSV-2. Significantly higher levels of HSV-2-specific IgG were detected in the sera of immunized and immunized/challenged mice than in the sera of naïve mice (*, P < 0.05; n = 3 naïve, 6 immunized, and 7 immunized and challenged mice). HSV-2-specific IgG was measured by ELISA.
Increased survival rates of mice with reconstituted human cell populations after immunization and challenge.
In order to determine whether the human cells in the mice could respond to immunization and protect against infection, mice with and without reconstituted human cell populations were immunized with HSV-2 Tk− and challenged 3 weeks later with a dose of wild-type HSV-2 that is lethal to mice without reconstituted human cell populations. The immunization and challenge were both delivered mucosally via the IVAG route. As shown in Fig. 7A, mice without reconstituted human cell populations did not survive the challenge whereas over half of the mice with reconstituted human cell populations survived the challenge (P < 0.005). In addition, vaginal wash samples were taken for 5 days following challenge and viral titers were measured. The mice with reconstituted human cell populations had significantly lower vaginal viral titers than the mice without reconstituted human cell populations (P < 0.05) (Fig. 7C).
FIG. 7.
Immunization confers protection against IVAG HSV-2 infection in mice with reconstituted cell populations. Mice with and without reconstituted cell populations were immunized with HSV-2 Tk− or a low dose (2 × 102 PFU) of wild-type HSV-2 and challenged 3 weeks later with a high dose of HSV-2. (A and B) Significantly higher survival rates were observed among HSV-2 Tk−-immunized mice (A) and low-dose HSV-2-immunized mice (B) than among control mice without reconstituted cell populations (P < 0.05). Survival was monitored for 21 days after infection (for panel A, n = 9 with and 5 without reconstituted cell populations; for panel B, n = 5 with and 3 without reconstituted cell populations). (C and D) Viral titers in vaginal wash fluids were measured for 5 days after infection of immunized mice. Graphs show significantly lower HSV-2 titers for HSV-2 Tk−-immunized mice (C) and low-dose HSV-2-immunized mice (D) than for mice without reconstituted cell populations (for panel C, n = 9 with and 6 without reconstituted cell populations; for panel D, n = 3 with and 6 without reconstituted cell populations). Virus was measured using a standard plaque assay, and graphs represent average viral titers (± SEM).
In a separate experiment, mice were given a low dose of wild-type HSV-2 and the surviving mice with reconstituted human cell populations were then rechallenged with a higher dose of HSV-2. None of the mice without reconstituted human cell populations survived the low- or high-dose challenge; however, all of the mice with reconstituted human cell populations that survived the low-dose challenge also survived the high-dose challenge (Fig. 7B). Furthermore, the vaginal viral titers in the mice with reconstituted human cell populations were significantly lower than those in the mice without reconstituted human cell populations (Fig. 7D).
DISCUSSION
A novel mouse model in which human immune responses to HSV-2 can be observed may be an important tool for preclinical evaluation of antiviral therapies and vaccines. This study has demonstrated the ability of an immunodeficient mouse strain to engraft human stem cells and support the development of human immune cells that are essential for innate and adaptive immunity to HSV-2. Importantly, the human immune cells can migrate to the vaginal tract and respond to mucosal primary infection, immunization, and secondary infection. The human immune response is robust enough to provide protection against a lethal dose of HSV-2 and significantly impair local viral replication.
In an attempt to identify the most appropriate murine recipient for human stem cells, we investigated three immunodeficient mouse strains for their abilities to support engraftment. The NOD/SCID strain has been used frequently to evaluate the health and repopulating ability of human stem cells (21, 36, 57). NOD/SCID mice lack murine T and B cells and have impaired innate immune functions, which allows for consistent and relatively high levels of human immune cell engraftment (as measured by the presence of human CD45+ cells in the bone marrow and spleen tissues). Although human immune cells from multiple myeloid, erythroid, and B-cell lineages have been detected in these mice, this study and others have shown that human T and NK cells do not develop (12, 20, 22, 26, 46). This lack of differentiation makes this model inappropriate for studies involving innate and adaptive immune responses, and it was therefore not used any further in this work. As an alternative to the NOD/SCID mutations, the RAG2−/− and γc−/− mutations confer a complete and permanent lack of T, B, and NK cells. RAG2−/− γc−/− mice with two different backgrounds, C57BL/6 and BALB/c, were tested. We evaluated both strains for their abilities to support human stem cell engraftment and differentiation. Interestingly, there was a complete contrast between the two strains, as the C57BL/6 mice did not support any engraftment while the BALB/c mice supported complete engraftment. In addition, the BALB/c RAG2−/− γc−/− mice developed a thymus which contained human T cells, as well as mesenteric lymph nodes containing human T, B, and NK cells. It is not clear why there is such a disparity between the reconstitution results for these strains. Since the RAG2−/− and γc−/− mutations are the same, differences in the background strain may be responsible. As the focus of this research was determining the most appropriate strain to use for studying the human innate and adaptive immune responses, we used the BALB/c RAG2−/− γc−/− mice for all future experiments.
Our knowledge of the immune responses generated against HSV-2 has been obtained primarily from in vivo studies using mouse models of HSV-2 and in vitro studies using human samples. This approach has limited our ability to apply the results to in vivo human responses and thus understand what components of the immune response are essential for protection in humans. The innate immune response to HSV-2 is not fully understood, and our knowledge of in vivo human NK cell functions, such as IFN-γ production, is lacking. In mice, NK and NKT cells are required for protection against mucosal HSV-2 infection (1) and IFN-γ is produced locally after infection (43, 63). In humans, NK cells accumulate at the site of HSV-2 infection (30), and in one study, patients with severe HSV infections were found to be lacking in NK cells (15). In a separate study, we have shown that human NK and NKT cells develop in the BALB/c RAG2−/− γc−/− mouse model and that these cells are functional (Kwant-Mitchell et al., submitted). They can produce human IFN-γ in response to IL-15 and can kill NK-sensitive K562 cells both in vitro and in vivo. In the present study, human NK cells developed in the spleens, thymuses, lymph nodes, and vaginal tracts of mice with reconstituted human cell populations. NK cells isolated from the mesenteric lymph nodes of infected mice produced human IFN-γ in response to stimulation with IL-15 and infection with HSV-2. In addition, the human innate immune response was capable of protecting mice against a primary genital HSV-2 infection and could significantly limit vaginal viral replication.
The adaptive immune response has been studied more extensively in an effort to understand which components are most important for protection against infection, limitation of symptoms, and prevention of transmission. In mice, the best protection against vaginal HSV-2 infection is achieved by immunizing with attenuated virus HSV-2 Tk−, delivered via the genital mucosa (52). CD4+ and CD8+ T-cell responses, as well as IFN-γ production, have been shown to play crucial roles in this protection (39, 41, 43, 44, 51). In addition, IgG has been shown to be the primary HSV-2-specific antibody in vaginal secretions following IVAG infection, whereas the role of secretory IgA seems to be less important (38, 42, 47, 49). In humans, it is not clear whether protective responses are mediated by T cells, antibodies, or some combination of the two. HSV-2-infected patients have been examined, and both HSV-2-specific antibodies and T cells have been detected in genital lesions (2, 14, 27, 28, 30, 54). However, results from several vaccine trials have indicated that the production of antibodies may not be sufficient for protection, and thus, cellular immunity is now regarded as an essential component of a protective human anti-HSV-2 immune response. In addition to the type of immune response that is important for protection, the best route of administration for a vaccine has been debated. Since HSV-2 infection is a mucosal infection that is initiated at the vaginal mucosa, many studies have been aimed at inducing local, genital immune responses as well as systemic ones. In mice, both intranasal and IVAG vaccine deliveries have proven to be effective at inducing genital immune responses, and there is evidence that intranasal immunization also elicits genital immune responses in humans (18, 19, 31, 32, 34, 60). In this study, we have shown that human T cells migrate to the vaginal tract after immunization and infection. In addition, iliac lymph nodes, which drain the genital tract, develop only after vaginal infection and also contain T cells. The human T cells from the spleen, lymph nodes, and vaginal tract are functional and respond specifically to HSV-2 antigens by replicating and producing human IFN-γ. Human B cells are also functional, as they produce antibodies which can be detected in the serum and are also able to make HSV-2-specific IgG after immunization and infection. Since engraftment, expansion, and homing of human cells is observed in our model, there is likely some degree of cross-species reactivity between mice and humans; however, this issue is known to represent a potential problem with current humanized mouse models. Indeed, several strategies to improve humanized mouse models involve making human-derived molecules more available to the engrafted cells.
The ultimate test of a vaccine is whether it can provide protection against infection with wild-type virus. In this study, two immunization approaches were used. One group of mice with reconstituted human cell populations was immunized IVAG with an attenuated strain of HSV-2, and another group was infected IVAG with a low dose of wild-type HSV-2. Both of these immunization strategies induced a human immune response that was strong enough to protect against a secondary IVAG wild-type HSV-2 infection. Furthermore, local viral titers in the genital tracts of immunized mice were kept significantly lower than those in the genital tracts of unimmunized mice, and many immunized mice showed no detectable virus. It is therefore clear from these studies that the human immune cells in the mice are functional and are able to mount antigen-specific systemic and mucosal immune responses after immunization and infection.
HSV-2 is becoming more prevalent in the human population and is emerging as a significant risk factor for HIV infection. There is no effective vaccine for HSV-2, and many candidates that showed promise in murine studies showed little effect in humans. The present study outlines a model that may potentially be used for preclinical testing of vaccines, as well as the study of human immune responses in an in vivo setting. Humanized BALB/c RAG2−/− γc−/− mice can mount both innate and adaptive immune responses to HSV-2, and human immune cells can migrate into the genital mucosa, where natural infection most often occurs.
Acknowledgments
We thank M. Ito at the Central Institute for Experimental Animals, Kawasaki, Japan, for generously providing us with BALB/c RAG2−/− γc−/− mice.
This work was supported by grants from the Canadian Institutes of Health Research (CIHR). K.L.R. is partially supported by a Career Scientist Award from the Ontario HIV Treatment Network (OHTN).
Footnotes
Published ahead of print on 5 August 2009.
REFERENCES
- 1.Ashkar, A. A., and K. L. Rosenthal. 2003. Interleukin-15 and natural killer and NKT cells play a critical role in innate protection against genital herpes simplex virus type 2 infection. J. Virol. 77:10168-10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashley, R. L., L. Corey, J. Dalessio, P. Wilson, M. Remington, G. Barnum, and P. Trethewey. 1994. Protein-specific cervical antibody responses to primary genital herpes simplex virus type 2 infections. J. Infect. Dis. 170:20-26. [DOI] [PubMed] [Google Scholar]
- 3.Ashley, R. L., F. M. Crisostomo, M. Doss, R. E. Sekulovich, R. L. Burke, M. Shaughnessy, L. Corey, N. L. Polissar, and A. G. Langenberg. 1998. Cervical antibody responses to a herpes simplex virus type 2 glycoprotein subunit vaccine. J. Infect. Dis. 178:1-7. [DOI] [PubMed] [Google Scholar]
- 4.Baenziger, S., R. Tussiwand, E. Schlaepfer, L. Mazzucchelli, M. Heikenwalder, M. O. Kurrer, S. Behnke, J. Frey, A. Oxenius, H. Joller, A. Aguzzi, M. G. Manz, and R. F. Speck. 2006. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted RAG2−/−γc−/− mice. Proc. Natl. Acad. Sci. USA 103:15951-15956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berges, B. K., S. R. Akkina, J. M. Folkvord, E. Connick, and R. Akkina. 2008. Mucosal transmission of R5 and X4 tropic HIV-1 via vaginal and rectal routes in humanized RAG2−/−γc−/− (RAG-hu) mice. Virology 373:342-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berges, B. K., W. H. Wheat, B. E. Palmer, E. Connick, and R. Akkina. 2006. HIV-1 infection and CD4 T cell depletion in the humanized RAG2−/−γc−/− (RAG-hu) mouse model. Retrovirology 3:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernstein, D. I., and L. R. Stanberry. 1999. Herpes simplex virus vaccines. Vaccine 17:1681-1689. [DOI] [PubMed] [Google Scholar]
- 8.Corey, L. 2007. Herpes simplex virus type 2 and HIV-1: the dialogue between the 2 organisms continues. J. Infect. Dis. 195:1242-1244. [DOI] [PubMed] [Google Scholar]
- 9.Corey, L. 2007. Synergistic copathogens—HIV-1 and HSV-2. N. Engl. J. Med. 356:854-856. [DOI] [PubMed] [Google Scholar]
- 10.Corey, L., A. G. Langenberg, R. Ashley, R. E. Sekulovich, A. E. Izu, J. M. Douglas, Jr., H. H. Handsfield, T. Warren, L. Marr, S. Tyring, R. DiCarlo, A. A. Adimora, P. Leone, C. L. Dekker, R. L. Burke, W. P. Leong, S. E. Straus, et al. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. JAMA 282:331-340. [DOI] [PubMed] [Google Scholar]
- 11.Corey, L., and A. Wald. 1999. Genital herpes, p. 285-312. In K. K. Holmes, P.-A. Mardh, P. F. Sparling, S. M. Lemon, W. E. Stamm, P. Piot, and J. N. Wasserheit (ed.), Sexually transmitted diseases. McGraw-Hill, New York, NY.
- 12.Cravens, P. D., M. W. Melkus, A. Padgett-Thomas, M. Islas-Ohlmayer, P. M. M. Del, and J. V. Garcia. 2005. Development and activation of human dendritic cells in vivo in a xenograft model of human hematopoiesis. Stem Cells 23:264-278. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham, A. L., A. L. Mindel, and D. E. Dwyer. 2001. Epidemiology of sexually transmitted diseases, p. 3-42. In L. R. Stanberry and D. I. Bernstein (ed.), Sexually transmitted diseases: vaccines, prevention and control. Academic Press, London, United Kingdom.
- 14.Cunningham, A. L., R. R. Turner, A. C. Miller, M. F. Para, and T. C. Merigan. 1985. Evolution of recurrent herpes simplex lesions. An immunohistologic study. J. Clin. Investig. 75:226-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalloul, A., E. Oksenhendler, O. Chosidow, P. Ribaud, G. Carcelain, S. Louvet, P. Massip, P. Lebon, and B. Autran. 2004. Severe herpes virus (HSV-2) infection in two patients with myelodysplasia and undetectable NK cells and plasmacytoid dendritic cells in the blood. J. Clin. Virol. 30:329-336. [DOI] [PubMed] [Google Scholar]
- 16.Dudley, K. L., N. Bourne, and G. N. Milligan. 2000. Immune protection against HSV-2 in B-cell-deficient mice. Virology 270:454-463. [DOI] [PubMed] [Google Scholar]
- 17.Freeman, E. E., H. A. Weiss, J. R. Glynn, P. L. Cross, J. A. Whitworth, and R. J. Hayes. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20:73-83. [DOI] [PubMed] [Google Scholar]
- 18.Gallichan, W. S., and K. L. Rosenthal. 1998. Long-term immunity and protection against herpes simplex virus type 2 in the murine female genital tract after mucosal but not systemic immunization. J. Infect. Dis. 177:1155-1161. [DOI] [PubMed] [Google Scholar]
- 19.Gallichan, W. S., R. N. Woolstencroft, T. Guarasci, M. J. McCluskie, H. L. Davis, and K. L. Rosenthal. 2001. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J. Immunol. 166:3451-3457. [DOI] [PubMed] [Google Scholar]
- 20.Guenechea, G., O. I. Gan, C. Dorrell, and J. E. Dick. 2001. Distinct classes of human stem cells that differ in proliferative and self-renewal potential. Nat. Immunol. 2:75-82. [DOI] [PubMed] [Google Scholar]
- 21.Hesselton, R. M., D. L. Greiner, J. P. Mordes, T. V. Rajan, J. L. Sullivan, and L. D. Shultz. 1995. High levels of human peripheral blood mononuclear cell engraftment and enhanced susceptibility to human immunodeficiency virus type 1 infection in NOD/LtSz-scid/scid mice. J. Infect. Dis. 172:974-982. [DOI] [PubMed] [Google Scholar]
- 22.Hogan, C. J., E. J. Shpall, O. McNulty, I. McNiece, J. E. Dick, L. D. Shultz, and G. Keller. 1997. Engraftment and development of human CD34+-enriched cells from umbilical cord blood in NOD/LtSz-scid/scid mice. Blood 90:85-96. [PubMed] [Google Scholar]
-
23.Ito, M., H. Hiramatsu, K. Kobayashi, K. Suzue, M. Kawahata, K. Hioki, Y. Ueyama, Y. Koyanagi, K. Sugamura, K. Tsuji, T. Heike, and T. Nakahata. 2002.
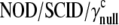 mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100:3175-3182. [DOI] [PubMed] [Google Scholar]
mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100:3175-3182. [DOI] [PubMed] [Google Scholar] - 24.Jones, C. A., and A. L. Cunningham. 2004. Vaccination strategies to prevent genital herpes and neonatal herpes simplex virus (HSV) disease. Herpes 11:12-17. [PubMed] [Google Scholar]
- 25.Kapiga, S. H., N. E. Sam, H. Bang, Q. Ni, T. T. Ao, I. Kiwelu, S. Chiduo, U. Ndibe, G. Seage III, P. Coplan, J. Shao, Z. F. Rosenberg, and M. Essex. 2007. The role of herpes simplex virus type 2 and other genital infections in the acquisition of HIV-1 among high-risk women in northern Tanzania. J. Infect. Dis. 195:1260-1269. [DOI] [PubMed] [Google Scholar]
- 26.Kerre, T. C., G. De Smet, M. De Smedt, A. Zippelius, M. J. Pittet, A. W. Langerak, J. De Bosscher, F. Offner, B. Vandekerckhove, and J. Plum. 2002. Adapted NOD/SCID model supports development of phenotypically and functionally mature T cells from human umbilical cord blood CD34+ cells. Blood 99:1620-1626. [DOI] [PubMed] [Google Scholar]
- 27.Koelle, D. M., H. Abbo, A. Peck, K. Ziegweid, and L. Corey. 1994. Direct recovery of herpes simplex virus (HSV)-specific T lymphocyte clones from recurrent genital HSV-2 lesions. J. Infect. Dis. 169:956-961. [DOI] [PubMed] [Google Scholar]
- 28.Koelle, D. M., and L. Corey. 2003. Recent progress in herpes simplex virus immunobiology and vaccine research. Clin. Microbiol. Rev. 16:96-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koelle, D. M., J. C. Gonzalez, and A. S. Johnson. 2005. Homing in on the cellular immune response to HSV-2 in humans. Am. J. Reprod. Immunol. 53:172-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koelle, D. M., C. M. Posavad, G. R. Barnum, M. L. Johnson, J. M. Frank, and L. Corey. 1998. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J. Clin. Investig. 101:1500-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozlowski, P. A., S. Cu-Uvin, M. R. Neutra, and T. P. Flanigan. 1997. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect. Immun. 65:1387-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozlowski, P. A., S. B. Williams, R. M. Lynch, T. P. Flanigan, R. R. Patterson, S. Cu-Uvin, and M. R. Neutra. 2002. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J. Immunol. 169:566-574. [DOI] [PubMed] [Google Scholar]
- 33.Kuruvilla, J. G., R. M. Troyer, S. Devi, and R. Akkina. 2007. Dengue virus infection and immune response in humanized RAG2−/−γc−/− (RAG-hu) mice. Virology 369:143-152. [DOI] [PubMed] [Google Scholar]
- 34.Kwant, A., and K. L. Rosenthal. 2004. Intravaginal immunization with viral subunit protein plus CpG oligodeoxynucleotides induces protective immunity against HSV-2. Vaccine 22:3098-3104. [DOI] [PubMed] [Google Scholar]
- 35.Langenberg, A. G., L. Corey, R. L. Ashley, W. P. Leong, S. E. Straus, et al. 1999. A prospective study of new infections with herpes simplex virus type 1 and type 2. N. Engl. J. Med. 341:1432-1438. [DOI] [PubMed] [Google Scholar]
- 36.Lowry, P. A., L. D. Shultz, D. L. Greiner, R. M. Hesselton, E. L. Kittler, C. Y. Tiarks, S. S. Rao, J. Reilly, J. H. Leif, H. Ramshaw, F. M. Stewart, and P. J. Quesenberry. 1996. Improved engraftment of human cord blood stem cells in NOD/LtSz-scid/scid mice after irradiation or multiple-day injections into unirradiated recipients. Biol. Blood Marrow Transplant. 2:15-23. [PubMed] [Google Scholar]
- 37.McClelland, R. S., C. C. Wang, J. Overbaugh, B. A. Richardson, L. Corey, R. L. Ashley, K. Mandaliya, J. Ndinya-Achola, J. J. Bwayo, and J. K. Kreiss. 2002. Association between cervical shedding of herpes simplex virus and HIV-1. AIDS 16:2425-2430. [DOI] [PubMed] [Google Scholar]
- 38.McDermott, M. R., L. J. Brais, and M. J. Evelegh. 1990. Mucosal and systemic antiviral antibodies in mice inoculated intravaginally with herpes simplex virus type 2. J. Gen. Virol. 71(Pt. 7):1497-1504. [DOI] [PubMed] [Google Scholar]
- 39.McDermott, M. R., P. L. Brais, G. C. Ploettsche, M. J. Evelegh, and C. H. Goldsmith. 1987. Expression of immunity to intravaginal herpes simplex virus type 2 infection in the genital tract and associated lymph nodes. Arch. Virol. 93:51-68. [DOI] [PubMed] [Google Scholar]
- 40.Mertz, G. J., O. Schmidt, J. L. Jourden, M. E. Guinan, M. L. Remington, A. Fahnlander, C. Winter, K. K. Holmes, and L. Corey. 1985. Frequency of acquisition of first-episode genital infection with herpes simplex virus from symptomatic and asymptomatic source contacts. Sex. Transm. Dis. 12:33-39. [DOI] [PubMed] [Google Scholar]
- 41.Milligan, G. N., and D. I. Bernstein. 1995. Analysis of herpes simplex virus-specific T cells in the murine female genital tract following genital infection with herpes simplex virus type 2. Virology 212:481-489. [DOI] [PubMed] [Google Scholar]
- 42.Milligan, G. N., and D. I. Bernstein. 1995. Generation of humoral immune responses against herpes simplex virus type 2 in the murine female genital tract. Virology 206:234-241. [DOI] [PubMed] [Google Scholar]
- 43.Milligan, G. N., and D. I. Bernstein. 1997. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology 229:259-268. [DOI] [PubMed] [Google Scholar]
- 44.Milligan, G. N., D. I. Bernstein, and N. Bourne. 1998. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J. Immunol. 160:6093-6100. [PubMed] [Google Scholar]
- 45.Nagot, N., A. Ouedraogo, V. Foulongne, I. Konate, H. A. Weiss, L. Vergne, M. C. Defer, D. Djagbare, A. Sanon, J. B. Andonaba, P. Becquart, M. Segondy, R. Vallo, A. Sawadogo, P. Van de Perre, and P. Mayaud. 2007. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N. Engl. J. Med. 356:790-799. [DOI] [PubMed] [Google Scholar]
- 46.Palucka, A. K., J. Gatlin, J. P. Blanck, M. W. Melkus, S. Clayton, H. Ueno, E. T. Kraus, P. Cravens, L. Bennett, A. Padgett-Thomas, F. Marches, M. Islas-Ohlmayer, J. V. Garcia, and J. Banchereau. 2003. Human dendritic cell subsets in NOD/SCID mice engrafted with CD34+ hematopoietic progenitors. Blood 102:3302-3310. [DOI] [PubMed] [Google Scholar]
- 47.Parr, E. L., J. J. Bozzola, and M. B. Parr. 1998. Immunity to vaginal infection by herpes simplex virus type 2 in adult mice: characterization of the immunoglobulins in vaginal mucus. J. Reprod. Immunol. 38:15-30. [DOI] [PubMed] [Google Scholar]
- 48.Parr, E. L., and M. B. Parr. 1998. Immunoglobulin G, plasma cells, and lymphocytes in the murine vagina after vaginal or parenteral immunization with attenuated herpes simplex virus type 2. J. Virol. 72:5137-5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parr, M. B., G. R. Harriman, and E. L. Parr. 1998. Immunity to vaginal HSV-2 infection in immunoglobulin A knockout mice. Immunology 95:208-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parr, M. B., and E. L. Parr. 2000. Immunity to vaginal herpes simplex virus-2 infection in B-cell knockout mice. Immunology 101:126-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parr, M. B., and E. L. Parr. 1998. Mucosal immunity to herpes simplex virus type 2 infection in the mouse vagina is impaired by in vivo depletion of T lymphocytes. J. Virol. 72:2677-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parr, M. B., and E. L. Parr. 2003. Vaginal immunity in the HSV-2 mouse model. Int. Rev. Immunol. 22:43-63. [DOI] [PubMed] [Google Scholar]
- 53.Patel, R., and D. R. Harper. 1998. Subclinical herpes virus reactivation and latency. Curr. Opin. Infect. Dis. 11:31-35. [DOI] [PubMed] [Google Scholar]
- 54.Posavad, C. M., D. M. Koelle, and L. Corey. 1998. Tipping the scales of herpes simplex virus reactivation: the important responses are local. Nat. Med. 4:381-382. [DOI] [PubMed] [Google Scholar]
- 55.Reynolds, S. J., A. R. Risbud, M. E. Shepherd, J. M. Zenilman, R. S. Brookmeyer, R. S. Paranjape, A. D. Divekar, R. R. Gangakhedkar, M. V. Ghate, R. C. Bollinger, and S. M. Mehendale. 2003. Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. J. Infect. Dis. 187:1513-1521. [DOI] [PubMed] [Google Scholar]
- 56.Schacker, T., A. J. Ryncarz, J. Goddard, K. Diem, M. Shaughnessy, and L. Corey. 1998. Frequent recovery of HIV-1 from genital herpes simplex virus lesions in HIV-1-infected men. JAMA 280:61-66. [DOI] [PubMed] [Google Scholar]
- 57.Shultz, L. D., P. A. Schweitzer, S. W. Christianson, B. Gott, I. B. Schweitzer, B. Tennent, S. McKenna, L. Mobraaten, T. V. Rajan, D. L. Greiner, et al. 1995. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J. Immunol. 154:180-191. [PubMed] [Google Scholar]
- 58.Smith, P. M., R. M. Wolcott, R. Chervenak, and S. R. Jennings. 1994. Control of acute cutaneous herpes simplex virus infection: T cell-mediated viral clearance is dependent upon interferon-gamma (IFN-gamma). Virology 202:76-88. [DOI] [PubMed] [Google Scholar]
- 59.Smyth, M. J., E. Cretney, M. H. Kershaw, and Y. Hayakawa. 2004. Cytokines in cancer immunity and immunotherapy. Immunol. Rev. 202:275-293. [DOI] [PubMed] [Google Scholar]
- 60.Staats, H. F., S. P. Montgomery, and T. J. Palker. 1997. Intranasal immunization is superior to vaginal, gastric, or rectal immunization for the induction of systemic and mucosal anti-HIV antibody responses. AIDS Res. Hum Retrovir. 13:945-952. [DOI] [PubMed] [Google Scholar]
- 61.Stanberry, L. R. 2004. Clinical trials of prophylactic and therapeutic herpes simplex virus vaccines. Herpes 11(Suppl. 3):161A-169A. [PubMed] [Google Scholar]
- 62.Sun, Z., P. W. Denton, J. D. Estes, F. A. Othieno, B. L. Wei, A. K. Wege, M. W. Melkus, A. Padgett-Thomas, M. Zupancic, A. T. Haase, and J. V. Garcia. 2007. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J. Exp. Med. 204:705-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Torseth, J. W., and T. C. Merigan. 1986. Significance of local gamma interferon in recurrent herpes simplex infection. J. Infect. Dis. 153:979-984. [DOI] [PubMed] [Google Scholar]
- 64.Traggiai, E., L. Chicha, L. Mazzucchelli, L. Bronz, J. C. Piffaretti, A. Lanzavecchia, and M. G. Manz. 2004. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 304:104-107. [DOI] [PubMed] [Google Scholar]
- 65.Watanabe, S., K. Terashima, S. Ohta, S. Horibata, M. Yajima, Y. Shiozawa, M. Z. Dewan, Z. Yu, M. Ito, T. Morio, N. Shimizu, M. Honda, and N. Yamamoto. 2007. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rγ null mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood 109:212-218. [DOI] [PubMed] [Google Scholar]
- 66.Yajima, M., K. Imadome, A. Nakagawa, S. Watanabe, K. Terashima, H. Nakamura, M. Ito, N. Shimizu, M. Honda, N. Yamamoto, and S. Fujiwara. 2008. A new humanized mouse model of Epstein-Barr virus infection that reproduces persistent infection, lymphoproliferative disorder, and cell-mediated and humoral immune responses. J. Infect. Dis. 198:673-682. [DOI] [PubMed] [Google Scholar]
- 67.Yasukawa, M., and J. M. Zarling. 1983. Autologous herpes simplex virus-infected cells are lysed by human natural killer cells. J. Immunol. 131:2011-2016. [PubMed] [Google Scholar]
- 68.Yu, C. I., M. Gallegos, F. Marches, G. Zurawski, O. Ramilo, A. Garcia-Sastre, J. Banchereau, and A. K. Palucka. 2008. Broad influenza-specific CD8+ T-cell responses in humanized mice vaccinated with influenza virus vaccines. Blood 112:3671-3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang, B., Z. Duan, and Y. Zhao. 2009. Mouse models with human immunity and their application in biomedical research. J. Cell. Mol. Med. 13:1043-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu, J., D. M. Koelle, J. Cao, J. Vazquez, M. L. Huang, F. Hladik, A. Wald, and L. Corey. 2007. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J. Exp. Med. 204:595-603. [DOI] [PMC free article] [PubMed] [Google Scholar]