Abstract
Recent findings suggest that most sexual transmission of human immunodeficiency virus type 1 (HIV-1) occurs during the acute phase of infection when viral replication is most intense. Here, we show that vaccine-elicited cellular immune responses can significantly reduce simian immunodeficiency virus levels in the semen during the period of primary infection in monkeys. A vaccine that decreases the quantity of HIV-1 in the semen of males during primary infection might decrease HIV-1 transmission in human populations and therefore affect the spread of AIDS.
An ideal AIDS vaccine would prevent the initiation of a human immunodeficiency virus type 1 (HIV-1) infection in a virus-exposed individual (7, 11, 12). However, a vaccine that does not confer sterilizing protection could have a significant impact on the AIDS epidemic if it decreases the rate of spread of HIV-1 (5, 12, 20). Recent studies have estimated that as many as 50% of all HIV-1 sexual transmission events occur when the individual transmitting the virus has recently become infected (1, 17, 19). This can be explained by the fact that HIV-1 replication is most intense during the period of primary infection. While a number of factors have been shown to increase HIV transmission (4, 8, 9, 21), the likelihood of transmitting the virus is largely determined by the level of virus replication in the already infected individual (10, 16, 18, 20).
Since no available immunogens induce antibodies that neutralize a variety of HIV-1 isolates, HIV-1 vaccine candidates that generate virus-specific cellular immune responses are currently being evaluated. While the immune responses elicited by these prototype vaccines do not confer sterilizing protection, studies of nonhuman primate models have shown that vaccine-elicited, virus-specific T-lymphocyte populations can suppress viral replication in the peripheral blood during the period of primary infection (13).
The present study was initiated to determine whether a vaccine-induced cellular immune response might decrease viral burden in the semen during the first weeks following simian immunodeficiency virus (SIV) challenge. Such a vaccine-associated diminished viral burden during the period of primary infection might confer protection against the spread of the AIDS virus by the newly infected individual (2, 15).
Rhesus monkeys were immunized using a heterologous prime/boost regimen that included both plasmid DNA and replication-defective recombinant adenovirus serotype 5 immunogens (13). One group of 13 male monkeys received these vaccine vectors expressing SIVmac239 Gag/Pol, and a second group of 13 male monkeys received sham vaccine constructs. Peak vaccine-induced peripheral blood lymphocyte gamma interferon enzyme-linked immunospot assay responses (mean spot-forming colony/106 lymphocytes ± standard error of the mean) were observed 2 weeks following the recombinant adenovirus serotype 5 boost (for Gag-Pol-vaccinated group, Gag, 2,088 ± 284, and Pol, 2,984 ± 669; for control group, Gag, 10 ± 2.6, and Pol, 5 ± 2.2); responses at the time of challenge were as follows: for Gag-Pol-vaccinated group, Gag, 942 ± 198, and Pol, 1,426 ± 106; for control group, Gag, 2 ± 0.8, and Pol, 70 ± 9.1.
Sixteen weeks after the last immunization, the monkeys were challenged by intravenous route with a highly pathogenic SIVmac251 quasispecies. Following SIVmac251 challenge, semen samples were obtained from the monkeys by a standard electroejaculation protocol on a weekly basis for 6 weeks, then at biweekly intervals for the duration of the study. An adequate semen specimen was not obtained following electroejaculation from all monkeys at every time point. Data were therefore generated for 7 to 13 monkeys in each experimental group at the time of each sampling. We followed the experimentally vaccinated and negative control monkeys for 16 weeks after infection to evaluate both the acute and the set point phase of SIV viremia. Median peripheral blood plasma virus RNA levels in these monkeys at the time of peak SIV replication as determined by bDNA assay (day 14 following SIV challenge) were significantly higher in the control group than in the vaccinated group (P = 0.035, Mann-Whitney test). The median virus load at day 14 (plasma RNA copies/ml) for the control group was 4.02 × 107 (interquartile range [IQR], 2.39 × 107 to 7.67 × 107), while the median day 14 virus load for the Gag-Pol-vaccinated group was 1.56 × 107 (IQR, 5.93 × 106 to 5.07 × 107).
Virus replication was also assessed in these two cohorts of monkeys by performing area under the curve (AUC) blood plasma virus RNA calculations through 112 days postchallenge. The median virus load AUC was significantly higher in the control group than in the Gag-Pol-vaccinated group (P = 0.045, Mann-Whitney test). The median AUC virus load for the control group was 3.63 × 108 (IQR, 1.71 × 108 to 5.30 × 108) while the median AUC virus load for the Gag-Pol-vaccinated group was 1.64 × 108 (IQR, 4.03 × 107 to 3.49 × 108).
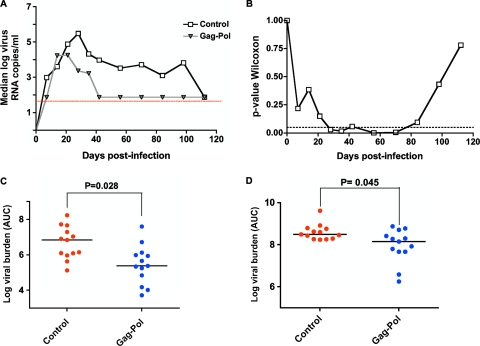
Virus loads in the seminal plasma were determined by a quantitative reverse transcription-PCR assay described previously (3, 22). Analysis of median seminal plasma virus RNA levels showed that SIV RNA was detected by day 7 following infection in the control group and day 14 following infection in the Gag-Pol-vaccinated group of monkeys (Fig. 1A). In the control group of monkeys, seminal plasma virus RNA levels were initially low or undetectable and reached a peak at day 28 postinfection (log-transformed median of 5.48 copies/ml). Importantly, the peak virus RNA level in the seminal plasma of the experimentally vaccinated group of monkeys was 1.25 logs lower than in the control group of monkeys, reaching a maximum at day 21 (log-transformed median of 4.25 copies/ml) and then declining to undetectable levels by day 42 after infection. The virus load in the semen of the experimentally vaccinated group of monkeys was consistently undetectable for the remaining 10 weeks of the study. The seminal plasma virus RNA levels in the experimentally vaccinated monkeys remained significantly lower than the levels in the control animals from days 28 to 80 after challenge as determined by the Mann-Whitney test (Fig. 1B). Interestingly, the loss of a statistically significant separation between these two groups of monkeys after day 80 reflects the loss of measurable seminal plasma virus in the control monkeys, not a waning of the effect of the vaccine in the Gag-Pol-immunized monkeys. Seminal plasma virus burden levels at peak (day 28 for the control group and day 21 for the experimental group) and set point (day 56) were also compared in these two groups of monkeys, using the nonparametric Mann-Whitney test, and highly significant differences were observed (day 28, P = 0.008; day 56, P = 0.005). Significant differences between these two groups were also observed for median AUC calculations in the seminal and peripheral blood plasma (Fig. 1C and D).
FIG. 1.
SIV RNA levels in the blood and seminal plasma of experimentally and sham-vaccinated rhesus monkeys during the first 16 weeks following SIVmac251 challenge. All animals were challenged by intravenous route with a pathogenic SIVmac251 quasispecies. (A) Median seminal plasma SIV RNA levels of the control and Gag-Pol-vaccinated groups of monkeys are shown. Monkeys were electroejaculated to obtain semen specimens. The presence of virus RNA in clarified seminal plasma was measured by quantitative reverse transcription-PCR and is expressed as log10 copies of RNA per ml. The assay limit of detection is indicated by the dashed horizontal line. (B). Seminal plasma virus RNA levels were compared at each time point by the Mann-Whitney test to determine if the values for the vaccinated and control monkeys differed. The associated P value for each comparison is shown in relation to the dashed line that represents a P value of 0.05. Median AUC comparisons are made between semen virus RNA levels (C) and total viral burden levels in the blood plasma (D). The comparisons of the Gag-Pol-vaccinated and control groups were analyzed using the Mann-Whitney nonparametric t test. P values shown exclude all monkeys expressing the major histocompatibility complex class I alleles Mamu-B*08 and Mamu-B*17, from calculation of significance.
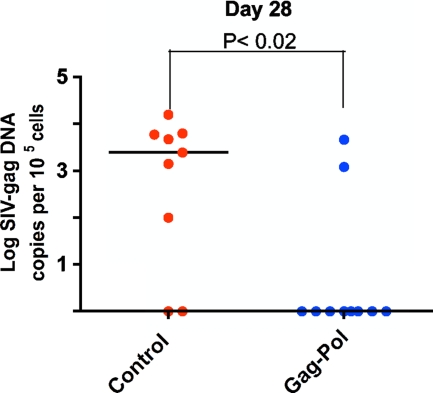
Semen cell-associated proviral SIV levels were determined by gag-specific real-time PCR, performed in parallel with a determination of albumin copy number. The sensitivity of the assay was determined by using the 3D8 CEMx174 cell clone harboring a single SIV proviral genome/cell as a positive control as described previously (6, 14). The absolute number of SIV gag DNA copies was calculated as described previously (6, 14). Importantly, the seminal cell-associated SIV DNA levels tracked with the seminal plasma RNA levels (Fig. 2), with a significant difference observed between the vaccinated and control monkeys by day 28 after infection.
FIG. 2.
SIV DNA in the semen-associated cell fraction. Comparisons between semen virus DNA levels at 28 days after virus challenge. The comparisons of the data from the Gag-Pol-vaccinated and control groups were analyzed using the Mann-Whitney nonparametric t test. Probability values of P < 0.05 were interpreted as significant. The absolute number of SIV gag DNA copies was calculated as described previously (6, 14) and is shown as log-transformed copies per 1 × 105 semen-associated cells.
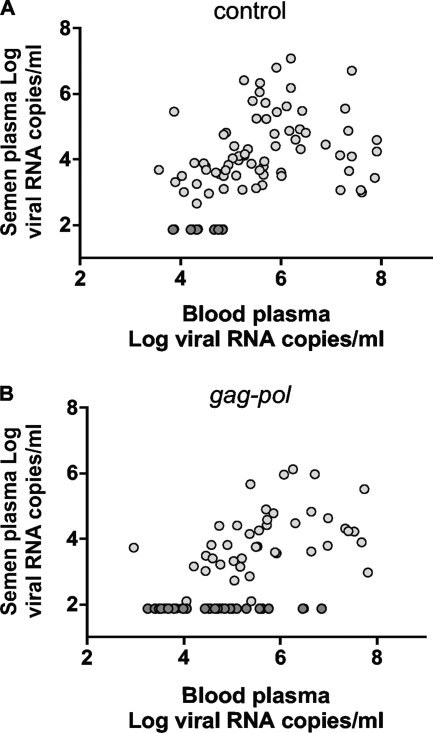
In view of the established importance of seminal plasma virus RNA levels as a predictor of HIV-1 transmission (2), we sought to determine the association between virus RNA levels in the blood and seminal plasma in these two groups of monkeys. A significant correlation was observed between the blood plasma virus RNA levels and virus RNA levels measured in seminal plasma in the control (r = 0.322, P = 0.006) (Fig. 3A) and in the vaccinated group of monkeys (r = −0.486, P = 0.001) (Fig. 3B). No significant correlation was observed between the seminal plasma virus RNA levels and proviral copies in seminal cells measured. This was seen both in the control (r = 0.11, P = not significant) (data not shown) and in the vaccinated group of monkeys (r = 0.13, P = not significant) (data not shown). These data indicate that virus RNA is detectable in the seminal plasma when virus in the blood plasma is in excess of 104 RNA copies/ml. This observation suggests that total virus RNA burden in the blood during the peak acute phase of SIV infection must surpass a threshold to be detected in the semen.
FIG. 3.
Association between SIV RNA levels in blood and semen samples. Associations are shown between levels in blood plasma and seminal plasma samples from control monkeys (r = 0.322, P = 0.006) (A) and blood and seminal plasma samples from vaccinated monkeys (r = 0.486, P = 0.001) (B). Data points denoted in dark gray were not included in calculation of significance.
The prevailing consensus among investigators is that HIV vaccine trials in nonhuman primates should be done using a protocol that closely models the sexual transmission of HIV-1, i.e., repeated low-dose mucosal challenge using an SIV isolate that is genetically disparate from the SIV used in the vaccine. The present study was done with a single, high-dose challenge by a virus that is similar in sequence to that used in the vaccine constructs. Since the vaccine employed in this study did not include envelope immunogens, no neutralizing antibodies specific for the challenge virus were elicited by the vaccination. In fact, the high-dose challenge model used in the present study was, therefore, a more rigorous test of the strategy of using T-cell vaccination to diminish the virus shed in semen than an evaluation using a repeated low-dose mucosal challenge.
If a large proportion of HIV-1 transmission events occur during the period of primary infection, a vaccine-associated damping of viral replication early after infection could impact HIV-1 transmission, even if that impact is of a brief duration. Both Modjarrad et al. (15) and Chakraborty et al. (2) have modeled the consequences of viral load reductions on transmission during acute infection and shown that modest reductions in viral load could significantly reduce transmission rates during this critical period of time. Studies of HIV-1 discordant couples have shown that heterosexual transmission is rare in persons with blood plasma HIV RNA levels below 1,500 copies/ml, and they suggest a possible association between viral load in the blood and transmissibility of HIV-1 in genital secretions (10, 20). These observations in humans are concordant with our findings.
The demonstration in the present study of a direct association between vaccination and diminished seminal plasma SIV RNA levels suggests that vaccination may be an effective measure for reducing HIV-1 transmission. As semen represents an important source of transmitted virus, these data suggest a possible strategy for limiting HIV transmission. These findings underscore the potential utility of widespread HIV vaccination programs using cell-based immunogens that do not induce sterilizing immunity.
Acknowledgments
This work was supported by the Intramural Research Program of the Vaccine Research Center, NIAID.
We thank Alan Dodson, Tammy Jenkins, and Wayne Stevens of Bioqual, Inc., for their expert assistance with nonhuman primate work.
Footnotes
Published ahead of print on 29 July 2009.
REFERENCES
- 1.Brenner, B. G., M. Roger, J. P. Routy, D. Moisi, M. Ntemgwa, C. Matte, J. G. Baril, R. Thomas, D. Rouleau, J. Bruneau, R. Leblanc, M. Legault, C. Tremblay, H. Charest, and M. A. Wainberg. 2007. High rates of forward transmission events after acute/early HIV-1 infection. J. Infect. Dis. 195:951-959. [DOI] [PubMed] [Google Scholar]
- 2.Chakraborty, H., P. K. Sen, R. W. Helms, P. L. Vernazza, S. A. Fiscus, J. J. Eron, B. K. Patterson, R. W. Coombs, J. N. Krieger, and M. S. Cohen. 2001. Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: a probabilistic empiric model. AIDS 15:621-627. [DOI] [PubMed] [Google Scholar]
- 3.Cline, A. N., J. W. Bess, M. Piatak, Jr., and J. D. Lifson. 2005. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J. Med. Primatol. 34:303-312. [DOI] [PubMed] [Google Scholar]
- 4.Cohen, M. S., I. F. Hoffman, R. A. Royce, P. Kazembe, J. R. Dyer, C. C. Daly, D. Zimba, P. L. Vernazza, M. Maida, S. A. Fiscus, J. J. Eron, Jr., et al. 1997. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet 349:1868-1873. [DOI] [PubMed] [Google Scholar]
- 5.Davenport, M. P., R. M. Ribeiro, D. L. Chao, and A. S. Perelson. 2004. Predicting the impact of a nonsterilizing vaccine against human immunodeficiency virus. J. Virol. 78:11340-11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douek, D. C., J. M. Brenchley, M. R. Betts, D. R. Ambrozak, B. J. Hill, Y. Okamoto, J. P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, Z. Grossman, M. Dybul, A. Oxenius, D. A. Price, M. Connors, and R. A. Koup. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417:95-98. [DOI] [PubMed] [Google Scholar]
- 7.Duerr, A., J. N. Wasserheit, and L. Corey. 2006. HIV vaccines: new frontiers in vaccine development. Clin. Infect. Dis. 43:500-511. [DOI] [PubMed] [Google Scholar]
- 8.Eron, J. J., Jr., B. Gilliam, S. Fiscus, J. Dyer, and M. S. Cohen. 1996. HIV-1 shedding and chlamydial urethritis. JAMA 275:36. [PubMed] [Google Scholar]
- 9.Fiscus, S. A., P. L. Vernazza, B. Gilliam, J. Dyer, J. J. Eron, and M. S. Cohen. 1998. Factors associated with changes in HIV shedding in semen. AIDS Res. Hum. Retrovir. 14(Suppl. 1):S27-S31. [PubMed] [Google Scholar]
- 10.Gray, R. H., M. J. Wawer, R. Brookmeyer, N. K. Sewankambo, D. Serwadda, F. Wabwire-Mangen, T. Lutalo, X. Li, T. vanCott, and T. C. Quinn. 2001. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 357:1149-1153. [DOI] [PubMed] [Google Scholar]
- 11.Johnston, M. I., and A. S. Fauci. 2007. An HIV vaccine—evolving concepts. N. Engl. J. Med. 356:2073-2081. [DOI] [PubMed] [Google Scholar]
- 12.Letvin, N. L. 2007. Correlates of immune protection and the development of a human immunodeficiency virus vaccine. Immunity 27:366-369. [DOI] [PubMed] [Google Scholar]
- 13.Letvin, N. L., J. R. Mascola, Y. Sun, D. A. Gorgone, A. P. Buzby, L. Xu, Z. Y. Yang, B. Chakrabarti, S. S. Rao, J. E. Schmitz, D. C. Montefiori, B. R. Barker, F. L. Bookstein, and G. J. Nabel. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 312:1530-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 15.Modjarrad, K., E. Chamot, and S. H. Vermund. 2008. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS 22:2179-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pilcher, C. D., J. J. Eron, Jr., P. L. Vemazza, M. Battegay, T. Harr, S. Yerly, S. Vom, and L. Perrin. 2001. Sexual transmission during the incubation period of primary HIV infection. JAMA 286:1713-1714. [DOI] [PubMed] [Google Scholar]
- 17.Pilcher, C. D., G. Joaki, I. F. Hoffman, F. E. Martinson, C. Mapanje, P. W. Stewart, K. A. Powers, S. Galvin, D. Chilongozi, S. Gama, M. A. Price, S. A. Fiscus, and M. S. Cohen. 2007. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS 21:1723-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilcher, C. D., D. C. Shugars, S. A. Fiscus, W. C. Miller, P. Menezes, J. Giner, B. Dean, K. Robertson, C. E. Hart, J. L. Lennox, J. J. Eron, Jr., and C. B. Hicks. 2001. HIV in body fluids during primary HIV infection: implications for pathogenesis, treatment and public health. AIDS 15:837-845. [DOI] [PubMed] [Google Scholar]
- 19.Pilcher, C. D., H. C. Tien, J. J. Eron, Jr., P. L. Vernazza, S. Y. Leu, P. W. Stewart, L. E. Goh, and M. S. Cohen. 2004. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J. Infect. Dis. 189:1785-1792. [DOI] [PubMed] [Google Scholar]
- 20.Quinn, T. C., M. J. Wawer, N. Sewankambo, D. Serwadda, C. Li, F. Wabwire-Mangen, M. O. Meehan, T. Lutalo, R. H. Gray, et al. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N. Engl. J. Med. 342:921-929. [DOI] [PubMed] [Google Scholar]
- 21.Schacker, T., A. J. Ryncarz, J. Goddard, K. Diem, M. Shaughnessy, and L. Corey. 1998. Frequent recovery of HIV-1 from genital herpes simplex virus lesions in HIV-1-infected men. JAMA 280:61-66. [DOI] [PubMed] [Google Scholar]
- 22.Whitney, J. B., C. L., S. Bao, A. Miura, S. S. Rao, J. R. Mascola, and N. L. Letvin. 2009. Monitoring HIV vaccine trial participants for primary infection: studies in the SIV/macaque model. AIDS 23:1453-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]