Abstract
New-generation gels that deliver potent antiretroviral drugs against human immunodeficiency virus type 1 have renewed hopes for topical prophylaxis as a prevention strategy. Previous preclinical research with monkey models suggested that high concentrations and drug combinations are needed for high efficacy. We evaluated two long-acting reverse transcriptase inhibitors, tenofovir (TFV) and emtricitabine (FTC), by using a twice-weekly repeat challenge macaque model and showed that a preexposure vaginal application of gel with 1% TFV alone or in combination with 5% FTC fully protected macaques from a total of 20 exposures to simian-human immunodeficiency virus SF162p3. FTC and TFV were detected in plasma 30 min after vaginal application, suggesting rapid absorption. FTC was detected more frequently than TFV and showed higher levels, reflecting the fivefold-higher concentration of this drug than of TFV. Two of 12 repeatedly exposed but protected macaques showed limited T-cell priming, which did not induce resistance to infection when macaques were rechallenged. Thus, single drugs with durable antiviral activity can provide highly effective topical prophylaxis and overcome the need for noncoital use or for drug combinations which are more complex and costly to formulate and approve.
Women continue to be disproportionately burdened by human immunodeficiency virus (HIV), particularly in sub- Saharan Africa, where 76% of new HIV infections occur in young women, aged 15 to 24 years (45). The proportion is as high as 90% in South Africa (41). Behavioral messages that encourage faithfulness in partnerships and condom use have had limited success in many countries throughout the world where marriage presents the greatest risk for HIV acquisition for women (7, 9, 15, 18, 34, 37, 46). In the absence of a preventive HIV vaccine, alternative biomedical interventions are urgently needed.
Vaginal microbicide gels hold great potential as a female-controlled prevention strategy, especially in circumstances where condom use cannot be negotiated (36, 47). Mathematical models predict that effective microbicides can substantially reduce HIV incidence and control HIV spread when widely implemented (1, 38). However, recent clinical trials with first-generation detergent or polyanionic microbicide products, such as cellulose sulfate and 1.0% C31G (Savvy) (4, 24), have not demonstrated efficacy in preventing or reducing HIV transmission in women or have even enhanced transmission, in the case of nonoxynol-9 (42). Incomplete adherence has further complicated the interpretation of efficacy results in these trials. Findings from a recent phase IIB study of the polyanion PRO 2000 gel in women has shown a 30% reduction in HIV infection (21). Although promising, this reduction was not statistically significant compared to that provided by a placebo gel. Overall, these studies highlight the need for different and more-potent microbicides.
New-generation topical gels containing antiretroviral drugs may be more promising because they can deliver potent and specific inhibitors of HIV replication that can block critical steps, including receptor binding and entry, reverse transcription, or integration. An expanding array of antiretroviral drugs are under consideration for gel formulation, either singly or in combination, providing many options for clinical trials with products containing drugs from different classes, ranging from CCR5 and fusion inhibitors to nucleoside and nonnucleoside reverse transcriptase (RT) or integrase inhibitors (4, 24).
Despite the many options for antiretroviral-based gels, little is known about what is required to achieve significant protection and whether single or combination drugs used either daily or intermittently are effective. Macaque models of vaginal and rectal transmission can be used to assess the efficacies of different products containing one or more drugs and to compare daily or exposure-driven modalities. Macaque studies can, thus, inform clinical trial designs by advancing highly promising products and modalities for vaginal and rectal use. Previous work with rhesus macaques provided the first evidence of the ability of topically applied gels with small-peptide or -molecule antagonists of the HIV CCR5 receptor to protect against vaginal infection (27, 43).
The nucleotide RT inhibitor tenofovir (TFV), due to its long intracellular half-life, low risk of resistance development, and success in treatment when paired with emtricitabine (FTC) as a once-daily oral formulation (Truvada), is a prime candidate for HIV prevention. Several studies of humans are under way to evaluate the safety and effectiveness of TFV as a gel formulation for use by both women and men (http://www.microbicide.org/cs/pipeline). Recent findings by Cranage et al., determined using a single high-dose challenge model with rhesus macaques, showed that 1% TFV gel applied rectally offered 67% protection (8). Less is known or reported about vaginal studies of macaques with topical TFV. However, there is increasing interest in developing topical gels with combination antiretroviral drugs to boost efficacy, since combination drugs have thus far been found to be more effective than single drugs in both treatment and prevention (11, 12). Macaque studies of systemic preexposure prophylaxis show higher protection with combination drugs. Oral TFV-FTC was found to be more protective than TFV or FTC alone in repeat low-dose rectal challenge studies of rhesus macaques (10, 39). Likewise, Veazey et al. demonstrated that topical gels containing combinations of two or three inhibitors of virus-cell fusion increased protection of macaques from vaginal infection (43). They also showed that the concentrations of inhibitors required for in vivo protection are about 1 million-fold higher those for in vitro inhibition, likely reflecting the need to shield a large vaginal surface for a significant period of time (43). While many antiretroviral drugs under consideration for topical prophylaxis are highly potent (with 50% inhibitory concentrations [IC50s] in the nM range), very few have the long intracellular half-life of TFV (>60 h) or FTC (∼40 h) that allows these drugs to persist in tissues after extracellular concentrations decrease (10). Long drug persistence can provide sustained and durable antiviral activity and may result in a higher efficacy.
In this study, we compared the efficacies of gels containing TFV alone or in combination with FTC in preventing vaginal simian-human immunodeficiency virus (SHIV) infection. We used a repeat low-dose challenge macaque model, for which transmission resembles human vaginal transmission in many important ways (23, 33). We used pig-tailed macaques, which have a menstrual cycle similar in frequency to that of humans under natural conditions without progesterone treatment. Animals were challenged repeatedly twice a week to mimic high-risk human exposure. We also used a lower challenge dose of SHIVSF162P3 that is more consistent with physiologic virus exposure levels than what is conventionally used for single high-dose challenge models. In addition, our SHIV inoculum contained an R5-tropic HIV type 1 (HIV-1) envelope similar to that of naturally transmitted human viruses. While single-dose challenge models measure protection against one transmission event per animal, this model can evaluate protection against multiple transmissions resulting from repeated virus exposures. Recent findings by Keele and others have shown that macaques infected intrarectally with a low dose of SIVmac251 or SIVsmE660 showed mucosal transmission and early virus diversification similar to those for HIV-1, thereby adding further support for the repeat low-dose transmission model (22). In this study, we also examined systemic drug exposure resulting from vaginal gel application and assessed SHIV-specific T-cell responses in repeatedly exposed but protected macaques and their impact on susceptibility to infection.
MATERIALS AND METHODS
Macaques.
All macaque studies adhered to the Guide for the Care and Use of Laboratory Animals (32a); all procedures were approved by the Institutional Animal Care and Use Committees (IACUC) of both the Centers for Disease Control and Prevention (CDC) and the Yerkes National Primate Research Center (Emory University). Seventeen normal-cycling female pig-tailed macaques (Macaca nemestrina) weighing 5 to 11 kg, at an average age of 6 years old, were utilized. All macaques tested negative for simian immunodeficiency virus, simian retroviruses, and simian T-cell leukemia viruses. Macaques were anesthetized using standard doses of ketamine hydrochloride. Animals were not treated with medroxyprogesterone acetate (Depo-Provera) or synchronized for menstrual cycle.
Study products.
TFV [(R)-9-(2-phosphonylmethoxypropyl) adenine] and FTC {5-fluoro-1-(2R,5S)-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine} were kindly provided by Gilead Sciences through a material transfer agreement. TFV (1%, wt/wt) was formulated in a 2% hydroxyethyl cellulose (HEC) gel as previously described, with minor modifications (30). A combination gel containing 1% TFV and 5% FTC (wt/wt) was prepared similarly. A placebo with 2% HEC was formulated in the same way. Gels were formulated at pH 6.5 to correlate with the average vaginal pH of pig-tailed macaques (28). Products were stored at room temperature throughout the study. The in vitro antiviral activity of the formulated product was confirmed using an MTT-based colorimetric drug susceptibility assay with MT-4 cells as previously described (49) (data not shown).
Virus stock.
SHIVSF162P3 (SIVmac239 backbone with an HIV-1 subtype B, CCR5-tropic envelope) (13, 14) was obtained from the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program and was propagated in peripheral blood mononuclear cells (PBMC) from pig-tailed macaques as previously described (23, 33). The virus stock was diluted to the challenge dose of 10 50% tissue culture infective doses (TCID50) calculated for pig-tailed macaque PBMC; this dose corresponds to 1.5 × 106 RNA copies per exposure.
Study design.
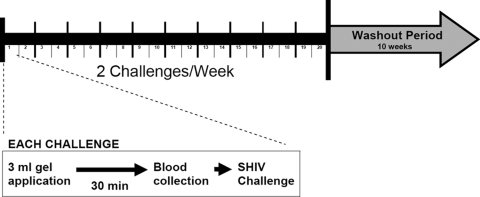
Initially, 14 naïve female pig-tailed macaques were assigned to a no-gel arm (n = 2), a 2% HEC placebo gel arm (n = 6), or an antiretroviral combination gel arm (5% FTC and 1% TFV, n = 6). Macaques were subjected to intravaginal gel application, followed by low-dose SHIV exposure given twice per week 3 to 4 days apart for up to 20 challenges (Fig. 1). Virus exposures were stopped when a macaque became SHIV RNA positive in plasma. Specifically, 3 ml of gel product was applied to the vaginal vault by use of sterile syringes attached to a gastric feeding tube that had been adjusted to a length appropriate for vaginal delivery. Virus inocula were administered 30 min after gel application by nontraumatic inoculation of 1 ml of SHIVSF162P3 into the vaginal vault via a sterile gastric feeding tube of adjusted length (33). Macaques remained recumbent and slightly elevated for at least 15 min after each intravaginal inoculation. All experiments were performed under highly controlled conditions, using the same virus stock, inoculum dose, and inoculation method.
FIG. 1.
Study design. Three milliliters of gel was applied intravaginally to each macaque. After 30 min, blood was collected intravenously and the animal was vaginally challenged with 10 TCID50 of SHIVSF162P3. Each macaque received 20 twice-weekly challenges and was followed for 10 weeks after the last challenge.
Systemic infection was monitored twice weekly by the detection of plasma SHIV RNA, using a real-time RT-PCR assay with an assay sensitivity of 50 RNA copies/ml (10, 39). PCR amplification of PBMC proviral DNA was done using primers and probes specific for SIVmac239 pol (10, 33). Serologic testing was performed using a synthetic-peptide enzyme immunoassay (Genetic Systems HIV-1/HIV-2 plus O; Bio-Rad). The time of infection was estimated as two challenges (7 days) prior to the first confirmed positive RNA RT-PCR to account for the lag period between virus inoculation and detection of SHIV RNA in plasma. Macaques were considered protected if they tested negative for viral RNA, proviral DNA, and antibodies during the course of the study and remained negative after 10 weeks of follow-up after the last virus challenge.
After a 10-week rest period, SHIV-negative, protected animals from the TFV-FTC gel arm were reassigned to an HEC placebo gel arm (n = 3) or a TFV-only gel arm (1% TFV in 2% HEC; n = 3). Three additional naïve animals were also assigned to the 1% TFV gel arm, resulting in a total of six macaques receiving the active gel product. Gel applications, virus challenges, and monitoring were all performed as described above.
Drug levels in plasma.
TFV and/or FTC plasma levels were measured using specimens collected from each macaque 30 min after vaginal administration of the gel product and just before virus exposure, resulting in the collection of 20 specimens from each macaque. Macaques that were reenrolled in a second protocol had 40 specimens collected and tested. TFV or FTC was extracted from 100 μl of plasma by protein precipitation with 350 μl of methanol containing 1,000 ng each of 13C-labeled TFV and FTC as internal standards. Supernatant containing the drugs from precipitation was evaporated to near dryness under vacuum and then resuspended in high-pressure liquid chromatography buffer containing 9.9 mM of acetic acid, 5.9 mM of ammonium hydroxide, and 9.4 mM of formic acid (pH of ∼3). Drug levels were analyzed by using liquid chromatography-mass spectrometry (25). Standard curves were generated from TFV and FTC spiked in normal human plasma over a range from 5 to 3,000 ng/ml. The assay had a limit of quantification (LOQ) of 10 ng/ml and standard-curve R2 values of greater than 0.99. Values reported are averages of two determinations from each specimen.
IFN-γ ELISPOT assays.
Gamma interferon (IFN-γ)-producing T-cell responses were enumerated using an enzyme-linked immunospot (ELISPOT) assay for the detection of macaque IFN-γ. All macaques were sampled during the follow-up period after SHIV challenges were completed. The sampling times corresponded to week 6 after virus challenges for protected macaques and varied from weeks 11 to 15 for infected macaques. PBMC were stimulated in duplicate wells at 2 × 105 cells/well by use of the following peptide pools at a final concentration of 1.5 μg/ml: SIVmac239 gag (gag1 pool, peptides 5211 to 5274; gag2 pool, peptides 5275 to 5335; NIH AIDS Research and Reference Reagent Program, catalog no. 6204) and SHIVSF162P3 env (env1 pool, peptides 7408 to 7477; env2 pool, peptides 7478 to 7548; env3 pool, peptides 7549 to 7618; NIH AIDS Research and Reference Reagent Program, catalog no. 7619; HIV-1 consensus B Tat peptide pool; NIH AIDS Research and Reference Reagent Program, catalog no. 5138). The mock peptide pool consisted of 10 Ebola virus-derived peptides. Results from each time point were used only if the assay control scored positive, defined as >100 spot-forming units (SFU)/106 cells, after stimulation with staphylococcal enterotoxin B (data not shown). To determine the frequency of virus-specific IFN-γ-producing T cells per 1 million PBMC, the number of background spots in the mock peptide pool control wells was subtracted from the number of spots in the peptide-stimulated wells. A cutoff for gag, env, and tat combined was established as the criterion for positive ELISPOT assay responses, based on results from nine unrelated, uninfected pig-tailed macaques (data not shown). Results represent the averages of two independent determinations from specimens collected at the same time point.
Statistical methods.
The cumulative probability of remaining uninfected after repeated low-dose virus exposures was computed and graphically displayed using a product limit estimator, and the log rank test statistic was used to nonparametrically compare Kaplan-Meier curves for the control group and both treatment groups (20). Uninfected macaques were right censored at the maximal exposure number (20 exposures). In addition, Fisher's exact test was used to compare the numbers of infections per total number of animal exposures by study group (2).
To compare ELISPOT assay reactivities to SHIV between protected and control macaques, we tested for differences in the sums of T-cell responses from all peptide pools by using the Wilcoxon rank sum statistical test. We also compared acute viremia for control animals that were treated previously to that for animals who were treatment naïve. Linear mixed-effects regression, with an autocorrelation covariance structure, was used to assess group differences in log10-transformed viral load measurements (44). Models included indicator variables (dummy coded) for equally spaced times (4 to 77 days) postinfection. Model predicted values based upon population fixed-parameter estimates were graphed to display changes in viral loads postinfection.
To assess systemic drug absorption following vaginal gel application, we evaluated plasma TFV and FTC concentrations 30 min after each gel application in animals receiving either the TFV-FTC gel or the TFV gel. The lower LOQ was 10 ng/ml. To account for the uncertainty due to repeated measurements below the LOQ, a left, interval-censoring mechanism was used in the estimation of mean plasma concentration per animal and per study group.
SAS version 9.2.1 or GraphPad Prism 5 for Windows (GraphPad Software, San Diego, CA) was used for all statistical analyses.
RESULTS
TFV-FTC gel fully protects macaques against vaginal SHIVSF162P3 infection.
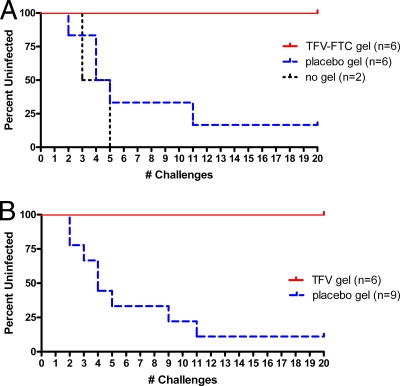
Figure 2A shows that of the six macaques that received the placebo gel (2% HEC), five became infected after challenges 2, 4, 4, 5, and 11. One macaque remained uninfected after 20 challenges. Of the two control macaques not given any intervention, one became infected after the third challenge, and the second became infected after the fifth challenge. The median number of challenges needed to infect control macaques (n = 2, no gel, and n = 6, placebo gel) was 4.5 challenges, or approximately 2.5 weeks. All infected macaques showed similar viral load kinetics (Fig. 3), and all had detectable proviral DNA (data not shown). Seroconversion occurred 1 to 3 weeks after the first detectable plasma SHIV RNA was observed in infected macaques (data not shown). These findings are similar to that seen with once-weekly vaginal challenge of pig-tailed macaques (23).
FIG. 2.
Gel with TFV-FTC combination or TFV alone fully protects macaques against vaginal SHIVSF162P3 infection. The Kaplan-Meier curves show data on the number (#) of twice-weekly challenges with 10 TCID50 of SHIVSF162P3 and the number of uninfected macaques. (A) TFV-FTC gel. The differences in infection between the TFV-FTC gel arm and the placebo gel arm were statistically significant (P = 0.004; log rank test). (B) TFV gel. The differences in infection between the TFV group and the placebo gel group were statistically significant (P = 0.001; log rank test).
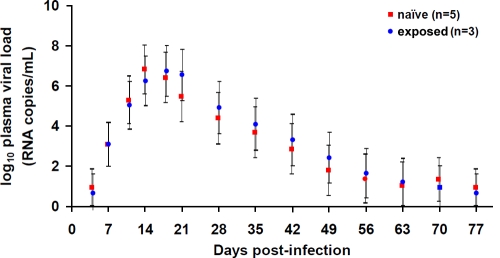
FIG. 3.
Acute viral loads in naïve and exposed macaques. Mixed-effects-model-based estimates of virus loads at times postinfection in macaques receiving placebo who were virus exposure naïve or had remained uninfected after 20 virus challenges in the TFV-FTC gel arm and were reenrolled in the placebo arm (exposed). Bars denote the standard errors of the means.
In contrast, all six macaques receiving the TFV-FTC gel remained uninfected after 20 challenges with SHIVSF162P3 (Fig. 2A), with each macaque showing no antibody reactivity and undetectable SHIV sequences in all plasma and PBMC samples collected during the study and throughout the 10-week washout period. Differences in infection outcome between the TFV-FTC group and the placebo gel group were statistically significant (P = 0.004; log rank test). Differences in infection rates between the TFV-FTC group (zero infections per 120 challenges) and the placebo gel group (five infections per 46 challenges) were also statistically significant (P = 0.001; Fisher's exact test).
TFV (1%) gel alone fully protects macaques against vaginal SHIVSF162P3 infection.
All three macaques who received the placebo gel became infected, after challenges 2, 3, and 9, respectively. In contrast, all six macaques treated with the TFV gel remained protected after 20 challenges, showing no antibody reactivity or SHIV sequences in any of the plasma or PBMC samples. Figure 2B shows the cumulative percentages of animals that remained uninfected, for the nine animals that received the placebo gel compared to the animals treated with the TFV gel. The median number of challenges needed to infect control animals was four challenges. The differences in infection outcome between the TFV group and placebo gel group were statistically significant (P = 0.001; log rank test). The differences in infection rate between the TFV group (zero infections per 120 challenges) and the placebo gel group (eight infections per 60 challenges) were statistically significant (P < 0.001; Fisher's exact test).
Virus-specific immune responses in protected animals and impact on susceptibility to infection.
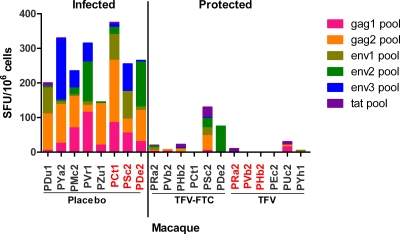
As shown in Fig. 4, eight infected macaques who received the placebo gel had IFN-γ-secreting gag-, env-, and tat-specific T-cell responses ranging from 145 to 375 SFU/106 PBMC. For 10 of 12 protected macaques, the reactivity to gag, env, and tat was below cutoff values. One protected macaque (PSc2) from the TFV-FTC group had gag-, env-, and tat-specific T-cell responses at a frequency of 130 SFU/106 PBMC, and another one (PDe2) from the same group had only an env-specific T-cell response at 75 SFU/106 PBMC (Fig. 4). None of the six protected animals in the TFV group had detectable ELISPOT assay reactivity, including the three animals (PRa2, PVb2, and PHb2) that were reenrolled from the TFV-FTC group. The difference in reactivity between infected and protected macaques was statistically significant (P = 0.002). To assess the impact of these responses on susceptibility to infection, both PSc2 and PDe2 were subsequently used in the placebo gel arm of the TFV gel study after a 10-week washout period. A similarly exposed and protected macaque (PCt1) that had undetectable ELISPOT assay reactivity was added to this placebo group. PSc2 and PDe2 became infected after two and three challenges, respectively, showing no evidence of resistance to infection. PCt1 became infected after nine SHIV challenges, which was within the range observed for naïve macaques. Levels of acute viremia in these three drug-experienced and five drug-naive SHIV-infected macaques were not statistically significantly different (Fig. 3).
FIG. 4.
SHIV-specific IFN-γ-secreting T-cell responses in protected and infected macaques. IFN-γ-secreting T-cell frequencies in PBMC from infected control animals were compared to those for animals protected by TFV-FTC or TFV gel. Results were measured by an ELISPOT assay and are expressed as SFU/1 million PBMC. The animal identifiers shown on the x axis are color coded as follows: black, naïve macaques; red, macaques previously protected by TFV-FTC gel and reenrolled in the placebo control group or the TFV group. The difference in reactivity between infected and protected macaques was statistically significant (P = 0.002; Wilcoxon rank sum test).
Plasma TFV and FTC concentrations in protected macaques.
All treated macaques had at least some drug detection. Overall, FTC or TFV was detected in 275 of 360 measurements (76%) from 12 macaques. As expected, given the fivefold difference in applied dosages, the plasma drug concentration of TFV 30 min after application, administered either alone or in combination with FTC, was consistently lower than that of FTC (Table 1). In addition, the proportion of measurements with values below the LOQ was higher for macaques administered TFV regimens than for macaques administered FTC. The mean plasma levels were 16.4, 38.7, and 160.2 ng/ml for TFV in the TFV-only group, TFV in the TFV-FTC group, and FTC in the TFV-FTC group, respectively. Given these plasma concentrations and assuming a plasma volume of 33 ml per kg of body weight, an estimated 0.014% TFV, 0.030% TFV (TFV-FTC group), and 0.025% FTC (TFV-FTC group) of applied gel (0.03 g TFV; 0.15 g FTC) were systemically absorbed, as determined 30 min after gel application (Table 1).
TABLE 1.
Plasma TFV and FTC levels
| Study group (regimen) and animal identifier | Animal wta (kg) | Animal ageb (mo) | No. of measurements below LOQc (%) | Mean plasma drug concn, ng/ml (95% confidence limit) | % Drug applied detected in plasma at 30 mind (95% confidence limit) |
|---|---|---|---|---|---|
| TFV (TFV only) | |||||
| PRa2 | 6.36 | 65 | 6 (30) | 22.4 (13.8-31.0) | 0.016 (0.010-0.022) |
| PVb2 | 6.96 | 61 | 9 (45) | 15.4 (10.0-20.9) | 0.012 (0.008-0.016) |
| PHb2 | 8.22 | 63 | 13 (65) | 13.6 (6.0-21.2) | 0.012 (0.005-0.019) |
| PEc2 | 6.54 | 60 | 10 (50) | 21.2 (9.7-32.7) | 0.015 (0.007-0.024) |
| PUc2 | 6.76 | 56 | 11 (55) | 15.3 (6.7-23.9) | 0.011 (0.005-0.018) |
| PYh1 | 11.62 | 146 | 9 (45) | 11.2 (8.9-13.5) | 0.014 (0.011-0.017) |
| Total | 7.74 | 75 | 58 (48) | 16.4 (13.1-19.7) | 0.014 (0.011-0.017) |
| TFV (TFV-FTC) | |||||
| PRa2 | 6.36 | 65 | 4 (20) | 34.7 (16.6-52.7) | 0.024 (0.012-0.037) |
| PVb2 | 6.96 | 61 | 2 (10) | 52.0 (17.6-86.4) | 0.040 (0.013-0.066) |
| PHb2 | 8.22 | 63 | 3 (15) | 25.3 (19.6-30.9) | 0.023 (0.018-0.028) |
| PCt1 | 8.00 | 95 | 4 (20) | 38.1 (17.4-58.8) | 0.034 (0.015-0.052) |
| PSc2 | 6.54 | 58 | 2 (10) | 49.2 (25.8-72.7) | 0.035 (0.019-0.052) |
| PDe2 | 5.86 | 50 | 6 (30) | 33.1 (14.0-52.2) | 0.021 (0.009-0.034) |
| Total | 6.99 | 65 | 21 (18) | 38.7 (29.6-47.8) | 0.030 (0.023-0.037) |
| FTC (TFV-FTC) | |||||
| PRa2 | 6.36 | 65 | 0 (0) | 133.6 (45.6-221.6) | 0.019 (0.006-0.031) |
| PVb2 | 6.96 | 61 | 2 (10) | 155.9 (41.2-270.7) | 0.024 (0.006-0.041) |
| PHb2 | 8.22 | 63 | 0 (0) | 124.6 (76.2-173.0) | 0.023 (0.014-0.031) |
| PCt1 | 8.00 | 95 | 4 (20) | 187.4 (43.2-331.6) | 0.033 (0.008-0.058) |
| PSc2 | 6.54 | 58 | 0 (0) | 228.3 (103.6-352.9) | 0.033 (0.015-0.051) |
| PDe2 | 5.86 | 50 | 0 (0) | 131.4 (36.7-226.2) | 0.017 (0.005-0.029) |
| Total | 6.99 | 65 | 6 (5) | 160.2 (116.1-204.3) | 0.025 (0.018-0.031) |
Baseline weight measured prior to first SHIV challenge.
Age of macaque at start of study.
The LOQ for both TFV and FTC was 10 ng/ml.
Calculated by dividing the weight-adjusted plasma drug concentration (estimated assuming a plasma volume of 33 ml/kg) (8) by the amount of drug applied (0.03 g TFV; 0.15 g FTC).
DISCUSSION
We evaluated the efficacy of gel containing TFV alone or in combination with FTC in preventing vaginal SHIV infection by using a repeat challenge macaque model that resembles human transmission. We demonstrate that topical application of a gel containing 1% TFV or a combination of 1% TFV and 5% FTC into the vaginal vault 30 min before virus exposure completely protected macaques against SHIV transmission. This suggests that the use of a 1% TFV gel close to the time of coitus may be sufficient to block transmission, without the need for application on noncoital days or the use of gels with drug combinations.
In our studies, we used female pig-tailed macaques (M. nemestrina), which have a menstrual cycle similar to that of humans, with a mean cycle length of 32.8 days (5). Pig-tailed macaques also have anatomy, pH, and bacterial microflora similar to those of humans (35). Therefore, we were able to measure protection under normal cycling conditions, as macaques were challenged vaginally twice a week for up to 20 challenges or 10 weeks without the use of menstrual cycle synchronization or progesterone treatment, which can alter the vaginal environment and the susceptibility to infection (29). The demonstration of high efficacy with repeated application of gels prior to exposure is reassuring because it does not indicate damage to the vaginal mucosa by the drugs or the gel formulation. Repeated use of nonoxynol-9 has led to cumulative mucosal toxicity, which may have neutralized the protective effect of this product or even increased virus transmission in humans (42).
Previous macaque studies of systemic antiretroviral prophylaxis have not shown a high level of protection with only TFV or FTC despite daily treatment, indicating that regimens combining TFV and FTC were required to improve efficacy (10, 39). These findings and similar results from monotherapy of infected persons suggested that drug combinations were likely needed for highly effective topical prophylaxis (11). Our findings that 1% TFV was sufficient to completely block transmission in this model are important and may be explained by the ability of the gel to deliver higher concentrations of drugs to vaginal tissues than are delivered via oral dosing. In addition, the twice-weekly dosing and the long intracellular half-life of TFV may both have helped in sustaining high and durable antiviral activity in vaginal tissues. Data comparing vaginal tissue drug levels in humans after oral or topical dosing will be highly informative (26). Despite the low potency of TFV (IC50 of ∼2 μM) (49), this gel product was more effective in macaques than were gels containing similar concentrations of more-potent drugs (IC50s of 1 to 20 nM) (43). Therefore, in addition to potency and concentration in the gel formulation, other drug characteristics, like long intracellular persistence, may be critical to efficacy.
Our gel formulation allowed for rapid drug absorption, as both TFV and FTC were frequently detected in plasma 30 min after application. This may have contributed to the high protection by effectively blocking early virus infection that can occur within the first hours after virus challenge (6, 31). While both TFV and FTC were detected in the blood plasma of all animals, the generally higher detection frequencies and levels of FTC may be due to the fivefold-higher concentration of this drug than of TFV. With extensive sampling for plasma drug levels following 20 gel applications in each macaque, we also assessed macaque-specific variability in drug levels 30 min after vaginal gel exposure. Based on findings with six macaques who received one rectal gel application and a single virus challenge, Cranage et al. found a positive association between the degree of protection and the concentration of TFV in plasma 15 min after gel use (8). We were unable to find a similar association between protection and plasma drug levels following vaginal gel application, since all animals were protected despite low levels of drug absorption. These data likely underscore the difficulty of identifying surrogate markers of protection from vaginal transmission based on plasma drug levels close to gel application.
Our model also provides an opportunity to examine immunologic priming in protected macaques that may have resulted from repeated virus exposures. Sequestration of virions by mucosal dendritic cells leading to cross-presentation of viral antigens may contribute to the development of such responses (17). We observed SHIV-specific T-cell ELISPOT assay reactivity in only 2 of 12 protected macaques, while Cranage et al. reported more-frequent Gag-specific IFN-γ-secreting T cells in four of seven macaques protected from a single rectal exposure to simian immunodeficiency virus (8). These responses may be either beneficial, because they reduce susceptibility to infection, or harmful, because they indicate T-cell activation and thus a higher susceptibility of target cells in the mucosa (16). To help clarify the impact of observed T-cell responses on susceptibility to infection, we rechallenged both of our ELISPOT assay-positive macaques with repeated low doses of SHIV in the presence of the placebo gel and found no evidence of resistance to infection. Peak viral loads were similar for naïve and rechallenged macaques. A lack of resistance to infection was also observed for macaques protected from repeated rectal SHIV challenges by preexposure prophylaxis, although T-cell responses were not evaluated for these macaques (10).
Our findings are in agreement with those of Cranage et al., who showed that a similar formulation of 1% TFV gel applied rectally up to 2 h before a single high-dose rectal challenge provided protection from infection with SIVmac251/32H in six of nine macaques (67%) (8). Differences between the two studies may be related to the use of distinct macaque models with different stringencies or may reflect biological factors, such as the need for more-potent gels to protect a larger colorectal mucosal surface against a more efficient mode of transmission (40). Further studies are needed to determine whether gels with combination drugs or higher TFV concentrations can enhance efficacy.
Our study is subject to several limitations. First, all viruses were inoculated in the absence of semen or semen-derived factors shown to enhance HIV infection in vitro (32). Viruses were also inoculated on apparently intact mucosa without trauma, concurrent genital ulcers, bacterial vaginosis, or other conditions which can increase the risk of HIV acquisition by increasing the number of susceptible cells in mucosal tissues or enhance systemic dissemination of virus or virus-infected cells (3, 48). The availability of macaque models with chemically induced transient ulcers of the lower female reproductive tract would provide an important tool to assess efficacy on nonintact mucosa (48). Second, since the contributions of cell-associated virus versus cell-free virus to establish infection in humans remain unclear, it may be prudent to confirm gel efficacy by using vaginal models of cell-associated transmission (19). TFV and FTC are expected to be active against cell-associated infection, since the mode of action for RT inhibitors occurs intracellularly (19, 50). Third, human trials currently assess the safety and efficacy of 40 mg TFV present in 4 ml of 1% TFV gel. We decreased the volume of gel tested to 3 ml (corresponding to a lower dose of 30 mg TFV) to accommodate the smaller vaginal cavity in macaques. Although even smaller volumes of gel can be evaluated with monkeys to better mimic gel distribution in the human vagina, efficacy data from such studies would require cautious interpretation. A reduction in gel volume would disproportionately decrease the applied TFV dose and reduce drug exposure. Additionally, macaques clear TFV more rapidly than humans, further reducing drug exposure (10).
Since all macaques in the TFV and TFV-FTC arms were fully protected from infection, drug resistance emergence in breakthrough infections could not be addressed in our study. Drug resistance concerns are important especially if gel products are not highly protective and include drugs that are used widely for treatment, as is the case with TFV and FTC evaluated in this study. However, whether a breakthrough infection would have resulted in the selection of drug-resistant virus is not known but may be less likely than expected because gel dosing results in lower levels of systemic drug exposure than oral dosing, which may not be sufficient for drug resistance selection. Therefore, because of differences in systemic drug exposure between oral and topical dosing with single drugs, a previous experience of drug resistance emergence from daily or intermittent monotherapy may be less relevant. Nevertheless, drug resistance emergence should be studied carefully with appropriate monkey models and current human trials by examining virus from plasma, rectum, and vagina.
In conclusion, we demonstrate that topical application of a gel containing 1% TFV or 1% TFV and 5% FTC 30 min before virus exposure completely protected macaques against numerous SHIV transmissions. Data from this model suggest that coital use of a 1% TFV gel may be sufficient to block HIV-1 transmission in humans, without the need for daily application or the use of gels containing drug combinations. This study highlights the high efficacy of this prevention strategy, supports clinical trials with humans, and informs the trial design by identifying a potentially highly effective modality.
Acknowledgments
We thank Stephanie Ehnert, Christopher Souder, Elizabeth Strobert, and the animal care staff from the Yerkes National Primate Center (Emory University) and James Mitchell from the Centers for Disease Control and Prevention for monitoring, maintaining, and performing animal procedures. We also thank Wei Luo and Debra Adams from the CDC for their excellent technical assistance performing ELISPOT assays for this study. We thank Jim Rooney from Gilead Sciences for providing TFV and FTC. The reagent SHIVSF162P3 was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS (DAIDS), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, from Janet Harouse, Cecilia Cheng-Mayer, and Ranajit Pal.
This work was supported in part by grant RR00165 (to the Yerkes Primate Research Center).
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. Use of trade names is for identification purposes only and does not constitute endorsement by the U.S. Centers for Disease Control and Prevention or the Department of Health and Human Services.
Footnotes
Published ahead of print on 5 August 2009.
REFERENCES
- 1.Abbas, U. L., R. M. Anderson, and J. W. Mellors. 2007. Potential impact of antiretroviral chemoprophylaxis on HIV-1 transmission in resource-limited settings. PLoS ONE 2:e875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agresti, A. 1996. An introduction to categorical data analysis. John Wiley & Sons, Inc., New York, NY.
- 3.Atashili, J., C. Poole, P. M. Ndumbe, A. A. Adimora, and J. S. Smith. 2008. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 22:1493-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balzarini, J., and L. Van Damme. 2007. Microbicide drug candidates to prevent HIV infection. Lancet 369:787-797. [DOI] [PubMed] [Google Scholar]
- 5.Blakley, G. B., T. W. Beamer, and W. R. Dukelow. 1981. Characteristics of the menstrual cycle in nonhuman primates. IV. Timed mating in Macaca nemestrina. Lab. Anim. 15:351-353. [DOI] [PubMed] [Google Scholar]
- 6.Boggiano, C., and D. R. Littman. 2007. HIV's vagina travelogue. Immunity 26:145-147. [DOI] [PubMed] [Google Scholar]
- 7.Chimbiri, A. M. 2007. The condom is an ‘intruder’ in marriage: evidence from rural Malawi. Soc. Sci. Med. 64:1102-1115. [DOI] [PubMed] [Google Scholar]
- 8.Cranage, M., S. Sharpe, C. Herrera, A. Cope, M. Dennis, N. Berry, C. Ham, J. Heeney, N. Rezk, A. Kashuba, P. Anton, I. McGowan, and R. Shattock. 2008. Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel. PLoS Med. 5:e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunkle, K. L., R. Stephenson, E. Karita, E. Chomba, K. Kayitenkore, C. Vwalika, L. Greenberg, and S. Allen. 2008. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet 371:2183-2191. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Lerma, J. G., R. A. Otten, S. H. Qari, E. Jackson, M.-E. Cong, S. Masciotra, W. Luo, C. Kim, D. R. Adams, M. Monsour, J. Lipscomb, J. A. Johnson, D. Delinsky, R. F. Schinazi, R. Janssen, T. M. Folks, and W. Heneine. 2008. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 5:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant, R. M., D. Hamer, T. Hope, R. Johnston, J. Lange, M. M. Lederman, J. Lieberman, C. J. Miller, J. P. Moore, D. E. Mosier, D. D. Richman, R. T. Schooley, M. S. Springer, R. S. Veazey, and M. A. Wainberg. 2008. Whither or wither microbicides? Science 321:532-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammer, S. M., D. A. Katzenstein, M. D. Hughes, H. Gundacker, R. T. Schooley, R. H. Haubrich, W. K. Henry, M. M. Lederman, J. P. Phair, M. Niu, M. S. Hirsch, and T. C. Merigan. 1996. A trial comparing nucleoside monotherapy with combination therapy in HIV-infected adults with CD4 cell counts from 200 to 500 per cubic millimeter. N. Engl. J. Med. 335:1081-1090. [DOI] [PubMed] [Google Scholar]
- 13.Harouse, J. M., A. Gettie, T. Eshetu, R. C. H. Tan, R. Bohm, J. Blanchard, G. Baskin, and C. Cheng-Mayer. 2001. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIVSF162P3. J. Virol. 75:1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harouse, J. M., A. Gettie, R. C. H. Tan, J. Blanchard, and C. Cheng-Mayer. 1999. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science 284:816-819. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch, J. S., S. Meneses, B. Thompson, M. Negroni, B. Pelcastre, and C. del Rio. 2007. The inevitability of infidelity: sexual reputation, social geographies, and marital HIV risk in rural Mexico. Am. J. Public Health 97:986-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hladik, F., and C. S. Dezzutti. 2008. Can a topical microbicide prevent rectal HIV transmission? PLoS Med. 5:e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hladik, F., P. Sakchalathorn, L. Ballweber, G. Lentz, M. Fialkow, D. Eschenbach, and M. J. McElrath. 2007. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 26:257-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacubowski, N. 2008. Marriage is not a safe place: heterosexual marriage and HIV-related vulnerability in Indonesia. Cult. Health Sex. 10:87-97. [DOI] [PubMed] [Google Scholar]
- 19.Kaizu, M., A. M. Weiler, K. L. Weisgrau, K. A. Vielhuber, G. May, S. M. Piaskowski, J. Furlott, N. J. Maness, T. C. Friedrich, J. T. Loffredo, A. Usborne, and E. G. Rakasz. 2006. Repeated intravaginal inoculation with cell-associated simian immunodeficiency virus results in persistent infection of nonhuman primates. J. Infect. Dis. 194:912-916. [DOI] [PubMed] [Google Scholar]
- 20.Kalbfleisch, J. D., and P. L. Prentice. 1980. The statistical analysis of failure time data. John Wiley & Sons, Inc., New York, NY.
- 21.Karim, S. S. A., A. Coletti, B. Richardson, G. Ramjee, I. Hoffman, M. Chirenje, T. Taha, M. Kapina, L. Maslankowski, and L. Soto-Torres. 2009. Safety and effectiveness of vaginal microbicides BufferGel and 0.5% PRO 2000/5 gel for the prevention of HIV infection in women: results of the HPTN 035 trial, abstr. 48LB, p. 82. 16th Conf. Retrovir. Opportun. Infect., Montreal, Quebec, Canada.
- 22.Keele, B. F., H. Li, G. H. Learn, P. Hraber, E. E. Giorgi, T. Grayson, C. Sun, Y. Chen, W. W. Yeh, N. L. Letvin, J. R. Mascola, G. J. Nabel, B. F. Haynes, T. Bhattacharya, A. S. Perelson, B. T. Korber, B. H. Hahn, and G. M. Shaw. 2009. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J. Exp. Med. 206:1117-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, C. N., D. R. Adams, S. Bashirian, S. Butera, T. M. Folks, and R. A. Otten. 2006. Repetitive exposures with simian/human immunodeficiency viruses: strategy to study HIV pre-clinical interventions in non-human primates. J. Med. Primatol. 35:210-216. [DOI] [PubMed] [Google Scholar]
- 24.Klasse, P. J., R. Shattock, and J. P. Moore. 2008. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu. Rev. Med. 59:455-471. [DOI] [PubMed] [Google Scholar]
- 25.Kuklenyik, Z., A. Martin, C.-P. Pau, J. G. Garcia-Lerma, W. Heneine, J. L. Pirkle, and J. B. Barr. 2009. Effect of mobile phase pH and organic content on liquid chromatography mass spectrometry analysis of nucleoside and nucleotide HIV reverse transcriptase inhibitors. J. Chromatogr. Sci. 47:365-372. [DOI] [PubMed] [Google Scholar]
- 26.Kwara, A., A. DeLong, N. Rezk, J. Hogan, H. Burtwell, S. Chapman, C. C. Moreira, J. Kurpewski, J. Ingersoll, A. M. Caliendo, A. Kashuba, and S. Cu-Uvin. 2008. Antiretroviral drug concentrations and HIV RNA in the genital tract of HIV-infected women receiving long-term highly active antiretroviral therapy. Clin. Infect. Dis. 46:719-725. [DOI] [PubMed] [Google Scholar]
- 27.Lederman, M. M., R. S. Veazey, R. Offord, D. E. Mosier, J. Dufour, M. Mefford, M. Piatak, J. D. Lifson, J. R. Salkowitz, B. Rodriguez, A. Blauvelt, and O. Hartley. 2004. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science 306:485-487. [DOI] [PubMed] [Google Scholar]
- 28.Lichtenwalner, A. B., D. L. Patton, S. J. Klebanoff, C. M. Headley, and S. L. Hillier. 2000. Vaginal myeloperoxidase and flora in the pig-tailed macaque. J. Med. Primatol. 29:36-41. [DOI] [PubMed] [Google Scholar]
- 29.Marx, P. A., A. I. Spira, A. Gettie, P. J. Dailey, R. S. Veazey, A. A. Lackner, C. J. Mahoney, C. J. Miller, L. E. Claypool, D. D. Ho, and N. J. Alexander. 1996. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat. Med. 2:1084-1089. [DOI] [PubMed] [Google Scholar]
- 30.Mayer, K. H., L. A. Maslankowski, F. Gai, W. M. El-Sadr, J. Justman, A. Kwiecien, B. Masse, S. H. Eshleman, C. Hendrix, K. Morrow, J. F. Rooney, and L. Soto-Torres. 2006. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS 20:543-551. [DOI] [PubMed] [Google Scholar]
- 31.Miller, C. J., Q. Li, K. Abel, E.-Y. Kim, Z.-M. Ma, S. Wietgrefe, L. La Franco-Scheuch, L. Compton, L. Duan, M. D. Shore, M. Zupancic, M. Busch, J. Carlis, S. Wolinksy, and A. T. Haase. 2005. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J. Virol. 79:9217-9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Münch, J., E. Rücker, L. Ständker, K. Adermann, C. Goffinet, M. Schindler, S. Wildum, R. Chinnadurai, D. Rajan, A. Specht, G. Giménez-Gallego, P. C. Sánchez, D. M. Fowler, A. Koulov, J. W. Kelly, W. Mothes, J.-C. Grivel, L. Margolis, O. T. Keppler, W.-G. Forssmann, and F. Kirchhoff. 2007. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell 131:1059-1071. [DOI] [PubMed] [Google Scholar]
- 32a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 33.Otten, R. A., D. R. Adams, C. N. Kim, E. Jackson, J. K. Pullium, K. Lee, L. A. Grohskopf, M. Monsour, S. Butera, and T. M. Folks. 2005. Multiple vaginal exposures to low doses of R5 simian-human immunodeficiency virus: strategy to study HIV preclinical interventions in nonhuman primates. J. Infect. Dis. 191:164-173. [DOI] [PubMed] [Google Scholar]
- 34.Parikh, S. A. 2007. The political economy of marriage and HIV: the ABC approach, “safe” infidelity, and managing moral risk in Uganda. Am. J. Public Health 97:1198-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patton, D. L., Y. T. C. Sweeny, K. Agnew, J. E. Balkus, L. K. Rabe, and S. L. Hillier. 2006. Development of a nonhuman primate model for Trichomonas vaginalis infection. Sex. Transm. Dis. 33:743-746. [DOI] [PubMed] [Google Scholar]
- 36.Potts, M. 1994. The urgent need for a vaginal microbicide in the prevention of HIV transmission. Am. J. Public Health 84:890-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silverman, J. G., M. R. Decker, N. A. Kapur, J. Gupta, and A. Raj. 2007. Violence against wives, sexual risk and sexually transmitted infection among Bangladeshi men. Sex. Transm. Infect. 83:211-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith, R. J., E. N. Bodine, D. P. Wilson, and S. M. Blower. 2005. Evaluating the potential impact of vaginal microbicides to reduce the risk of acquiring HIV in female sex workers. AIDS 19:413-421. [DOI] [PubMed] [Google Scholar]
- 39.Subbarao, S., R. A. Otten, A. Ramos, C. Kim, E. Jackson, M. Monsour, D. R. Adams, S. Bashirian, J. Johnson, V. Soriano, A. Rendon, M. G. Hudgens, S. Butera, R. Janssen, L. Paxton, A. E. Greenberg, and T. M. Folks. 2006. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. J. Infect. Dis. 194:904-911. [DOI] [PubMed] [Google Scholar]
- 40.Subbarao, S., A. Ramos, C. Kim, D. Adams, M. Monsour, S. Butera, T. Folks, and R. A. Otten. 2007. Direct stringency comparison of two macaque models (single-high vs. repeat-low) for mucosal HIV transmission using an identical anti-HIV chemoprophylaxis intervention. J. Med. Primatol. 36:238-243. [DOI] [PubMed] [Google Scholar]
- 41.UNAIDS. 2008. 2008 Report on the global AIDS epidemic. http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp.
- 42.Van Damme, L., G. Ramjee, M. Alary, B. Vuylsteke, V. Chandeying, H. Rees, P. Sirivongrangson, L. M. Tshibaka, V. Ettiègne-Traoré, C. Uaheowitchai, S. S. A. Karim, B. Mâsse, J. Perriëns, and M. Laga. 2002. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 360:971-977. [DOI] [PubMed] [Google Scholar]
- 43.Veazey, R. S., P. J. Klasse, S. M. Schader, Q. Hu, T. J. Ketas, M. Lu, P. A. Marx, J. Dufour, R. J. Colonno, R. J. Shattock, M. S. Springer, and J. P. Moore. 2005. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature 438:99-102. [DOI] [PubMed] [Google Scholar]
- 44.Verbeke, G., and G. Molenberghs. 2000. Linear mixed models for longitudinal data. Springer, New York, NY.
- 45.Voelker, R. 2005. Women shoulder growing HIV/AIDS burden. JAMA 293:281-282. [DOI] [PubMed] [Google Scholar]
- 46.Wardlow, H. 2007. Men's extramarital sexuality in rural Papua New Guinea. Am. J. Public Health 97:1006-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weber, J., K. Desai, and J. Darbyshire. 2005. The development of vaginal microbicides for the prevention of HIV transmission. PLoS Med. 2:e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiler, A. M., Q. Li, L. Duan, M. Kaizu, K. L. Weisgrau, T. C. Friedrich, M. R. Reynolds, A. T. Haase, and E. G. Rakasz. 2008. Genital ulcers facilitate rapid viral entry and dissemination following intravaginal inoculation with cell-associated simian immunodeficiency virus SIVmac239. J. Virol. 82:4154-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Witvrouw, M., C. Pannecouque, W. M. Switzer, T. M. Folks, E. De Clercq, and W. Heneine. 2004. Susceptibility of HIV-2, SIV and SHIV to various anti-HIV-1 compounds: implications for treatment and postexposure prophylaxis. Antivir. Ther. 9:57-65. [PubMed] [Google Scholar]
- 50.Zhu, T., N. Wang, A. Carr, D. S. Nam, R. Moor-Jankowski, D. A. Cooper, and D. D. Ho. 1996. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J. Virol. 70:3098-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]