Abstract
Borna disease virus (BDV), the prototypic member of the Bornaviridae family within the order Mononegavirales, exhibits high neurotropism and provides an important and unique experimental model system for studying virus-cell interactions within the central nervous system. BDV surface glycoprotein (G) plays a critical role in virus cell entry via receptor-mediated endocytosis, and therefore, G is a critical determinant of virus tissue and cell tropism. However, the specific cell pathways involved in BDV cell entry have not been determined. Here, we provide evidence that BDV uses a clathrin-mediated, caveola-independent cell entry pathway. We also show that BDV G-mediated fusion takes place at an optimal pH of 6.0 to 6.2, corresponding to an early-endosome compartment. Consistent with this finding, BDV cell entry was Rab5 dependent but Rab7 independent and exhibited rapid fusion kinetics. Our results also uncovered a key role for microtubules in BDV cell entry, whereas the integrity and dynamics of actin cytoskeleton were not required for efficient cell entry of BDV.
Borna disease virus (BDV) causes central nervous system disease in a variety of vertebrate species that is frequently manifested by behavioral abnormalities (27, 59). BDV is the causative agent of Borna disease, an often fatal immune-mediated neurological disease naturally occurring mainly in horses and sheep (21, 26, 47). However, current evidence indicates that the natural host range, prevalence, and geographic distribution of BDV are wider than originally thought (25, 31). Experimentally, BDV has a wide host range, and both host and viral factors contribute to a variable period of incubation and heterogeneity in the symptoms and pathology associated with BDV infection (20, 23, 34, 50). Notably, cases of proventricular dilatation disease affecting different species of psittacine birds have recently been linked to infection with avian bornaviruses (24, 29), a finding that expands the natural host range of bornavirus infections associated with clinical manifestations.
BDV is an enveloped virus with a nonsegmented negative-strand RNA genome (11, 33, 53, 55) whose gene organization [3′-N-P-p10 (X)-M-G-L-5′] is characteristic of mononegaviruses. However, on the basis of its unique genetic and biological features, BDV is considered to be the prototypic member of a new virus family, Bornaviridae, within the order Mononegavirales.
The BDV surface glycoprotein G plays a key role in receptor recognition and cell entry (20, 46). The G gene directs the synthesis of a precursor, GPC, with a predicted Mr of ca. 56 kDa, but due to its extensive glycosylation, GPC migrates with an Mr of 84 to 94 kDa. GPC is posttranslationally cleaved by the cellular protease furin into GP-1 and GP-2, corresponding to the N-and C-terminal regions, respectively, of G (2, 8, 19, 49). GP-1 has been shown to be sufficient for virus cell entry via receptor-mediated endocytosis (46), whereas GP-2 likely mediates the pH-dependent fusion event between BDV and cell membranes required for a BDV productive infection (19). In vivo, neurons are the initial target of BDV, suggesting a restricted expression pattern of a yet-unidentified virus receptor. Late in infection, BDV is detected in many tissues and organs as a consequence of its centrifugal spread via the axoplasm of peripheral nerve tissues. Receptor-independent mechanisms also contribute to cell-to-cell propagation of BDV (8).
The paucity of cell-free virus associated with BDV infection has hindered studies aimed at the elucidation of the mechanisms involved in BDV cell entry. To overcome this problem, we generated a replication-competent recombinant vesicular stomatitis virus expressing BDV G (rVSVΔG*/BDVG) (45). Cells infected with rVSVΔG*/BDVG produced high titers (107 PFU/ml) of cell-free virus progeny. Notably, rVSVΔG*/BDVG recreated the cell tropism and entry pathway of bona fide BDV, thus providing a unique tool for the investigation of BDV G-mediated cell entry.
Viruses that enter cells via receptor-mediated endocytosis mainly use trafficking pathways mediated by either clathrin or caveola, although alternative entry pathways have been also reported (36). Nevertheless, clathrin-mediated endocytosis (CME) is the route most commonly used by enveloped viruses for cell internalization (35). The initial virus-cell surface receptor interaction results in the activation of different signaling pathways leading to the accumulation of clathrin coated-pits and subsequent formation of endocytotic vesicles (43). Another major endocytotic pathway used by several viruses, including Ebola virus (16) and SV40 (44), uses caveolae for viral internalization into the cell. This endocytotic pathway is strictly dependent on recruitment of lipid rafts to the cell surface, an event mediated by cholesterol. In this regard, we have recently documented the requirements of cholesterol and structural integrity of cell surface lipid rafts for efficient cell entry of BDV (9).
In this work, we provide evidence for the first time that BDV cell entry follows a CME-dependent, caveola-independent pathway. Moreover, we show that BDV entry is Rab5 dependent but Rab7 independent and that BDV G-mediated fusion has a rapid kinetics and an optimal pH between 6.0 and 6.2. These findings indicate that BDV G-mediated fusion occurs within the early-endosome compartment. We also provide evidence that microtubules, but not actin dynamics, play a role in BDV cell entry likely by mediating trafficking of BDV-containing endosomes to the subcellular location where viral and endosomal membranes fuse.
MATERIALS AND METHODS
Cells and virus.
Ol, an established human oligodendroglial cell line (5), and Vero E6 cells (ATCC CRL-1586) were maintained in Dulbecco's modified Eagle's medium supplemented with 2 mM glutamine, 10 mM HEPES, and 10% heat-inactivated fetal bovine serum. Huh-7 cells (38) were kindly provided by Stefan Wieland (The Scripps Research Institute, La Jolla, CA) and were maintained in Dulbecco's modified Eagle's medium supplemented with 2 mM glutamine, 10 mM HEPES, and 10% heat-inactivated fetal bovine serum. Recombinant vesicular stomatitis viruses (rVSV) rVSVΔ*/BDVG and rVSVΔ*/VSVG have previously been described (45). The BDV, VSV, and lymphocytic choriomeningitis virus (LCMV) strains utilized were He80 (54), Indiana (28), and Armstrong (32), respectively.
Analysis of dominant mutants of dynamins I and II, Eps15, caveolin-1, Rab5, and Rab7.
Plasmids expressing hemagglutinin (HA)-tagged versions of the human dynamin I wild-type (WT), human dynamin I (K44A), rat dynamin II WT, and rat dynamin II (K44A) constructs (1) were kindly provided by Sandra Schmid (The Scripps Research Institute, La Jolla, CA). Plasmids for expression of Eps15DIIIΔ2 (4) and the dominant-negative (DN) Eps15Δ95/295 (3) green fluorescent protein (GFP)-tagged versions were provided by Alice Daurty-Varsat and Nathalie Sauvonnet (Institut Pasteur, Paris, France). Plasmids expressing the GFP-tagged versions of both WT caveolin-1 (44) and DN caveolin-1 (Y14F) (10) were obtained from Jeffrey M. Bergelson (University of Pennsylvania). GFP-tagged expression vectors of Rab5 and Rab7 and the respective DN Rab5 (S34N) and Rab7 (T22N) mutants (56, 57) were provided by Craig Roy (Yale University School of Medicine, New Haven, CT). Ol cells were transfected with Amaxa nucleofector technology in accordance with predefined protocols. For Ol cells, 2 μg of DNA was mixed with 106 Ol cells resuspended in 100 μl of nucleofection buffer (buffer V) and the nucleofection was performed utilizing the U-23 program. Finally, cells were plated in M12 plates with covers. At 24 h postinfection (p.i.), transfected cells were infected at 0.1 PFU/cell with either VSV or LCMV or 0.1 focus-forming unit/cell with BDV, and the cells were fixed at 8, 24, or 48 h p.i., respectively, and analyzed by an immunofluorescence assay (IFA) utilizing antisera against VSV N (10G4 [antibody to VSV N]), LCMV N (monoclonal antibody 113), or rabbit serum to BDV N. For detection of transiently expressed dynamin constructs, we used a monoclonal antibody to the HA epitope (HA probe F-7; Santa Cruz). Numbers of infected cells in each case were estimated by determining the average number of virus antigen-positive cells after examining eight different and randomly selected fields. Data shown correspond to the averages of results from three independent experiments.
RNAi-mediated knockdown of cellular genes.
Ol cells (4 × 104) were reverse transfected with RNA interference (RNAi; 10 nM) using Lipofectamine RNAiMAX transfection agent (Invitrogen). We used RNAi against caveolin 1 (CAV1) (5′-CCUUCACUGUGACGAAAUATT-3′) and clathrin heavy chain (CLTC) (5′-GAUUAUCAAUUACCGUACATT-3′) that had previously been validated. Efficiency of knockdown was determined by comparing the protein expression levels of cells transfected with a specific RNAi and those transfected with a nonspecific control RNAi, as determined by Western blot analysis using specific sera to tubulin (Sigma), CLTC, or CAV1 (BD Biosciences). Quantification of Western blot signals was done by scanning the films with a GS-800 densitometer (Bio-Rad) and using the ImageQuant image analysis software program (Molecular Dynamics, Palo Alto, CA).
Drug treatments.
Ol cells were pretreated for 1 h in the presence of chlorpromazine or nocodazole (Noc) or for 30 min in the presence of latrunculin A (latA) at the indicated concentrations and infected (multiplicity of infection [MOI] = 0.3) with either rVSVΔ*/BDVG or rVSVΔ*/VSVG. At 7 h p.i., single-cell preparations were made and fixed (4% paraformaldehyde in phosphate-buffered saline). GFP-expressing cells were determined by fluorescence-activated cell sorting (FACS) analysis using a FACSCalibur flow cytometer (Becton Dickinson) and analyzed using FlowJo (Treestar) software. The viability of drug-treated cells was determined by staining of single-cell preparations with trypan blue.
Fusion experiments.
Uninfected Ol cells and Ol cells persistently infected with BDV were washed twice and incubated for 5 min with the fusion buffer (10 mM NaH2PO4, 10 mM MES [morpholineethanesulfonic acid], 10 mM HEPES, 150 mM NaCl 150) at different pHs (7.0, 6.5, 6.25, 6.0, 5.75, 5.5, and 5.0) (18). After incubation with the fusion buffer, cells were washed three times with medium and incubated for 45 min at 37°C. Syncytium formation was observed by light microscopy.
NH4Cl treatment.
Ol cells were incubated at 4°C with medium containing BDV (MOI = 0.1) for 1.5 h. After removal of the virus inoculum, cell monolayers were washed twice and incubated with medium prewarmed at 37°C to initiate virus cell entry. NH4Cl (20 mM final concentration) was added at the indicated times after addition of warmed medium and kept throughout the infection. At 48 h p.i., cells were fixed and expression of BDV antigen (N protein) was examined by immunofluorescence (IF) using a rabbit polyclonal serum to BDV N.
Detection of BDV mRNA.
Ol cells were infected with BDV (MOI = 0.3), and at 8 h p.i., total cellular RNA was isolated and subjected to reverse transcription-PCR (RT-PCR) analysis. The RT reaction was primed with oligo(dT) and the PCR done using primers 3.1 and 3.2, whose sequences flank intron I of the BDV genome and allow for the generation of PCR DNA products of 470 and 377 bp, corresponding to unspliced and spliced BDV mRNA species, respectively (19). As a control, an aliquot of the RT reaction was subjected to PCR using primers to amplify a segment of the cellular gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase).
RESULTS
Contribution of clathrin-and caveola-dependent pathways to BDV cell entry.
Clathrin and caveola routes are the receptor-mediated endocytosis pathways most frequently utilized by enveloped viruses to enter cells. We identified cholesterol as a key factor for BDV entry (9), a finding compatible with a clathrin or caveola-mediated cell entry pathway for BDV. To assess the contribution of one or both of these two endocytotic pathways to BDV cell entry, we first examined the role of dynamin I (Dyn I) and Dyn II in BDV cell entry. Dyn I and Dyn II are GTPases responsible for scission of endocytotic vesicles from the cell surface membrane, a process required for cell entry of virus by either clathrin-mediated or caveloar endocytosis (14, 40). Ol cells expressing, via transfection, HA-tagged versions of well-characterized (K44A) DN versions of Dyn I and Dyn II exhibited increased resistance to BDV, whereas overexpression of the corresponding wt forms of Dyn I and Dyn II did not affect the efficiency of BDV infection (Fig. 1).
FIG. 1.
DN mutants of dynamin inhibit BDV cell entry. Ol cells expressing the HA-tagged version of either WT dynamin I, DN dynamin I (K44A), WT dynamin II, or DN dynamin II (K44A) were infected with BDV (MOI = 0.1). (A) At 48 h p.i., cells were fixed and analyzed by IFA using anti-BDV N. (B) Both GFP-positive cells and GFP- and virus antigen-positive cells were counted and plotted. Normalization was achieved by considering the ratio of GFP- and virus antigen-positive cells/total GFP-positive cells in each viral system assessed 100%. * indicates P values of ≤0.05 by Student's t test.
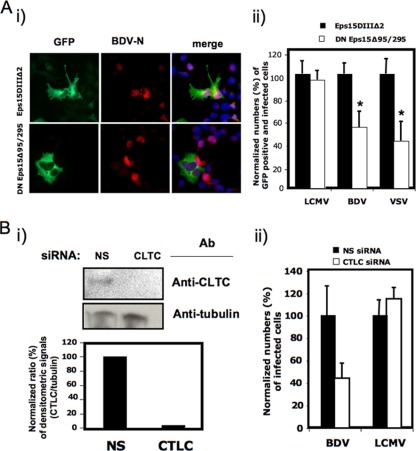
To assess the specific contribution of CME to BDV cell entry, we examined the effect on BDV infection of the expression of a well-characterized DN mutant (Eps15Δ95/295) of the clathrin coat-associated protein Eps15 (Eps15Δ95/295), known to specifically interfere with clathrin-coated pit assembly without affecting clathrin-independent pathways (3). For this, we transfected Ol cells with GFP-tagged versions of WT and DN forms of Eps15 and at 24 h posttransfection infected them with BDV (MOI = 0.1). As controls, Ol-transfected cells were also infected with VSV, whose cell entry is clathrin dependent (60) or LCMV, which uses a clathrin- and caveola-independent pathway for cell entry (48, 51). The use of GFP-tagged versions of WT and DN Eps15 proteins allowed us to correlate the expression of these proteins with virus infection at the single-cell level. Infections with BDV and VSV, but not LCMV, were significantly inhibited in cells expressing the DN form of Eps15 (Fig. 2A). As a complementary genetic approach, we examined the effect of RNAi-mediated knockdown of clathrin heavy chain (CLTC) on BDV infection. Cells transfected with an RNAi specific to CLTC exhibited over 90% reduction in expression levels of CLTC protein in comparison to cells transfected with a nonspecific control RNAi as determined by Western blot analysis (Fig. 2Bi). BDV infection was inhibited in cells with reduced expression levels of CLTC, whereas infection with LCMV, a virus that follows a CME-independent cell entry pathway, remained largely unaffected (Fig. 2Bii).
FIG. 2.
Genetic targeting of clathrin inhibits BDV cell entry. (A) Effect of DN mutant forms of Eps15 on BDV cell entry. Ol cells expressing the GFP-tagged version of Eps15DIIIΔ2 or DN Eps15Δ95/295 were infected with BDV (MOI = 0.1). (Ai) Expression of GFP-tagged proteins and BDV antigen. At 48 h p.i., cells were fixed and GFP-expressing cells were identified by direct epifluorescence, whereas virus-infected cells were identified by IF using a rabbit polyclonal serum to BDV N. (Aii) Normalized values for virus-infected cells also expressing GFP. As a control, Ol cells expressing the GFP-tagged versions of Eps15DIIIΔ2 and DN Eps15Δ95/295 were infected with VSV and LCMV (MOI = 0.1). Virus-infected cells were identified by IF using either a rabbit serum to VSV N or a mouse monoclonal antibody to LCMV N. Normalized values were determined by considering the ratio of GFP- and virus antigen-positive cells/total GFP-positive cells in each viral system assessed 100%. (B) Effect of RNAi-mediated knockdown of CLTC on BDV cell entry. Ol cells were transfected with both RNAi against CLTC and a nonspecific (NS) control RNAi and then infected (MOI = 0.1) with either BDV or LCMV. (Bi) Expression levels of CLTC were determined by Western blot analysis, and specific signals were scanned and quantified by densitometry. siRNA, small interfering RNA; Ab, antibody. (Bii) Cells transfected with NS and CLTC siRNA were infected with LCMV or BDV (MOI = 0.1) and at 24 (LCMV) or 48 (BDV) h p.i. fixed and subjected to IF for identification of virus-infected cells. * indicates P values of ≤0.05 by Student's t test.
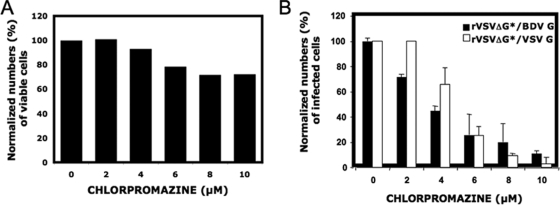
To further confirm the contribution of CME to BDV cell entry, we pursued chemical inhibition studies using chlorpromazine, a drug inhibitor of assembly of coated pits. For this, we took advantage of a recombinant VSV expressing GFP in which BDV G substituted for VSV G (rVSVΔG*/BDVG). This rVSVΔG*/BDVG virus was shown to accurately recreate the cell tropism and cell entry features of bona fide BDV but has a replication kinetics corresponding to VSV (45). The shorter p.i. time required to readily detect VSV protein expression than for BDV allowed us to avoid cell toxicity associated with long-term exposure to chlorpromazine. We treated Ol cells with increasing concentrations of chlorpromazine and infected them (MOI = 0.3) with either rVSVΔ*/BDVG or, as a control, rVSVΔG*/VSVG. At 7 h p.i., cells were fixed and GFP-expressing cells detected by FACS. Cell viability was assessed in parallel to determine chlorpromazine-induced cell toxicity under our experimental conditions (Fig. 3A), and we used this information to normalize numbers of infected cells in the presence of different drug concentrations. We observed a dose-dependent inhibition by chlorpromazine of rVSVΔG*/BDVG infection (Fig. 3B).
FIG. 3.
Chemical inhibition of CME prevents BDV cell entry. Vero cells were treated with increasing amounts of chlorpromazine, starting at 2 h prior infection, and then infected with either rVSVΔ*/BDVG or rVSVΔ*/VSVG (MOI = 0.3). Drug was maintained along the course of infection, and its effect on cell viability (A) was tested as described in Materials and Methods. At 7 h p.i., single-cell suspensions were prepared, fixed, and subjected to FACS analysis to assess levels of GFP expression.
To assess the possible contribution of caveolar endocytosis to BDV cell entry, we used a DN mutant of caveolin-1 (CAV1 Y14F), which has been shown to prevent caveola-mediated cell entry of other viruses (51). We transfected Ol cells with GFP-tagged versions of WT and DN CAV1 and subsequently infected them with BDV (MOI = 0.1). At 48 h p.i., BDV-infected cells were assessed based on expression of BDV N as determined by IFA (Fig. 4A). Cells expressing GFP-tagged versions of WT or DN CAV1 proteins were equally susceptible to BDV infection, suggesting a caveola-independent BDV cell entry pathway. As a complementary approach, we also examined the effect of RNAi-mediated knockdown of CAV1 on BDV infection. BDV infection was not significantly inhibited in cells transfected with an RNAi to CAV1 in comparison to cells transfected with a nonspecific control RNAi (Fig. 4B). We also examined the ability of BDV to infect Huh-7 cells, a cell line known to have an impaired caveolar endocytotic pathway due to only marginal expression levels of CAV1 (15). Huh-7 cells were fully susceptible to both BDV and rVSVΔG*/BDVG as determined by levels of virus antigen expression detected by IF (Fig. 5).
FIG. 4.
Role of caveolae in BDV cell entry. (A) Effect DN mutant forms of CAV1 on BDV cell entry. (Ai) Ol cells expressing the GFP-tagged versions of both WT CAV1 and DN CAV1 Y14F were infected with BDV or VSV (MOI = 0.1). At 48 h p.i., cells were fixed and analyzed for GFP and virus antigen expression. Viral antigens were detected by IF-specific sera to BDV N and VSV N. (Aii) Normalized numbers of GFP-positive cells expressing viral antigen. Normalization was done by considering the number of cells expressing GFP-tagged WT CAV1 that were also positive for viral antigen 100%. (B) Effect of RNAi-mediated knockdown of CAV1 on BDV cell entry. Ol cells were transfected with CAV1 or nonspecific (NS) siRNA. (Bi) Expression levels of CAV1 protein were determined by Western blot analysis. The results of the Western blot analysis were quantified as described elsewhere in the text (see Materials and Methods and Fig. 2B). siRNA, small interfering RNA; Ab, antibody. (Bii) After RNAi gene silencing, transfected cells were infected with BDV (MOI = 0.1). At 48 h p.i., cells were fixed and subjected to IF to determine numbers of BDV-infected cells.
FIG. 5.
BDV and rVSVΔG*/BDVG can efficiently infect Huh-7 cells. (A) Huh-7 cells and Ol cells were infected with either BDV or rVSVΔ*/BDVG (MOI = 0.1). At 7 h p.i. (rVSVΔG*/BDVG) or 48 h p.i. (BDV), cells were fixed and viral antigen was detected by IF using specific sera to BDV and VSV. (B) Infected BDV or rVSVΔ*/BDVG cells were counted. Normalization was done by considering the number of infected cells per field in Ol cells 100%. Values represent the averages (and standard deviations) of results from three independent fields.
Role of Rab5 and Rab7 in efficient BDV infection.
Consistent with a BDV cell entry pathway via receptor-mediated endocytosis, previous studies documented the requirement of a pH-dependent fusion event between cellular and viral membranes for the release of BDV RNPs into the cell cytoplasm (19) and their subsequent trafficking to the cell nucleus, where virus RNA replication takes place (5, 12). This previous study, however, examined only the effect of a very acidic environment (pH 5) on BDV G-mediated fusion and hence did not address whether the fusion event could have occurred at a higher pH during BDV trafficking through the early-endosome compartment. To further investigate this issue, we examined the roles in BDV infection of the small GTPases Rab5 and Rab7, known to be involved in vesicular trafficking to the early- and late-endosomal compartments, respectively (17, 22). For this, we use the DN S34N (Rab5) and T22N (Rab7) mutants, which have previously been validated in studies examining trafficking of different viruses within the endosomal compartment (30, 48, 56). Ol cells were transfected with plasmids expressing GFP-tagged versions of WT or DN (S34N) forms of Rab5 as well as WT and DN (T22N) forms of Rab7. At 24 h posttransfection, cells were infected with BDV (MOI = 0.1), and 48 h later, BDV N-positive cells were detected by IF (Fig. 6). BDV infection was significantly inhibited in cells expressing DN Rab5 but not in cells expressing DN Rab7.
FIG. 6.
Role of Rab5 and Rab7 in BDV cell entry. Ol cells expressing the GFP-tagged versions of WT and DN Rab5 and Rab7 (Rab5 S34N and Rab7 T22N) were infected with BDV (MOI = 0.1) and at 48 h p.i. fixed and analyzed by IF using an antibody to BDV N. (A) Representative fields of cells transfected with each construct and infected with BDV. (B) Normalized numbers of infected cells. GFP-positive and GFP- and virus antigen-positive cells were counted, and normalized numbers of infected cells were obtained for each virus system by considering the number of GFP- and virus antigen-positive cells/total number of GFP-positive cells 100%. * indicates P values of ≤0.05 by Student's t test.
These results were consistent with a cell entry pathway where the fusion event between BDV and cellular membranes occurred within early endosomes. However, these data did not formally rule out the alternative that the fusion event happened only once BDV reached, in a Rab7-independent manner, the late-endosomal compartment. To distinguish between these possibilities, we conducted studies aimed at defining more precisely the pH required for optimal BDV G-mediated fusion. For this, both noninfected Ol cells and Ol cells persistently infected with BDV were exposed to a range of different pHs and syncytium formation was determined (Fig. 7). We observed a dramatic increase in syncytium formation by Ol-BDV cells upon a drop in pH from 6.25 to 6.0, suggesting that BDV G-mediated fusion takes place in the early-intermediate endosome, a finding consistent with a Rab5-dependent but Rab7-independent cell entry pathway. A model proposing that BDV exits from an early-endosomal compartment would predict a relatively rapid fusion kinetics, which corresponds to the time BDV would require from cell attachment to escape from the endosome compartment. To examine this question, we determined the time required for BDV to become resistant to the lysosomotropic agent ammonium chloride (NH4Cl). Treatment of cells with 20 mM NH4Cl raises the endosomal pH rapidly and prevents low pH-dependent cellular processes without causing significant cell toxicity (41). Addition of NH4Cl at times later than 30 min did not further increase levels of BDV infection (data not shown). Results from these studies revealed that BDV-induced fusion occurs within 10 min of virus adsorption to cells (Fig. 7C).
FIG. 7.
Determination of the pH required for optimal fusion induced by BDV G and kinetics of fusion. (A) Noninfected Ol cells and Ol cells persistently infected with BDV were treated with buffer at the indicated different pHs for 5 min, and at 45 min posttreatment, cells were fixed and nuclei stained with DAPI (4′,6-diamidino-2-phenylindole). (B) Numbers of syncytia induced at different pHs were determined by light microscopy. (C) Ol cells infected with BDV (MOI = 0.1) at 4°C were transferred to 37°C, and at the indicated times, NH4Cl (20 mM) was added to inhibit endosomal acidification. At 48 h p.i., cells were fixed and subjected to IFA using a rabbit polyclonal serum to BDV N to identify virus-infected cells.
Role of actin cytoskeleton and microtubules in BDV cell entry.
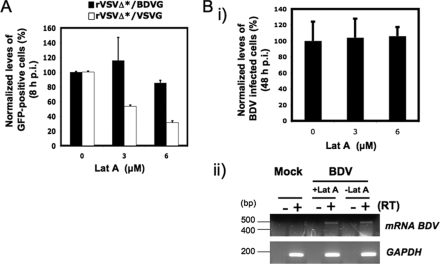
Actin plays key roles in a large number of different cellular functions and many intracellular pathogens, including viruses interacting with actin and actin-regulating signaling pathways at several points in the life cycle of the pathogens, including internalization and intracellular movement. Accordingly, actin cytoskeleton has been implicated in cell entry of several viruses (13). To determine whether actin cytoskeleton played a role during early stages of BDV internalization, we examined the effect of latA and jasplakinolide on BDV G-mediated cell entry. latA is an agent that disrupts actin fibers (58), whereas jasplakinolide is an actin polymer-stabilizing drug that blocks the normal dynamics of actin filaments (6). Ol cells were pretreated with either latA or jasplakinolide, followed by infection with rVSVΔG*/BDVG or, as a control, rVSVΔG*/VSVG (Fig. 8). Consistent with recent published data (13), we observed that latA had a dose-dependent inhibitory effect on rVSVΔG*/VSVG infection (Fig. 8A). In contrast, infection with rVSVG*/BDVG (Fig. 8A) or with bona fide BDV (Fig. 8B) was not significantly affected by latA treatment (Fig. 8A). We observed similar results (data not shown) in cells treated with jasplakinolide (500 nM).
FIG. 8.
Role of actin cytoskeleton in BDV G-mediated cell entry. (A) Ol cells were pretreated with latA at the indicated concentrations for 30 min prior to infection with the indicated rVSV (MOI = 0.1). LatA was maintained throughout the infection. At 8 h p.i., cells were trypsinized, fixed, and subjected to FACS analysis for detection of GFP expression. (B) Ol cells were infected with BDV (MOI = 0.5) in the presence of latA at the indicated concentrations. At 8 h p.i., cells were washed and incubated with medium without latA for the rest of the infection. (Bi) At 48 h p.i., cells were fixed and virus-infected cells identified by IF using a rabbit polyclonal serum to BDV N. (Bii) Cells pretreated with latA (5 μM) for 30 min were infected with BDV (MOI = 0.5) in the presence of latA, and at 8 h p.i., total cellular RNA was isolated from BDV- and mock-infected control cells and subjected to RT-PCR as described in Materials and Methods to detect BDV-specific mRNA species.
BDV antigen expression in infected cells is readily detected only after 36 to 48 h p.i., but exposure of cells to latA for longer than 8 h was associated with significant toxicity. The relatively long time (>40 h) between removal of latA (to prevent confounding factors related to toxicity) and detection of viral antigen raised the possibility that a complete regeneration of actin fibers accounted for the lack of effect of latA on BDV infection. To overcome this limitation, we used RT-PCR to examine whether latA treatment influenced levels of BDV mRNA species amplified by primers 3.1 and 3.2, which can be detected by 8 h p.i. (19). Synthesis of virus mRNA species can proceed only after the fusion event between viral and cellular membranes required for the release and subsequent nuclear transport of BDV RNP (5). Treatment with latA (5 μM) did not affect levels of BDV mRNA (Fig. 8C), indicating that actin fibers were not required for the exit of BDV from the endosomal compartment. Levels of intron I spliced BDV mRNA species are extremely low, often undetectable, at early times p.i., and therefore, levels of the corresponding 377-bp PCR product remained below detection levels under the experimental conditions of our RT-PCR assay.
Microtubules contribute to regulation of cargo trafficking within endosomal compartments and serve as scaffolding structures for a variety of cellular proteins, including Rab5 (39). Consistent with this, microtubules have been implicated in cell entry of several viruses, including hepatitis C virus (52). To examine the potential role of microtubules in BDV productive infection, we assessed the effect of Noc on BDV infection. Noc disrupts microtubules by binding β-tubulin and preventing formation of one of the two interchain disulfide linkages (61). We treated Ol cells with Noc at different times before, during, and after infection with rVSVΔG*/BDVG and, as a control, rVSVΔG*/VSVG (Fig. 9). Treatment of cells with Noc prior to infection significantly affected BDV G-mediated, but not VSV G-mediated, virus cell entry, whereas drug treatment initiated during or after virus adsorption had no effect on either BDV G- or VSV G-mediated virus cell entry.
FIG. 9.
Role of microtubules in BDV G-mediated cell entry. Vero cells were treated with Noc (10 μM) for 1 hour at different times prior to and after infection. Cells were also infected with rVSVΔ*/BDVG and rVSVΔ*/VSVG (MOI 0.3). At 7 h p.i., single-cell suspensions were prepared and fixed for detection of GFP-positive cells by FACS.
DISCUSSION
Previous studies have documented that BDV enters cells via receptor-mediated endocytosis (19) and that cholesterol plays a critical role in BDV cell entry (9). However, many of the details related to BDV cell entry have not been investigated yet. In this report, we have presented evidence for the first time that BDV uses a dynamin- and clathrin-dependent, but caveola-independent, endocytotic cell entry pathway. CME is a pathway used by a variety of viruses, including VSV (60) and influenza virus, which, like BDV, also replicates in the nucleus (57).
The formation of lipid rafts, mediated by cholesterol, is strictly required for caveola-mediated virus cell entry (42). However, other viruses, including West Nile virus (37), that follow a CME pathway may also depend on cholesterol and its role as a key factor for lipid raft formation within the surface of the cell.
Results obtained using DN forms of Rab5 and Rab7, together with the finding that BDV G-mediated fusion was optimal between pH 6.2 and 6.0, support the view that upon CME, BDV follows a Rab5-mediated trafficking through the early endosome, where fusion between viral and cellular membranes takes place. VSV fuses also in the early endosome and exhibits a Rab5-dependent, Rab7-independent entry pathway (56). However, VSV replicates in the cell cytoplasm, whereas BDV RNPs need to be further transported from the location of virus-cell envelope fusion within the early-endosome compartment of the cell to the nucleus, where BDV RNA replication and transcription take place. Cell entry of influenza virus, another virus with nuclear replication, is both Rab5 and Rab7 dependent, and fusion between influenza virus and cellular membranes happens within late endosomes (56), whose proximity to the nuclear envelope may facilitate the transport of viral RNPs to the nucleus. In contrast, BDV RNPs are likely released into the cell cytoplasm from the early-endosome compartment. The mechanisms by which BDV RNPs are transported to the nucleus remain to be determined.
A Rab5-dependent, Rab7-independent CME pathway also involving a critical role for microtubules has been described for several viruses, including West Nile virus (7, 30). We also found that microtubules play an important role during very early steps of BDV cell entry prior to the fusogenic event in the early endosome. This finding is consistent with the role previously assigned to Rab5 as a regulator of motility of early endosomes via interaction with the microtubular network (39). Once BDV RNPs are released following the fusion event, microtubules may play an additional role to facilitate the transport of BDV RNPs to the nucleus. Likewise, recent evidence has documented that actin plays a key role during internalization of VSV via CME (13). Notably, we observed that the internalization of not only BDV but also rVSVΔG*/BDVG did not require the integrity or dynamics of actin cytoskeleton, suggesting that besides the virus particle size, the nature of BDVG protein displayed at the virion surface can also influence the requirement of actin for efficient virus internalization.
In summary, our results have provided evidence for the first time that BDV cell entry follows a clathrin-mediated pathway that is independent of the structural integrity and dynamics of actin cytoskeleton. In addition, we have provided evidence that BDV traffics to early endosomes in a process that requires the participation of Rab5 and microtubules. Upon a fusion between viral and cellular membranes within the early endosomes, the virus RNPs are released and subsequently transported to the nucleus by mechanisms that remain to be determined.
Acknowledgments
We thank Sandra Schmid (The Scripps Research Institute, La Jolla, CA), Alice Daurty-Varsat and Nathalie Sauvonnet (Institut Pasteur, Paris, France), Jeffrey M. Bergelson (University of Pennsylvania), and Craig Roy (Yale University School of Medicine, New Haven, CT) for providing us with plasmids expressing the different DN mutant proteins used in this work. We thank Joanna Porras for technical support and Stefan Kunz for valuable comments and scientific discussion.
This work was supported by a fellowship of the Ministerio de Educacion y Ciencia of Spain to R.C. and NIH grant R56 AI073297-01A1 to J.C.D.L.T. This is publication 19978 of Immunology and Microbial Science.
Footnotes
Published ahead of print on 5 August 2009.
REFERENCES
- 1.Altschuler, Y., S. M. Barbas, L. J. Terlecky, K. Tang, S. Hardy, K. E. Mostov, and S. L. Schmid. 1998. Redundant and distinct functions for dynamin-1 and dynamin-2 isoforms. J. Cell Biol. 143:1871-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bajramovic, J. J., S. Munter, S. Syan, U. Nehrbass, M. Brahic, and D. Gonzalez-Dunia. 2003. Borna disease virus glycoprotein is required for viral dissemination in neurons. J. Virol. 77:12222-12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303-1311. [DOI] [PubMed] [Google Scholar]
- 4.Benmerah, A., C. Lamaze, B. Begue, S. L. Schmid, A. Dautry-Varsat, and N. Cerf-Bensussan. 1998. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J. Cell Biol. 140:1055-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briese, T., J. C. de la Torre, A. Lewis, H. Ludwig, and W. I. Lipkin. 1992. Borna disease virus, a negative-strand RNA virus, transcribes in the nucleus of infected cells. Proc. Natl. Acad. Sci. USA 89:11486-11489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bubb, M. R., A. M. Senderowicz, E. A. Sausville, K. L. Duncan, and E. D. Korn. 1994. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J. Biol. Chem. 269:14869-14871. [PubMed] [Google Scholar]
- 7.Chu, J. J., and M. L. Ng. 2004. Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J. Virol. 78:10543-10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemente, R., and J. C. de la Torre. 2007. Cell-to-cell spread of Borna disease virus proceeds in the absence of the virus primary receptor and furin-mediated processing of the virus surface glycoprotein. J. Virol. 81:5968-5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemente, R., A. de Parseval, M. Perez, and J. C. de la Torre. 2009. Borna disease virus requires cholesterol in both cellular membrane and viral envelope for efficient cell entry. J. Virol. 83:2655-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coyne, C. B., and J. M. Bergelson. 2006. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell 124:119-131. [DOI] [PubMed] [Google Scholar]
- 11.Cubitt, B., and J. C. de la Torre. 1994. Borna disease virus (BDV), a nonsegmented RNA virus, replicates in the nuclei of infected cells where infectious BDV ribonucleoproteins are present. J. Virol. 68:1371-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cubitt, B., C. Oldstone, J. Valcarcel, and J. Carlos de la Torre. 1994. RNA splicing contributes to the generation of mature mRNAs of Borna disease virus, a non-segmented negative strand RNA virus. Virus Res. 34:69-79. [DOI] [PubMed] [Google Scholar]
- 13.Cureton, D. K., R. H. Massol, S. Saffarian, T. L. Kirchhausen, and S. P. Whelan. 2009. Vesicular stomatitis virus enters cells through vesicles incompletely coated with clathrin that depend upon actin for internalization. PLoS Pathog. 5:e1000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damke, H., T. Baba, D. E. Warnock, and S. L. Schmid. 1994. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 127:915-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damm, E. M., L. Pelkmans, J. Kartenbeck, A. Mezzacasa, T. Kurzchalia, and A. Helenius. 2005. Clathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolae. J. Cell Biol. 168:477-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Empig, C. J., and M. A. Goldsmith. 2002. Association of the caveola vesicular system with cellular entry by filoviruses. J. Virol. 76:5266-5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng, Y., B. Press, and A. Wandinger-Ness. 1995. Rab 7: an important regulator of late endocytic membrane traffic. J. Cell Biol. 131:1435-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fredericksen, B. L., and M. A. Whitt. 1995. Vesicular stomatitis virus glycoprotein mutations that affect membrane fusion activity and abolish virus infectivity. J. Virol. 69:1435-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Dunia, D., B. Cubitt, and J. C. de la Torre. 1998. Mechanism of Borna disease virus entry into cells. J. Virol. 72:783-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez-Dunia, D., B. Cubitt, F. A. Grasser, and J. C. de la Torre. 1997. Characterization of Borna disease virus p56 protein, a surface glycoprotein involved in virus entry. J. Virol. 71:3208-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Dunia, D., C. Sauder, and J. C. de la Torre. 1997. Borna disease virus and the brain. Brain Res. Bull. 44:647-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorvel, J. P., P. Chavrier, M. Zerial, and J. Gruenberg. 1991. rab5 controls early endosome fusion in vitro. Cell 64:915-925. [DOI] [PubMed] [Google Scholar]
- 23.Hatalski, C. G., A. J. Lewis, and W. I. Lipkin. 1997. Borna disease. Emerg. Infect. Dis. 3:129-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honkavuori, K. S., H. L. Shivaprasad, B. L. Williams, P. L. Quan, M. Hornig, C. Street, G. Palacios, S. K. Hutchison, M. Franca, M. Egholm, T. Briese, and W. I. Lipkin. 2008. Novel borna virus in psittacine birds with proventricular dilatation disease. Emerg. Infect. Dis. 14:1883-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornig, M., and W. I. Lipkin. 2001. Infectious and immune factors in the pathogenesis of neurodevelopmental disorders: epidemiology, hypotheses, and animal models. Ment. Retard. Dev. Disabil. Res. Rev. 7:200-210. [DOI] [PubMed] [Google Scholar]
- 26.Hornig, M., H. Weissenbock, N. Horscroft, and W. I. Lipkin. 1999. An infection-based model of neurodevelopmental damage. Proc. Natl. Acad. Sci. USA 96:12102-12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikuta, K., M. S. Ibrahim, T. Kobayashi, and K. Tomonaga. 2002. Borna disease virus and infection in humans. Front. Biosci. 7:d470-d495. [DOI] [PubMed] [Google Scholar]
- 28.Johnson, K. M., J. E. Vogel, and P. H. Peralta. 1966. Clinical and serological response to laboratory-acquired human infection by Indiana type vesicular stomatitis virus (VSV). Am. J. Trop. Med. Hyg. 15:244-246. [DOI] [PubMed] [Google Scholar]
- 29.Kistler, A. L., A. Gancz, S. Clubb, P. Skewes-Cox, K. Fischer, K. Sorber, C. Y. Chiu, A. Lublin, S. Mechani, Y. Farnoushi, A. Greninger, C. C. Wen, S. B. Karlene, D. Ganem, and J. L. DeRisi. 2008. Recovery of divergent avian bornaviruses from cases of proventricular dilatation disease: identification of a candidate etiologic agent. Virol. J. 5:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnan, M. N., B. Sukumaran, U. Pal, H. Agaisse, J. L. Murray, T. W. Hodge, and E. Fikrig. 2007. Rab 5 is required for the cellular entry of dengue and West Nile viruses. J. Virol. 81:4881-4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lancaster, K., D. M. Dietz, T. H. Moran, and M. V. Pletnikov. 2007. Abnormal social behaviors in young and adult rats neonatally infected with Borna disease virus. Behav. Brain Res. 176:141-148. [DOI] [PubMed] [Google Scholar]
- 32.Lee, K. J., I. S. Novella, M. N. Teng, M. B. Oldstone, and J. C. de La Torre. 2000. NP and L proteins of lymphocytic choriomeningitis virus (LCMV) are sufficient for efficient transcription and replication of LCMV genomic RNA analogs. J. Virol. 74:3470-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipkin, W. I., and T. Briese. 2007. Bornaviridae, p. 1829-1851. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 34.Lipkin, W. I., C. G. Hatalski, and T. Briese. 1997. Neurobiology of Borna disease virus. J. Neurovirol. 3(Suppl. 1):S17-S20. [PubMed] [Google Scholar]
- 35.Marsh, M., and A. Helenius. 2006. Virus entry: open sesame. Cell 124:729-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayor, S., and R. E. Pagano. 2007. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 8:603-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medigeshi, G. R., A. J. Hirsch, D. N. Streblow, J. Nikolich-Zugich, and J. A. Nelson. 2008. West Nile virus entry requires cholesterol-rich membrane microdomains and is independent of αvβ3 integrin. J. Virol. 82:5212-5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakabayashi, H., K. Taketa, K. Miyano, T. Yamane, and J. Sato. 1982. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 42:3858-3863. [PubMed] [Google Scholar]
- 39.Nielsen, E., F. Severin, J. M. Backer, A. A. Hyman, and M. Zerial. 1999. Rab5 regulates motility of early endosomes on microtubules. Nat. Cell Biol. 1:376-382. [DOI] [PubMed] [Google Scholar]
- 40.Oh, P., D. P. McIntosh, and J. E. Schnitzer. 1998. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J. Cell Biol. 141:101-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohkuma, S., and B. Poole. 1981. Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. J. Cell Biol. 90:656-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pelkmans, L. 2005. Secrets of caveolae- and lipid raft-mediated endocytosis revealed by mammalian viruses. Biochim. Biophys. Acta 1746:295-304. [DOI] [PubMed] [Google Scholar]
- 43.Pelkmans, L., E. Fava, H. Grabner, M. Hannus, B. Habermann, E. Krausz, and M. Zerial. 2005. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature 436:78-86. [DOI] [PubMed] [Google Scholar]
- 44.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3:473-483. [DOI] [PubMed] [Google Scholar]
- 45.Perez, M., R. Clemente, C. S. Robison, E. Jeetendra, H. R. Jayakar, M. A. Whitt, and J. C. de la Torre. 2007. Generation and characterization of a recombinant vesicular stomatitis virus expressing the glycoprotein of Borna disease virus. J. Virol. 81:5527-5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perez, M., M. Watanabe, M. A. Whitt, and J. C. de la Torre. 2001. N-terminal domain of Borna disease virus G (p56) protein is sufficient for virus receptor recognition and cell entry. J. Virol. 75:7078-7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pletnikov, M. V., T. H. Moran, and K. M. Carbone. 2002. Borna disease virus infection of the neonatal rat: developmental brain injury model of autism spectrum disorders. Front. Biosci. 7:d593-d607. [DOI] [PubMed] [Google Scholar]
- 48.Quirin, K., B. Eschli, I. Scheu, L. Poort, J. Kartenbeck, and A. Helenius. 2008. Lymphocytic choriomeningitis virus uses a novel endocytic pathway for infectious entry via late endosomes. Virology 378:21-33. [DOI] [PubMed] [Google Scholar]
- 49.Richt, J. A., T. Furbringer, A. Koch, I. Pfeuffer, C. Herden, I. Bause-Niedrig, and W. Garten. 1998. Processing of the Borna disease virus glycoprotein gp94 by the subtilisin-like endoprotease furin. J. Virol. 72:4528-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richt, J. A., I. Pfeuffer, M. Christ, K. Frese, K. Bechter, and S. Herzog. 1997. Borna disease virus infection in animals and humans. Emerg. Infect. Dis. 3:343-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rojek, J. M., M. Perez, and S. Kunz. 2008. Cellular entry of lymphocytic choriomeningitis virus. J. Virol. 82:1505-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roohvand, F., P. Maillard, J. P. Lavergne, S. Boulant, M. Walic, U. Andreo, L. Goueslain, F. Helle, A. Mallet, J. McLauchlan, and A. Budkowska. 2009. Initiation of hepatitis C virus infection requires the dynamic microtubule network: role of the viral nucleocapsid protein. J. Biol. Chem. 284:13778-13791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneemann, A., P. A. Schneider, R. A. Lamb, and W. I. Lipkin. 1995. The remarkable coding strategy of Borna disease virus: a new member of the nonsegmented negative strand RNA viruses. Virology 210:1-8. [DOI] [PubMed] [Google Scholar]
- 54.Schneider, P. A., T. Briese, W. Zimmermann, H. Ludwig, and W. I. Lipkin. 1994. Sequence conservation in field and experimental isolates of Borna disease virus. J. Virol. 68:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneider, U. 2005. Novel insights into the regulation of the viral polymerase complex of neurotropic Borna disease virus. Virus Res. 111:148-160. [DOI] [PubMed] [Google Scholar]
- 56.Sieczkarski, S. B., and G. R. Whittaker. 2003. Differential requirements of Rab5 and Rab7 for endocytosis of influenza and other enveloped viruses. Traffic 4:333-343. [DOI] [PubMed] [Google Scholar]
- 57.Sieczkarski, S. B., and G. R. Whittaker. 2002. Influenza virus can enter and infect cells in the absence of clathrin-mediated endocytosis. J. Virol. 76:10455-10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spector, I., N. R. Shochet, Y. Kashman, and A. Groweiss. 1983. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science 219:493-495. [DOI] [PubMed] [Google Scholar]
- 59.Staeheli, P., C. Sauder, J. Hausmann, F. Ehrensperger, and M. Schwemmle. 2000. Epidemiology of Borna disease virus. J. Gen. Virol. 81:2123-2135. [DOI] [PubMed] [Google Scholar]
- 60.Sun, X., V. K. Yau, B. J. Briggs, and G. R. Whittaker. 2005. Role of clathrin-mediated endocytosis during vesicular stomatitis virus entry into host cells. Virology 338:53-60. [DOI] [PubMed] [Google Scholar]
- 61.Vasquez, R. J., B. Howell, A. M. Yvon, P. Wadsworth, and L. Cassimeris. 1997. Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Mol. Biol. Cell 8:973-985. [DOI] [PMC free article] [PubMed] [Google Scholar]