Abstract
Type I interferons (IFN) inhibit several steps of the human immunodeficiency virus type 1 (HIV) replication cycle. Some HIV proteins, like Vif and Vpu, directly counteract IFN-induced restriction factors. Other mechanisms are expected to modulate the extent of IFN inhibition. Here, we studied the impact of IFN on various aspects of HIV replication in primary T lymphocytes. We confirm the potent effect of IFN on Gag p24 production in supernatants. Interestingly, IFN had a more limited effect on HIV spread, measured as the appearance of Gag-expressing cells. Primary isolates displayed similar differences in the inhibition of p24 release and virus spread. Virus emergence was the consequence of suboptimal inhibition of HIV replication and was not due to the selection of resistant variants. Cell-to-cell HIV transfer, a potent means of virus replication, was less sensitive to IFN than infection by cell-free virions. These results suggest that IFN are less active in cell cultures than initially thought. They help explain the incomplete protection by naturally secreted IFN during HIV infection and the unsatisfactory outcome of IFN treatment in HIV-infected patients.
The inhibition of human immunodeficiency virus type 1 (HIV) replication by type I interferons (IFN) was demonstrated soon after the discovery of the virus (4, 16, 20, 23, 49, 71). Different steps of the virus cycle are sensitive to IFN (reviewed in references 24, 25, and 47). IFN treatment of primary T lymphocytes, macrophages, and some T-cell lines efficiently inhibits early phases of infection, including HIV-induced cell fusion and reverse transcription (15, 16, 26, 27, 46, 60, 61, 70). IFN also impair later steps of the HIV replication cycle, ranging from reduced virus protein processing and stability to altered virion release and composition (2, 8, 17, 19, 20, 23, 26, 28, 39, 49, 63, 71, 72). In these early reports, the experimental systems were optimized to measure the effect of IFN on either early or late steps of the virus replication cycle but did not fully explore the long-term efficacy of IFN.
Some potential IFN-induced anti-HIV effectors were identified. Both the protein kinase R and the 2′,5′-oligoadenylate synthetase-directed RNase L pathways are activated by HIV components and may participate in the inhibition of HIV replication (32, 37, 59). IFN-α treatment also enhances the expression of TRIM5α and APOBEC3G (6, 53), two cellular factors that mediate potent intrinsic immunity against HIV and other viruses (reviewed in references 33 and 66). Moreover, recent studies have characterized IFN-induced antiviral factors that decrease virus particle release. In particular, IFN-stimulated gene 15 (ISG15) interferes with the recruitment of the cellular machinery required for viral assembly (43), TRIM22 appears to perturb virus precursor protein trafficking (3), and BST-2/CD317/HM1.24 (also called tetherin) acts by tethering mature virus particles to the cell surface (41, 67).
HIV counteracts some of these antiviral systems, confirming that endogenous IFN exert a selective pressure on virus replication in vivo. For instance, the viral protein Tat masks the oligoadenylate synthetase-sensitive TAR sequence (32) and binds to protein kinase R (37); an RNAse L inhibitor is induced by HIV replication (36); Vpu prevents virus tethering to the cell membrane by down-modulating BST-2 (41, 67); Vif degrades members of the APOBEC3 cytidine deaminase family (33); Vpr and Vif induce the degradation of IFN regulatory factor 3 (42). Nef can also be considered as acting indirectly against IFN by decreasing the surface levels of major histocompatibility complex class I molecules (57), which are upregulated by IFN (58).
The impact of endogenous and exogenous IFN on virus load in patients is difficult to evaluate. Obviously, endogenous IFN do not fully abrogate virus dissemination during the acute phase of infection. IFN appear even to contribute to the pathogenesis of infection, both in patients and in animal models (9, 22, 34). The effect of exogenously administered IFN on HIV load in plasma is often masked by concomitant use of anti-HIV molecules in most trials. Interestingly, two recent short-term trials reported a rapid and marked decrease of viremia (1 log/ml) that was attributed to IFN-α (1, 21). These studies confirmed earlier reports on the anti-HIV activity observed when very high doses of IFN were administered to patients with Kaposi's sarcoma (7, 13, 31), at least in the short term. In longer-term studies (associating IFN and a reverse transcriptase inhibitor), responsive patients showed a similar reduction in viremia in the first few weeks of treatment. This effect, however, was transient, and viremia increased afterwards with variable slopes (10, 12, 14, 44).
Therefore, the antiviral potency of IFN reported in vitro generally contrasts with their insufficient clinical efficacy. Various parameters may explain why cell culture systems do not recapitulate the in vivo situation. For instance, most experiments have been conducted with relatively low virus doses to optimize the readout of the effects of IFN, or virus infections were initiated with cell-free virions and not by infected cells, a much more efficient mode of virus spread. Moreover, analysis was generally limited to a few days (or even hours) after virus exposure.
Here, we analyzed further the effects of IFN on replication of various HIV strains in T-cell cultures. We first confirmed that pretreatment of primary T lymphocytes with IFN strongly inhibited virus particle release. Virus spread in culture, measured by following Gag-expressing cells, was less potently inhibited by IFN. In particular, a gradual increase in the percentage of infected cells over time was observed in cultures exposed to high virus doses. Virus emergence was the consequence of suboptimal inhibition of HIV replication and was not due to the selection of resistant variants. Cell-to-cell transfer, an efficient mode of virus spread, was only partially sensitive to IFN. Our results indicate that IFN are less active in cell cultures than initially thought.
MATERIALS AND METHODS
Cells and viruses.
T-cell lines (MT4R5, Jurkat, HUT-78, and CEM) and primary CD4+ T lymphocytes were grown in RPMI medium with 10% fetal bovine serum and penicillin (100 IU/ml)-streptomycin (100 μg/ml). Buffy-coat peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated by Ficoll centrifugation. CD4+ T lymphocytes were isolated by negative selection using magnetic beads (Miltenyi Biotec). For activation, primary CD4+ T lymphocytes were treated with phytohemagglutinin (1 mg/ml) for 24 h at 37°C and cultured in interleukin-2-containing medium (100 IU/ml).
Virus production based on transfection of pNL4-3-based proviral constructs in 293-T cells and amplification in MT4 cells has been described previously (35). The Vpu-defective (ΔVpu) pNL4-3 provirus was kindly provided by Klaus Strebel (56). The primary HIV isolates KAS and BON were obtained by spinoculation of donor PBMCs with plasma from HIV-infected patients. For each HIV-infected plasma sample, 5 ×106 cells were mixed with 0.5 ml of plasma in complete RPMI medium supplemented with 1% (vol/vol) dimethyl sulfoxide and 2 μg/ml DEAE-dextran in a final volume of 1.2 ml. The plates were centrifuged (at 860 × g for 2 h at 22°C), and cells were recovered and washed once. Cultures were maintained in complete RPMI medium with interleukin-2. Supernatants from infected cultures were collected before peak virus production and frozen at −80°C. BX08 and 132W primary strains were directly isolated from PBMCs of HIV-infected patients and were kindly provided by Gianfranco Pancino, Pasteur Institute.
IFN treatment and HIV infection.
Cells were treated with 0, 10, 100, 1,000 or 10,000 IU/ml of recombinant human IFN-α of different subtypes (1b, 2a, and 2b) or IFN-β1a (ImmunoTools) or with pegylated IFN-α2a (Roche). At 24 h after IFN treatment cells were exposed to the viruses indicated in the figure legends.
Infection of CD4+ T lymphocytes was performed by incubating 1, 10, or 100 ng of p24/0.5 ml/106 cells at 37°C for 2 to 4 h in the presence of DEAE-dextran (4 μg/ml) and HEPES (10 mM). CD4+ T lymphocytes were then washed and seeded in 96-well plates at a concentration of 2 × 106/ml and maintained with the indicated doses of IFN. T-cell lines (Jurkat, HUT-78, CEM, and MT4R5) were infected with 0.1 or 1 ng of p24/ml/106 cells with DEAE (2 μg/ml) and HEPES (10 mM) for 2 h at 37°C. Cells were then washed and seeded in 96-well plates at a concentration of 0.8 × 106/ml. IFN was maintained in cultures. For comparison of wild-type (WT) and ΔVpu viruses, IFN-treated lymphocytes were infected with 10, 50, and 100 ng of p24/0.5 ml/106 cells.
For postinfection treatment, IFN was added to cultures when approximately 1% of cells expressed HIV Gag proteins (48 h after virus exposure). When stated, zidovudine (AZT; 5 μM) and nevirapine (NVP; 6.25 μM) were added 3 h before infection (pretreatment) or at the same time as IFN for postinfection treatment. IFN or AZT/NVP was then maintained throughout the course of the assay. Viral p24 production in supernatants was measured by an enzyme-linked immunosorbent assay (ELISA) (Perkin-Elmer Life Science).
Flow cytometry analysis.
HIV Gag protein expression in infected cells was measured after permeabilization and intracellular staining with anti-Gag p24-phycoerythrin monoclonal antibody (KC57; Coulter). An isotype-matched monoclonal antibody was used as a negative control. To test the effect of IFN on cell proliferation, 104 beads (Beckman Coulter, Villepinte, France) were added to IFN-treated and untreated cultures. The number of cells for a given number of beads was counted by flow cytometry. The percentage of nonviable cells in culture treated by increasing concentrations of IFN and/or exposed to HIV was assessed by staining with the nucleic acid dye 7-amino-actinomycin D (7-AAD) (BD Biosciences) according to the manufacturer's instructions. Flow-cytometry data were acquired using a FACSCalibur instrument (Becton Dickinson) with CellQuest software and analyzed using Flowjo software (Treestar).
Analysis of cell-to-cell HIV transfer.
Primary T lymphocytes were infected with NL4-3 and used a few days later as donor cells when between 15 and 30% of cells were Gag-positive (Gag+). Target cells (primary T lymphocytes) were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) (2.5 μM; Molecular Probes) for 10 min at 37°C. Donor and target cells were then mixed at the indicated ratios in 96-well plates at a final concentration of 1 × 106 cells/ml in a final volume of 200 μl. At the time points indicated on the figures, cells were stained for intracellular Gag expression as described above and analyzed by flow cytometry.
Data analysis.
Results of experiments are expressed as means ± standard deviations (SDs). Comparisons between groups were performed using a Kruskal-Wallis test. Posttest comparisons, performed only for P values <0.05, were made using Dunn's multiple comparison test.
RESULTS
Inhibition of HIV replication by type I IFN in primary T lymphocytes.
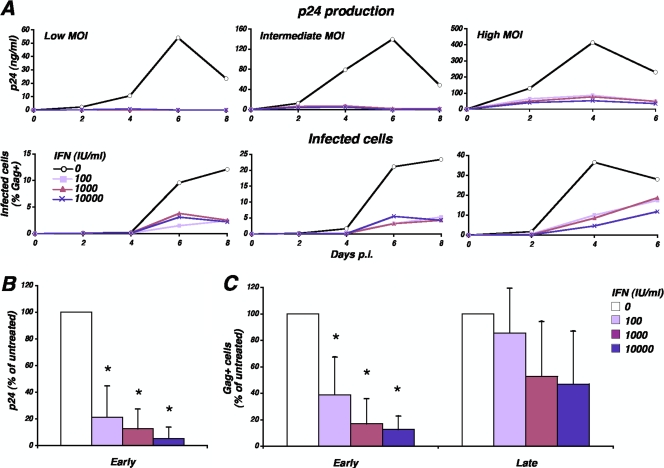
IFN is known to inhibit different steps of the HIV replication cycle and, in particular, to reduce the release of HIV particles from infected cells. We examined the effect of IFN on HIV replication in primary T lymphocytes. Cells were pretreated with increasing concentrations of IFN-α1b (100, 1,000 and 10,000 IU/ml) for 24 h and then exposed to different multiplicities of infection (MOIs) of HIV (low, intermediate, and high MOIs, corresponding to 1, 10, and 100 ng of p24/0.5 ml/106 cells, respectively). We then measured both the amount of p24 antigen released in the supernatant (Fig. 1A upper panels) and the appearance of Gag+ cells in the culture (Fig. 1A, lower panels) at different days postinfection. As expected, in the absence of IFN, the accumulation of p24 in the supernatant over time depended on virus input. Treatment by IFN-α abrogated p24 production in the supernatant of cultures exposed to low and intermediate MOIs and markedly decreased p24 levels in cultures infected at high MOIs. In four independent experiments using different MOIs, we observed a significant (P < 0.01) and IFN dose-dependent inhibition of p24 production at the day of peak virus replication, which could exceed 90% inhibition for the highest IFN concentration (Fig. 1B). These findings are in agreement with previous reports on the strong effect of IFN on HIV replication, as assessed by the number of virus particles in supernatants (40, 41, 67).
FIG. 1.
Inhibition of HIV replication in primary T lymphocytes by IFN-α. CD4+ T lymphocytes were cultured in the presence of increasing concentrations of IFN-α (0 to 10,000 IU/ml) for 24 h and then exposed to HIV (1, 10, or 100 ng of p24/0.5 ml/106 cells). IFN was maintained in the cultures. The accumulation of Gag p24 in the culture supernatant over time was measured by ELISA, and the percentage of Gag+ cells in the culture was determined by flow cytometry (A). Data are representative of four independent experiments. (B) Means and SDs of Gag p24 levels in the supernatants, measured in four independent experiments. The value 100% corresponds to the amount of Gag p24 obtained in the absence of IFN the day of peak virus replication (day 4 or day 6 postinfection). (C) Means and SD of the percentage of Gag+ cells at early time points (peak virus replication in the absence of IFN) and late time points (2 days postpeak), measured in four independent experiments using in each experiment 1, 10, and 100 ng of p24/0.5 ml/106 cells (corresponding to low, intermediate, and high MOIs, respectively). The asterisks indicate significant pairwise differences compared to infections of untreated cells (P < 0.01). p.i., postinfection.
We also measured the percentage of cells expressing Gag p24 antigen (Gag+ cells) over time by flow cytometry (Fig. 1A, lower panels). In untreated cultures, the percentage of Gag+ cells augmented over time with kinetics reflecting virus inputs. In IFN-treated cultures, we observed a delay in the appearance of Gag+ cells, after which the extent of virus spread largely depended on the MOI. At a low MOI, HIV spread was affected by IFN for several days. At a high MOI, the percentage of Gag+ cells increased substantially over time. In four independent experiments using different MOIs, we show that IFN had a stronger effect at early time points (at the peak of virus replication) while 48 h later virus replication was less efficiently controlled (Fig. 1C). Similar inhibition patterns were observed with different IFN-α subtypes (1b, 2a, and 2b), with pegylated IFN-α2a (not shown), and with IFN-β (data available at http://www.pasteur.fr/ip/easysite/go/03b-00003g-063/virus-and-immunity/supplemental-material). Overall, the potent effect of IFN to reduce HIV production in supernatants was paralleled by a saturable capacity to control virus spread in culture.
We next analyzed whether an effect of IFN on cell growth or survival could account for our observations on HIV replication. IFN may affect cell proliferation in culture, and it was reported that IFN exposure may lead to the selective death of HIV-infected cells (22). In uninfected cultures, untreated and IFN-treated cells were counted by flow cytometry, using reference beads. We did not observe an effect of IFN on the number of cells over time (data not shown). The effect of IFN and of HIV infection on the survival of primary T lymphocytes was measured by staining nonviable cells with the nucleic acid dye 7-AAD, followed by flow cytometry analysis (Fig. 2A). In noninfected cell cultures, only a small percentage of cells (1 to 3%) were 7-AAD positive over the course of the analysis (Fig. 2A, upper panel). Treatment by IFN (10 to 10,000 IU/ml) did not change the proportion of nonviable cells. By contrast, the proportion of 7-AAD-positive cells increased over time in HIV-infected cultures (lower panel), following the curve of virus replication (Fig. 2B) with a 2- to 3-day delay and thus reflecting the cytopathic effect induced by the virus. Treatment of cells by IFN before exposure to the virus reduced the extent of virus replication (Fig. 2B) and, consequently, the proportion of 7-AAD-positive cells (Fig. 2A). As reported above, the extent of inhibition of HIV replication was strong at early time points (until day 7) and then decreased (Fig. 2B). Pretreatment of cells by AZT/NVP efficiently prevented virus infection (Fig. 2B) and HIV-induced cell death (Fig. 2A, lower panel). Overall, IFN did not affect the proportion of nonviable cells in uninfected cultures while it reduced the extent of the HIV-induced cell death in a dose-dependent manner.
FIG. 2.
Effects of IFN and of HIV infection on primary T lymphocytes survival. (A) The percentage of nonviable cells (primary CD4+ T lymphocytes) was followed over time in cultures treated with the indicated concentrations of IFN-α, by staining with the nucleic acid dye 7-AAD. The lower panel shows cell cultures exposed to HIV (low MOI of 1 ng of p24/0.5 ml/106 cells). (B) The percentage of productively infected cells in HIV-exposed cultures was followed over time by intracellular Gag staining. Overall, IFN did not affect the proportion of nonviable cells in uninfected cultures while it reduced the extent of the HIV-induced cell death in a dose-dependent manner. p.i., postinfection.
Inhibition of Vpu-defective HIV.
The viral protein Vpu promotes virion release from infected cells (56, 65) by counteracting host restriction factors (40, 68, 69). At least one of these cellular inhibitors (tetherin) is induced by IFN (41, 67). It may thus be expected that Vpu facilitates virus spread in the presence of IFN. On the other hand, a virus lacking a functional Vpu protein was selected in an assay aimed at identifying fast-growing strains (18). A Vpu-defective virus may thus efficiently replicate without important virus release, most likely by cell-to-cell transfer. Such virus could be less susceptible to IFN inhibition. To discriminate between these two possibilities, we studied the replication of a Vpu-defective HIV strain (ΔVpu) (56) in the absence and in the presence of IFN. We first confirmed that the ΔVpu provirus produced normal levels of p24 upon transfection of tetherin-negative 293-T cells and low levels in tetherin-positive HeLa cells (approximately 80% decrease [data not shown]). As expected, infection of primary T lymphocytes by ΔVpu in the absence of IFN resulted in reproducibly lower production of p24 than infection with the WT virus (Fig. 3A). IFN-α potently decreased p24 production in cultures exposed to the Vpu-defective virus (Fig. 3B and C). We also measured the appearance of Gag+ cells in culture infected by the two viruses. In the absence of IFN, similar kinetics of appearance of Gag+ cells were observed for WT and ΔVpu virus (Fig. 3D), confirming that a ΔVpu virus may efficiently spread in culture despite reduced virus release (18). In IFN-α-treated lymphocytes, a strong inhibition of virus spread was observed at early time points for both WT and ΔVpu virus (Fig. 3E). At later time points, ΔVpu appeared slightly more affected by IFN than WT HIV. Data from three independent experiments confirmed this trend (Fig. 3F), but the difference between WT and ΔVpu did not appear significant. In conclusion, Vpu seems to confer only a small advantage to the virus in the presence of IFN in this multiple-cycle system of virus replication. This may result from an equilibrium between the different effects of Vpu, which, on the one hand, counteracts IFN-induced factors but, on the other hand, may limit virus cell-to-cell spread.
FIG. 3.
Inhibition of a Vpu-defective HIV strain by IFN-α. CD4+ T lymphocytes were cultured in the absence or in the presence of IFN-α (1,000 IU/ml) for 24 h and then exposed to WT or ΔVpu HIV. The accumulation of Gag p24 in the culture supernatants over time was measured by ELISA in untreated cells (A) and in IFN-treated cultures (B). The percentage of Gag+ cells in untreated lymphocyte cultures (D) or in IFN-treated cells (E) was determined by flow cytometry. The depicted experiment corresponds to infection using 50 ng of p24, whereas means and SDs for p24 (C) and Gag+ cells (F) were calculated in three independent experiments using 10, 50, and 100 ng of p24. The value 100% corresponds to cultures infected by WT virus. The asterisks indicate significant pairwise differences compared to infections of untreated cells by the WT virus (for panel C, P < 0.01; for panel F, P < 0.05). p.i., postinfection.
Inhibition of primary isolates by IFN.
Primary HIV isolates differ in their sensitivity to IFN (10, 29). We examined the behavior of four primary isolates in our experimental system. Primary CD4+ T lymphocytes, cultured in the presence or absence of IFN-α (1,000 IU/ml), were exposed to these isolates. We chose a relatively low MOI that induced a peak of p24 production at day 7 or 10 postinfection. The amounts of p24 in the culture supernatants and appearance of Gag+ cells were followed over time (Fig. 4). For all virus strains, IFN markedly decreased or delayed virus production in supernatants. By day 10 postinfection, however, detectable p24 levels were measured in all supernatants, showing incomplete suppression of virus infection by IFN. As with the reference strain, the use of lower MOIs resulted in a more potent inhibition of virus particle production (data not shown).
FIG. 4.
Inhibition of primary HIV isolates by IFN-α. Replication of four primary HIV isolates (BX08, 132W, BON, and KAS) in CD4+ T lymphocytes was measured in the absence and presence of 1,000 IU/ml of IFN-α. The accumulation of Gag p24 in the culture supernatant (left panels) and the percentage of Gag+ cells in the cultures (right panels) were followed over time. Data shown were obtained using the following amounts of p24/0.5 ml/106 cells for infection: BX08, 0.5 ng; 132W, 5 ng; BON, 5 ng; KAS, 10 ng. Data are representative of three independent experiments. p.i., postinfection.
The appearance of Gag+ cells in culture was delayed by IFN treatment (Fig. 4) in an MOI-dependent fashion (data not shown), confirming the only partial inhibition of virus spread for these isolates. Overall, for primary isolates as well as for the reference virus NL4-3, IFN-α displayed a stronger effect on virus production than on virus spread in culture.
Inhibition of HIV replication in different T-cell lines.
Type I IFN inhibit HIV replication in some T-cell lines (2, 28, 40, 60, 67, 71). To identify a convenient model in which we could follow the effect of IFN on HIV replication, we infected four cell lines commonly used in HIV studies, Jurkat, CEM, HUT-78, and MT4R5, in the presence of IFN-α or -β. We used a low MOI to avoid masking inhibition by IFN. Gag p24 production in the supernatants appeared not to be sensitive to IFN in Jurkat, CEM, and HUT-78 cell lines (data available at http://www.pasteur.fr/ip/easysite/go/03b-00003g-063/virus-and-immunity/supplemental-material) even at very high IFN concentrations (10,000 IU/ml). By contrast, in MT4R5 cells, IFN-α and -β induced a strong reduction in the p24 level for several days. This was followed by a rapid increase in the p24 level at the IFN dose of 1,000 IU/ml while no evidence of virus replication was detected at 10,000 IU/ml IFN (data available at http://www.pasteur.fr/ip/easysite/go/03b-00003g-063/virus-and-immunity/supplemental-material).
In agreement with data on p24 production, IFN did not delay the appearance of Gag+ cells in Jurkat, CEM, and HUT-78 infected cultures. In MT4R5 cells, a strong but incomplete inhibition of the appearance of Gag+ cells in the presence of 1,000 IU/ml IFN-α or -β was instead observed. Higher IFN concentrations (10,000 IU/ml) fully abrogated virus spread in MT4R5 cultures. In conclusion, HIV replication was sensitive to IFN in MT4R5 cells, which represent a valuable model, and insensitive in Jurkat, CEM, and HUT-78 cell lines. This difference deserves further exploration.
Virus emergence in IFN-treated cultures is not due to selection of resistant HIV.
The detection of productive infection in IFN-treated primary lymphocytes was relatively rapid and depended on the virus input. This suggested that virus emergence was the result of breakthrough due to incomplete IFN suppression rather than to the selection of less susceptible variants in culture. To test this hypothesis, we compared the susceptibility to IFN-α of virus preparations from IFN-treated lymphocyte cultures (1,000 IU/ml) to those of viruses from untreated cultures. The IFN-naive and IFN-treated viruses were comparably affected in the presence of 100 and 1,000 IU/ml IFN-α (Fig. 5A). Similar IFN sensitivities were also measured for IFN-naive and IFN-treated viruses issued from, and tested in, MT4R5 cells (Fig. 5B). Therefore, virus emergence in IFN-treated cells is likely due to an incomplete antiviral effect of the cytokine.
FIG. 5.
HIV emerging in treated cultures remains susceptible to IFN. Comparison of the susceptibility to IFN of viruses issued from IFN-treated or untreated cultures. Primary T lymphocytes or MT4R5 cells were cultured in the presence of 1,000 IU/ml IFN-α and infected by HIV (not shown). Viruses produced in the supernatants were used to infect primary T lymphocytes (A) or MT4R5 cells (B) that had been pretreated with increasing concentrations if IFN-α. By comparison, IFN-naive viruses, collected from T lymphocytes and MT4R5-infected cultures, were used in parallel to infect untreated or IFN-treated cells (right panels). In each cell type, IFN-naive and IFN-treated viruses displayed comparable levels of inhibition by IFN. p.i., postinfection.
Effects of IFN on the establishment and spread of virus infection.
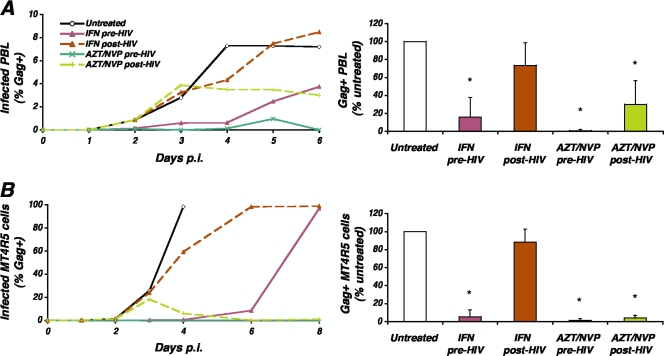
We next analyzed the impact of the time of IFN-α addition on the inhibition of HIV replication. Primary CD4+ T lymphocytes were either treated with IFN-α (1,000 IU/ml) 24 h before being exposed to HIV (pretreatment, as above) or 48 h after infection, when approximately 1% of cells expressed HIV Gag proteins (postinfection treatment). As in previous experiments, pretreatment with IFN-α resulted in the control of virus spread for several days, after which emergence of Gag+ cells was observed (Fig. 6A). In contrast, postinfection treatment only delayed by 1 day the peak of infection. By comparison, postinfection treatment with AZT and NVP stopped virus spread within 24 h of addition of the inhibitors, demonstrating that virus spread can be arrested, provided that strong antivirals are used. As a control, pretreatment of cells with AZT/NVP largely prevented productive infection (appearance of Gag+ cells).
FIG. 6.
Effect of the time of addition of IFN on the inhibition of virus spread. Primary CD4+ T lymphocytes (A) or MT4R5 cells (B) were treated with IFN-α (1,000 IU/ml) 24 h before exposure to HIV (pretreatment) or 48 h postinfection (postinfection treatment). Virus spread was followed over time by determining the percentage of Gag+ cells. Postinfection treatment induced only a marginal delay in virus spread. Pretreatment was performed also using a potent combination of reverse transcriptase inhibitors (AZT/NVP; green line), which successfully inhibited the appearance of Gag+ cells, while postinfection treatment with reverse transcriptase inhibitors prevented further virus spread. Data are representative of four independent experiments for which means and SDs at the time of peak virus replication are shown. Asterisks indicate significant pairwise differences compared to virus replication in untreated cells (P < 0.05). p.i., postinfection; PBL, peripheral blood lymphocytes.
In four independent experiments, inhibition of virus spread by pretreatment with IFN, but not by postinfection treatment, was statistically significant (P < 0.01) (Fig. 6A). Similar results were obtained with MT4R5 cells (Fig. 6B). Altogether, these data confirm that pretreatment with IFN-α potently inhibits HIV replication while treatment of cells shortly after the establishment of infection is largely ineffective at limiting virus spread.
Inhibition of cell-to-cell transmission of HIV by IFN.
Cell-to-cell transfer is a more potent and rapid means of virus propagation than infection by cell-free virus (54), and in culture it represents the main HIV transfer process (64). The partial control of virus spread by IFN may result from its low efficacy in preventing cell-to-cell transmission. We thus examined the effect of IFN on cell-to-cell HIV transfer. To this end, we used a flow cytometry-based cell-to-cell virus transfer assay developed in our laboratory (64). Infected primary CD4+ T lymphocytes (15 to 30% Gag+) were cocultured with autologous IFN-treated or untreated target cells. Target cells were labeled with CFSE to distinguish them from donors. The percentage of Gag+ cells among CFSE-labeled lymphocytes was followed at multiple time points over 2 days to measure early productive virus transfer events. We have previously shown that within this time frame, free virions are negligibly involved in the emergence of Gag+ target cells (64). Also, as previously reported (64), the low fraction of Gag signal in target cells is not due to productive infection but likely represents capture of incoming viral material. This signal is indeed detected as soon as 2 to 4 h after coculture (64) and is not abrogated by AZT/NVP treatment (Fig. 7A and B). Since the efficiency of cell-to-cell transfer depends on the ratio of donor to target cells (64), we used both low donor-to-target cell ratios (1:4 to 1:9) and high ratios (1:1 or 1:2).
FIG. 7.
Inhibition of cell-to-cell HIV transfer by IFN. Cell-to-cell virus transfer among primary T lymphocytes was measured by a flow cytometry-based assay. Cocultures were established between HIV-infected donor cells and target cells. In the experiment shown, approximately 25% of the donor cell population was productively infected. We used a low donor-to-target (D:T) cell ratio (C; 1 donor to 8 targets) and a high ratio (C; 1 donor to 2 targets). Before the coculture, target cells were treated with different concentrations of IFN-α. The increase in the percentage of Gag+ cells among target T lymphocytes was followed over time. The mean effect and SD measured at 48 h in five independent experiments using low (B; 1:4 to 1:9) or high (O; 1:1 to 1:2) ratios of donor to target cells are reported. Asterisks indicate significant pairwise differences compared to coculture with untreated cells (P < 0.05).
In untreated cultures, the proportion of Gag+ target cells increased between 16 and 48 h (Fig. 7). As expected, higher donor-to-target cell ratios resulted in more rapid kinetics of appearance of Gag+ target cells (Fig. 7A and C). Treatment of target cells with IFN affected the efficacy of HIV transfer. In particular, in experiments with low donor-to-target ratios (Fig. 7A and B), a potent and dose-dependent inhibition of HIV transfer was observed, which reached statistical significance at the IFN concentration of 1,000 IU/ml. Even for high IFN concentrations, however, inhibition of virus transfer did not exceed 60%. Using higher ratios of donor to target cells (Fig. 7C and D), virus transfer was clearly less susceptible to IFN. A slightly reduced HIV transfer was observed only with high IFN concentrations (10,000 IU/ml). Of note, a similar efficacy of inhibition was obtained by treating donor or target cells with IFN (data not shown). Altogether, these results show that IFN inhibits HIV cell-to-cell transfer in a dose-dependent manner although even at high IFN concentrations inhibition remained incomplete. The potency of inhibition vanishes with the increase in the proportion of infected cells in culture.
DISCUSSION
Like other virus species, HIV encounters the antiviral effects of type I IFN in infected individuals and has evolved multiple strategies to overcome these constraints. Some viral proteins, including Vpu and Vif, directly counteract IFN-induced restriction factors. Other mechanisms are likely regulating the battle between the virus and IFN. Here, we studied the impact of IFN on various aspects of HIV replication in cell culture systems using both reference strains and primary virus isolates. We report that IFN potently decrease HIV release in the extracellular milieu while their impact on the appearance of newly infected cells in culture was more limited. We show that parameters like the infectious dose or the time of addition of IFN strongly influence virus susceptibility to the cytokines. Moreover, we demonstrate that cell-to-cell HIV transfer is less sensitive to IFN than infection by cell-free virus particles.
Limited effect of IFN on virus spread despite consistent reduction of virus particle production.
IFN markedly reduce the amount of Gag p24 in the supernatants of infected lymphocytes (40, 67). This reflects the cumulative effect of IFN on early and late steps of virus replication (15, 16, 26, 27, 46, 60, 61, 70). In particular, IFN directly prevent virus release by inducing the accumulation of virions at the cell surface (3, 41, 42). We show here that this potent and prolonged inhibition of virus production in supernatants was accompanied by a less sustained reduction in the propagation of Gag+ cells in culture. This discrepancy was particularly marked in cells infected at high MOIs, where IFN delayed by only a few days the appearance of productively infected cells. An immediate consequence of this observation is that follow-up of virus replication based only on quantification of viral material in culture supernatants may lead to an overestimation of the potency of IFN.
In the absence of IFN, as expected, ΔVpu release was restricted in T lymphocytes, which likely express low levels of tetherin. However, ΔVpu promoted normal levels of Gag+ cells in T-cell cultures. This suggests that ΔVpu may replicate without important virus release and, hence, through direct cell-to-cell transfer. It is interesting that Gummuluru et al. selected a virus lacking a functional Vpu protein in an assay aimed at identifying fast-growing strains (18). Here, the impact of IFN on virus spread was slightly stronger for the ΔVpu virus than for the WT, but the defective virus finally managed to spread in the presence of the cytokine. This suggests that restriction factors counteracted by Vpu, like tetherin and calcium-modulating cyclophilin ligand (41, 68), likely impair viral cell-to-cell transfer but do not totally block this mode of virus replication. Additionally, IFN treatment in cell cultures may only induce suboptimal or saturable levels of these antiviral proteins. Finally, the advantage conferred by Vpu may be partially compensated in the ΔVpu virus by its higher cell-to-cell transmission efficacy, at least under certain culture conditions (18). This will deserve further investigations.
Interestingly, we show that viruses emerging in IFN-treated cultures remained sensitive to IFN in secondary assays. This indicates that IFN treatment did not result in the selection of less susceptible or even IFN-resistant strains, which was unlikely to occur within the few days of culture. Rather, this rapid emergence supports the hypothesis that IFN act suboptimally on HIV replication and cell-to-cell transfer.
Partial control of HIV cell-to-cell transfer by IFN.
We also compared HIV replication in cells treated by IFN before or after infection. Previous studies showed that IFN remain active if added a few hours after virus exposure but not at later times (2, 38, 60). We added IFN when a low percentage of cells were Gag+ (1 to 3%). Under these conditions, high doses of IFN only slightly delayed (1 to 2 days) the progression of infection. This result, together with the incomplete inhibition of virus spread when cells were infected at high MOIs, suggested that IFN restrict infection by cell-free virus but are less efficient in the presence of infected cells, when HIV can spread by direct cell-to-cell transfer.
This hypothesis was explored using an assay designed to quantify the efficiency of cell-to-cell virus transfer (64). In this assay, we follow the appearance of Gag+ cells among targets after coculture with productively infected cells. We show here that high concentrations of IFN are required to inhibit the direct transfer of HIV from cell to cell and that inhibition is largely incomplete. It will be worth exploring directly the impact of various ISGs on the efficiency of cell-to-cell virus transfer. We also report that the efficiency of IFN inhibition was inversely correlated with the proportion of donor cells in the cocultures. This finding helps explain why the incomplete abrogation of the initial infection by IFN ineluctably leads to the loss of control of virus spread over time.
Impact of the infectious dose on susceptibility to IFN.
An effect of the virus dose on sensitivity to IFN has been previously described (8, 20, 60). By comparison, inhibition of HIV by neutralizing antibodies or antiviral drugs targeting viral enzymes is less dependent on the MOI (45, 51). This observation suggests that some of the anti-HIV ISGs can be saturated. On the other hand, most drug and antibody susceptibility assays rely on single-cycle virus infection (51, 55). Classic single-cycle assays, however, may not apprehend the global antiviral effect of IFN, which acts on both early and late steps and whose overall inhibition may be synergistic. In addition, single-cycle analysis would mask the intrinsic long-term loss of control of virus replication due to ineffective inhibition of cell-to-cell virus transfer.
Low efficacy of IFN in HIV-infected patients.
Before the advent of highly active antiretroviral treatment, IFN were tested in HIV-infected patients. Several clinical trials have been conducted in which IFN was added to treatment regimens that would be considered suboptimal today. A modest clinical benefit was associated with the administration of IFN in some of these studies but not in others (11, 12, 14, 30, 52, 62). IFN are not currently used in HIV-infected patients, with the notable exception of patients with concomitant hepatitis virus infection, because of the inconclusive clinical benefit, the potential secondary effects, and the availability of effective treatment alternatives (5, 48). IFN treatment generally provokes a potent (1 log) but short-lived reduction of HIV viremia (1, 10, 12, 14, 21, 44). In view of our findings and of previous studies, the rapid decrease of viremia under IFN treatment may be in part attributed to the reduction of virus release and to the relatively strong inhibition of infection by cell-free virions. Our work suggests that cell-to-cell HIV transfer may participate in the subsequent loss of control of virus replication in patients. Alternative, but not mutually exclusive, explanations include the in vivo selection of less susceptible or resistant virus variants, a frequent event under other incompletely suppressive antiretroviral treatment (50), or the insensitivity or inaccessibility of some infected cells or tissues to IFN.
To summarize, our results show that pretreatment of cells by IFN efficiently reduces the rate of infection by cell-free virions. The efficiency of inhibition largely depends on the virus dose. The fraction of cells infected despite treatment by IFN produces markedly reduced amounts of virus particles. HIV spread in culture, however, proceeds essentially by cell-to-cell virus transfer. As the proportion of infected cells increases, the extent of IFN inhibition fades. Inhibition of this potent means of virus transfer requires very high concentrations of IFN and is not absolute. Cell-to-cell transfer likely represents an escape strategy from innate host defenses.
Acknowledgments
This work was supported by grants from the French national agency for AIDS and hepatitis research (ANRS), Sidaction, European Community (EC) (FP7 contract 201412), and Institut Pasteur. D.V. is a fellow of ANRS. M.S. was supported by the EC contract and by Fondation pour la recherche médicale. V.P. is supported by Sidaction.
We thank Florence Guivel for expert technical assistance; Alice Lepelley, Nicoletta Casartelli, and Nathalie Sol-Foulon for critically reading the manuscript; and Klaus Strebel and Gianfranco Pancino for providing reagents.
Footnotes
Published ahead of print on 12 August 2009.
REFERENCES
- 1.Aguilar Marucco, D., L. Veronese, D. G. de Requena, S. Bonora, A. Calcagno, I. Cavecchia, A. Sinicco, F. G. De Rosa, G. Cariti, and G. Di Perri. 2007. Antiretroviral activity of pegylated interferon alfa-2a in patients co-infected with HIV/hepatitis C virus. J. Antimicrob. Chemother. 59:565-568. [DOI] [PubMed] [Google Scholar]
- 2.Agy, M. B., R. L. Acker, C. H. Sherbert, and M. G. Katze. 1995. Interferon treatment inhibits virus replication in HIV-1- and SIV-infected CD4+ T-cell lines by distinct mechanisms: evidence for decreased stability and aberrant processing of HIV-1 proteins. Virology 214:379-386. [DOI] [PubMed] [Google Scholar]
- 3.Barr, S. D., J. R. Smiley, and F. D. Bushman. 2008. The interferon response inhibits HIV particle production by induction of TRIM22. PLoS Pathog. 4:e1000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bednarik, D. P., J. D. Mosca, N. B. Raj, and P. M. Pitha. 1989. Inhibition of human immunodeficiency virus (HIV) replication by HIV-trans-activated alpha 2-interferon. Proc. Natl. Acad. Sci. USA 86:4958-4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borden, E. C., G. C. Sen, G. Uze, R. H. Silverman, R. M. Ransohoff, G. R. Foster, and G. R. Stark. 2007. Interferons at age 50: past, current and future impact on biomedicine. Nat. Rev. Drug Discov. 6:975-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, K., J. Huang, C. Zhang, S. Huang, G. Nunnari, F. X. Wang, X. Tong, L. Gao, K. Nikisher, and H. Zhang. 2006. Alpha interferon potently enhances the anti-human immunodeficiency virus type 1 activity of APOBEC3G in resting primary CD4 T cells. J. Virol. 80:7645-7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Wit, R., J. K. Schattenkerk, C. A. Boucher, P. J. Bakker, K. H. Veenhof, and S. A. Danner. 1988. Clinical and virological effects of high-dose recombinant interferon-alpha in disseminated AIDS-related Kaposi's sarcoma. Lancet 2:1214-1217. [DOI] [PubMed] [Google Scholar]
- 8.Dianzani, F., C. Castilletti, M. Gentile, H. R. Gelderblom, F. Frezza, and M. R. Capobianchi. 1998. Effects of IFN alpha on late stages of HIV-1 replication cycle. Biochimie 80:745-754. [DOI] [PubMed] [Google Scholar]
- 9.Diop, O. M., M. J. Y. Ploquin, L. Mortara, A. Faye, B. Jacquelin, D. Kunkel, P. Lebon, C. Butor, A. Hosmalin, F. Barre-Sinoussi, and M. C. Muller-Trutwin. 2008. Plasmacytoid dendritic cell dynamics and alpha interferon production during Simian immunodeficiency virus infection with a nonpathogenic outcome. J. Virol. 82:5145-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edlin, B. R., R. A. Weinstein, S. M. Whaling, C. Y. Ou, P. J. Connolly, J. L. Moore, and J. D. Bitran. 1992. Zidovudine-interferon-alpha combination therapy in patients with advanced human immunodeficiency virus type 1 infection: biphasic response of p24 antigen and quantitative polymerase chain reaction. J. Infect. Dis. 165:793-798. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Cruz, E., J. M. Lang, J. Frissen, V. Furner, M. Chateauvert, C. A. Boucher, P. Dowd, J. Stevens, et al. 1995. Zidovudine plus interferon-alpha versus zidovudine alone in HIV-infected symptomatic or asymptomatic persons with CD4+ cell counts > 150 × 106/L: results of the Zidon trial. AIDS 9:1025-1035. [PubMed] [Google Scholar]
- 12.Fischl, M. A., D. D. Richman, M. Saag, T. C. Meng, K. E. Squires, J. Holden-Wiltse, and P. M. Meehan. 1997. Safety and antiviral activity of combination therapy with zidovudine, zalcitabine, and two doses of interferon-alpha2a in patients with HIV. AIDS Clinical Trials Group Study 197. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 16:247-253. [DOI] [PubMed] [Google Scholar]
- 13.Frissen, P. H., F. de Wolf, P. Reiss, P. J. Bakker, C. H. Veenhof, S. A. Danner, J. Goudsmit, and J. M. Lange. 1997. High-dose interferon-alpha2a exerts potent activity against human immunodeficiency virus type 1 not associated with antitumor activity in subjects with Kaposi's sarcoma. J. Infect. Dis. 176:811-814. [DOI] [PubMed] [Google Scholar]
- 14.Frissen, P. H., M. E. van der Ende, C. H. ten Napel, H. M. Weigel, G. S. Schreij, R. H. Kauffmann, P. P. Koopmans, A. I. Hoepelman, J. B. de Boer, G. J. Weverling, and et al. 1994. Zidovudine and interferon-alpha combination therapy versus zidovudine monotherapy in subjects with symptomatic human immunodeficiency virus type 1 infection. J. Infect. Dis. 169:1351-1355. [DOI] [PubMed] [Google Scholar]
- 15.Gendelman, H. E., L. Baca, J. A. Turpin, D. C. Kalter, B. D. Hansen, J. M. Orenstein, R. M. Friedman, and M. S. Meltzer. 1990. Restriction of HIV replication in infected T cells and monocytes by interferon-alpha. AIDS Res. Hum. Retrovir. 6:1045-1049. [DOI] [PubMed] [Google Scholar]
- 16.Gendelman, H. E., L. M. Baca, J. Turpin, D. C. Kalter, B. Hansen, J. M. Orenstein, C. W. Dieffenbach, R. M. Friedman, and M. S. Meltzer. 1990. Regulation of HIV replication in infected monocytes by IFN-alpha. Mechanisms for viral restriction. J. Immunol. 145:2669-2676. [PubMed] [Google Scholar]
- 17.Gottlinger, H. G., T. Dorfman, J. G. Sodroski, and W. A. Haseltine. 1991. Effect of mutations affecting the p6 Gag protein on human immunodeficiency virus particle release. Proc. Natl. Acad. Sci. USA 88:3195-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gummuluru, S., C. M. Kinsey, and M. Emerman. 2000. An in vitro rapid-turnover assay for human immunodeficiency virus type 1 replication selects for cell-to-cell spread of virus. J. Virol. 74:10882-10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen, B. D., P. L. Nara, R. K. Maheshwari, G. S. Sidhu, J. G. Bernbaum, D. Hoekzema, M. S. Meltzer, and H. E. Gendelman. 1992. Loss of infectivity by progeny virus from alpha interferon-treated human immunodeficiency virus type 1-infected T cells is associated with defective assembly of envelope gp120. J. Virol. 66:7543-7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartshorn, K. L., D. Neumeyer, M. W. Vogt, R. T. Schooley, and M. S. Hirsch. 1987. Activity of interferons alpha, beta, and gamma against human immunodeficiency virus replication in vitro. AIDS Res. Hum. Retrovir. 3:125-133. [DOI] [PubMed] [Google Scholar]
- 21.Hatzakis, A., P. Gargalianos, V. Kiosses, M. Lazanas, V. Sypsa, C. Anastassopoulou, V. Vigklis, H. Sambatakou, C. Botsi, D. Paraskevis, and C. Stalgis. 2001. Low-dose IFN-alpha monotherapy in treatment-naive individuals with HIV-1 infection: evidence of potent suppression of viral replication. J. Interferon Cytokine Res. 21:861-869. [DOI] [PubMed] [Google Scholar]
- 22.Herbeuval, J. P., and G. M. Shearer. 2007. HIV-1 immunopathogenesis: how good interferon turns bad. Clin. Immunol. 123:121-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho, D. D., K. L. Hartshorn, T. R. Rota, C. A. Andrews, J. C. Kaplan, R. T. Schooley, and M. S. Hirsch. 1985. Recombinant human interferon alfa-A suppresses HTLV-III replication in vitro. Lancet 1:602-604. [DOI] [PubMed] [Google Scholar]
- 24.Hosmalin, A., and P. Lebon. 2006. Type I interferon production in HIV-infected patients. J. Leukoc. Biol. 80:984-993. [DOI] [PubMed] [Google Scholar]
- 25.Karpov, A. V. 2001. Endogenous and exogenous interferons in HIV-infection. Eur. J. Med. Res. 6:507-524. [PubMed] [Google Scholar]
- 26.Kornbluth, R. S., P. S. Oh, J. R. Munis, P. H. Cleveland, and D. D. Richman. 1989. Interferons and bacterial lipopolysaccharide protect macrophages from productive infection by human immunodeficiency virus in vitro. J. Exp. Med. 169:1137-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kornbluth, R. S., P. S. Oh, J. R. Munis, P. H. Cleveland, and D. D. Richman. 1990. The role of interferons in the control of HIV replication in macrophages. Clin. Immunol. Immunopathol. 54:200-219. [DOI] [PubMed] [Google Scholar]
- 28.Korth, M. J., M. D. Taylor, and M. G. Katze. 1998. Interferon inhibits the replication of HIV-1, SIV, and SHIV chimeric viruses by distinct mechanisms. Virology 247:265-273. [DOI] [PubMed] [Google Scholar]
- 29.Kunzi, M. S., H. Farzadegan, J. B. Margolick, D. Vlahov, and P. M. Pitha. 1995. Identification of human immunodeficiency virus primary isolates resistant to interferon-alpha and correlation of prevalence to disease progression. J. Infect. Dis. 171:822-828. [DOI] [PubMed] [Google Scholar]
- 30.Lane, H. C., V. Davey, J. A. Kovacs, J. Feinberg, J. A. Metcalf, B. Herpin, R. Walker, L. Deyton, R. T. Davey, Jr., J. Falloon, et al. 1990. Interferon-alpha in patients with asymptomatic human immunodeficiency virus (HIV) infection. A randomized, placebo-controlled trial. Ann. Intern. Med. 112:805-811. [DOI] [PubMed] [Google Scholar]
- 31.Lane, H. C., J. A. Kovacs, J. Feinberg, B. Herpin, V. Davey, R. Walker, L. Deyton, J. A. Metcalf, M. Baseler, and N. Salzman. 1988. Anti-retroviral effects of interferon-alpha in AIDS-associated Kaposi's sarcoma. Lancet 2:1218-1222. [DOI] [PubMed] [Google Scholar]
- 32.Maitra, R. K., N. A. McMillan, S. Desai, J. McSwiggen, A. G. Hovanessian, G. Sen, B. R. Williams, and R. H. Silverman. 1994. HIV-1 TAR RNA has an intrinsic ability to activate interferon-inducible enzymes. Virology 204:823-827. [DOI] [PubMed] [Google Scholar]
- 33.Malim, M. H., and M. Emerman. 2008. HIV-1 accessory proteins—ensuring viral survival in a hostile environment. Cell Host Microbe 3:388-398. [DOI] [PubMed] [Google Scholar]
- 34.Mandl, J. N., A. P. Barry, T. H. Vanderford, N. Kozyr, R. Chavan, S. Klucking, F. J. Barrat, R. L. Coffman, S. I. Staprans, and M. B. Feinberg. 2008. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat. Med. 14:1077-1087. [DOI] [PubMed] [Google Scholar]
- 35.Marechal, V., F. Clavel, J. M. Heard, and O. Schwartz. 1998. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J. Virol. 72:2208-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinand, C., C. Montavon, T. Salehzada, M. Silhol, B. Lebleu, and C. Bisbal. 1999. RNase L inhibitor is induced during human immunodeficiency virus type 1 infection and down regulates the 2-5A/RNase L pathway in human T cells. J. Virol. 73:290-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMillan, N. A., R. F. Chun, D. P. Siderovski, J. Galabru, W. M. Toone, C. E. Samuel, T. W. Mak, A. G. Hovanessian, K. T. Jeang, and B. R. Williams. 1995. HIV-1 Tat directly interacts with the interferon-induced, double-stranded RNA-dependent kinase, PKR. Virology 213:413-424. [DOI] [PubMed] [Google Scholar]
- 38.Meylan, P. R., J. C. Guatelli, J. R. Munis, D. D. Richman, and R. S. Kornbluth. 1993. Mechanisms for the inhibition of HIV replication by interferons-alpha, -beta, and -gamma in primary human macrophages. Virology 193:138-148. [DOI] [PubMed] [Google Scholar]
- 39.Michaelis, B., and J. A. Levy. 1989. HIV replication can be blocked by recombinant human interferon beta. AIDS 3:27-31. [PubMed] [Google Scholar]
- 40.Neil, S. J., V. Sandrin, W. I. Sundquist, and P. D. Bieniasz. 2007. An interferon-alpha-induced tethering mechanism inhibits HIV-1 and Ebola virus particle release but is counteracted by the HIV-1 Vpu protein. Cell Host Microbe 2:193-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neil, S. J., T. Zang, and P. D. Bieniasz. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425-430. [DOI] [PubMed] [Google Scholar]
- 42.Okumura, A., T. Alce, B. Lubyova, H. Ezelle, K. Strebel, and P. M. Pitha. 2008. HIV-1 accessory proteins VPR and Vif modulate antiviral response by targeting IRF-3 for degradation. Virology 373:85-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okumura, A., G. Lu, I. Pitha-Rowe, and P. M. Pitha. 2006. Innate antiviral response targets HIV-1 release by the induction of ubiquitin-like protein ISG15. Proc. Natl. Acad. Sci. USA 103:1440-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orholm, M., C. Pedersen, L. Mathiesen, P. Dowd, and J. O. Nielsen. 1989. Suppression of p24 antigen in sera from HIV-infected individuals with low-dose alpha-interferon and zidovudine: a pilot study. AIDS 3:97-100. [DOI] [PubMed] [Google Scholar]
- 45.Petropoulos, C. J., N. T. Parkin, K. L. Limoli, Y. S. Lie, T. Wrin, W. Huang, H. Tian, D. Smith, G. A. Winslow, D. J. Capon, and J. M. Whitcomb. 2000. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 44:920-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinto, L. A., V. Blazevic, B. K. Patterson, C. Mac Trubey, M. J. Dolan, and G. M. Shearer. 2000. Inhibition of human immunodeficiency virus type 1 replication prior to reverse transcription by influenza virus stimulation. J. Virol. 74:4505-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pitha, P. M. 1994. Multiple effects of interferon on the replication of human immunodeficiency virus type 1. Antivir. Res. 24:205-219. [DOI] [PubMed] [Google Scholar]
- 48.Pol, S., and V. Soriano. 2008. Management of chronic hepatitis C virus infection in HIV-infected patients. Clin. Infect. Dis. 47:94-101. [DOI] [PubMed] [Google Scholar]
- 49.Poli, G., J. M. Orenstein, A. Kinter, T. M. Folks, and A. S. Fauci. 1989. Interferon-alpha but not AZT suppresses HIV expression in chronically infected cell lines. Science 244:575-577. [DOI] [PubMed] [Google Scholar]
- 50.Richman, D. D. 2006. Antiviral drug resistance. Antivir. Res. 71:117-121. [DOI] [PubMed] [Google Scholar]
- 51.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 100:4144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rivero, J., M. Limonta, A. Aguilera, M. Fraga, and P. Lopez Saura. 1994. Use of recombinant interferon-alpha in human immunodeficiency virus (HIV)-infected individuals. Biotherapy 8:23-31. [DOI] [PubMed] [Google Scholar]
- 53.Sakuma, R., A. A. Mael, and Y. Ikeda. 2007. Alpha interferon enhances TRIM5α-mediated antiviral activities in human and rhesus monkey cells. J. Virol. 81:10201-10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sattentau, Q. 2008. Avoiding the void: cell-to-cell spread of human viruses. Nat. Rev. Microbiol. 6:815-826. [DOI] [PubMed] [Google Scholar]
- 55.Schmidt, B., H. Walter, N. Zeitler, and K. Korn. 2002. Genotypic drug resistance interpretation systems—the cutting edge of antiretroviral therapy. AIDS Rev. 4:148-156. [PubMed] [Google Scholar]
- 56.Schubert, U., K. A. Clouse, and K. Strebel. 1995. Augmentation of virus secretion by the human immunodeficiency virus type 1 Vpu protein is cell type independent and occurs in cultured human primary macrophages and lymphocytes. J. Virol. 69:7699-7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 58.Seliger, B., F. Ruiz-Cabello, and F. Garrido. 2008. IFN inducibility of major histocompatibility antigens in tumors. Adv. Cancer Res. 101:249-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.SenGupta, D. N., and R. H. Silverman. 1989. Activation of interferon-regulated, dsRNA-dependent enzymes by human immunodeficiency virus-1 leader RNA. Nucleic Acids Res. 17:969-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shirazi, Y., and P. M. Pitha. 1992. Alpha interferon inhibits early stages of the human immunodeficiency virus type 1 replication cycle. J. Virol. 66:1321-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shirazi, Y., and P. M. Pitha. 1993. Interferon alpha-mediated inhibition of human immunodeficiency virus type 1 provirus synthesis in T-cells. Virology 193:303-312. [DOI] [PubMed] [Google Scholar]
- 62.Skillman, D. R., J. L. Malone, C. F. Decker, K. F. Wagner, R. L. Mapou, M. J. Liao, D. Testa, and M. S. Meltzer. 1996. Phase I trial of interferon alfa-n3 in early-stage human immunodeficiency virus type 1 disease: evidence for drug safety, tolerance, and antiviral activity. J. Infect. Dis. 173:1107-1114. [DOI] [PubMed] [Google Scholar]
- 63.Smith, M. S., R. J. Thresher, and J. S. Pagano. 1991. Inhibition of human immunodeficiency virus type 1 morphogenesis in T cells by alpha interferon. Antimicrob. Agents Chemother. 35:62-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sourisseau, M., N. Sol-Foulon, F. Porrot, F. Blanchet, and O. Schwartz. 2007. Inefficient human immunodeficiency virus replication in mobile lymphocytes. J. Virol. 81:1000-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strebel, K., T. Klimkait, and M. A. Martin. 1988. A novel gene of HIV-1, vpu, and its 16-kilodalton product. Science 241:1221-1223. [DOI] [PubMed] [Google Scholar]
- 66.Towers, G. J. 2007. The control of viral infection by tripartite motif proteins and cyclophilin A. Retrovirology 4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Damme, N., D. Goff, C. Katsura, R. L. Jorgenson, R. Mitchell, M. C. Johnson, E. B. Stephens, and J. Guatelli. 2008. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3:245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Varthakavi, V., E. Heimann-Nichols, R. M. Smith, Y. Sun, R. J. Bram, S. Ali, J. Rose, L. Ding, and P. Spearman. 2008. Identification of calcium-modulating cyclophilin ligand as a human host restriction to HIV-1 release overcome by Vpu. Nat. Med. 14:641-647. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Varthakavi, V., R. M. Smith, S. P. Bour, K. Strebel, and P. Spearman. 2003. Viral protein U counteracts a human host cell restriction that inhibits HIV-1 particle production. Proc. Natl. Acad. Sci. USA 100:15154-15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wells, D. E., S. Chatterjee, M. J. Mulligan, and R. W. Compans. 1991. Inhibition of human immunodeficiency virus type 1-induced cell fusion by recombinant human interferons. J. Virol. 65:6325-6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamada, O., N. Hattori, T. Kurimura, M. Kita, and T. Kishida. 1988. Inhibition of growth of HIV by human natural interferon in vitro. AIDS Res. Hum. Retrovir. 4:287-294. [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto, J. K., F. Barre-Sinoussi, V. Bolton, N. C. Pedersen, and M. B. Gardner. 1986. Human alpha- and beta-interferon but not gamma- suppress the in vitro replication of LAV, HTLV-III, and ARV-2. J. Interferon Res. 6:143-152. [DOI] [PubMed] [Google Scholar]