CK1 puts the brakes on dynein activity when added to purified axonemes in vitro, presumably to regulate how flagella bend.
Abstract
Experimental analysis of isolated ciliary/flagellar axonemes has implicated the protein kinase casein kinase I (CK1) in regulation of dynein. To test this hypothesis, we developed a novel in vitro reconstitution approach using purified recombinant Chlamydomonas reinhardtii CK1, together with CK1-depleted axonemes from the paralyzed flagellar mutant pf17, which is defective in radial spokes and impaired in dynein-driven microtubule sliding. The CK1 inhibitors (DRB and CK1-7) and solubilization of CK1 restored microtubule sliding in pf17 axonemes, which is consistent with an inhibitory role for CK1. The phosphatase inhibitor microcystin-LR blocked rescue of microtubule sliding, indicating that the axonemal phosphatases, required for rescue, were retained in the CK1-depleted axonemes. Reconstitution of depleted axonemes with purified, recombinant CK1 restored inhibition of microtubule sliding in a DRB– and CK1-7–sensitive manner. In contrast, a purified “kinase-dead” CK1 failed to restore inhibition. These results firmly establish that an axonemal CK1 regulates dynein activity and flagellar motility.
Introduction
Motile cilia and flagella are capable of complex, carefully coordinated movements and have diverse roles in embryonic development, fertilization, and function of epithelia (Satir and Christensen, 2007; Basu and Brueckner, 2008; Marshall, 2008; Sharma et al., 2008). Ciliary and flagellar movement is mediated by the axoneme, a highly ordered “9 + 2” microtubule scaffold composed of hundreds of conserved proteins (Avidor-Reiss et al., 2004; Li et al., 2004b; Pazour et al., 2005). Within the axoneme, spatial and temporal regulation of dynein-driven microtubule sliding is required for production of the complex bends that characterize ciliary and flagellar motility (Satir, 1968; Summers and Gibbons, 1971; Shingyoji et al., 1977; Brokaw, 1991b). However, the mechanisms that regulate dynein and modulate the size and shape of the axonemal bend are poorly understood (Salathe, 2007; Brokaw, 2009).
Analyses of isolated Chlamydomonas reinhardtii axonemes have revealed that the central pair–radial spoke structures (CP/RS) regulate dynein-driven microtubule sliding by a control mechanism involving axonemal protein phosphorylation (Porter and Sale, 2000; Smith and Yang, 2004; Wirschell et al., 2007). Additional evidence for such a control system has come from characterization of bypass suppressor mutations that restore motility to paralyzed CP/RS mutants without restoring the missing structures (for review see Porter and Sale, 2000). These experiments have revealed regulatory systems that, in the absence of the CP/RS, result in inhibition of axonemal dyneins. Consistent with this interpretation, isolated axonemes lacking the CP/RS can undergo microtubule sliding (Witman et al., 1978); however, the rate of microtubule sliding is significantly reduced compared with wild-type axonemes (Smith and Sale, 1992a). In vitro assays have demonstrated that the changes in microtubule sliding velocity are mediated by phosphorylation of the inner dynein arm proteins (Smith and Sale, 1992b; Howard et al., 1994; Habermacher and Sale, 1996; Habermacher and Sale, 1997; King and Dutcher, 1997). These studies also revealed that the protein kinases and phosphatases responsible for control of dynein phosphorylation, including casein kinase I (CK1), are physically anchored in the axoneme (Yang et al., 2000; for review see Porter and Sale, 2000).
In addition, the CP/RS phospho-regulatory pathway also requires the assembly of an inner arm dynein called “I1 dynein” (“dynein-f”), a dynein subform important for control of flagellar waveform (Wirschell et al., 2007). The key phospho-protein in I1 dynein is IC138. This conclusion is based on direct analysis of IC138 phosphorylation (Habermacher and Sale, 1997; Yang and Sale, 2000; Hendrickson et al., 2004) and on mutants defective in either IC138 phosphorylation (King and Dutcher, 1997; Hendrickson et al., 2004; Dymek and Smith, 2007; Wirschell et al., 2009) or in IC138 assembly (Bower et al., 2009). For example, rescue of microtubule sliding by protein kinase inhibitors requires assembly of I1 dynein and the IC138 subcomplex (Habermacher and Sale, 1997; Yang and Sale, 2000, Wirschell et al., 2009; Bower et al., 2009). Pharmacological experiments also revealed a role for the protein kinase CK1 in the regulatory pathway (Yang and Sale, 2000).
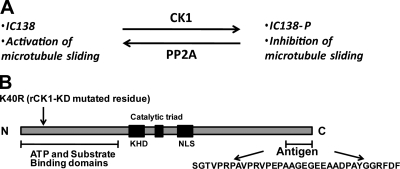
CK1 belongs to a family of serine/threonine kinases that are highly conserved and have diverse and vital cellular functions including regulation of the cell cycle, control of circadian rhythm, regulation of motility and organelle transport, and regulation of development (Knippschild et al., 2005). Several of these functions involve interaction of CK1 with the cytoskeleton, presumably for localization of CK1 and specificity of substrate phosphorylation (Gross and Anderson, 1998; Behrend et al., 2000; Sillibourne et al., 2002; Li et al., 2004a; Ben-Nissan et al., 2008). However, the mechanisms for targeting CK1 within the cell are not well understood. CKI is also located in the flagellar axoneme (Yang and Sale, 2000; Pazour et al., 2005). These studies have led to a model (Fig. 1 A) implicating an axonemal CK1 in control of IC138 phosphorylation and microtubule sliding, and a failure in regulation of CK1, resulting in defective flagellar motility. Tests of this model require direct analysis of axonemal CK1.
Figure 1.
Model for regulation of I1 dynein and the CK1 protein. (A) Analysis of wild-type and mutant axonemes has revealed that microtubule sliding activity is regulated by phosphorylation of the I1 dynein subunit IC138 (Wirschell et al., 2007). The data predicts that IC138 is phosphorylated by the axonemal kinase CK1, and that phosphorylation inhibits dynein-driven microtubule sliding activity. The model also indicates that axonemal phosphatase PP2A is required to rescue microtubule sliding activity (Yang and Sale, 2000). (B) C. reinhardtii CK1 is highly conserved and contains characteristic CK1 domains including the N-terminal ATP and substrate-binding domains, the kinesin homology domain (KHD), the catalytic triad, and the nuclear localization signal (NLS). To generate rCK1-KD, K 40, shown to be required for kinase activity (Gao et al., 2002), was replaced by R. A CK1-specific antibody was made to the polypeptide at the C terminus.
Here, we cloned the C. reinhardtii CK1 gene, characterized the predicted protein, and generated a CK1-specific antibody. CK1 is localized along the length of each axoneme and can be solubilized by 0.3 M NaCl, a condition that does not disrupt the dynein arm, radial spoke, or central pair structures. To directly test the role of CK1 in control of microtubule sliding, we used “CK1-depleted axonemes” for in vitro reconstitution with purified recombinant CK1 (rCK1). We demonstrate that the CK1 rebinds to the depleted axonemes, and is required to inhibit I1 dynein-dependent microtubule sliding. The results support a model in which CK1 and I1 dynein function together to control flagellar waveform.
Results and discussion
Characterization of an axonemal CK1
A candidate CK1 protein was identified in the C. reinhardtii flagellar proteome (Pazour et al., 2005; NCBI Protein database accession no. 137286). 15 separate peptides, spanning the entire protein, were found in the salt-soluble KCl fraction, indicating that CK1 is an axonemal protein, and defined the gene that maps to LG XII/XIII (Fig. S1A; Pazour et al., 2005). CK1 mutants have not been identified, likely because of additional essential functions in cell growth, flagellar assembly, and circadian control (Schmidt et al., 2006). CK1 is a polypeptide with a predicted mass of 38.4 kD and a high degree of homology to other CK1 isoforms, particularly the mammalian CK1 δ (79% identity) and ϵ (78% identity; Fig. 1 B). CK1 δ is enriched in ciliated cells of the mouse respiratory tract and rat testis, suggesting that CK1 has analogous functions in mammalian cilia and flagella (Löhler et al., 2009).
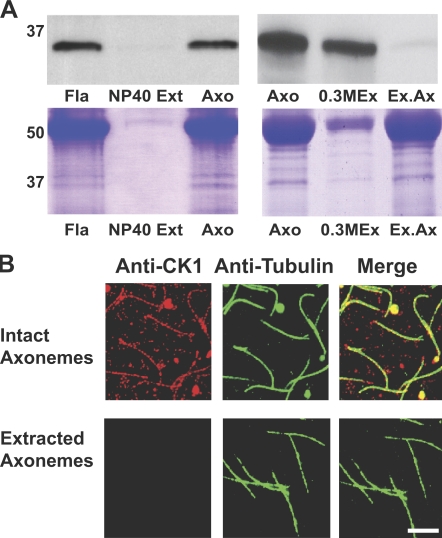
To confirm that the CK1 is an axonemal protein, a polyclonal antibody was produced to a unique C-terminal polypeptide (Fig. 1 B). The antibody detected a single 36.5-kD band in isolated flagella or axonemes, confirming that CK1 is in the axoneme (Fig. 2 A, left; and Fig. S1 B). Analysis of flagellar mutants confirmed that CK1 is not localized to the CP/RS, the dynein regulatory complex (DRC), I1 dynein, or the outer dynein arm structures (Fig. S1 C). Therefore, CK1 is associated with the outer doublet microtubules, presumably near I1 dynein (Yang and Sale, 2000). Immunofluorescent localization revealed a uniform distribution of CK1 along the length of isolated axonemes (Fig. 2 B, top). The axonemal CK1 was readily extracted with 0.3 M NaCl buffer (Fig. 2 A, right). Consistent with the biochemistry, axonemes depleted of CK1 do not react with the CK1 antibody by immunofluorescence (Fig. 2 B, bottom).
Figure 2.
CK1 is an axonemal protein located along the length of the axoneme and is extractable in 0.3 M NaCl buffers. (A) Flagella (Fla) were fractionated into a Nonidet P40 soluble membrane/matrix fraction (NP-40 Ext) and axonemal fraction (Axo), then analyzed by an immunoblot probed with the anti-CK1 antibody (top left). Axonemal CK1 was extracted with 0.3 M NaCl (top right). The bottom panels show Coomassie-stained proteins of the same samples and those used as a loading control. Positions of molecular mass standards are indicated in kD. (B) CK1 localizes along the length of the axoneme (top, “Intact Axonemes”). Biochemical depletion of CK1 removes all detectable CK1 (bottom, “Extracted Axonemes”). Bar, 5 µm.
In keeping with a dynein regulatory function, we propose that CK1 is positioned near I1 dynein and the IC138 subcomplex at regular intervals of 96 nm (Bower et al., 2009; Wirschell et al., 2009). Demonstration of this periodic spacing will require direct localization by immunoelectron microscopy. Currently, there is no known mechanism for the targeting of CK1 to specific sites along the outer doublet microtubule. However, we speculate that CK1 may be targeted by a type of CK1-anchoring protein analogous to AKAPs (A-kinase anchoring proteins), which are predicted to target PKA in the axoneme (Gaillard et al., 2001, 2006). It is possible that PP2A and CK1 are targeted to the same site and anchored by the same protein near the IC138 complex (e.g., Reinhardt et al., 2007) in order to generate a localized “switch,” allowing tight and local control of dynein activity.
Depletion of CK1 rescues microtubule sliding in RS mutant axonemes, and rescue requires I1 dynein
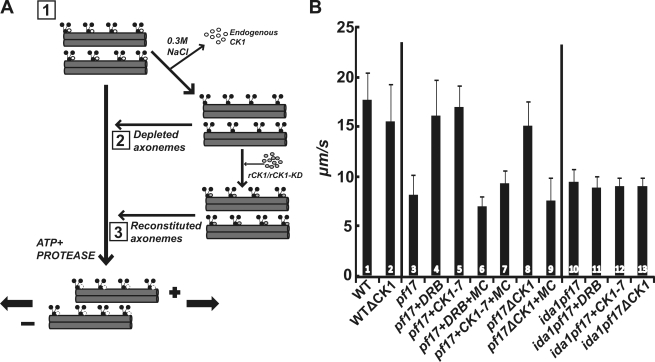
To test the hypothesis that CK1 inhibits dynein-driven microtubule sliding, we developed a novel approach of measuring microtubule sliding in CK1-depleted axonemes (Fig. 3 A). We predicted that biochemical removal of CK1 from pf17 axonemes would mimic treatment with the kinase inhibitors 5, 6-dichloro-1-b-D-ribofuranosylbenzimidazole (DRB) and N-(2-aminoethyl)-5-chloroisoquinoline-8-sulfonamide (CK1-7), and rescue microtubule sliding. As shown in Fig. 3 B, the CK1 inhibitors restore microtubule sliding velocity in isolated pf17 axonemes (Fig. 3 B, compare bar 3 with 4 and 5; Yang and Sale, 2000). The results indicated that flagellar paralysis in pf17 is a consequence of uniform, CK1-dependent inhibition of dynein activity through phosphorylation (Fig. 1 A). Consistent with this interpretation, addition of the phosphatase inhibitor, microcystin-LR, blocked the CK1 inhibitor–mediated rescue of microtubule sliding in pf17 axonemes (Fig. 3 B, bars 6 and 7). Microtubule sliding velocity was also rescued in CK1-depleted pf17 axonemes (Fig. 3 B, compare bar 3 with 8), which mimics DRB/CK1-7 treatment and is consistent with the hypothesis that failure in regulation of CK1 in pf17 axonemes results in inhibition of dynein-driven microtubule sliding. The phosphatase inhibitor microcystin-LR blocked rescue of microtubule sliding in the CK1-depleted pf17 axonemes (Fig. 3 B, compare bars 8 and 9). This result established that the CK1-depletion procedure did not remove the microcystin-LR–sensitive phosphatases required for rescue of microtubule sliding (Fig. 1 A). Kinase inhibitors do not alter microtubule sliding velocity in wild-type axonemes (unpublished data); thus, we predicted that CK1 depletion would not alter sliding in wild-type axonemes. As expected, depletion of CK1 from wild-type axonemes had no effect on microtubule sliding velocity (Fig. 3 B, compare bars 1 and 2). We postulate that dynein, particularly I1 dynein, is fully active in wild-type axonemes, and thus, in these assays, removal of CK1 would have no further effect (for review see Wirschell et al., 2007).
Figure 3.
Biochemical depletion of CK1 rescues microtubule sliding in isolated pf17 axonemes, and this rescue requires I1 dynein. (A) Experimental strategy to test the role of CK1 in microtubules. (B) ATP-induced microtubule sliding velocity was measured in isolated axonemes, CK1-depleted axonemes, or CK1-depleted axonemes reconstituted with purified rCK1 (Okagaki and Kamiya, 1986; Wirschell et al., 2009). The effect of DRB/CK1-7 and the phosphatase inhibitor microcystin-LR (MC) was also examined. The bars represent: (1) wild-type (WT) axonemes; (2) WT axonemes depleted of CK1 (note that there is no change in velocity); (3) pf17 axonemes (note the slow, baseline sliding velocity); (4) pf17 axonemes plus DRB; (5) pf17 axonemes plus CK1-7; (6) pf17 axonemes plus DRB and microcystin-LR; (7) pf17 axonemes plus CK1-7 and microcystin-LR; (8) pf17 axonemes depleted of CK1 (note the rescue of microtubule sliding); (9) pf17 axonemes depleted of CK1 plus microcystin-LR; (10) ida1pf17 axonemes; (11) ida1pf17 axonemes plus DRB; (12) ida1pf17 axonemes plus CK1-7; and (13) ida1pf17 axonemes depleted of CK1 (note the failure in rescue of sliding). Microtubule sliding velocity is expressed as µm/s, and means and standard deviations (error bars) were calculated from at least three independent experiments with a minimum sample size of 75 axonemes.
Rescue of microtubule sliding also required the assembly of I1 dynein and its regulatory intermediate chain IC138 (Habermacher and Sale, 1997; Yang and Sale, 2000; Bower et al., 2009; Wirschell et al., 2009). To determine whether I1 dynein assembly is required for rescue of microtubule sliding in the CK1-depletion experiments, we analyzed a double mutant ida1pf17 defective in I1 dynein and radial spoke assembly. Microtubule sliding in the ida1pf17 axonemes is slow and neither DRB nor CK1-7 rescues sliding (Fig. 3 B, bars 11 and 12). Similarly, depletion of CK1 fails to rescue microtubule sliding (Fig. 3 B, bar 13). Therefore, assembly of I1 dynein is required for rescue of microtubule sliding in the CP/RS pathway.
Exogenous CK1 restores inhibition of microtubule sliding in a DRB/CK1-7–sensitive manner
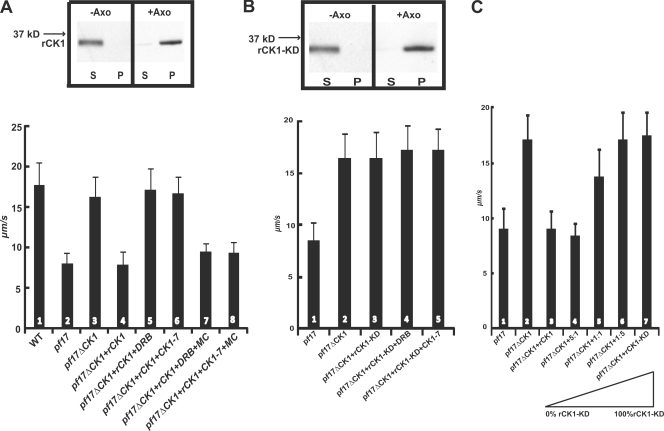
To determine whether rescue of microtubule sliding is specifically caused by CK1 depletion, we reconstituted the CK1-depleted pf17 axonemes with purified rCK1 (Figs. 3 A and 4A). We predicted that the rCK1 will functionally rebind CK1-depleted axonemes and restore inhibition of microtubule sliding to the original slow velocity that is characteristic of pf17 axonemes. Additionally, we designed a recombinant “kinase-dead” CK1 (rCK1-KD) predicted to also rebind to CK1-depleted axonemes but fail to restore CK1-dependent inhibition of microtubule sliding (see Fig. S2 for characterization of rCK1 and rCK1-KD). The purified rCK1 rebound to CK1-depleted axonemes (Fig. 4 A, immunoblot), and inhibition of microtubule sliding velocity was restored (Fig. 4 A, compare bars 3 and 4). Treatment of the rCK1-reconstituted axonemes with DRB or CK1-7 blocked this restoration of sliding inhibition (Fig. 4 A, bars 5 and 6), and microcystin-LR blocked rescue of microtubule sliding by the kinase inhibitors DRB and CK1-7 in the rCK1-reconstituted axonemes (Fig. 4 A, bars 7 and 8). These results demonstrated that exogenous CK1 binds to the axoneme and inhibits I1 dynein–dependent microtubule sliding velocity in pf17 axonemes. The results also demonstrate that rescue of microtubule sliding in pf17 axonemes depends upon an axonemal, microcystin-LR–dependent phosphatase (Fig. 1 A).
Figure 4.
CK1 kinase activity is required for regulation of microtubule sliding. (A) Addition of rCK1 restores inhibition in CK1-depleted pf17 axonemes (bars 3 and 4) and rebinds to CK1-depleted pf17 axonemes (top). DRB and CK1-7 block restoration of inhibition (bars 5 and 6), and microcystin-LR blocks the effects of the kinase inhibitors (bars 7 and 8). (B) rCK1-KD rebinds to CK1-depleted pf17 axonemes (top) but fails to restore inhibition of microtubule sliding velocity (compare bars 2 and 3). Treatment with CK1 inhibitors had no effect (bars 4 and 5). P, pellet; S, supernatant. (C) rCK1 and rCK1-KD were reconstituted at different ratios to CK1-depleted pf17 axonemes (illustrated below bars 3–7). With increasing amounts of rCK1-KD, there is a corresponding increase in microtubule sliding velocity, indicating that rCK1-KD competes for binding and inhibits the effect of rCK1 on microtubule sliding. Microtubule sliding velocity is expressed as µm/s, and averages and standard deviations (error bars) were calculated from at least three independent experiments with a minimum sample size of 75 axonemes.
CK1 kinase activity is required for inhibition of microtubule sliding
For further investigation, we used rCK1-KD, which lacks kinase activity but is otherwise capable of binding to the CK1-depleted axonemes (Fig. 4 B, immunoblot; and Fig. S2 B). Reconstitution of CK1-depleted axonemes with rCK1-KD did not alter rescued microtubule sliding velocity (Fig. 4 B, bar 3), and, as expected, DRB or CK1-7 treatment did not have any further effect (Fig. 4 B, bars 4 and 5). To test whether rCK1-KD functionally rebinds to the CK1-depleted axonemes, we performed competition experiments using a mixture of different ratios of rCK1 and rCK1-KD. We predicted that rCK1-KD would compete with rCK1 for binding to the axoneme and block inhibition of microtubule sliding. As the amount of rCK1-KD protein was increased, there was a corresponding increase in microtubule sliding (Fig. 4 C, bars 3–7). This result demonstrated that rCK1 and rCK1-KD compete equivalently for axonemal binding and that rCK1-KD can block the effect of rCK1 kinase activity in restoring inhibition of microtubule sliding. The intermediate sliding velocity is predicted to be a result of a mixture of active and inactive I1 dynein. This observation may be consistent with biophysical analysis that has indicated that I1 dynein can operate, in part, to resist microtubule sliding generated by other dyneins (Kotani et al., 2007).
Here, we present a novel in-vitro assay that reveals coupling of CK1 kinase activity to the CP/RS phospho-regulatory pathway that controls dynein activity (Fig. 1 A). The central question is how the CP/RS phospho-regulatory pathway contributes to axonemal motility. The CP/RS phospho-regulatory mechanism does not appear to be required for initiation of bending and bend oscillation, features that may be inherent to the dynein motors and to mechanical feedback control mechanisms that are not well understood (Hayashibe et al., 1997; Lindemann and Hunt, 2003; Morita and Shingyoji, 2004; Aoyama and Kamiya, 2005; Brokaw, 2009; Hayashi and Shingyoji, 2008). Rather, this regulatory mechanism appears to be required for controlling the form of forward and reverse bends important for normal flagellar movement. Consistent with this interpretation, mutations that lead to a failure in I1 dynein assembly are defective in flagellar waveform (Brokaw and Kamiya, 1987) and fail to undergo normal phototaxis (King and Dutcher, 1997; Okita et al., 2005). Bypass suppressor mutants that restore motility in the absence of radial spoke assembly fail to generate normal flagellar waveform (Brokaw et al., 1982). In wild-type axonemes, signals from the central pair are presumably asymmetrically distributed to radial spokes anchored on individual or subsets of outer doublet microtubules. Predictably, such signals locally regulate dynein activity on individual or groups of outer doublet microtubules, altering the form of the axonemal bend (Wirschell et al., 2007).
It is likely that the CP/RS phospho-regulatory mechanism responds to changes in second messengers including cyclic nucleotides and calcium (Walczak and Nelson, 1994; Bannai et al., 2000; Smith, 2002a; Salathe, 2007; Hayashi and Shingyoji, 2009). Consistent with this idea, changes in calcium affect the flagellar waveform, and the mechanism involves direct interaction of calcium with calcium-binding proteins in the axoneme (Bessen et al., 1980; Kamiya and Witman, 1984; Omoto and Brokaw, 1985; Brokaw, 1991a; Yang et al., 2001, 2004; Patel-King et al., 2004). Changes in calcium have been shown to regulate microtubule sliding by a mechanism that involves the central pair (Smith, 2002b; Nakano et al., 2003), and may require assembly of I1 dynein (Smith, 2002b). Dymek and Smith (2007) have identified a calmodulin-containing complex called calmodulin-and spoke-associated complex (CSC) that interacts with the radial spokes and functionally interacts with I1 dynein. The CSC could also interact with CK1 and PP2A and mediate signaling from the radial spokes, as well as calcium, for the control of I1 dynein.
We have shown that C. reinhardtii expresses a conserved CK1 that localizes to the flagellar axoneme outer doublet microtubules presumably near I1 dynein and that regulates I1 dynein–dependent microtubule sliding. A new functional assay, which couples in vitro reconstitution and microtubule sliding, revealed that unregulated axonemal CK1 kinase activity results in inhibition of microtubule sliding. Thus, based on these data, we postulate that the axonemal CK1 is a “downstream” component of the CP/RS phospho-regulatory pathway that involves phosphorylation of the I1 dynein IC138 (Porter and Sale, 2000; Wirschell et al., 2007). This regulatory mechanism is presumably highly controlled in wild-type axonemes and is required for precise regulation of the size and shape of the flagellar bend. Challenges include determining how physical and chemical activity of the CP/RS structures regulate axonemal protein kinases and phosphatases (Smith and Yang, 2004; Yang et al., 2004).
Materials and methods
Strains and culture conditions
C. reinhardtii strains used include CC125 (wild type), pf17 (lacks radial spoke head), pf18 (lacks central pair apparatus), pf28pf30ssh1 (lacks outer dynein arms and I1 dynein), pf3 (lacks DRC), and ida1pf17 (lacks I1 dynein and radial spoke head). All strains were obtained from the Chlamydomonas Genetics Center (University of Minnesota, St. Paul, MN) with the exception of pf28pf30ssh1(Freshour et al., 2007) and ida1pf17 (recovered from nonparental tetrad). Cells were grown in tris-acetate-phosphate (TAP) medium with aeration on a 14:10 h light/dark cycle.
Molecular approaches
The CK1 coding sequence was PCR cloned into the pCR 2.1 TOPO cloning vector according to the manufacturer's instructions (Invitrogen) to yield plasmid pAGCK1-FL. The insert in pAGCK1-FL was excised by digestion with BamH1 and HindIII and cloned into the pET28A (EMD). The resulting expression construct, pAGHisCK1, was transformed into strain BL21(DE3) pLysS (Agilent Technologies), and expression was induced with 1 mM IPTG. The His-tagged CK1 protein was purified using Talon metal affinity resin (Clontech Laboratories, Inc.). Site-directed mutagenesis was performed on the pAGCK1-FL plasmid to make the amino acid substitution, K40R, using the QuikChange in vitro Site-Directed Mutagenesis System (Agilent Technologies) to produce the plasmid pAGCK1-KD. The His-tagged CK1-KD was induced, expressed, and purified as described above.
Kinase assays were performed with rCK1 and rCK1-KD (Fig. S2 B; Yang and Sale, 2000) using specific CK1 peptide substrates (C2335; Sigma-Aldrich). For CK1 activity, 2-µl samples from chromatography fractions or purified rCK1 fractions were added to a final volume of 20 µl in reaction buffer (50 mM Tris, pH 8.0, 0.1 mM EDTA, 0.2% β-mercaptoethanol, 7 mM magnesium acetate, 0.02% Brij 35, 20 mM NaCl, and 100 mM sodium orthovanadate), 0.5 µg/µl of CKI-specific substrate, and 40 µM [γ-32P]ATP (2,000 cpm/pmol). After 30 min at 30°C, the reactions were terminated by adding 1 µl of 100% trichloroacetic acid. 10 µl from each reaction was applied in duplicate to discs of P-81 filter paper (GE Healthcare), washed extensively with 75 mM phosphoric acid, and rinsed with acetone. The radioactivity of the dried P-81 paper discs was measured by scintillation counting.
Axoneme isolation
Flagella were isolated by the dibucaine method in buffer (10 mM Hepes, 30 mM NaCl, 5 mM MgSO4, 1 mM DTT, 0.5 mM EDTA, 0.1 M PMSF, and 0.6 trypsin inhibitory unit [TIU] aprotinin, pH 7.4) and demembranated in the same buffer with 1% Nonidet for subsequent isolation of axonemes by centrifugation (Witman, 1986). For CK1 extraction, 1 mg/ml of axonemes was treated with buffer (10 mM Hepes, 5 mM MgSO4, 1 mM DTT, 0.5 mM EDTA, 0.1 M PMSF, and 0.6 TIU aprotinin, pH 7.4) containing 0.3 M NaCl for 20 min on ice, then centrifuged for 20 min at 12,000 rpm in an SS34 rotor (Sorvall). The supernatant was collected and is referred to as the “0.3 M NaCl extract”; the resulting axonemes are referred to as “extracted axonemes.” Axonemal fractions were fixed for SDS-PAGE at a concentration of 1 mg/ml, and 20 µg of protein was used for analysis.
Kinase inhibitors and reagents
DRB (BIOMOL International L.P.) was stored as a 50-mM stock solution in ethanol, and CK1-7 (Toronto Research Chemicals) was stored as a 50-mM stock solution in DMSO. Microcystin-LR (EMD) was stored as a 500-µM stock solution in methanol.
Antibody preparation and immunoblotting
The last 102 base pairs of the CK1-coding region (encoding the C-terminal 34 amino acids; Fig. 1 B) were cloned into the pCR2.1 vector to yield plasmid pAGCK1. The insert was excised with Xba1 and HindIII and subcloned into the pMAL-c (New England Biolabs, Inc.) to obtain plasmid pAGCK1-MBP. The expression construct was transformed into strain BL21 (DE3) pLysS cells (Agilent Technologies), and protein expression was induced as described above. The CK1 fusion protein was purified by amylose affinity chromatography (New England Biolabs, Inc.) and was used as an antigen to immunize two rabbits (Spring Valley Laboratories, Inc.). The CK1 antibodies were blot affinity purified using recombinant His-CK1 protein and subsequently used for immunoblotting and immunofluorescence analyses. Protein samples were separated by SDS-PAGE and transferred to a nitrocellulose membrane (Bio-Rad Laboratories). The membrane was blocked with 5% nonfat dry milk followed by incubation with primary antibodies (anti-CK1 antibody 1:10,000–1:20,000) followed by incubation with HRP-conjugated secondary antibodies (1:10,000; Bio-Rad Laboratories). The antibody reactivity was detected by chemiluminescence (Thermo Fischer Scientific).
Immunofluorescence microscopy
Isolated flagella or axonemes were processed for immunofluorescence as described previously (Yang and Sale, 1998) with the following modifications. Isolated flagella or axonemes were immobilized on poly-l-lysine–coated coverslips and fixed by immersion in −20°C methanol for 10 min. After fixation, coverslips were immersed in a blocking buffer (2% BSA, 1% fish skin gelatin, 0.02% saponin, and 15% horse serum in PBS) at room temperature. Antibodies were diluted in blocking buffer and used at the following concentrations: 1:100 affinity purified CK1, 1:500 acetylated α-tubulin (611B1), and 1:1,000 Alexa Fluor secondary antibodies (Invitrogen). After incubation and wash steps, samples were mounted on coverslips in ProLong Gold antifade reagent (Invitrogen). Microscopy was performed at 21°C, and images were captured and processed with Simple PCI software (Hamamatsu Photonics) using a wide-field fluorescence microscope (DMR-E; Leica), a 100× Plan-Apochromat lens (1.4 NA; Leica), and a digital camera (Orca-ER; Hamamatsu Photonics). Images were processed using Photoshop 9.0 (Adobe) and figures were assembled using Illustrator CS2 (Adobe).
Microtubule sliding assay and reconstitution experiments
Measurement of microtubule sliding velocity was performed as described previously (Okagaki and Kamiya, 1986; Wirschell et al., 2009). In brief, isolated flagella were resuspended in a motility buffer (10 mM Hepes, 50 mM potassium acetate, 5 mM MgSO4, 1 mM DTT, 0.5 mM EDTA, and 1% 20,000 polyethylene glycol) without protease inhibitors, demembranated with buffer containing 1% Nonidet P40, and added to perfusion chambers. Microtubule sliding was initiated by the addition of buffer containing 1 mM ATP and 5 µg/ml subtilisin A type VIII protease (Sigma-Aldrich). Sliding was recorded using an Axiovert 35 microscope (Carl Zeiss, Inc.) equipped with a 40× Plan-Apochromat lens (Carl Zeiss, Inc.), dark field condenser, and a silicon intensified camera (VE-1000; Dage-MTI). The video images were converted to a digital format using LabVIEW 7.1 software (National Instruments). Sliding velocity was determined manually by measuring microtubule displacement on tracings calibrated with a micrometer. For CK1-depletion experiments, flagella were demembranated and extracted in buffer containing 1% Nonidet P40 and 0.3 M NaCl for ∼2 min, and the axonemes were applied to the perfusion chamber. For inhibition studies, 50 µM DRB, 50 µM CK1-7, or 2 µM of a mixture of DRB/CK1-7 and microcystin-LR was introduced to the perfusion chamber, and sliding was then initiated with 1 mM ATP and subtilisin (5 µg/ml). For reconstitution experiments, buffer containing 0.5 µg of recombinant proteins (rCK1, rCK1-KD, or a mixture of rCK1 and rCK1-KD) was perfused through the chamber containing CK1-depleted axonemes for 2 min, and unbound fusion protein was washed away. The reconstituted axonemes were treated with inhibitors, and microtubule sliding was induced as described above.
Online supplemental material
Fig. S1 details the C. reinhardtii CK1 protein sequence, the specificity of the CK1 antibody used in this study, and the localization of CK1 in central pair, radial spoke, DRC, and an I1–outer dynein arm double mutant. Fig. S2 shows the purified, recombinant proteins (rCK1 and rCK1-KD) used in this study and the results of in vitro kinase assays on the recombinant proteins. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200906168/DC1.
Supplementary Material
Acknowledgments
We wish to thank Maureen Powers, Victor Y. Faundez, Pinfen Yang, Laura Fox, and Candice Elam for discussion and helpful suggestions on the manuscript.
These studies were supported by the March of Dimes and National Institutes of Health (NIH) grants (GM051173) to W.S. Sale, an NIH training grant (EY070911) to A. Gokhale, and an NIH National Research Service Award postdoctoral fellowship (GM075446) to M. Wirschell.
Footnotes
Abbreviations used in this paper:
- CK1
- casein kinase I
- CK1-7
- N-(2-aminoethyl)-5-chloroisoquinoline-8-sulfonamide
- CP/RS
- central pair–radial spoke
- DRB
- 5, 6-dichloro-1-b-d-ribofuranosylbenzimidazole
- DRC
- dynein regulatory complex
- rCK1
- recombinant CK1
- rCK1-KD
- kinase-dead rCK1
References
- Aoyama S., Kamiya R.. 2005. Cyclical interactions between two outer doublet microtubules in split flagellar axonemes.Biophys. J. 89:3261–3268. doi: 10.1529/biophysj.105.067876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidor-Reiss T., Maer A.M., Koundakjian E., Polyanovsky A., Keil T., Subramaniam S., Zuker C.S.. 2004. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis.Cell. 117:527–539. doi: 10.1016/S0092-8674(04)00412-X [DOI] [PubMed] [Google Scholar]
- Bannai H., Yoshimura M., Takahashi K., Shingyoji C.. 2000. Calcium regulation of microtubule sliding in reactivated sea urchin sperm flagella.J. Cell Sci. 113:831–839. [DOI] [PubMed] [Google Scholar]
- Basu B., Brueckner M.. 2008. Cilia multifunctional organelles at the center of vertebrate left-right asymmetry.Curr. Top. Dev. Biol. 85:151–174. doi: 10.1016/S0070-2153(08)00806-5 [DOI] [PubMed] [Google Scholar]
- Behrend L., Stöter M., Kurth M., Rutter G., Heukeshoven J., Deppert W., Knippschild U.. 2000. Interaction of casein kinase 1 delta (CK1delta) with post-Golgi structures, microtubules and the spindle apparatus.Eur. J. Cell Biol. 79:240–251. doi: 10.1078/S0171-9335(04)70027-8 [DOI] [PubMed] [Google Scholar]
- Ben-Nissan G., Cui W., Kim D.J., Yang Y., Yoo B.C., Lee J.Y.. 2008. Arabidopsis casein kinase 1-like 6 contains a microtubule-binding domain and affects the organization of cortical microtubules.Plant Physiol. 148:1897–1907. doi: 10.1104/pp.108.129346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen M., Fay R.B., Witman G.B.. 1980. Calcium control of waveform in isolated flagellar axonemes of Chlamydomonas.J. Cell Biol. 86:446–455. doi: 10.1083/jcb.86.2.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower R., VanderWaal K., O'Toole E., Fox L., Perrone C., Mueller J., Wirschell M., Kamiya R., Sale W.S., Porter M.E.. 2009. IC138 defines a subdomain at the base of the I1 dynein that regulates microtubule sliding and flagellar motility.Mol. Biol. Cell. 20:3055–3063. doi: 10.1091/mbc.E09-04-0277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokaw C.J. 1991a. Calcium sensors in sea urchin sperm flagella.Cell Motil. Cytoskeleton. 18:123–130. doi: 10.1002/cm.970180207 [DOI] [PubMed] [Google Scholar]
- Brokaw C.J. 1991b. Microtubule sliding in swimming sperm flagella: direct and indirect measurements on sea urchin and tunicate spermatozoa.J. Cell Biol. 114:1201–1215. doi: 10.1083/jcb.114.6.1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokaw C.J. 2009. Thinking about flagellar oscillation.Cell Motil. Cytoskeleton. 66:425–436. doi: 10.1002/cm.20313 [DOI] [PubMed] [Google Scholar]
- Brokaw C.J., Kamiya R.. 1987. Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function.Cell Motil. Cytoskeleton. 8:68–75. doi: 10.1002/cm.970080110 [DOI] [PubMed] [Google Scholar]
- Brokaw C.J., Luck D.J., Huang B.. 1982. Analysis of the movement of Chlamydomonas flagella:” the function of the radial-spoke system is revealed by comparison of wild-type and mutant flagella.J. Cell Biol. 92:722–732. doi: 10.1083/jcb.92.3.722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymek E.E., Smith E.F.. 2007. A conserved CaM- and radial spoke–associated complex mediates regulation of flagellar dynein activity.J. Cell Biol. 179:515–526. doi: 10.1083/jcb.200703107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freshour J., Yokoyama R., Mitchell D.R.. 2007. Chlamydomonas flagellar outer row dynein assembly protein ODA7 interacts with both outer row and I1 inner row dyneins.J. Biol. Chem. 282:5404–5412. doi: 10.1074/jbc.M607509200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard A.R., Diener D.R., Rosenbaum J.L., Sale W.S.. 2001. Flagellar radial spoke protein 3 is an A-kinase anchoring protein (AKAP).J. Cell Biol. 153:443–448. doi: 10.1083/jcb.153.2.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard A.R., Fox L.A., Rhea J.M., Craige B., Sale W.S.. 2006. Disruption of the A-kinase anchoring domain in flagellar radial spoke protein 3 results in unregulated axonemal cAMP-dependent protein kinase activity and abnormal flagellar motility.Mol. Biol. Cell. 17:2626–2635. doi: 10.1091/mbc.E06-02-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z.H., Seeling J.M., Hill V., Yochum A., Virshup D.M.. 2002. Casein kinase I phosphorylates and destabilizes the beta-catenin degradation complex.Proc. Natl. Acad. Sci. USA. 99:1182–1187. doi: 10.1073/pnas.032468199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S.D., Anderson R.A.. 1998. Casein kinase I: spatial organization and positioning of a multifunctional protein kinase family.Cell. Signal. 10:699–711. doi: 10.1016/S0898-6568(98)00042-4 [DOI] [PubMed] [Google Scholar]
- Habermacher G., Sale W.S.. 1996. Regulation of flagellar dynein by an axonemal type-1 phosphatase in Chlamydomonas.J. Cell Sci. 109:1899–1907. [DOI] [PubMed] [Google Scholar]
- Habermacher G., Sale W.S.. 1997. Regulation of flagellar dynein by phosphorylation of a 138-kD inner arm dynein intermediate chain.J. Cell Biol. 136:167–176. doi: 10.1083/jcb.136.1.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Shingyoji C.. 2008. Mechanism of flagellar oscillation-bending-induced switching of dynein activity in elastase-treated axonemes of sea urchin sperm.J. Cell Sci. 121:2833–2843. doi: 10.1242/jcs.031195 [DOI] [PubMed] [Google Scholar]
- Hayashi S., Shingyoji C.. 2009. Bending-induced switching of dynein activity in elastase-treated axonemes of sea urchin sperm—roles of Ca2+ and ADP.Cell Motil. Cytoskeleton. 66:292–301. doi: 10.1002/cm.20360 [DOI] [PubMed] [Google Scholar]
- Hayashibe K., Shingyoji C., Kamiya R.. 1997. Induction of temporary beating in paralyzed flagella of Chlamydomonas mutants by application of external force.Cell Motil. Cytoskeleton. 37:232–239. doi: [DOI] [PubMed] [Google Scholar]
- Hendrickson T.W., Perrone C.A., Griffin P., Wuichet K., Mueller J., Yang P., Porter M.E., Sale W.S.. 2004. IC138 is a WD-repeat dynein intermediate chain required for light chain assembly and regulation of flagellar bending.Mol. Biol. Cell. 15:5431–5442. doi: 10.1091/mbc.E04-08-0694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D.R., Habermacher G., Glass D.B., Smith E.F., Sale W.S.. 1994. Regulation of Chlamydomonas flagellar dynein by an axonemal protein kinase.J. Cell Biol. 127:1683–1692. doi: 10.1083/jcb.127.6.1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya R., Witman G.B.. 1984. Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas.J. Cell Biol. 98:97–107. doi: 10.1083/jcb.98.1.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S.J., Dutcher S.K.. 1997. Phosphoregulation of an inner dynein arm complex in Chlamydomonas reinhardtii is altered in phototactic mutant strains.J. Cell Biol. 136:177–191. doi: 10.1083/jcb.136.1.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knippschild U., Gocht A., Wolff S., Huber N., Löhler J., Stöter M.. 2005. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes.Cell. Signal. 17:675–689. doi: 10.1016/j.cellsig.2004.12.011 [DOI] [PubMed] [Google Scholar]
- Kotani N., Sakakibara H., Burgess S.A., Kojima H., Oiwa K.. 2007. Mechanical properties of inner-arm dynein-f (dynein I1) studied with in vitro motility assays.Biophys. J. 93:886–894. doi: 10.1529/biophysj.106.101964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Yin H., Kuret J.. 2004a. Casein kinase 1 delta phosphorylates tau and disrupts its binding to microtubules.J. Biol. Chem. 279:15938–15945. doi: 10.1074/jbc.M314116200 [DOI] [PubMed] [Google Scholar]
- Li J.B., Gerdes J.M., Haycraft C.J., Fan Y., Teslovich T.M., May-Simera H., Li H., Blacque O.E., Li L., Leitch C.C., et al. 2004b. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene.Cell. 117:541–552. doi: 10.1016/S0092-8674(04)00450-7 [DOI] [PubMed] [Google Scholar]
- Lindemann C.B., Hunt A.J.. 2003. Does axonemal dynein push, pull, or oscillate? Cell Motil. Cytoskeleton. 56:237–244. doi: 10.1002/cm.10148 [DOI] [PubMed] [Google Scholar]
- Löhler J., Hirner H., Schmidt B., Kramer K., Fischer D., Thal D.R., Leithäuser F., Knippschild U.. 2009. Immunohistochemical characterisation of cell-type specific expression of CK1delta in various tissues of young adult BALB/c mice.PLoS One. 4:e4174. doi: 10.1371/journal.pone.0004174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W.F. 2008. The cell biological basis of ciliary disease.J. Cell Biol. 180:17–21. doi: 10.1083/jcb.200710085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y., Shingyoji C.. 2004. Effects of imposed bending on microtubule sliding in sperm flagella.Curr. Biol. 14:2113–2118. doi: 10.1016/j.cub.2004.11.028 [DOI] [PubMed] [Google Scholar]
- Nakano I., Kobayashi T., Yoshimura M., Shingyoji C.. 2003. Central-pair-linked regulation of microtubule sliding by calcium in flagellar axonemes.J. Cell Sci. 116:1627–1636. doi: 10.1242/jcs.00336 [DOI] [PubMed] [Google Scholar]
- Okagaki T., Kamiya R.. 1986. Microtubule sliding in mutant Chlamydomonas axonemes devoid of outer or inner dynein arms.J. Cell Biol. 103:1895–1902. doi: 10.1083/jcb.103.5.1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita N., Isogai N., Hirono M., Kamiya R., Yoshimura K.. 2005. Phototactic activity in Chlamydomonas ‘non-phototactic’ mutants deficient in Ca2+-dependent control of flagellar dominance or in inner-arm dynein.J. Cell Sci. 118:529–537. doi: 10.1242/jcs.01633 [DOI] [PubMed] [Google Scholar]
- Omoto C.K., Brokaw C.J.. 1985. Bending patterns of Chlamydomonas flagella: II. Calcium effects on reactivated Chlamydomonas flagella.Cell Motil. 5:53–60. doi: 10.1002/cm.970050105 [DOI] [PubMed] [Google Scholar]
- Patel-King R.S., Gorbatyuk O., Takebe S., King S.M.. 2004. Flagellar radial spokes contain a Ca2+-stimulated nucleoside diphosphate kinase.Mol. Biol. Cell. 15:3891–3902. doi: 10.1091/mbc.E04-04-0352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour G.J., Agrin N., Leszyk J., Witman G.B.. 2005. Proteomic analysis of a eukaryotic cilium.J. Cell Biol. 170:103–113. doi: 10.1083/jcb.200504008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter M.E., Sale W.S.. 2000. The 9 + 2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility.J. Cell Biol. 151:F37–F42. doi: 10.1083/jcb.151.5.F37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt J., Ferandin Y., Meijer L.. 2007. Purification of CK1 by affinity chromatography on immobilised axin.Protein Expr. Purif. 54:101–109. doi: 10.1016/j.pep.2007.02.020 [DOI] [PubMed] [Google Scholar]
- Salathe M. 2007. Regulation of mammalian ciliary beating.Annu. Rev. Physiol. 69:401–422. doi: 10.1146/annurev.physiol.69.040705.141253 [DOI] [PubMed] [Google Scholar]
- Satir P. 1968. Studies on cilia. 3. Further studies on the cilium tip and a “sliding filament” model of ciliary motility.J. Cell Biol. 39:77–94. doi: 10.1083/jcb.39.1.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P., Christensen S.T.. 2007. Overview of structure and function of mammalian cilia.Annu. Rev. Physiol. 69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236 [DOI] [PubMed] [Google Scholar]
- Schmidt M., Gessner G., Luff M., Heiland I., Wagner V., Kaminski M., Geimer S., Eitzinger N., Reissenweber T., Voytsekh O., et al. 2006. Proteomic analysis of the eyespot of Chlamydomonas reinhardtii provides novel insights into its components and tactic movements.Plant Cell. 18:1908–1930. doi: 10.1105/tpc.106.041749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N., Berbari N.F., Yoder B.K.. 2008. Ciliary dysfunction in developmental abnormalities and diseases.Curr. Top. Dev. Biol. 85:371–427. doi: 10.1016/S0070-2153(08)00813-2 [DOI] [PubMed] [Google Scholar]
- Shingyoji C., Murakami A., Takahashi K.. 1977. Local reactivation of Triton-extracted flagella by iontophoretic application of ATP.Nature. 265:269–270. doi: 10.1038/265269a0 [DOI] [PubMed] [Google Scholar]
- Sillibourne J.E., Milne D.M., Takahashi M., Ono Y., Meek D.W.. 2002. Centrosomal anchoring of the protein kinase CK1delta mediated by attachment to the large, coiled-coil scaffolding protein CG-NAP/AKAP450.J. Mol. Biol. 322:785–797. doi: 10.1016/S0022-2836(02)00857-4 [DOI] [PubMed] [Google Scholar]
- Smith E.F. 2002a. Regulation of flagellar dynein by calcium and a role for an axonemal calmodulin and calmodulin-dependent kinase.Mol. Biol. Cell. 13:3303–3313. doi: 10.1091/mbc.E02-04-0185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E.F. 2002b. Regulation of flagellar dynein by the axonemal central apparatus.Cell Motil. Cytoskeleton. 52:33–42. doi: 10.1002/cm.10031 [DOI] [PubMed] [Google Scholar]
- Smith E.F., Sale W.S.. 1992a. Regulation of dynein-driven microtubule sliding by the radial spokes in flagella.Science. 257:1557–1559. doi: 10.1126/science.1387971 [DOI] [PubMed] [Google Scholar]
- Smith E.F., Sale W.S.. 1992b. Structural and functional reconstitution of inner dynein arms in Chlamydomonas flagellar axonemes.J. Cell Biol. 117:573–581. doi: 10.1083/jcb.117.3.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E.F., Yang P.. 2004. The radial spokes and central apparatus: mechano-chemical transducers that regulate flagellar motility.Cell Motil. Cytoskeleton. 57:8–17. doi: 10.1002/cm.10155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers K.E., Gibbons I.R.. 1971. Adenosine triphosphate-induced sliding of tubules in trypsin-treated flagella of sea-urchin sperm.Proc. Natl. Acad. Sci. USA. 68:3092–3096. doi: 10.1073/pnas.68.12.3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak C.E., Nelson D.L.. 1994. Regulation of dynein-driven motility in cilia and flagella.Cell Motil. Cytoskeleton. 27:101–107. doi: 10.1002/cm.970270202 [DOI] [PubMed] [Google Scholar]
- Wirschell M., Hendrickson T., Sale W.S.. 2007. Keeping an eye on I1: I1 dynein as a model for flagellar dynein assembly and regulation.Cell Motil. Cytoskeleton. 64:569–579. doi: 10.1002/cm.20211 [DOI] [PubMed] [Google Scholar]
- Wirschell M., Yang C., Yang P., Fox L., Yanagisawa H.A., Kamiya R., Witman G.B., Porter M.E., Sale W.S.. 2009. IC97 is a novel intermediate chain of I1 dynein that interacts with tubulin and regulates interdoublet sliding.Mol. Biol. Cell. 20:3044–3054. doi: 10.1091/mbc.E09-04-0276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman G.B. 1986. Isolation of Chlamydomonas flagella and flagellar axonemes.Methods Enzymol. 134:280–290. doi: 10.1016/0076-6879(86)34096-5 [DOI] [PubMed] [Google Scholar]
- Witman G.B., Plummer J., Sander G.. 1978. Chlamydomonas flagellar mutants lacking radial spokes and central tubules. Structure, composition, and function of specific axonemal components.J. Cell Biol. 76:729–747. doi: 10.1083/jcb.76.3.729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Sale W.S.. 1998. The Mr 140,000 intermediate chain of Chlamydomonas flagellar inner arm dynein is a WD-repeat protein implicated in dynein arm anchoring.Mol. Biol. Cell. 9:3335–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Sale W.S.. 2000. Casein kinase I is anchored on axonemal doublet microtubules and regulates flagellar dynein phosphorylation and activity.J. Biol. Chem. 275:18905–18912. doi: 10.1074/jbc.M002134200 [DOI] [PubMed] [Google Scholar]
- Yang P., Fox L., Colbran R.J., Sale W.S.. 2000. Protein phosphatases PP1 and PP2A are located in distinct positions in the Chlamydomonas flagellar axoneme.J. Cell Sci. 113:91–102. [DOI] [PubMed] [Google Scholar]
- Yang P., Diener D.R., Rosenbaum J.L., Sale W.S.. 2001. Localization of calmodulin and dynein light chain LC8 in flagellar radial spokes.J. Cell Biol. 153:1315–1326. doi: 10.1083/jcb.153.6.1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Yang C., Sale W.S.. 2004. Flagellar radial spoke protein 2 is a calmodulin binding protein required for motility in Chlamydomonas reinhardtii.Eukaryot. Cell. 3:72–81. doi: 10.1128/EC.3.1.72-81.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.