Abstract
CD40 is a Tumor Necrosis Factor Receptor superfamily member expressed by immune and non-immune cells. CD40:CD154 interactions mediate T-dependent B cell responses and efficient T cell priming. Thus, CD40 is a likely candidate to play roles in autoimmune diseases in which activated T and B cells cause pathology. Diseases in which CD40 plays a pathogenic role include autoimmune thyroiditis, type 1 diabetes, inflammatory bowel disease, psoriasis, multiple sclerosis, rheumatoid arthritis, and systemic lupus erythematosus. This review discusses the role of CD40:CD154 interaction in human and mouse autoimmunity, human polymorphisms associated with disease incidence, and disrupting CD40:CD154 interactions as an autoimmune therapy.
Keywords: CD40, CD154, autoimmune disease, polymorphism, T lymphocyte, B lymphocyte
CD40:CD154 expression and function
CD40 is a member of the Tumor Necrosis Factor Receptor (TNFR) superfamily which is constitutively or inducibly expressed on the surface of a variety of immune and non-immune cell types including B cells, macrophages, dendritic cells (DC), microglia, endothelial cells, epithelial cells, and keratinocytes[1, 2]. Its ligand, CD154, is transiently expressed on the surface of activated CD4+ T cells, but can also be upregulated on other cell types in the context of autoimmune disease[3, 4]. CD40 has a short cytoplasmic tail of only 64 amino acids, with no intrinsic enzymatic activity[1]. The CD40-CD154 interaction results in movement of CD40 into cholesterol-rich membrane microdomains and the binding of TNFR Associated Factors (TRAFs) to its cytoplasmic tail [1, 5]. CD40 directly binds TRAF2, TRAF3, TRAF5, and TRAF6, and indirectly associates with TRAF1 [5]. These interactions result in activation of mitogen and stress-activated protein kinase (MAPK/SAPK) cascades, transcription factor activation, cytokine secretion, proliferation, differentiation of B cells into Ig-secreting plasma cells, and the formation of humoral memory. Specific TRAF molecules are associated with overlapping and distinct CD40-mediated functions [5]. For example, in B cells TRAF6 is required for CD40-mediated JNK activation and IL-6 production, while TRAF2 is required for activation of NF-kB, and TRAF3 serves as a negative regulator of CD40 signaling [1].
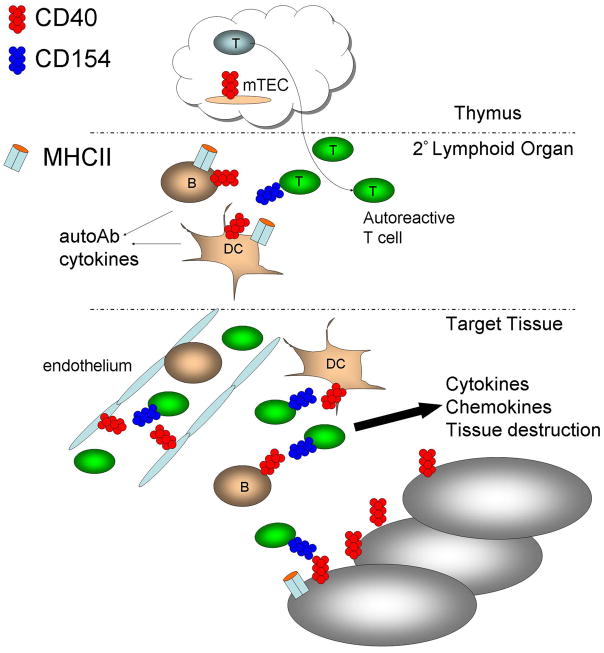
Because of the centrality of CD40 in generating effective immune responses, it also plays an important role in the pathogenesis of autoimmune disease. CD40 potentially contributes to T-cell dependent autoimmune diseases in several ways (Figure 1). First, CD40 signaling could function at the level of T cell selection in the thymus. Medullary thymic epithelial cells (mTECs) mediate negative selection of potentially autoreactive T cells by expressing peripheral tissue-restricted antigens. While the TNFR family member RANK is critically important in embryonic mTEC development, CD40 cooperates with RANK in promoting mTEC development after birth and thus self-tolerance[6]. Disruption of CD40-CD154 interactions in mTECs could potentially contribute to failure of central tolerance. Secondly, CD40 signaling results in the production of pro-inflammatory cytokines, such as IL-6, which can influence T cell differentiation to Th17 cells [7]. CD40 is also upregulated upon antigen presenting cell (APC) activation. Increased levels of CD40, either constitutive or induced, could contribute to increased strength of CD40-CD154 interactions [7]. Another mechanism could be aberrant expression of CD40 in tissues where it is normally undetectable. It has been hypothesized that aberrant expression of MHC class II molecules on endocrine tissues could contribute to the initiation of autoimmune disease [8]. Aberrant CD40 expression on such tissues has also been proposed as a contributing factor to the initiation of autoimmunity in Grave’s disease [9], and in the production of inflammatory cytokines contributing to the failure of pancreatic islet cell transplants [10]. Finally, CD40 bearing CD4+ T cells play a role in type 1 diabetes in humans and mice [11, 12]. CD40+ T cells are reviewed in a separate chapter in this volume.
Figure 1. Potential mechanisms by which CD40:CD154 interactions contribute to autoimmune disease.
CD40 signaling may potentially contribute to autoimmune disease in several ways. 1) At the level of T cell selection in the thymus, potentially allowing autoreactive T cell clones to escape deletion. 2) In the secondary lymphoid organs, where T cells are primed by B cells or other APC. 3) Within the target tissue, where CD40 signaling leads to production of pro-inflammatory cytokines and chemokines which contribute to tissue destruction and inflammatory cell influx.
Consequently, CD40 is an attractive candidate receptor for contributing to a variety of autoimmune processes in which B and T cell activation play a role in pathogenesis. This review will discuss the role of CD40 in the pathogenesis of a variety of human autoimmune diseases and their respective mouse models, human polymorphisms in CD40 associated with autoimmune diseases, and the validity of CD40:CD154 antagonists as therapeutic targets for autoimmune disease.
Autoimmune Thyroiditis
Autoimmune thyroid disease is common, affecting up to 5% of humans [13]. Grave’s disease (GD) is characterized by thyrotoxicosis, lymphocytic infiltration of the thyroid gland, goiter, and presence of stimulatory autoantibodies against the thyroid stimulating hormone receptor. Hashimoto’s thyroiditis (HT) is characterized by more severe lymphocytic infiltration of the thyroid gland, goiter, and loss of thyroid function. HT is often associated with autoantibodies to thyroglobulin and thyroid peroxidase. Both HT and GD have a strong genetic component [13].
CD40, CD80 and MHC class II molecules are aberrantly expressed on thyroid epithelial cells in GD [14] and other thyroid diseases [15], possibly contributing to the presentation of thyroid auto-antigens to T cells, as well as providing co-stimulatory signals.
The C allele of a C/T single nucleotide polymorphism in the Kozak sequence of the CD40 gene has been shown to be associated with increased risk for Grave’s disease [13]. Healthy people with the CC version of the CD40 Kozak sequence have elevated resting B cell CD40 expression compared to CT or TT sequences. This increase in CD40 protein levels reflects enhanced translation [16]. Interestingly, the CC allele is not associated with myasthenia gravis, another autoimmune disease characterized by pathogenic autoantibodies [9]. It is postulated that the CC genotype in GD could contribute to increased CD40 expression on B cells and thyrocytes in the context of inflammation, and could play a role in the development of GD.
In contrast to GD, no CD40 gene polymorphisms have been associated with HT. Blocking anti-CD154 Abs prevent experimental thyroiditis induced by injection with thyroglobulin (experimental autoimmune thyroiditis, or EAT), implicating CD40-CD154 interactions in HT[17]. Further, both thyroglobulin-specific B cells and T cells primed in a mouse with intact CD40 and CD154 are required for the induction of EAT [18].
Type I Diabetes (T1D)
Insulin-dependent diabetes, or T1D, results from the destruction of the beta cells in the islets of Langerhans in the pancreas [19]. CD4+ and CD8+ T cells specific for beta cell antigens are essential for beta cell destruction.
One of the most studied models of autoimmune diabetes is the NOD mouse, which spontaneously develops diabetes secondary to insulitis and islet infiltration and destruction. Disrupting CD40-CD154 interactions using antagonistic anti-CD154 Abs delays diabetes in NOD mice, consistent with a role for CD40-CD154 interactions in disease development [20]. This treatment is even more effective if combined with anti-ICOS Abs [21].
B cells play an important role in NOD disease pathogenesis, as B cell-deficient NOD mice do not develop diabetes [22]. When B cell development is blocked, NOD mice do not develop diabetes [23]. Accumulation of activated B cells expressing high levels of MHC class II in the draining lymph nodes of NOD mice occurs prior to beta cell destruction [24]. These B cells may have a role as APC in promoting diabetogenic T cell proliferation in NOD mice [19], and CD40-CD154 interactions may be important at this stage of the diabetes development.
Autoantibodies directed at beta cell antigens, including insulin and glutamic acid decarboxylase (GAD) are found in the NOD mouse and human diabetes patients[19, 25], but their role in pathogenesis is unclear. Because B cell CD40 plays an essential role in antibody class switching and affinity maturation, CD40-CD154 blockade could affect the production of pathogenic autoantibodies.
Blockade of the CD40-CD154 pathway in Bio-Breeding (BB) diabetes-prone rats delays diabetes onset, and CD28 costimulation also appears to be involved [26]. BB diabetes-resistant rats develop diabetes after treatment with poly I:C coupled with regulatory T cell depletion. In this model, treatment with anti-CD154 blocking mAb also delays diabetes onset, but there is no apparent contribution by other costimulatory pathways [26]. However, CD134-CD134L blockade prevents autoimmune destruction of islet cell transplants in BB diabetes resistant rats [26].
Human pancreatic beta cells express CD40 after isolation, and CD40 is constitutively expressed in mouse islets. Inflammatory cytokines (IL1β, IFN-γ and TNFα) can upregulate CD40 on human beta cells [27], and signaling through CD40 expressed on human or non-human primate islet cells can lead to the production of inflammatory cytokines [10].
The role of CD40+ T cells in diabetes has been investigated recently. Stimulation of NOD T cells through the T cell receptor and CD40 results in upregulation of T cell CD40 and prevents CTLA4 upregulation [28]. Pretreatment of diabetogenic T cell clones with anti-CD40, but not with anti-CD28 blocking Abs, abrogates the ability to transfer diabetes to NOD:SCID recipients [28]. CD40+ T cells are present at increased frequency in the peripheral blood of people with T1D, relative to CD40+ T cells in controls, or people with type 2 diabetes [12]. CD40+ T cells are discussed in further detail in another chapter in this volume.
CD40 and Neuroinflammatory Diseases
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the brain and spinal cord in which CD4+ and CD8+ T cells, B cells, macrophages, and activated microglia infiltrate the CNS and destroy myelin. This results in motor and sensory dysfunction [29]. Much of what is known regarding MS pathogenesis has been learned from either examining human post-mortem brain lesions or through studies of a mouse model, experimental autoimmune encephalomyelitis (EAE), in which immune cells infiltrate the CNS following immunization of mice with myelin components[29].
Most CD154+ cells found in the brains of MS patients are CD4+ T cells, and most of the CD40+ cells are either CD11b+ macrophages/microglia or B cells [30]. T cells from the peripheral blood of MS patients induce more IL-12 secretion from either normal or MS-derived APCs in a CD40-dependent manner[31]. MS patients have a higher frequency of CD154+ T cells than healthy controls, which decrease following treatment with interferon-β [32].
In EAE, CD40 is expressed in the spinal cord during acute disease and relapse, while CD154 is expressed highly only during relapse. Expression of these molecules correlates with Th1 cytokine production within the CNS [33]. CD154−/− mice do not develop EAE due to a defect in APC activation [34]. Administration of an antagonistic anti-CD154 mAb at the time of EAE induction prevents disease[30], perhaps by skewing immune responses toward non-pathogenic Th2 responses [35] or preventing the retention or expansion of Th1 cells within the CNS [36–38]. If treatment is given at the peak of acute disease, mice have fewer relapses of shorter duration, correlating with decreased Th1 cell differentiation and fewer inflammatory cells within the CNS [37, 39]. In other studies, however, anti-CD154 treatment is ineffective at alleviating EAE if given >7 days post-immunization [40]. Thus, CD40 signals appear to be more critical during the priming stages of EAE than during established disease. Microglia express CD40, and upregulate CD40 further following IFN-γ treatment [41]. CD40 signaling in microglia results in the production of TNF and IL-12, microglial activation, and neuronal cell death[41–43]. Indeed, CD40 expression on microglia is critical for EAE progression, as illustrated by experiments using bone-marrow chimeras [43, 44]. Mice lacking expression of CD40 only in the radioresistant CNS compartment have less severe EAE disease, characterized by fewer CNS-infiltrating encephalitogenic T cells and less demyelination [43, 44]. Therefore, CD40 signals are required not only for initial priming of T cell responses in the periphery, but also for optimal T cell expansion and/or retention in the target tissue.
Although MS has a strong genetic component, the CD40 gene does not lie within any of the identified genomic risk regions [29, 45]. A small case-control study in a heterogeneous population of MS vs. Huntington’s disease patients found no association of the Kozak sequence SNP (C/T−1) with MS susceptibility or disease course [46], although this SNP has been associated with Graves’ disease (see above) [47, 48].
CD40 and Psoriasis
Psoriasis is an autoinflammatory skin disease affecting 2–3% of Caucasians [49]. Thick, scaly plaques affect extensor surfaces of the skin, containing hyperproliferative keratinocytes and a complex immune infiltrate of T cells, macrophages, neutrophils and DC [50]. Arthritis and arthralgias are also present in some patients, a condition called psoriatic arthritis [49, 50].
Activated T cells and TNF- and iNOS producing DC (Tip-DC)[51] are essential for plaque formation, although the antigen driving T cell activation is unknown[49]. Indeed, Tip-DC found in human psoriatic skin biopsies express CD40 and as the near-exclusive producers of iNOS and TNF within the psoriatic plaque, play an important role in plaque maintenance [51]. Human keratinocytes and endothelial cells express CD40 within psoriatic plaques, adjacent to T cells and ICAM-1-expressing cells [52, 53], with more pronounced staining in early vs. chronic lesions. CD40 is further upregulated by IFN-γ [52]. Keratinocytes treated with soluble CD154 + IFN-γ upregulate ICAM-1, the anti-apoptotic protein Bcl-x, and chemokines IL-8 [52], CCL20 [54], RANTES, and MCP-1 [55], which recruit T cells and monocytes into the inflammatory lesion.
CD154 is overexpressed on peripheral blood T cells isolated from patients with psoriatic arthritis [56]. The importance of T cell CD80/86 costimulation in psoriasis pathogenesis is further illustrated by the ability of CTLA-4-Ig treatment to decrease lesion activity and the expression of CD40 and ICAM-1 within psoriatic plaques [57]. These data suggest that CD40 signaling recruits and activates T cells, monocytes, and DC which contribute to ongoing inflammation and keratinocyte activation. No data exist on the role of CD40 in mouse models of psoriasis, in part because no small animal model fully recapitulates the human disease [58]. However, mice expressing a CD154 transgene in basal keratinocytes develop skin lesions containing CD4+ and CD8+ T cells and macrophages within 6 weeks of age, indicating that aberrant CD40 signaling within the skin is sufficient to initiate autoimmunity [59].
Although polymorphisms in a growing number of human genes are associated with psoriasis and psoriatic arthritis, the CD40 gene does not lie within any identified susceptibility regions [49]. This suggests that differences in CD40 expression or function do not play a role in initiating psoriasis. However, CD40 signaling may contribute to psoriasis severity once the autoimmune response is initiated. The success of TNF antagonists in psoriasis treatment suggests that inflammatory cytokines downstream of CD40 signaling are required for plaque maintenance[50].
CD40 and Inflammatory Bowel Disease (IBD)
Crohn’s Disease (CD) and ulcerative colitis are the two main subtypes of IBD, characterized by relapsing inflammation of the small and large intestine [60]. T cells, B cells, and macrophages infiltrate the intestinal epithelium and disrupt barrier function, resulting in diarrhea, abdominal pain, rectal bleeding, and malnutrition [60].
The first clues that CD40 may play a role in IBD came from studies in mice. IBD can be modeled in mice by administration of the hapten 2,4,6,-trinitrobenzene sulfonic acid (TNBS) or by adoptive transfer of activated CD45RBhighCD4+ T cells into Rag−/− recipients. In both models, administration of an antagonistic anti-CD154 mAb at the time of colitis induction prevents disease and lymphocytic infiltration and decreases IFN-γ production by gut-infiltrating T cells, effects which can be reversed by co-administration of IL-12 [61, 62]. Blocking CD154 four weeks after T cell transfer ameliorates established disease, but has no effect in the TNBS-induced colitis model. These studies suggest that CD40-CD154 interactions are crucial for IBD initiation but not required for ongoing inflammatory responses [61].
Transgenic mice expressing CD154 driven by the Lck promoter develop lymphocytic infiltrates in multiple organs and lethal IBD by 3–6 weeks of age, illustrating the importance of tightly controlled CD154 expression for preventing autoimmunity [63]. CD40 is expressed by normal human colonic fibroblasts and can be further upregulated by IFN-γ. CD40 signaling in these cells results in the activation of NF-κB and secretion of IL-6, MCP-1 and IL-8 [64]. CD40 is also overexpressed on microvascular endothelial cells in the inflamed mucosa of CD patients [65]. An increased percentage of CD40+ DCs are found within the intestinal mucosa of CD patients[66], although most of the CD40+ cells are B cells or macrophages [67, 68]. CD154 is overexpressed on peripheral blood [65] and lamina propria CD4+ T cells[67, 68] and can be reversed by treatment with TNF antagonists [65].
Similar to MS and psoriasis, multiple genetic associations and disease-causing alleles have been identified for IBD [45, 60]. Although none of the genomic loci associated with IBD incidence contain the CD40 gene [45], polymorphisms in genes related to the Th17 pathway including IL12B, STAT3, and IL23R confer increased risk of developing the disease [45, 60]. CD40 signaling in multiple cell types leads to the production of IL-6, IL-12, and IL-23 and may therefore contribute to disease initiation and/or progression in susceptible individuals[7]. Indeed, a small study treating CD patients with a chimeric antagonistic anti-CD40 mAb showed some benefit, with 77% of patients responding to the study drug and 22% entering remission [69] (see further discussion of CD40-directed therapeutics below).
CD40 and Rheumatoid Arthritis (RA)
RA is a chronic inflammatory arthritis affecting ~1% of the world’s population and leading to joint destruction if untreated [70]. Cells of the innate and adaptive immune system infiltrate the joint space, driving local production of pro-inflammatory Th1 and Th17-type cytokines, chemokines, and matrix metalloproteinases by infiltrating monocytes and synovial cells [71]. Synovial cells also proliferate, leading to thickening of the synovium and degradation of the underlying cartilage and bone [70].
CD40 is functionally expressed on smooth muscle fibroblasts from normal and RA patients[72] and RA synovial cells [73], and can be upregulated by pro-inflammatory cytokines including IFN-γ and TNFα. CD40 signals in these cell types results in fibroblast proliferation, adhesion molecule upregulation [72], and secretion of pro-inflammatory cytokines and chemokines. These include IL-6, GM-CSF, and MIP-1α [72, 73]. When cultured with activated T cells from RA patients, fibroblast-like synovial cells secrete increased amounts of IL-15, TNF, and IL-17, as well as IL-8 and MCP-1 [74] in a CD40-dependent manner [74, 75]. Similar results are observed when CD40-activated monocytes are cultured with fibroblast-like synovial cells [76]. These data suggest that CD40 signaling on either monocytes or synovial fibroblasts sets up a complex network of pro-inflammatory cytokine and chemokine secretion which contributes to joint destruction [70, 71]. In support of this scenario, CD40 signaling in fibroblast-like synovial cells induces expression of RANKL, which stimulates osteoclast-mediated bone resorption [77]. A nurse-cell like population in the bone marrow and synovium of RA patients which is thought to support B cell survival upregulates CD40 in response to IFN-γ treatment; however, the functional consequences of CD40 signaling in these cells is unknown [78]. The adherent fraction of synovial tissue cells, which likely contains macrophages and DC, secretes TNF in response to CD40 ligation [79, 80]. DC-derived TNF contributes directly to collagen destruction in ex vivo cultures [81]. A subset of RA patients most prone to severe disease have detectable antibodies against citrulline-containing peptides (anti-CCP Abs)[82]. CD40 signals are required to induce IgM anti-CCP Ab secretion by B cells from either healthy controls or RA patients, but only B cells from anti-CCP seropositive patients secrete anti-CCP Abs spontaneously ex vivo, suggesting that these cells already received CD40 signals within the synovial compartment[82].
CD154 is upregulated faster and to a higher degree on peripheral blood and synovial T cells from RA patients compared to healthy controls, induces more Ig production by B cells, and is required for IL-12 production by synovial DC and macrophages [79, 80, 83, 84]. Overexpression of CD154 on T cells correlates with higher disease activity and fewer remissions [85].
Treating mice with an antagonistic anti-CD154 mAb prior to induction of collagen-induced arthritis (CIA, a mouse RA model), or at the time pathogenic autoantibodies are transferred in the K/B×N mouse model of inflammatory arthritis prevents [86] or ameliorates [87] disease. However, treatment of mice in either model with anti-CD154 mAb after arthritis has developed does not reverse or stabilize established disease [86, 87]. Furthermore, transferring serum from arthritic K/BxN mice into CD154−/− mice still results in severe arthritis [87]. Administering agonistic anti-CD40 Abs to mice at the time of CIA induction exacerbates disease, correlating with increased IFN-γ production by collagen-specific T cells [88]. These data suggest that CD40 signals are important at the initial stages of arthritis induction, but are not required once disease is established and pathogenic antibodies are already present.
Multiple genome association studies have been performed in large cohorts of RA patients, identifying multiple genomic loci associated with RA incidence [45]. The CD40 locus is associated with juvenile RA by genome linkage analysis[89]. A SNP within the CD40 locus has recently been associated with disease incidence in RA patients of European [90], but not Korean ancestry [91]. This study also identified several other SNPs in components of the CD40 signaling pathway, including TNFAIP3 (also known as A20, an E3-ubiquitin ligase) and TRAF1-C5. TRAF1 is an adaptor protein that cooperates with TRAF2 to enhance CD40 signals [1]. Whether these SNPs result in changes in expression levels of CD40, A20, or TRAF1 or in altered protein functions is an exciting and important area for future study. A SNP in the 3′ untranslated region of CD154 (24CAs) is underrepresented in female RA patients from Spain compared to healthy controls; this association was not found in male RA patients [92]. The 24CAs allele has a longer mRNA half-life, but a lower percentage of CD4+ T cells bearing the 24CAs allele express CD154 following stimulation than non-24CAs individuals[92]. How this particular allele protects against RA development or progression remains to be elucidated [92].
CD40 and Systemic Lupus Erythematosus (SLE)
SLE is a systemic autoimmune disease in which autoantibodies to dsDNA and other nuclear components form immune complex deposits in small blood vessels throughout the body, affecting the skin, joints, lungs, heart, brain, and kidneys[93]. The disease has multiple manifestations, and a patient need manifest only 4/11 criteria to meet the diagnosis of SLE [93].
CD154 is overexpressed on CD4+ and CD8+ T cells from SLE patients with active disease, and is ectopically expressed on monocytes and B cells[3, 4, 94, 95]. B cell CD154 is functional, because CD154+ B cells from SLE patients spontaneously produce antibodies in vitro in a CD154-dependent manner [3]. Transgenic mice expressing B cell CD154 develop a lupus-like disease with age, suggesting that ectopic expression of CD154 on B cells may be sufficient to cause autoimmunity[96]. Treatment with Rituximab, an anti-CD20 mAb which depletes B cells, decreases the frequency of remaining B cells expressing CD40 and T cells expressing CD154, suggesting that some of the benefit of this drug in treating lupus patients may be secondary to decreased activation of the CD40 signaling pathway[97, 98]. SLE patients also have elevated levels of sCD154 in serum which correlates with disease activity[99]. Human kidney mesangial cells express CD40 and upregulate this receptor in response to IFN-γ treatment or activated CD154+ platelets from SLE patients[100]. CD40 signaling in mesangial cells results in proliferation and production of the pro-fibrotic cytokine TGF-β[100], which may contribute to kidney disease. SLE patients have a decreased frequency of CD34+ hematopoietic progenitor cells in the bone marrow, and an increased degree of apoptosis in these cells compared to controls. CD40 signaling in CD34+ cells leads to the upregulation of Fas and Fas-induced apoptosis which may contribute to the pan-cytopenias often found in lupus patients[101].
The (NZB × NZW)F1 and (SWR × NZB)F1 mouse strains develop SLE-like symptoms spontaneously. Treatment of either strain with anti-CD154 Abs prior to onset of symptoms delays or prevents proteinuria, prolongs survival, ameliorates or prevents kidney disease and decreases anti-DNA Ab titers which usually rebound once therapy is stopped [102–104]. Treatment after moderate to severe proteinuria manifests prolongs survival and ameliorates kidney disease and immune complex deposition [104–107]. Even a short course of anti-CD154 mAb treatment can have long-lasting beneficial effects on survival, anti-dsDNA Ab production, and kidney disease, especially when combined with anti-CTLA4Ig [108]. However, trials of anti-CD154 mAb treatment for SLE patients have had mixed results (see below).
Multiple genome linkage analyses have identified regions in humans and in mice which are associated with SLE [45, 93]. The CD40 gene lies on human chromosome 20q11.2-13.1, a region identified as suggestive for linkage to SLE incidence [93, 109]. A particular missense SNP, rs11086998 G, results in a proline-to-alanine substitution at amino acid 227 of the CD40 protein (CD40-P227A), within the cytoplasmic tail of CD40 and three residues upstream of the TRAF6 binding site [110]. Interestingly, rs11086998 G is overrepresented in individuals of Mexican and South American descent [110]. Although this allele is not associated with SLE incidence in Hispanic populations, patients of Hispanic ethnicity have a tendency toward increased severity of SLE symptoms, particularly lupus nephritis [111]. Interestingly, CD40-P227A exhibits gain-of-function signaling capacity when compared to CD40-Wt, namely, increased antibody and pro-inflammatory cytokine secretion due to hyperactivation of the JNK pathway[110]. Therefore, CD40-P227A may contribute to SLE severity or exacerbation in those who have already developed the disease. Whether CD40-P227A is associated with increased incidence or severity of the other autoimmune diseases discussed here remains to be tested.
CD40:CD154 Interactions as a Therapeutic Target
The preceding sections establish that CD40 signals make important contributions to initiation and progression of many autoimmune diseases (Table 1). Strategies for alleviating these diseases by inhibiting CD40 signaling have been ongoing for over a decade, recently reviewed in [112]. Space does not permit discussion of all studies involved, but we will briefly discuss major approaches, outcomes and challenges.
Table 1.
Disease in which CD40:CD154 interactions play a role in pathogenesis
| Ectopic CD154 expression | SLE |
|---|---|
| CD154 overexpressed on T cells | MS Psoriasis IBD RA SLE |
| CD40 overexpressed and/or ectopically expressed | EAT Type 1 DM Psoriasis IBD RA |
| Decreased disease activity upon CD40:CD154 blockade (mice) | EAT NOD mice/BB rats EAE IBD CIA SLE |
The majority of approaches to intervening in CD40-CD154 interactions used CD154-specific blocking mAbs. This strategy is highly efficacious in mouse models as discussed above and in [112]. While overall benefit of blocking CD40-CD154 association is decreased when therapy is given after disease establishment, anti-CD154 therapy also prevents relapses of ongoing disease, and/or halts pathologic progression in models of RA, SLE, MS, IBD, diabetes and inflammatory heart disease (above and [112]. In a model of pemphigus vulgaris, anti-CD154 Ab pre-treatment effectively induces tolerance to the major autoantigen, desmogelin-3. However, the treatment is ineffective in established disease [113]. Similarly, anti-CD154 Ab treatment from birth of mice prone to develop a disorder similar to human systemic sclerosis prevents disease development [114].
Although the major mechanism of anti-CD154 Ab therapy is assumed as physical blocking of CD40-CD154 interaction and preventing T cell priming, additional activities have been implicated. In the MS model EAE, anti-CD154 prevents disease development, but also blocks relapse of established disease, possibly by inhibiting Th1 differentiation and/or effector T cell CNS migration and activity [37]. Similarly, delayed anti-CD154 treatment in mouse IBD shows significant clinical efficacy correlative with reduced cytokines, suggesting the effector phase of disease is affected [62, 115]. Anti-CD154 treatment may affect a small subset of CD40+ T cells implicated in pathogenesis of autoimmunity (see M.E. Munroe, this volume). Such T cells could be depleted via complement fixation and binding to FcγR on effector cells.
Despite the considerable promise shown for CD154 blocking Abs in mouse models, clinical experience has been mixed. A particular challenge has been thromboembolism [112, 116]. The reasons for this complication in humans have not been defined, but one significant difference is the presence of the FcγRIIA on human, but not mouse platelets [117]. Thus, as CD154 is expressed on activated platelets [118], anti-CD154 mAb could engage FcγRIIA on the same or adjacent platelets, causing aggregation. CD154 may also stabilize arterial thrombi by binding platelet integrins [119].
The first anti-CD154 mAb used in clinical trials was humanized 5c8 Ab, ruplizumab. Ruplizumab treatment induced partial therapeutic responses in some SLE patients, but trials stopped early due to thromboses in some recipients [120, 121]. Both ruplizumab and a second anti-CD154 mAb, toralizumab, induced partial responses in some patients with refractory ITP, and no thrombotic complications were seen, possibly because ITP patients have low platelet numbers[122]. However, no significant improvement was seen in a toralizumab trial of SLE patients [123], and Crohn’s disease trials were halted after a thrombosis developed in a recipient [112]. A third anti-CD154 mAb, ABI793, binds to a different CD154 epitope, and showed promise in primates, but thromboembolism occurred during organ transplant trials [112]. Interestingly, this indicates that this complication occurs independently of CD154 epitope.
Given recurring concerns about thromboembolism with therapies using intact anti-CD154 mAbs, alternatives are needed to exploit the benefits of blocking CD40 signals to alleviate autoimmunity. One possibility is engineering Abs that won’t bind FcRs or activate complement (C′). As this will not allow C′-mediated T cell elimination, such Abs may not prevent transplant rejection, but may still benefit autoimmune diseases. An aglycosyl ruplizumab with decreased FcR and C′ binding cannot prevent transplant rejection in monkeys, but prolongs survival and reduces autoantibody production in mouse SLE [124]. Aglycosyl mAbs remain effective in alleviating symptoms in mouse EAE [125]. Another approach used blocking peptide mimics that have the functional CD40 binding site but block association of CD154 [126, 127]. Some of these peptides have efficacy in relieving symptoms of mouse EAE [126], but only at high concentrations [126, 127], a challenge for practical applications.
A promising alternative uses mAbs against CD40, rather than CD154. While many anti-CD40 mAbs exist, and vary considerably in specific epitope recognition and binding affinities, no consistent correlations have emerged between affinity, ability to block CD154 binding, and agonistic activity (reviewed in [112]. However, several mAbs antagonistic to CD40 activation signals show promising initial results as autoimmune disease treatments. The human anti-CD40 mAb HCD122 blocks and competes with CD154 for binding to CD40, but does not itself induce CD40 signaling, and mediates antibody-dependent cellular cytotoxicity against CD40+ myeloma cells [128]. However, it isn’t clear if this mAb would be useful in autoimmune disease therapy. Another humanized anti-human CD40 antagonist mAb, ch5D12, showed promise in a phase I/IIa Crohn’s disease study [69]. Thus, future clinical development of antagonistic anti-CD40 mAbs is desirable.
The preceding overview emphasizes the multiple ways in which CD40:CD154 interactions, critical for normal adaptive immunity, can also play important roles in the establishment and pathogenesis of a wide variety of autoimmune disease. New ways of intervention that spare normal immune function while blocking damaging effects of CD40 signaling are a key future goal.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bishop GA, Moore CR, Xie P, Stunz LL, Kraus ZJ. TRAF proteins in CD40 signaling. Adv Exp Biol Med. 2007;597:131–51. doi: 10.1007/978-0-387-70630-6_11. [DOI] [PubMed] [Google Scholar]
- 2.Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001;58(1):4–43. doi: 10.1007/PL00000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grammer AC, Slota R, Fischer R, Gur H, Girschick H, Yarboro C, et al. Abnormal GC reactions in SLE demonstrated by blockade of CD154-CD40 interactions. J Clin Invest. 2003;112(10):1506–20. doi: 10.1172/JCI19301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katsiari CG, Liossis SN, Souliotis VL, Dimopoulos AM, Manoussakis MN, Sfikakis PP. Aberrant expression of the costimulatory molecule CD40L on monocytes from patients with SLE. Clin Immunol. 2002;103(1):54–62. doi: 10.1006/clim.2001.5172. [DOI] [PubMed] [Google Scholar]
- 5.Bishop GA. The multifaceted roles of TRAFs in the regulation of B cell function. Nat Rev Immunol. 2004;4(Oct):775–86. doi: 10.1038/nri1462. [DOI] [PubMed] [Google Scholar]
- 6.Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, et al. The TNFRs RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29(3):423–37. doi: 10.1016/j.immuni.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Iezzi G, Sonderegger I, Ampenberger F, Schmitz N, Marsland BJ, Kopf M. CD40-CD40L cross-talk integrates strong antigenic signals and microbial stimuli to induce development of IL-17-producing CD4+ T cells. Proc Natl Acad Sci U S A. 2009;106(3):876–81. doi: 10.1073/pnas.0810769106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bottazzo GF, Pujol-Borrell R, Hanafusa T, Feldmann M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983;2(8359):1115–9. doi: 10.1016/s0140-6736(83)90629-3. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson EM, Huber AK, Akeno N, Sivak M, Li CW, Concepcion E, et al. A CD40 Kozak sequence polymorphism and susceptibility to antibody-mediated autoimmune conditions: the role of CD40 tissue-specific expression. Genes Immun. 2007;8(3):205–14. doi: 10.1038/sj.gene.6364375. [DOI] [PubMed] [Google Scholar]
- 10.Barbe-Tuana FM, Klein D, Ichii H, Berman DM, Coffey L, Kenyon NS, et al. CD40-CD40L interaction activates proinflammatory pathways in pancreatic islets. Diabetes. 2006;55(9):2437–45. doi: 10.2337/db05-1673. [DOI] [PubMed] [Google Scholar]
- 11.Wagner DH, Vaitaitis G, Sanderson R, Poulin M, Dobbs C, Haskins K. Expression of CD40 identifies a unique pathogenic T cell population in T1D. Proc Natl Acad Sci (USA) 2002;99(6):3782–7. doi: 10.1073/pnas.052247099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waid DM, Wagner RJ, Putnam A, Vaitaitis GM, Pennock ND, Calverley DC, et al. A unique T cell subset described as CD4loCD40+ T cells in human T1D. Clin Immunol. 2007;124(2):138–48. doi: 10.1016/j.clim.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson EM, Tomer Y. The CD40, CTLA-4, thyroglobulin, TSH receptor, and PTPN22 gene quintet and its contribution to thyroid autoimmunity: back to the future. J Autoimmun. 2007;28(2–3):85–98. doi: 10.1016/j.jaut.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faure GC, Bensoussan-Lejzerowicz D, Bene MC, Aubert V, Leclere J. Coexpression of CD40 and class II antigen HLA-DR in GD thyroid epithelial cells. Clin Immunol Immunopathol. 1997;84(2):212–5. doi: 10.1006/clin.1997.4391. [DOI] [PubMed] [Google Scholar]
- 15.Smith TJ, Sciaky D, Phipps RP, Jennings TA. CD40 expression in human thyroid tissue: evidence for involvement of multiple cell types in autoimmune and neoplastic diseases. Thyroid. 1999;9(8):749–55. doi: 10.1089/thy.1999.9.749. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson EM, Concepcion E, Oashi T, Tomer Y. A GD-associated Kozak sequence SNP enhances the efficiency of CD40 gene translation: a case for translational pathophysiology. Endocrinology. 2005;146(6):2684–91. doi: 10.1210/en.2004-1617. [DOI] [PubMed] [Google Scholar]
- 17.Carayanniotis G, Masters SR, Noelle RJ. Suppression of murine thyroiditis via blockade of the CD40-CD40L interaction. Immunology. 1997;90(3):421–6. doi: 10.1111/j.1365-2567.1997.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson KE, Braley-Mullen H. CD40L is necessary for the priming of effector cells for lymphocytic and granulomatous EAT. J Autoimmun. 1999;12(1):1–12. doi: 10.1006/jaut.1998.0256. [DOI] [PubMed] [Google Scholar]
- 19.Marino E, Grey ST. A new role for an old player: do B cells unleash the self-reactive CD8+ T cell storm necessary for the development of T1D? J Autoimmun. 2008;31(3):301–5. doi: 10.1016/j.jaut.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Balasa B, Krahl T, Paststone G, Lee J, Tisch R, McDevitt HO, et al. CD40L-CD40 interactions are necessary for the initiation of insulitis and diabetes in NOD mice. J Immunol. 1997;159:4620–7. [PubMed] [Google Scholar]
- 21.Nanji SA, Hancock WW, Luo B, Schur CD, Pawlick RL, Zhu LF, et al. Costimulation blockade of both ICOS and CD40L induces dominant tolerance to islet allografts and prevents spontaneous autoimmune diabetes in the NOD mouse. Diabetes. 2006;55(1):27–33. [PubMed] [Google Scholar]
- 22.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B lymphocytes are critical APC for the initiation of T cell-mediated autoimmune diabetes in NOD mice. J Immunol. 1998;161(8):3912–8. [PubMed] [Google Scholar]
- 23.Marino E, Villanueva J, Walters S, Liuwantara D, Mackay F, Grey ST. CD4+ CD25+ T cells control autoimmunity in the absence of B cells. Diabetes. 2009 doi: 10.2337/db08-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marino E, Batten M, Groom J, Walters S, Liuwantara D, Mackay F, et al. MZB-cells of NOD mice expand with diabetes onset, invade the pancreatic LN, and present autoantigen to diabetogenic T-cells. Diabetes. 2008;57(2):395–404. doi: 10.2337/db07-0589. [DOI] [PubMed] [Google Scholar]
- 25.Taplin CE, Barker JM. Autoantibodies in T1D. Autoimmunity. 2008;41(1):11–8. doi: 10.1080/08916930701619169. [DOI] [PubMed] [Google Scholar]
- 26.Beaudette-Zlatanova BC, Whalen B, Zipris D, Yagita H, Rozing J, Groen H, et al. Costimulation and autoimmune diabetes in BB rats. Am J Transplant. 2006;6(5 Pt 1):894–902. doi: 10.1111/j.1600-6143.2005.01227.x. [DOI] [PubMed] [Google Scholar]
- 27.Klein D, Barbe-Tuana F, Pugliese A, Ichii H, Garza D, Gonzalez M, et al. A functional CD40 receptor is expressed in pancreatic beta cells. Diabetologia. 2005;48(2):268–76. doi: 10.1007/s00125-004-1645-7. [DOI] [PubMed] [Google Scholar]
- 28.Baker RL, Wagner DH, Jr, Haskins K. CD40 on NOD CD4 T cells contributes to their activation and pathogenicity. J Autoimmun. 2008;31(4):385–92. doi: 10.1016/j.jaut.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Sospedra M, Martin R. Immunology of MS. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 30.Gerritse K, Laman JD, Noelle RJ, Aruffo A, Ledbetter JA, Boersma WJA, et al. CD40-CD40L interactions in EAE and MS. Proc Natl Acad Sci (USA) 1996;93(March):2499–504. doi: 10.1073/pnas.93.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balashov KE, Smith DR, Khoury SJ, Hafler DA, Weiner HL. Increased IL-12 production in pMS: induction by activated CD4+ T cells via CD40L. Proc Natl Acad Sci U S A. 1997;94(2):599–603. doi: 10.1073/pnas.94.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teleshova N, Bao W, Kivisakk P, Ozenci V, Mustafa M, Link H. Elevated CD40L-expressing blood T-cell levels in MS are reversed by interferon-beta treatment. Scand J Immunol. 2000;51(3):312–20. doi: 10.1046/j.1365-3083.2000.00688.x. [DOI] [PubMed] [Google Scholar]
- 33.Issazadeh S, Navikas V, Schaub M, Sayegh M, Khoury S. Kinetics of expression of costimulatory molecules and their ligands in murine rEAE in vivo. J Immunol. 1998;161(3):1104–12. [PubMed] [Google Scholar]
- 34.Grewal IS, Foellmer HG, Grewal KD, Xu J, Hardardottir F, Baron JL, et al. Requirement for CD40L in costimulation induction, T cell activation, and EAE. Science. 1996;273(27 Sept):1864–7. doi: 10.1126/science.273.5283.1864. [DOI] [PubMed] [Google Scholar]
- 35.Samoilova EB, Horton JL, Zhang H, Chen Y. CD40L blockade prevents autoimmune encephalomyelitis and hampers TH1 but not TH2 pathway of T cell differentiation. J Mol Med. 1997;75(8):603–8. doi: 10.1007/s001090050145. [DOI] [PubMed] [Google Scholar]
- 36.Howard LM, Miller SD. Autoimmune intervention by CD154 blockade prevents T cell retention and effector function in the target organ. J Immunol. 2001;166(3):1547–53. doi: 10.4049/jimmunol.166.3.1547. [DOI] [PubMed] [Google Scholar]
- 37.Howard LM, Miga AJ, Vanderlugt CL, Dal Canto MC, Laman JD, Noelle RJ, et al. Mechanisms of immunotherapeutic intervention by anti-CD40L antibody in an animal model of MS. J Clin Invest. 1999;103(2):281–90. doi: 10.1172/JCI5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abromson-Leeman S, Maverakis E, Bronson R, Dorf ME. CD40-mediated activation of T cells accelerates, but is not required for, encephalitogenic potential of MBP-recognizing T cells in a model of pEAE. Eur J Immunol. 2001;31(2):527–38. doi: 10.1002/1521-4141(200102)31:2<527::aid-immu527>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 39.Howard LM, Dal Canto MC, Miller SD. Transient anti-CD154-mediated immunotherapy of ongoing rEAE induces long-term inhibition of disease relapses. J Neuroimmunol. 2002;129(1–2):58–65. doi: 10.1016/s0165-5728(02)00175-3. [DOI] [PubMed] [Google Scholar]
- 40.Schaub M, Issazadeh S, Stadlbauer TH, Peach R, Sayegh MH, Khoury SJ. Costimulatory signal blockade in murine rEAE. J Neuroimmunol. 1999;96(2):158–66. doi: 10.1016/s0165-5728(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 41.Tan J, Town T, Paris D, Placzek A, Parker T, Crawford F, et al. Activation of microglial cells by the CD40 pathway: relevance to multiple sclerosis. J Neuroimmunol. 1999;97(1–2):77–85. doi: 10.1016/s0165-5728(99)00053-3. [DOI] [PubMed] [Google Scholar]
- 42.Becher B, Blain M, Antel JP. CD40 engagement stimulates IL-12p70 production by human microglial cells: basis for Th1 polarization in the CNS. J Neuroimmunol. 2000;102(1):44–50. doi: 10.1016/s0165-5728(99)00152-6. [DOI] [PubMed] [Google Scholar]
- 43.Ponomarev ED, Shriver LP, Dittel BN. CD40 expression by microglial cells is required for their completion of a two-step activation process during CNS autoimmune inflammation. J Immunol. 2006;176(3):1402–10. doi: 10.4049/jimmunol.176.3.1402. [DOI] [PubMed] [Google Scholar]
- 44.Becher B, Durell BG, Miga AV, Hickey WF, Noelle RJ. The clinical course of EAE and inflammation is controlled by the expression of CD40 within the CNS. J Exp Med. 2001;193(8):967–74. doi: 10.1084/jem.193.8.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lettre G, Rioux JD. Autoimmune diseases: insights from genome-wide association studies. Hum Mol Genet. 2008;17(R2):R116–21. doi: 10.1093/hmg/ddn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buck D, Kroner A, Rieckmann P, Maurer M, Wiendl H. Analysis of the C/T(−1) SNP in the CD40 gene in MS. Tissue Antigens. 2006;68(4):335–8. doi: 10.1111/j.1399-0039.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 47.Tomer Y, Concepcion E, Greenberg DA. A C/T SNP in the region of the CD40 gene is associated with GD. Thyroid. 2002;12(12):1129–35. doi: 10.1089/105072502321085234. [DOI] [PubMed] [Google Scholar]
- 48.Kurylowicz A, Kula D, Ploski R, Skorka A, Jurecka-Lubieniecka B, Zebracka J, et al. Association of CD40 gene polymorphism with susceptibility and phenotype of GD. Thyroid. 2005;15(10):1119–24. doi: 10.1089/thy.2005.15.1119. [DOI] [PubMed] [Google Scholar]
- 49.Bowcock AM, Krueger JG. Getting under the skin: the immunogenetics of psoriasis. Nat Rev Immunol. 2005;5(9):699–711. doi: 10.1038/nri1689. [DOI] [PubMed] [Google Scholar]
- 50.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–73. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 51.Lowes MA, Chamian F, Abello MV, Fuentes-Duculan J, Lin SL, Nussbaum R, et al. Increase in TNF-alpha and iNOS-expressing DC in psoriasis and reduction with efalizumab. Proc Natl Acad Sci U S A. 2005;102(52):19057–62. doi: 10.1073/pnas.0509736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Denfeld RW, Hollenbaugh D, Fehrenbach A, Weiss JM, von Leoprechting A, Mai B, et al. CD40 is functionally expressed on human keratinocytes. Eur J Immunol. 1996;26(10):2329–34. doi: 10.1002/eji.1830261009. [DOI] [PubMed] [Google Scholar]
- 53.Ohta Y, Hamada Y. In situ Expression of CD40 and CD40L in psoriasis. Dermatology. 2004;209(1):21–8. doi: 10.1159/000078582. [DOI] [PubMed] [Google Scholar]
- 54.Homey B, Dieu-Nosjean MC, Wiesenborn A, Massacrier C, Pin JJ, Oldham E, et al. Up-regulation of CCL20 and CCR6 in psoriasis. J Immunol. 2000;164(12):6621–32. doi: 10.4049/jimmunol.164.12.6621. [DOI] [PubMed] [Google Scholar]
- 55.Pasch MC, Timar KK, van Meurs M, Heydendael VM, Bos JD, Laman JD, et al. In situ demonstration of CD40- and CD154-positive cells in psoriatic lesions and keratinocyte production of chemokines by CD40 ligation in vitro. J Pathol. 2004;203(3):839–48. doi: 10.1002/path.1581. [DOI] [PubMed] [Google Scholar]
- 56.Daoussis D, Antonopoulos I, Andonopoulos AP, Liossis SN. Increased expression of CD40L on stimulated T-cells from patients with PsA. Rheumatology (Oxford) 2007;46(2):227–31. doi: 10.1093/rheumatology/kel229. [DOI] [PubMed] [Google Scholar]
- 57.Abrams JR, Kelley SL, Hayes E, Kikuchi T, Brown MJ, Kang S, et al. Blockade of T lymphocyte costimulation with CTLA4Ig reverses the cellular pathology of psoriatic plaques, including the activation of keratinocytes, DC, and endothelial cells. J Exp Med. 2000;192(5):681–94. doi: 10.1084/jem.192.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boehncke WH, Schon MP. Animal models of psoriasis. Clin Dermatol. 2007;25(6):596–605. doi: 10.1016/j.clindermatol.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 59.Mehling A, Loser K, Varga G, Metze D, Luger TA, Schwarz T, et al. Overexpression of CD40L in murine epidermis results in chronic skin inflammation and systemic autoimmunity. J Exp Med. 2001;194(5):615–28. doi: 10.1084/jem.194.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cho JH. IBD: genetic and epidemiologic considerations. World J Gastroenterol. 2008;14(3):338–47. doi: 10.3748/wjg.14.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stuber E, Strober W, Neurath M. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of Th1 cells through the inhibition of IL-12 secretion. J Exp Med. 1996;183(2):693–8. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Z, Geboes K, Colpaert S, Overbergh L, Mathieu C, Heremans H, et al. Prevention of experimental colitis in SCID mice reconstituted with CD45RBhigh CD4+ T cells by blocking the CD40-CD154 interactions. J Immunol. 2000;164:6005–14. doi: 10.4049/jimmunol.164.11.6005. [DOI] [PubMed] [Google Scholar]
- 63.Clegg CH, Rulffes JT, Haugen HS, Hoggatt H, Aruffo A, Durham SK, et al. Thymus dysfunction and chronic inflammatory disease in gp39 transgenic mice. International Immunology. 1997;9(8):1111–22. doi: 10.1093/intimm/9.8.1111. [DOI] [PubMed] [Google Scholar]
- 64.Gelbmann CM, Leeb SN, Vogl D, Maendel M, Herfarth H, Scholmerich J, et al. Inducible CD40 expression mediates NFkappaB activation and cytokine secretion in human colonic fibroblasts. Gut. 2003;52(10):1448–56. doi: 10.1136/gut.52.10.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Danese S, Sans M, Scaldaferri F, Sgambato A, Rutella S, Cittadini A, et al. TNF-a blockade downregulates the CD40/CD40L pathway in the mucosal microcirculation: a novel anti-inflammatory mechanism of infliximab in CD. J Immunol. 2006;176(4):2617–24. doi: 10.4049/jimmunol.176.4.2617. [DOI] [PubMed] [Google Scholar]
- 66.Hart AL, Al-Hassi HO, Rigby RJ, Bell SJ, Emmanuel AV, Knight SC, et al. Characteristics of intestinal DC in IBD. Gastroenterology. 2005;129(1):50–65. doi: 10.1053/j.gastro.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 67.Liu Z, Colpaert S, D’Haens GR, Kasran A, de Boer M, Rutgeerts P, et al. Hyperexpression of CD154 in IBD and its contribution to pathogenic cytokine production. J Immunol. 1999;163(7):4049–57. [PubMed] [Google Scholar]
- 68.Battaglia E, Biancone L, Resegotti A, Emanuelli G, Fronda GR, Camussi G. Expression of CD40 and CD40L in intestinal lesions of CD. Am J Gastroenterol. 1999;94(11):3279–84. doi: 10.1111/j.1572-0241.1999.01538.x. [DOI] [PubMed] [Google Scholar]
- 69.Kasran A, Boon L, Wortel CH, Van Hogezand RA, Schreiber S, Goldin E, et al. Safety and tolerability of antagonist anti-human CD40 mAb ch5D12 in patients with moderate to severe CD. Aliment Pharmacol Ther. 2005;22:111–22. doi: 10.1111/j.1365-2036.2005.02526.x. [DOI] [PubMed] [Google Scholar]
- 70.Noss EH, Brenner MB. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in RA. Immunol Rev. 2008;223:252–70. doi: 10.1111/j.1600-065X.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- 71.Brennan FM, McInnes IB. Evidence that cytokines play a role in RA. J Clin Invest. 2008;118(11):3537–45. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yellin MJ, Winikoff S, Fortune SM, Baum D, Crow MK, Lederman S, et al. Ligation of CD40 on fibroblasts induces CD54 and CD106 upregulation, IL-6 production, and proliferation. J Leuk Biol. 1995;58:209–16. doi: 10.1002/jlb.58.2.209. [DOI] [PubMed] [Google Scholar]
- 73.Rissoan MC, Van Kooten C, Chomarat P, Galibert L, Durand I, Thivolet-Bejui F, et al. The functional CD40 antigen of fibroblasts may contribute to the proliferation of rheumatoid synovium. Clin Exp Immunol. 1996;106:481–90. doi: 10.1046/j.1365-2249.1996.d01-858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Min DJ, Cho ML, Lee SH, Min SY, Kim WU, Min JK, et al. Augmented production of chemokines by the interaction of type II collagen-reactive T cells with rheumatoid synovial fibroblasts. Arthritis Rheum. 2004;50(4):1146–55. doi: 10.1002/art.20133. [DOI] [PubMed] [Google Scholar]
- 75.Cho ML, Yoon CH, Hwang SY, Park MK, Min SY, Lee SH, et al. Effector function of type II collagen-stimulated T cells from RA patients: crosstalk between T cells and synovial fibroblasts. Arthritis Rheum. 2004;50:776–84. doi: 10.1002/art.20106. [DOI] [PubMed] [Google Scholar]
- 76.Harigai M, Hara M, Kawamoto M, Kawaguchi Y, Sugiura T, Tanaka M, et al. Amplification of the synovial inflammatory response through activation of MAPKs and NF-kB using ligation of CD40 on CD14+ synovial cells from patients with RA. Arthritis Rheum. 2004;50(7):2167–77. doi: 10.1002/art.20340. [DOI] [PubMed] [Google Scholar]
- 77.Lee HY, Jeon HS, Song EK, Han MK, Park SI, Lee SI, et al. CD40 ligation of rheumatoid synovial fibroblasts regulates RANKL-mediated osteoclastogenesis: evidence of NF-kB-dependent, CD40-mediated bone destruction in RA. Arthritis Rheum. 2006;54(6):1747–58. doi: 10.1002/art.21873. [DOI] [PubMed] [Google Scholar]
- 78.Shimaoka Y, Attrep JF, Hirano T, Ishihara K, Suzuki R, Toyosaki T, et al. Nurse-like cells from bone marrow and synovium of patients with RA promote survival and enhance function of human B cells. J Clin Invest. 1998;102(3):606–18. doi: 10.1172/JCI3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harigai M, Hara M, Nakazawa S, Fukasawa C, Ohta S, Sugiura T, et al. Ligation of CD40 induced TNF-alpha in RA: a novel mechanism of activation of synoviocytes. J Rheumatol. 1999;26(5):1035–43. [PubMed] [Google Scholar]
- 80.Liu MF, Chao SC, Wang CR, Lei HY. Expression of CD40 and CD40L among cell populations within rheumatoid synovial compartment. Autoimmunity. 2001;34(2):107–13. doi: 10.3109/08916930109001958. [DOI] [PubMed] [Google Scholar]
- 81.Lakey RL, Morgan TG, Rowan AD, Isaacs JD, Cawston TE, Hilkens CM. A novel paradigm for DC as effectors of cartilage destruction. Rheumatology (Oxford) 2009;48(5):502–7. doi: 10.1093/rheumatology/kep040. [DOI] [PubMed] [Google Scholar]
- 82.Reparon-Schuijt CC, van Esch WJ, van Kooten C, Schellekens GA, de Jong BA, van Venrooij WJ, et al. Secretion of anti-CCP antibody by B lymphocytes in RA. Arthritis Rheum. 2001;44(1):41–7. doi: 10.1002/1529-0131(200101)44:1<41::AID-ANR6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 83.MacDonald KP, Nishioka Y, Lipsky PE, Thomas R. Functional CD40L is expressed by T cells in RA. J Clin Invest. 1997;100(9):2404–14. doi: 10.1172/JCI119781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kitagawa M, Mitsui H, Nakamura H, Yoshino S, Miyakawa S, Ochiai N, et al. Differential regulation of rheumatoid synovial cell interleukin-12 production by TNF-a and CD40 signals. Arthritis Rheum. 1999;42(9):1917–26. doi: 10.1002/1529-0131(199909)42:9<1917::AID-ANR18>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 85.Berner B, Wolf G, Hummel KM, Muller GA, Reuss-Borst MA. Increased expression of CD154 on CD4+ T cells as a marker of disease activity in RA. Ann Rheum Dis. 2000;59(3):190–5. doi: 10.1136/ard.59.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Durie FH, Fava RA, Foy TM, Aruffo AA, Ledbetter JA, Noelle RJ. Prevention of CIA with an antibody to gp39, the ligand for CD40. Science. 1993;261:1328–30. doi: 10.1126/science.7689748. [DOI] [PubMed] [Google Scholar]
- 87.Kyburz D, Carson DA, Corr M. The role of CD40L and TNF-alpha signaling in the transgenic K/BxN mouse model of RA. Arthritis Rheum. 2000;43(11):2571–7. doi: 10.1002/1529-0131(200011)43:11<2571::AID-ANR26>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 88.Tellander AC, Michaelsson E, Brunmark C, Andersson M. Potent adjuvant effect by anti-CD40 in CIA. Enhanced disease is accompanied by increased production of collagen type-II reactive IgG2a and IFN-gamma. J Autoimmun. 2000;14(4):295–302. doi: 10.1006/jaut.2000.0374. [DOI] [PubMed] [Google Scholar]
- 89.Thompson SD, Moroldo MB, Guyer L, Ryan M, Tombragel EM, Shear ES, et al. A genome-wide scan for jRA in affected sibpair families provides evidence of linkage. Arthritis Rheum. 2004;50(9):2920–30. doi: 10.1002/art.20425. [DOI] [PubMed] [Google Scholar]
- 90.Raychaudhuri S, Remmers EF, Lee AT, Hackett R, Guiducci C, Burtt NP, et al. Common variants at CD40 and other loci confer risk of RA. Nat Genet. 2008;40(10):1216–23. doi: 10.1038/ng.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee HS, Korman BD, Le JM, Kastner DL, Remmers EF, Gregersen PK, et al. Genetic risk factors for RA differ in Caucasian and Korean populations. Arthritis Rheum. 2009;60(2):364–71. doi: 10.1002/art.24245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martin-Donaire T, Losada-Fernandez I, Perez-Chacon G, Rua-Figueroa I, Erausquin C, Naranjo-Hernandez A, et al. Association of the microsatellite in the 3′-UTR of the CD154 gene with RA in females from a Spanish cohort: a case-control study. Arthritis Res Ther. 2007;9(5):R89. doi: 10.1186/ar2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wakeland EK, Liu K, Graham RR, Behrens TW. Delineating the genetic basis of SLE. Immunity. 2001;15(3):397–408. doi: 10.1016/s1074-7613(01)00201-1. [DOI] [PubMed] [Google Scholar]
- 94.Desai-Mehta A, Lu L, Ramsey-Goldman R, Datta SK. Hyperexpression of CD40L by B and T cells in human lupus and its role in pathogenic autoantibody production. J Clin Invest. 1996;97(9):2063–73. doi: 10.1172/JCI118643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koshy M, Berger D, Crow MK. Increased expression of CD40 ligand on SLE lymphocytes. J Clin Invest. 1996;98:826–32. doi: 10.1172/JCI118855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Higuchi T, Aiba Y, Nomura T, Matsuda J, Mochida K, Suzuki M, et al. Cutting edge: Ectopic expression of CD40L on B cells induces lupus-like autoimmune disease. J Immunol. 2002;168(1):9–12. doi: 10.4049/jimmunol.168.1.9. [DOI] [PubMed] [Google Scholar]
- 97.Tokunaga M, Saito K, Kawabata D, Imura Y, Fujii T, Nakayamada S, et al. Efficacy of rituximab for refractory SLE involving the CNS. Ann Rheum Dis. 2007;66(4):470–5. doi: 10.1136/ard.2006.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tamimoto Y, Horiuchi T, Tsukamoto H, Otsuka J, Mitoma H, Kimoto Y, et al. A dose-escalation study of rituximab for treatment of SLE and Evans’ syndrome: immunological analysis of B cells, T cells and cytokines. Rheumatology (Oxford) 2008;47(6):821–7. doi: 10.1093/rheumatology/ken071. [DOI] [PubMed] [Google Scholar]
- 99.Goules A, Tzioufas AG, Manousakis MN, Kirou KA, Crow MK, Routsias JG. Elevated levels of sCD40L in serum of patients with systemic autoimmune diseases. J Autoimmun. 2006;26(3):165–71. doi: 10.1016/j.jaut.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 100.Delmas Y, Viallard JF, Solanilla A, Villeneuve J, Pasquet JM, Belloc F, et al. Activation of mesangial cells by platelets in SLE via a CD154-dependent induction of CD40. Kidney Int. 2005;68(5):2068–78. doi: 10.1111/j.1523-1755.2005.00663.x. [DOI] [PubMed] [Google Scholar]
- 101.Pyrovolaki K, Mavroudi I, Sidiropoulos P, Eliopoulos AG, Boumpas DT, Papadaki HA. Increased expression of CD40 on bone marrow CD34+ HPCs in patients with SLE: contribution to Fas-mediated apoptosis. Arthritis Rheum. 2009;60(2):543–52. doi: 10.1002/art.24257. [DOI] [PubMed] [Google Scholar]
- 102.Mohan C, Shi Y, Laman JD, Datta SK. Interaction between CD40 and its ligand gp39 in the development of murine lupus nephritis. J Immunol. 1995;154(3):1470–80. [PubMed] [Google Scholar]
- 103.Early G, Zhao W, Burns C. Anti-CD40L antibody treatment prevents the development of lupus-like nephritis in a subset of NZB×NZW mice. J Immunol. 1996;157:3159–64. [PubMed] [Google Scholar]
- 104.Wang X, Huang W, Schiffer LE, Mihara M, Akkerman A, Hiromatsu K, et al. Effects of anti-CD154 treatment on B cells in murine SLE. Arthritis Rheum. 2003;48(2):495–506. doi: 10.1002/art.10929. [DOI] [PubMed] [Google Scholar]
- 105.Kalled SL, Cutler AH, Datta SK, Thomas DW. Anti-CD40L Ab treatment of SNF1 mice with established nephritis: preservation of kidney function. J Immunol. 1998;160:2158–65. [PubMed] [Google Scholar]
- 106.Kalled SL, Cutler AH, Ferrant JL. Long-term anti-CD154 dosing in nephritic mice is required to maintain survival and inhibit mediators of renal fibrosis. Lupus. 2001;10(1):9–22. doi: 10.1191/096120301668384751. [DOI] [PubMed] [Google Scholar]
- 107.Quezada SA, Eckert M, Adeyi OA, Schned AR, Noelle RJ, Burns CM. Distinct mechanisms of action of anti-CD154 in early versus late treatment of murine lupus nephritis. Arthritis Rheum. 2003;48(9):2541–54. doi: 10.1002/art.11230. [DOI] [PubMed] [Google Scholar]
- 108.Wang X, Huang W, Mihara M, Sinha J, Davidson A. Mechanism of action of combined short-term CTLA4Ig and anti-CD40L in murine SLE. J Immunol. 2002;168(4):2046–53. doi: 10.4049/jimmunol.168.4.2046. [DOI] [PubMed] [Google Scholar]
- 109.Gaffney PM, Langefeld CD, Graham RR, Ortmann WA, Williams AH, Rodine PR, et al. Fine-mapping chromosome 20 in 230 SLE sib-pair and multiplex families: evidence for genetic epistasis with chromosome 16q12. Am J Hum Genet. 2006;78(5):747–58. doi: 10.1086/503686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peters AL, Plenge R, Graham R, Altshuler D, Moser K, Gaffney PM, et al. A novel polymorphism of the human CD40 receptor with enhanced function. Blood. 2008;112:1863–71. doi: 10.1182/blood-2008-02-138925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fernandez M. A multiethnic, multicenter cohort of patients with SLE as a model for the study of ethnic disparities in SLE. Arthritis Rheum. 2007;57:576–84. doi: 10.1002/art.22672. [DOI] [PubMed] [Google Scholar]
- 112.Law C-L, Grewal IS. Therapeutic interventions targeting CD40L and CD40: The opportunities and challenges. In: Grewal IS, editor. Therapeutic Targets of the TNF Superfamily: Landes Bioscience. 2009. pp. 8–36. [DOI] [PubMed] [Google Scholar]
- 113.Liu Z, Li N, Diaz LA. Inhibition of pemphigus vulgaris by targeting of the CD40-CD154 co-stimulatory pathway: A step toward antigen-specific therapy? J Invest Dermatol. 2006;126(1):11–3. doi: 10.1038/sj.jid.5700059. [DOI] [PubMed] [Google Scholar]
- 114.Komura K, Fujimoto M, Yanaba K, Matsushita T, Matsushita Y, Horikawa M, et al. Blockade of CD40-CD40L interactions attenuates skin fibrosis and autoimmunity in the tight-skin mouse. Ann Rheum Dis. 2008;67:867–72. doi: 10.1136/ard.2007.073387. [DOI] [PubMed] [Google Scholar]
- 115.De Jong YP, Comiskey M, Kalled SL, Mizoguchi E, Flavell RA, Bhan AK, et al. Chronic murine colitis is dependent on the CD154/CD40 pathway and can be attenuated by anti-CD154 administration. Gastroenterology. 2000;119:715–23. doi: 10.1053/gast.2000.16485. [DOI] [PubMed] [Google Scholar]
- 116.Toubi E, Shoenfeld Y. The role of CD40-CD154 interaction in autoimmunity and the benefit of disrupting this pathway. Autoimmunity. 2004;37:457–64. doi: 10.1080/08916930400002386. [DOI] [PubMed] [Google Scholar]
- 117.Roth GA, Zuckermann A, Klepetko W, Wolner E, Ankersmit HJ, Moser B. Thrombophilia associated with anti-Cd154 mAb treatment and its prophylaxis in nonhuman primates. Transplantation. 2004;78(8):1238. doi: 10.1097/01.tp.0000135457.69220.5b. [DOI] [PubMed] [Google Scholar]
- 118.Freedman JE. CD40-CD40L and platelet function: beyond hemostasis. Circ Res. 2003;92:944–6. doi: 10.1161/01.RES.0000074030.98009.FF. [DOI] [PubMed] [Google Scholar]
- 119.Andre P, Prasad KS, Denis CV, He M, Papalia JM, Hynes RO, et al. CD40L stabilizes arterial thrombi by a b3 integrin-dependent mechanism. Nat Med. 2002;8:247–52. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 120.Huang W, Sinha J, Newman J, Reddy B, Budhai L, Furie R, et al. The effect of anti-CD40L Ab on B cells in human SLE. Arth Rheum. 2002;46(6):1554–62. doi: 10.1002/art.10273. [DOI] [PubMed] [Google Scholar]
- 121.Boumpas DT, Furie R, Manzi S, Illei GG, Wallace DJ, Balow JE, et al. A short course of BG9588 improves serologic activity and decreases hematuria in patients with proliferative lupus GN. Arth Rheum. 2003;48(3):719–27. doi: 10.1002/art.10856. [DOI] [PubMed] [Google Scholar]
- 122.Patel VL, Schwartz J, Bussel JB. The effect of anti-CD40L in ITP. Brit J Hematol. 2008;141:545–8. doi: 10.1111/j.1365-2141.2008.07039.x. [DOI] [PubMed] [Google Scholar]
- 123.Kalunian KC, Davis JC, Merrill JT, Totoritis MC, Wofsy D. Treatment of SLE by inhibition of T cell costimulation with anti-CD154: A randomized, double-blind, placebo-controlled trial. Arth Rheum. 2002;46(12):3251–8. doi: 10.1002/art.10681. [DOI] [PubMed] [Google Scholar]
- 124.Ferrant JL, Benjamin CD, Cutler AH, Kalled SL, Hsu Y-M, Garber EA, et al. The contribution of Fc effector mechanisms in the efficacy of anti-CD154 immunotherapy depends on the nature of the immune challenge. Int Immunol. 2004;16(11):1583–94. doi: 10.1093/intimm/dxh162. [DOI] [PubMed] [Google Scholar]
- 125.Nagelkerken L, Haspels I, van Rijs W, Blauw B, Ferrant JL, Hess DM, et al. FcR interactions do not play a major role in inhibition of EAE by anti-CD154 mAbs. J Immunol. 2004;173:993–9. doi: 10.4049/jimmunol.173.2.993. [DOI] [PubMed] [Google Scholar]
- 126.Allen SD, Rawale SV, Whitacre CC, Kaumaya PTP. Therapeutic peptidomimetic strategies for autoimmune diseases: costimulation blockade. J Peptide Res. 2005;65(6):591–604. doi: 10.1111/j.1399-3011.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 127.Kitagawa M, Goto D, Mamura M, Matsumoto I, Ito S, Tsutsumi A, et al. Identification of three novel peptides that inhibit CD40-CD154 interaction. Mod Rheumatol. 2005;15:423–6. doi: 10.1007/s10165-005-0442-6. [DOI] [PubMed] [Google Scholar]
- 128.Tai Y-T, Li X, Tong X, Santos D, Otsuki T, Catley L, et al. Human anti-CD40 antagonist Ab triggers significant antitumor activity against human MM. Cancer Res. 2005;65(13):5898–906. doi: 10.1158/0008-5472.CAN-04-4125. [DOI] [PubMed] [Google Scholar]