I. INTRODUCTION
Approximately two thirds of patients with heart failure (HF) have underlying coronary artery disease (CAD), the presence of which is associated with worse long-term outcomes.[1, 2] In addition to providing prognostic information, the detection of CAD in the setting of HF can also result in a number of therapeutic management alterations including revascularization and the use of statin and antiplatelet drugs.[1] In the setting of ischemic heart disease, cardiovascular magnetic resonance (CMR) has demonstrated usefulness in two manners: firstly for the detection of CAD, and secondly for the assessment of myocardial viability in consideration for revascularization. In fact, CMR is now widely considered the gold-standard for the latter; this will be addressed in the following section on myocardial viability and revascularization. This chapter will discuss use to CMR for the detection of CAD.
Currently there are a number of CMR approaches to the detection of CAD: 1) coronary magnetic resonance angiography (MRA), 2) pharmacologic stress CMR with dobutamine (to assess contractile reserve and inducible wall motion abnormalities), and 3) pharmacologic stress CMR with adenosine (to assess myocardial perfusion reserve). The purpose of this article will be to provide the reader with a brief overview of each of the CMR techniques, their relative strengths, and their relative weakness. As adenosine stress CMR is currently the most widely used clinically, it will be the primary focus of this article.
II. CORONARY MRA
Coronary MRA may be used to directly visualize coronary anatomy and morphology. However, coronary MRA is technically demanding for several reasons. The coronary arteries are small (3–5 mm) and tortuous compared with other vascular beds that are imaged by MRA, and there is nearly constant motion during both the respiratory and cardiac cycles. In order to counter these difficulties a number of technical advancements have been made in recent years to improve the reliability of coronary MRA. These include the advent of ultrafast SSFP sequences that offer superior signal-to-noise ratio in combination with whole-heart approaches[3, 4] analogous to multi-detector CT, and parallel imaging to reduce scan times. These sequences typically can be run with submillimeter in-plane spatial resolution (0.8 × 1.0 mm) and slice thickness just over 1 mm. Additionally, with the use of modifications that compensate for respiratory drift,[5] imaging can usually be completed in under 10 minutes.
A. Anomalous Coronary Imaging
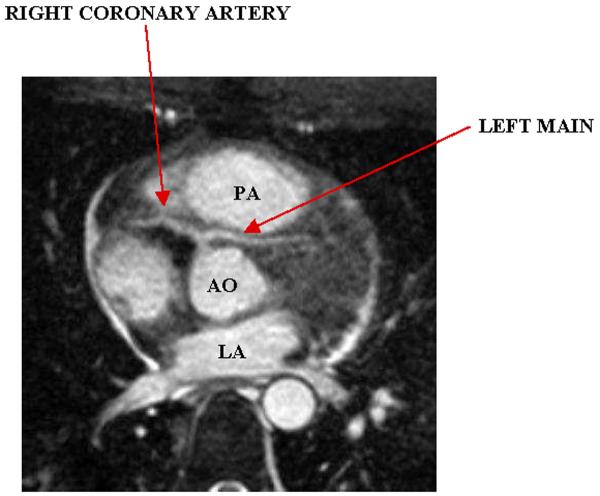
With recent advances, the ability of coronary MRA to reliably identify the major coronary arteries immediately provides for use in the identification and characterization of anomalous coronary anatomy. While most coronary anomalies are benign, situations in which the anomalous segment courses anterior to the aorta and posterior to the pulmonary artery (referred to as “intra-arterial course”), can result in myocardial ischemia and sudden cardiac death, see figure 1.[6] Multiple published series exist of patients who underwent blinded comparison of coronary MRA with x-ray angiography[7–10]; these studies uniformly reported excellent accuracy, including several studies in which coronary MRA was determined to be superior to x-ray angiography.[8, 9] For these reasons, as well as radiation-protection concerns, coronary MRA is the preferred test for patients in whom an anomalous artery origin is suspected or a known anomalous coronary artery origin needs to be further clarified.[11]
Figure 1.
Anomalous left main arising from the right coronary artery and traveling between the aorta and pulmonary artery (intra-arterial course). PA, pulmonary artery; AO, aorta; LA, left atrium.
B. Identification of Native Vessel Coronary Stenosis
Although excellent for evaluation of anomalous coronaries, coronary MRA is still in evolution for detection of native vessel stenosis and therefore it is not recommended for routine assessment in symptomatic patients, or for “screening” in high-risk populations.[11] A recent multicenter single-vendor study did demonstrate a high sensitivity (100%) and negative predictive value (100%) for detection of left main and triple vessel disease.[12] Therefore, there may be a role for coronary MRA in the evaluation of patients who present with dilated cardiomyopathy/congestive heart failure in the absence of clinical infarction in who discrimination between ischemic or nonischemic cardiomyopathy is sought.[11, 13] This is an area that requires further investigation however.
C. Consensus Recommendation for Coronary MRA
Recently a consensus document from the American Heart Association addressed the current role of coronary MRA in clinical practice.[11] The panel assigned a Class IIa recommendation (i.e. weight of evidence/opinion is in favor of usefulness/efficacy) to use of coronary MRA for identification of coronary anomalies and furthermore indicated that radiation-protection concerns indicate that coronary MRA is preferred over coronary computed tomography angiography (CTA) for this indication.
III. DOBUTAMINE STRESS CMR
Whereas coronary MRA provide detail concerning anatomy, stress testing with imaging of myocardial contraction can provide information concerning the presence and functional significance of coronary lesions. Dobutamine stress CMR to detect ischemia-induced wall-motion abnormalities is an established technique for the diagnosis of coronary disease. It yields higher diagnostic accuracy than dobutamine echocardiography[14] and can be effective in patients not suited for echocardiography because of poor acoustic windows.[15] Since the publication of these studies, MR image quality has improved with the widespread availability of SSFP imaging. Parallel imaging techniques that use spatial information from arrays of radiofrequency detector coils to accelerate imaging are expected to improve image quality further. Nonetheless, logistic issues regarding patient safety and adequate monitoring are nontrivial matters that require thorough planning and experienced personnel.
IV. ADENOSINE STRESS CMR
With recent technical and clinical advances, adenosine stress perfusion CMR has evolved from a promising research tool to an everyday clinical tool that is considered a competitive first line test for common indications like the evaluation of ischemic heart disease. In 2006, a consensus panel from the American College of Cardiology Foundation deemed the following indications as appropriate uses of stress perfusion CMR: (1) evaluating chest pain syndromes in patients with intermediate probability of coronary artery disease (CAD) and (2) ascertaining the physiologic significance of indeterminate coronary artery lesions[16]. In part, this report reflects the growing clinical experience with stress perfusion CMR. In dedicated CMR clinical centers, perfusion stress-testing is often the fastest growing component of the clinical volume and can comprise nearly half of all referrals[17].
A. Overview
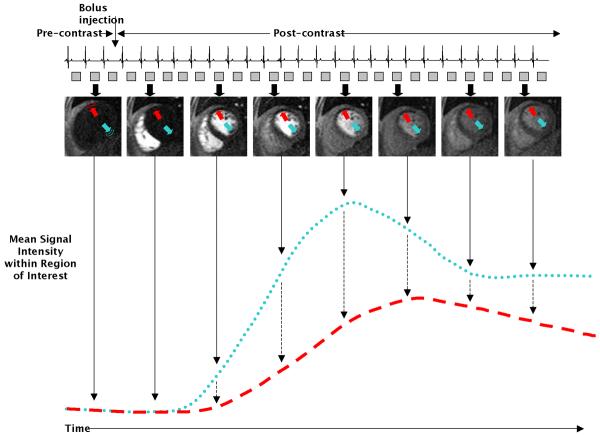
The “goal” of perfusion CMR is to create a movie of the transit of contrast media (typically gadolinium based) with the blood during its initial pass through the LV myocardium (“first-pass contrast-enhancement”). Myocardial perfusion by CMR may be assessed quantitatively or semi-quantitatively by measuring dynamic signal intensities within the myocardium in consecutive images (Figure 2). During pharmacological vasodilation (e.g. adenosine), myocardial blood flow increases four to five- fold downstream of normal coronary arteries, but does not increase downstream of severely diseased arteries since the arteriolar beds are already maximally vasodilated. These physiological differences result in both lower peak myocardial signal intensity and lengthening in the measures of myocardial contrast transit time (e.g. signal upslope, arrival time, time to peak signal, mean transit time) in regions supplied by diseased vessels (Figure 2)[18]. Signal intensity parameters can be plotted with respect to time and, with some assumptions, quantitatively modeled to provide absolute tissue blood flow in milliliters per minutes per gram or utilized in a semi-quantitative fashion to index relative differences in regional flow[18]. Alternatively, the images can be interpreted visually for the presence or absence of perfusion defects.
Figure 2.
Signal-intensity time curves from two myocardial regions. In this example, there is hypoperfusion of the anterior wall (red arrow and dashed red line), while the perfusion of the inferior wall (blue arrow and dotted blue line) is normal. From these curves various parameters can be extracted to derive quantitative measures of blood flow.
Compared with competing technologies such as radionuclide imaging, perfusion CMR has many potential advantages: more than an order of magnitude improvement in spatial resolution (typical voxel dimensions, CMR 3.0×1.8×8mm =43 mm3 versus SPECT 10×10×10 mm =1000 mm3); the ability to identify regional differences in flow over the full range of coronary vasodilation (i.e. no plateau in signal at high flow rates, as seen with radionuclide tracers)[19, 20]; the lack of ionizing radiation; and an examination time of 30–45 minutes versus 2–3 hours.
B. Pre-clinical Validation
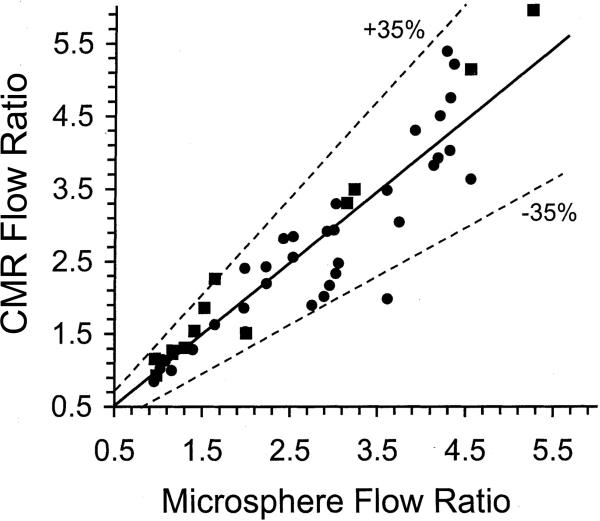
Several studies have shown a good correlation between semi-quantitative and quantitative CMR indices of perfusion with tissue perfusion in animal models[21–25]. In a porcine model with ligation of the left circumflex coronary (LCx) artery, Wilke et al performed MR perfusion studies both at rest and during vasodilation with adenosine[21]. The authors found a linear correlation between relative CMR perfusion indices and true perfusion as measured by radioactive microspheres. Similarly, in a chronically instrumented canine model, Klocke et al produced regional differences in flow with selective LCx infusion of graded doses of adenosine or partial LCx obstruction using a hydraulic occlusion device[23]. Regional differences in the area under the upslope of the CMR signal intensity curve linearly correlated with flow differences measured by fluorescent microspheres (Figure 3). Moreover, regional flow differences of ≥ 2-fold were consistently discerned by perfusion CMR, suggesting that clinically relevant coronary stenoses of 70% or greater could be reliably detected.
Figure 3.
CMR vs. microsphere flow ratios in a canine model. Solid line is linear regression (CMR ratio=0.96 microsphere +0.07); dotted lines indicate 95% confidence limits for individual values. ●, Ratios of LCx to remote CMR areas and relative microsphere flows during LCx adenosine infusion; ∎, ratios of remote to LCx CMR areas and relative microsphere flows during LCx constriction in the presence of global LV vasodilation. From Klocke et al. Limits of detection of regional differences in vasodilated flow in viable myocardium by first-pass magnetic resonance perfusion imaging. Circulation 2001;104:2412–2416, with permission.
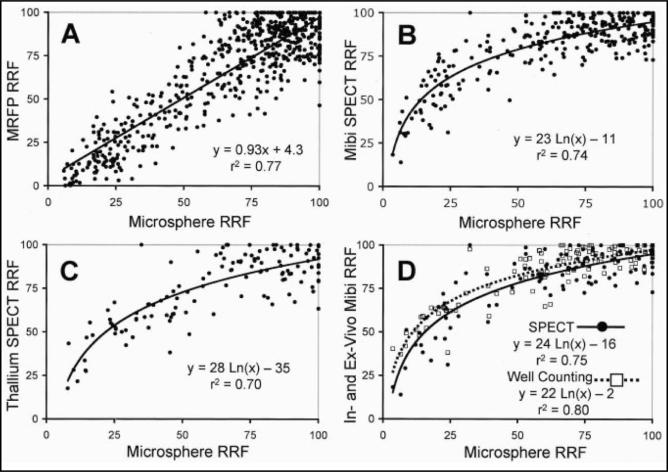
Extending these observations are the findings from Lee et al[24]. In this study, perfusion CMR was compared to technetium-99m (99mTc) sestamibi and 201-Thallium (201Tl) SPECT imaging in the quantification of regional differences in vasodilated blood flow in viable myocardium. The authors utilized a canine model where a hydraulic occluder was placed in the left circumflex coronary artery to produce graded reductions in regional flows. When circumflex microsphere flow was reduced by ≥50%, perfusion defects were apparent on the MR images both by visual inspection and by analysis of the signal intensity curves. Moreover, flows derived from the initial areas under the CMR signal intensity time curves were linearly related to reference microsphere flows over the full range of vasodilation. In contrast, with SPECT imaging, perfusion defects were not evident until flow was reduced by at least 85%, and the relationships between both 99mTc and 201Tl activity and microsphere flows were curvilinear, plateauing as flows increased (Figure 4).
Figure 4.
Comparisons of perfusion CMR, radionuclide, and microsphere flows. CMR signal intensity-time curves were linearly related to reference microsphere flows over the full range of vasodilation. Relationships between 99mTc-sestamibi and 201Thallium activity and microsphere flows were curvilinear, plateauing as flows increased. Data suggests that perfusion CMR, unlike radionuclide imaging, has the potential for detecting stenoses producing only moderate limitations in flow reserve. (A) Normalized magnetic resonance first-pass perfusion (MRFP) imaging and full-thickness microsphere relative regional flows (RRF). (B) Normalized 99mTc-sestamibi and full-thickness microsphere RRFs. (C) Normalized 201Tl and full-thickness microsphere RRFs. (D) In-vivo SPECT and ex-vivo well counting values of 99mTc-sestamibi versus microsphere RRFs. From Lee et al. Magnetic resonance versus radionuclide pharmacological stress perfusion imaging for flow-limiting stenoses of varying severity. Circulation 2004;110:58–65, with permission.
More recently, Christian et al used a Fermi function deconvolution method to quantify absolute perfusion in a canine model of coronary artery stenosis[25]. Vessel occluders or intracoronary adenosine infusion catheters were used to produce a wide range of coronary flows. These authors derived myocardial flow in both the endocardial and epicardial layers of the heart by perfusion CMR. They showed that quantitative coronary flow by CMR in both layers was linearly related to flow by fluorescent microspheres (without plateauing at higher flow rates) in the corresponding locations. These findings and those from others[22] demonstrate that perfusion CMR, with its advantage of high spatial resolution, has the potential to discern differences in endocardial and epicardial flow. This may have value in evaluation of 3 vessel CAD with “balanced ischemia,” syndrome X, or for the detection of subtle abnormalities such as hypertensive heart disease.
C. Diagnostic Performance in Patients
The diagnostic performance of stress perfusion CMR has been evaluated in a number of studies in humans[26–44]. Overall, these studies have shown good correlations with radionuclide imaging and x-ray coronary angiography, although there have been some variable results. Table 1 summarizes the published stress perfusion CMR studies in humans with coronary angiography comparison. A total of 21 studies have been completed, consisting of 1233 patients with known or suspected CAD. On average, the sensitivity and specificity of perfusion CMR for detecting obstructive CAD were 84% (range, 44–93%) and 80% (range, 60–100%), respectively. Likely on the basis of these studies, the most recent consensus report on clinical indications for CMR classified perfusion imaging as a Class II indication for the assessment of CAD (provides clinically relevant information and is frequently useful)[45].
Table 1.
Stress Perfusion CMR Studies in Humans with Coronary Angiography Comparison.
| Year | Author | Reference | n | Pts with known CAD excluded | MRI Perfusion Protocol1 | Protocol Gadolinium Dose (mmol/kg) | Pulse-Sequence | X-Ray Angiography (CAD definition) | Analysis Method2 | Sens | Spec |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1993 | Klein | AJR 161(2):257–63 | 5 | no | Stress only | 0.05 | IR-GRE | >50 | prospective | 81* | 100* |
| 1994 | Hartnell | AJR 163(5):1061–7 | 18 | no | Rest/Stress | 0.04 | IR-GRE | ≥70 | prospective | 83 | 100 |
| 1994 | Eichenberger | JMRI 4(3):425–31 | 10 | no | Rest/Stress | 0.05 | GRE | >75 | retrospective | 44* | 80* |
| 2000 | Al-Saadi | Circ 101(12):1379–83 | 34 | yes | Rest/Stress | 0.025 | IR-GRE | ≥75 | prospective3 | 90 | 83 |
| 2001 | Bertschinger | JMRI 14(5):556–62 | 14 | no | Stress only | 0.1 | SR-EPI | ≥50 | retrospective | 85 | 81 |
| 2001 | Schwitter | Circ 103(18):2230–5 | 48 | yes | Stress only | 0.1 | SR-GRE-EPI | ≥50 | retrospective | 87 | 85 |
| 2001 | Panting | JMRI 13(2):192–200 | 22 | no | Rest/Stress | 0.05 | IR Spin Echo-EPI | >50 | retrospective | 79 | 83 |
| 2002 | Sensky | Int J CV Imaging 18(5):373–383 | 30 | no | Rest/Stress | 0.025 | IR-GRE | >50 | prospective | 93* | 60* |
| 2002 | Ibrahim | JACC 39(5):864–870 | 25 | no | Rest/Stress | 0.05 | SR-GRE-EPI | >75 | retrospective | 69* | 89* |
| 2003 | Chiu | Radiology 226(3):717–722 | 13 | no4 | Rest/Stress | 0.05 | IR-SSFP | >50 | NS | 92* | 92* |
| 2003 | Ishida | Radiology 229(1):209–216 | 104 | no | Stress/Rest | 0.075 | SR-GRE-EPI | ≥70 | prospective | 90 | 85 |
| 2003 | Nagel | Circ 108(4):432–437 | 84 | no | Rest/Stress | 0.025 | SR-GRE-EPI | ≥75 | retrospective | 88 | 90 |
| 2003 | Doyle | JCMR 5(3):475–85 | 138 | no | Rest/Stress | 0.04 | SR-GRE | ≥70 | prospective3 | 57 | 85 |
| 2004 | Wolff | Circ 110(6):732–737 | 75 | no | Stress/Rest | 0.05–0.15 | SR-GRE-EPI | ≥70 | prospective5 | 93 | 75 |
| 2004 | Giang | EHJ 25(18):1657–65 | 80 | no | Stress only | 0.05–0.15 | SR-GRE-EPI | ≥50 | retrospective5 | 93 | 75 |
| 2004 | Paetsch | Circ 110(7):835–842 | 79 | no | Stress/Rest | 0.05 | SR-GRE-EPI | >50 | prospective | 91 | 62 |
| 2004 | Plein | JACC 44(11):2173–81 | 68 | no4 | Rest/Stress | 0.05 | SR-GRE6 | ≥70 | prospective | 88 | 83 |
| 2005 | Plein | Radiology 235(2):423–430 | 92 | no | Rest/Stress | 0.05 | SR-GRE6 | >70 | retrospective | 88 | 82 |
| 2006 | Klem | JACC 47(8):1630–38 | 100 | yes | Stress/Rest | 0.063 | SR-GRE6 | ≥70 | prospective | 84† | 58† |
| 2006 | Cury | Radiology 240(1):39–45 | 47 | no | Stress/Rest | 0.1 | SR-GRE-EPI | ≥70 | prospective | 81§ | 87§ |
| 2008 | Klem | J Am Coll Cardiol Img, 2008; 1:436–445 | 147 | yes | Stress/Rest | 0.07 | SR-GRE6 | ≥70 | prospective | 84 | 88 |
| Total Weighted Average | 21 | 1233 | 84 | 80 |
CMR, cardiovascular magnetic resonance; CAD, coronary artery disease; n, number of patients; n, number of patients; IR, inversion recovery pre-pulse; SR, saturation recovery pre-pulse; GRE, gradient-recalled echo; EPI, echo-planar imaging; SSFP, steady-state free precession; DE-CMR, delayed enhancement CMR; Sens, sensitivity; Spec, specificity; NS, not stated.
numbers based on a regional rather than per patient analysis
when both rest and stress imaging were performed the order is as listed
prospective studies were those in which the criteria for test abnormality were prespecified before data analysis
pilot study performed first to determine the best threshold for test abnormality
at enrollment all patients had the clinical diagnosis of non-ST elevation MI or acute coronary syndrome
reported sensitivity and specificity are from a fraction of the total cohort, a subgroup with the best results
with parallel imaging acceleration
sensitivity/specificity were higher after incorporating DE-CMR (89% and 87%, respectively)
sensitivity/specificity were higher after incorporating DE-CMR (87% and 89%, respectively)
Adapted from Kim HW, Rehwald W, White, JA et al: Magnetic Resonance Imaging of the Heart. In Fuster V, O'Rourke, RA (eds): Hurst's The Heart. New York: McGraw-Hill Medical, In Press.
Despite the mostly favorable results of these studies, a number of issues should be considered. Some studies are of limited clinical applicability, because they required central venous catheters[30, 34], imaged only 1 slice per heartbeat[30], or excluded patients with diabetes[39]. Many studies had small sample sizes—eight had 30 or fewer patients. Most included patients already known to have CAD or known to have prior myocardial infarction. In these studies there is pretest referral or “spectrum” bias, which can artificially raise test sensitivity and/or specificity[46, 47]. Importantly, in many studies after the data were collected, several methods of analysis were tested and different thresholds for test abnormality were appraised. For these studies, the reported sensitivity and specificity values are optimistic since the endpoints were chosen retrospectively and they represent optimized values.
Two practical issues also limit clinical applicability. First, there is no consensus regarding the optimal pulse sequence or imaging protocol. The studies in Table 1 are very heterogeneous in terms of the techniques and methods employed. For example, the dose of gadolinium contrast administered varied six-fold, with doses ranging from 0.025 to 0.15 mmol/kg. The inconsistent results in the literature likely reflect the lack of a standard method for performing perfusion CMR. Second, many of the studies used a quantitative approach for diagnostic assessment. Although a quantitative approach has the advantage, potentially, of allowing absolute blood flow to be measured or parametric maps of perfusion to be generated, the approach is laborious and requires extensive interactive post-processing. At present, a quantitative approach is not feasible for everyday clinical use.
In contrast, image interpretation by simple visual assessment would be a realistic approach for a clinical CMR practice. Unfortunately, the results in the literature regarding visual assessment of perfusion CMR are mixed, generally demonstrating adequate sensitivity but relatively poor specificity for the detection of CAD. In large part, image artifacts are responsible for reduced specificity. In this context, it is noteworthy that recently an interpretation algorithm that combines data from perfusion CMR and delayed enhancement CMR (DE-CMR) has been introduced that substantially improves the specificity and accuracy of rapid visual assessment for the detection of CAD[44, 48]. Based on these data, we have adopted a multi-component approach to stress testing, which permits rapid visual image interpretation with high diagnostic accuracy.
D. Multi-Component CMR Stress Testing Protocol
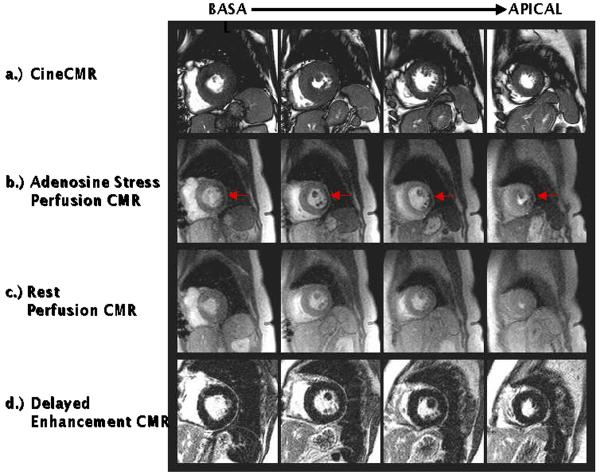
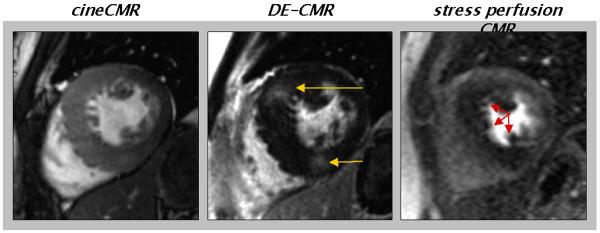
The multi-component approach to CMR stress testing includes the following: (a.) cineCMR for the assessment of cardiac morphology and regional and global systolic function at baseline, (b.) stress perfusion CMR to visualize regions of myocardial hypoperfusion during vasodilation (e.g. with adenosine infusion), (c.) rest perfusion CMR to aid in distinguishing true perfusion defects from image artifacts, and (d.) delayed enhancement CMR (DE-CMR) for the determination of myocardial infarction (Figure 5). The timeline of the multi-component CMR stress test is displayed in Figure 6. Details regarding cineCMR and DE-CMR are discussed elsewhere in this issue of Heart Failure Clinics.
Figure 5.
Components of the multi-component CMR stress test. Cine CMR (a.), stress (b.) and rest perfusion (c.) CMR, and delayed enhancement CMR (d.) are performed at identical short axis locations. During image interpretation, the different components are analyzed side-by-side to facilitate differentiation of perfusion defects due to infarction, ischemia, or artifact. Arrows points to perfusion defects seen during adenosine infusion, but not at rest consistent with the presence of ischemic heart disease.
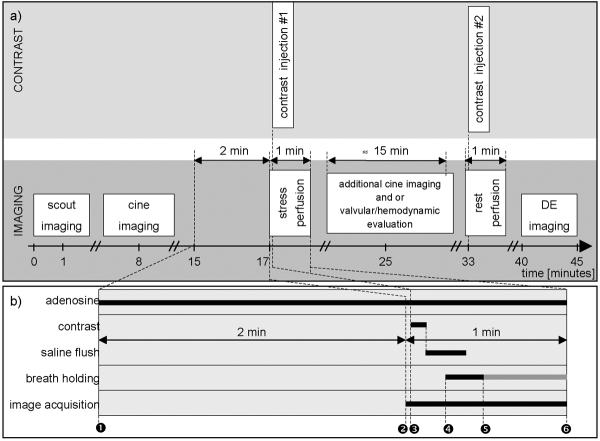
Figure 6.
Timeline for the multi-component CMR stress test. See text for details.
Stress perfusion imaging is performed after scouting and cine imaging. Typically, prior to adenosine administration, the patient table is partially pulled out of bore of the magnet to allow direct observation and full access to the patient. Adenosine (140 μg•kg−1•min−1) is then infused under continuous electrocardiography and blood pressure monitoring for at least two minutes. The perfusion sequence is then applied by the scanner operator, which automatically re-centers the patient back in the scanner bore and commences imaging. Gadolinium contrast (0.075 to 0.10 mmol/kg body weight) is then administered followed by a saline flush (≈ 50 ml) at a rate of at least 3 ml/s via an antecubital vein. On the console, the perfusion images are observed as they are acquired, with breath-holding starting from the appearance of contrast in the right ventricular cavity. If the scanner software does not provide real-time image display, breath-holding should be started no more than 5–6 seconds after beginning gadolinium injection. Breath-holding is performed to ensure the best possible image quality (i.e. no artifacts due to respiratory motion) during the initial wash-in of contrast into the LV myocardium. Once the contrast bolus has transited the LV myocardium, adenosine is stopped and imaging is completed 5–10 seconds later. Typically, the total imaging time is 40–50 seconds, and the total time of adenosine infusion is 3 to 3.5 minutes. During vasodilation, direct access to the patient is limited only during imaging of the first pass.
Prior to the rest perfusion scan, a waiting period of about 15 minutes is required for gadolinium to sufficiently clear from the blood pool. During this time, additional cine scans and or velocity/flow imaging for valvular or hemodynamic evaluation can be performed. For the rest perfusion scan an additional dose of 0.075–0.10 mmol/kg gadolinium is given, and the imaging parameters are identical to the stress scan. Approximately 5 minutes after rest perfusion, delayed enhancement imaging can be performed. The total scan time for a comprehensive CMR stress test, including cine imaging, stress and rest perfusion, and delayed enhancement is usually well under 45 minutes.
Unlike vasodilator radionuclide imaging in which adenosine is typically infused for 6 minutes (tracer injection at 3 minutes), stress perfusion CMR is performed using an abbreviated adenosine protocol (<3 minutes total) since the requirements for imaging are different[44]. With radionuclide imaging, maintaining a vasodilated state for 2–3 minutes after tracer injection is necessary to allow time for tracer uptake into myocytes. In contradistinction, with CMR currently available gadolinium media are inert, extracellular agents that do not cross sarcolemmal membranes[52], and vasodilation needs to be maintained only for the initial first-pass through the myocardium. Although severe reactions to adenosine are rare, a shortened protocol is relevant because moderate reactions that affect patient tolerability are relatively commonplace[53]. A minimum 2-minute infusion duration was chosen on the basis of physiological studies in humans demonstrating that maximum coronary blood flow is reached, on average, 1 minute after the start of intravenous adenosine infusion (140 μg•kg−1•min−1) and in nearly all patients by 2 minutes[54].
E. Image Interpretation
Interpretation Algorithm for Coronary Artery Disease
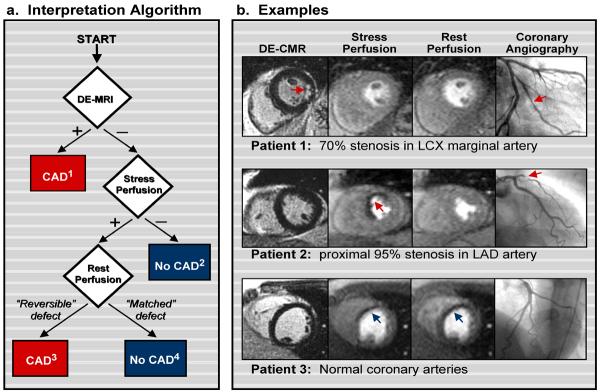
An overview of the interpretation algorithm that facilitates rapid visual interpretation for a multi-component CMR stress test is presented with examples in Figure 7. Using this stepwise algorithm, a CMR stress test is deemed “positive for CAD” if myocardial infarction is present on DE-CMR OR if perfusion defects are present during stress imaging, but absent at rest (“reversible” defect) in the absence of infarction. Conversely, the test is deemed “negative for CAD” if no abnormalities are found (e.g. no MI and no stress/rest perfusion defects) OR if perfusion defects are seen at both stress and rest imaging (“matched” defect) in the absence of infarction. In the latter, matched defects are regarded as artifacts and not suggestive of CAD with rare exceptions (see next section). When both DE-CMR and stress perfusion CMR are abnormal, the test is scored positive for ischemia if the perfusion defect is larger than the area of infarction.
Figure 7.
Interpretation algorithm for incorporating delayed enhancement imaging (DE-CMR) with stress and rest perfusion CMR for the detection of coronary disease. Modified from Klem I, Heitner JF, Shah DJ, et al. Improved detection of coronary artery disease by stress perfusion cardiovascular magnetic resonance with the use of delayed enhancement infarction imaging. J Am Coll Cardiol 2006; 47: 1630–8. CAD=coronary artery disease.
(a) Schema of the interpretation algorithm. (1) Positive DE-CMR Study: Hyperenhanced myocardium consistent with a prior myocardial infarction (MI) is detected. Does not include isolated midwall or epicardial hyperenhancement which can occur in nonischemic disorders. (2) Standard Negative Stress Study: No evidence of prior MI or inducible perfusion defects. (3) Standard Positive Stress Study: No evidence of prior MI but perfusion defects are present with adenosine that are absent or reduced at rest. (4) Artifactual Perfusion Defect: Matched stress and rest perfusion defects without evidence of prior MI on DE-CMR.
(b) Patient Examples. Top row: Patient with a positive DE-CMR study demonstrating an infarct in the inferolateral wall (red arrow) although perfusion CMR is negative. The interpretation algorithm (step 1) classified this patient as positive for CAD. Coronary angiography verified disease in the left circumflex (LCX) marginal artery. Cine CMR demonstrated normal contractility. Middle row: Patient with a negative DE-CMR study but with a prominent reversible defect in the anteroseptal wall on perfusion CMR (arrow). The interpretation algorithm (step 3) classified this patient as positive for CAD. Coronary angiography demonstrated a proximal 95% left anterior descending (LAD) stenosis. Bottom row: Patient with a matched stress-rest perfusion defect (arrows) but without evidence of prior MI on DE-CMR. The interpretation algorithm (step 4) classified the perfusion defects as artifactual. Coronary angiography demonstrated normal coronary arteries.
The interpretation algorithm is based on two simple principles. First, with perfusion CMR and DE-CMR, there are two independent methods to obtain information regarding the presence or absence of myocardial infarction (MI). Thus, one method could be used to confirm the results of the other. Second, DE-CMR image quality (e.g. signal-to-noise ratio) is far better than perfusion CMR since it is less demanding in terms of scanner hardware (DE-CMR images can be built up over several seconds rather than in 0.1 seconds as is required for first-pass perfusion)[55]. Thus, DE-CMR should be more accurate for the diagnosis of MI[55], and the presence of infarction on DE-CMR favors the diagnosis of CAD, irrespective of the perfusion CMR results. Conceptually, it then follows that perfusion defects that have similar intensity and extent during both stress and rest (“matched defect”) but do not have infarction on DE-CMR are artifactual and should not be considered positive for CAD with rare exceptions.
Klem et al reported that the determination of CAD using the multi-component CMR stress test and interpretation algorithm significantly improved diagnostic performance[44]. In that study, the interpretation algorithm yielded a sensitivity of 89%, specificity of 87%, and diagnostic accuracy of 88% for the detection of CAD (major coronary artery with stenosis ≥70% or left main stenosis ≥50%). In comparison, when stress/rest perfusion was considered alone (without DE-CMR), the sensitivity, specificity, and diagnostic accuracy were 84%, 58%, and 68% respectively. Thus, the interpretation algorithm had markedly higher specificity and diagnostic accuracy than perfusion CMR alone (p<0.0001 for both). Notably, the higher specificity with the interpretation algorithm was primarily the result of correctly changing the diagnosis from positive to negative for CAD in 12 patients in whom infarction was not observed on DE-CMR even though perfusion CMR demonstrated matched stress-rest perfusion defects. Importantly, in this study, the imaging protocol and interpretation algorithm was prespecified, and all patients were consecutively recruited prospectively from a pool referred for elective coronary angiography. Patients with known CAD (e.g. prior MI or revascularization) were excluded to reduce pretest referral or “spectrum” bias. Moreover, to avoid post-test referral bias, all patients underwent angiography within 24 hours of CMR without regard to the CMR findings. Thus, it is likely that these results reflect the actual real world performance of a multi-component CMR stress-test with appropriate image interpretation.
Artifacts
Image artifacts often occur at the interface between the left ventricular cavity and the endocardium (arising from susceptibility effects or rapid cardiac motion) and may mimic true perfusion defects[56]. Characteristics that may be useful in distinguishing between artifact and true perfusion defects include the following: (1) artifacts are more common in the phase-encode direction; true perfusion defects should follow coronary artery distribution territories, (2) artifacts are transitory, varying in signal intensity in consecutive images during the transit of contrast media through the myocardium; true perfusion defects often linger for multiple image frames and should follow smooth image intensity trajectories, and (3) artifacts are generally present at both stress and rest imaging; true perfusion defects generally appear only during vasodilator stress.
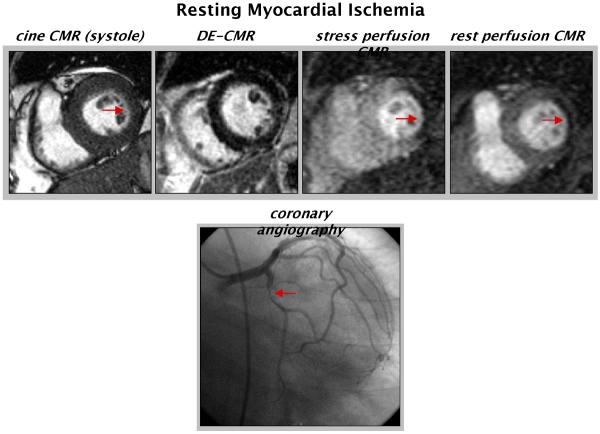
Concerning this latter point, it is important to recognize that the interpretation of stress/rest perfusion CMR is not analogous to stress/rest SPECT imaging. For instance, matched perfusion defects on perfusion CMR are far more likely to represent artifact than prior myocardial infarction. Additionally, we have also observed that severe, but matched perfusion defects can occur in the setting of critical resting ischemia (Figure 8). Unlike artifacts, these perfusion defects are transmural (or nearly transmural) and persist for nearly the entire first-pass and are associated with wall motion abnormalities in the same location as the perfusion defects. Although extremely rare, recognition of true perfusion defects occurring at both stress and rest with limited or absent myocardial infarction on DE-CMR is important, as they are associated with total or subtotal occlusions and are potentially reversible following revascularization.
Figure 8.
Example of resting myocardial ischemia. CineCMR demonstrates hypokinesis of the lateral wall without evidence of myocardial infarction on DE-CMR. Dense, nearly transmural perfusion defects are present at both stress and rest (though larger with stress). Coronary angiography demonstrates a high-grade lesion in the proximal left circumflex coronary artery. Arrows point to the abnormalities.
Perfusion Defects due to Microvascular Dysfunction
Data regarding the use of multi-component CMR stress testing in patients with microvascular dysfunction are limited. However, the high spatial resolution of perfusion CMR, which allows the identification of perfusion defects that primarily affect the subendocardium, may be useful in patients with potential microvascular dysfunction, such as hypertrophic cardiomyopathy (HCM)[57] or aortic stenosis, and also possibly in cardiac syndrome X[58] although the latter is somewhat controversial.[59] For example, in patients with HCM, we have observed stress induced perfusion defects in the absence of epicardial coronary disease (Figure 9). These perfusion defects are most apparent in the more hypertrophied portions of the myocardium and co-localize with regions of scarring on DE-CMR (presented elsewhere in this issue of Heart Failure Clinics). The clinical significance of these CMR findings has yet to be determined. However, since both scarring and ischemia are likely to have prognostic implications, multi-component CMR stress testing may have utility in risk stratification.
Figure 9.
Evaluation of Hypertrophic Cardiomyopathy by CMR. Cine CMR demonstrates asymmetric septal hypertrophy. On DE-CMR, there is evidence of scarring in the ventricular septum at the right ventricular insertion sites. The stress perfusion MR images show a dense perfusion defect in the septum as well, though the region of ischemia is larger than the area of scarring on DE-CMR. This patient had normal epicardial coronary arteries on angiography. Modified from Shah DJ, Judd RM, Kim RJ. Technology insight: MRI of the myocardium. Nat Clin Pract Cardiovasc Med 2005; 2:597–605.
Reporting
At our institutions, CMR stress tests are scored regionally using the American Heart Association 17 segment model[60]. For the determination of the presence of CAD, the components are scored while viewing the images side-by-side (Figure 5). Myocardial infarction is scored from DE-CMR when hyperenhancement is present, unless the hyperenhancement is isolated to the midwall or subepicardium[44, 61, 62]. As previously described, these latter patterns are found in non-ischemic rather than ischemic disorders[63, 64]. Stress and rest perfusion images are scored for perfusion defects in 16 segments (segment 17 at the apex is usually not visualized) using the interpretation algorithm on a four point scale: 0, normal; 1, probably normal; 2, probably abnormal; and 3, definitely abnormal[44]. The corresponding coronary artery territory is assigned based on the distribution of abnormal segments.
F. Stress CMR as Prognostic Tool
In addition to diagnostic accuracy in comparison to coronary angiography, a number of studies have evaluated the prognostic value of stress perfusion CMR.[49–51] In a study evaluating 135 patients presenting to the emergency department with chest pain, Ingkanisorn et. al[49], demonstrated that adenosine perfusion abnormalities had 100% sensitivity and 93% specificity for detection of significant CAD based on any of the following: coronary artery stenosis < 50% on angiography, abnormal correlative stress test, new myocardial infarction (MI), or death. Additionally in this study an abnormal stress CMR added significant prognostic value in predicting future diagnosis of CAD, MI, or death over clinical risk factors.[49] In a more recent study Jahnke et. al.,[50] performed combined stress perfusion CMR and dobutamine stress CMR in a series of 513 patients with known or suspected CAD; they demonstrated a 97.7% rate of survival free from cardiovascular death or nonfatal MI at 3 years in patients with a normal stress perfusion CMR. These data along with another series from Bodi et. al.,[51] demonstrate that stress perfusion CMR is useful not only for detection of CAD, but that it also can provide important prognostic information. Although further confirmatory studies are required early studies suggest that a normal stress perfusion CMR is associated with a low likelihood of future cardiovascular events, at least in the short term and intermediate term.
V. Cost Implications of CMR
In the current medical environment with rising health care costs, any new cardiac imaging modality would need to be more efficacious and more cost effective than alternative testing. There seems to be a general perception by numerous authors and societies that CMR stress testing is more expense than current common tests such as SPECT[16, 45, 65, 66]. To our knowledge, there have been no analyses that have looked at the direct CMS (Center for Medicare & Medicaid Services) costs of stress CMR to alternative tests. Therefore, we investigated this by tabulating the CMS reimbursement rates for both stress SPECT and stress perfusion CMR. This was performed by visiting the website for TrailBlazer Health Enterprises, LLC (a contracted administrator for CMS) at www.trailblazerhealth.com on one given day (October 20, 2008). Table 2 lists the CMS reimbursement rates for both the professional and technical imaging components of a stress SPECT and stress perfusion CMR examination. Although there is considerable variation in the Medicare reimbursement rates from region to region, stress CMR is consistently 40–50% less expensive than stress SPECT. A large component of the difference can be explained by the expense for the radiopharmaceutical, which accounts for almost $400 of the expense for stress SPECT, and which is not required for stress perfusion CMR.
Table 2. CMS Reimbursement for SPECT and CMR.
CMS reimbursement data for 50 United States, District of Columbia, and territories of Puerto Rico and Virgin Islands for 2008. Obtained from http://www.trailblazerhealth.com/. Accessed online October 20, 2008. Technical reimbursement rates are for hospital outpatient prospective payment system (HOPPS). In states where reimbursements vary between different localities the average for all localities is listed. SPECT, single photon emission computed tomography; CMR, cardiac magnetic resonance.
| SPECT | CMR¶ | |||||
|---|---|---|---|---|---|---|
| STATE* | TECHNICALψ | PROFESSIONALΩ | TOTAL | TECHNICAL | PROFESSIONAL | TOTAL |
| Alabama | $1,023.51 | $111.26 | $1,134.77 | $440.73 | $147.81 | $588.54 |
| Alaska | $1,209.27 | $122.99 | $1,332.26 | $570.12 | $163.44 | $733.56 |
| Arizona | $1,125.48 | $117.25 | $1,242.73 | $511.45 | $155.72 | $667.17 |
| Arkansas | $1,008.66 | $110.21 | $1,118.87 | $430.62 | $146.49 | $577.11 |
| California | $1,332.95 | $131.35 | $1,464.30 | $656.76 | $174.75 | $831.51 |
| Colorado | $1,137.57 | $117.63 | $1,255.20 | $520.22 | $156.32 | $676.54 |
| Connecticut | $1,271.40 | $128.12 | $1,399.52 | $613.34 | $170.25 | $783.59 |
| Delaware | $1,160.75 | $119.82 | $1,280.57 | $536.32 | $159.21 | $695.53 |
| District of Columbia | $1,312.96 | $131.15 | $1,444.11 | $642.31 | $174.26 | $816.57 |
| Florida | $1,187.17 | $122.06 | $1,309.22 | $552.85 | $161.71 | $714.56 |
| Georgia | $1,116.90 | $117.06 | $1,233.96 | $505.53 | $155.48 | $661.01 |
| Hawaii | $1,233.18 | $122.96 | $1,356.14 | $586.96 | $163.45 | $750.41 |
| Idaho | $1,037.53 | $111.88 | $1,149.41 | $450.69 | $148.69 | $599.38 |
| Illinois | $1,170.64 | $121.40 | $1,292.03 | $541.96 | $160.98 | $702.95 |
| Indiana | $1,063.84 | $113.34 | $1,177.18 | $469.04 | $150.64 | $619.68 |
| Iowa | $1,032.75 | $111.63 | $1,144.38 | $447.35 | $148.34 | $595.69 |
| Kansas | $1,046.28 | $112.51 | $1,158.79 | $456.61 | $149.50 | $606.11 |
| Kentucky | $1,033.90 | $112.00 | $1,145.90 | $447.77 | $148.75 | $596.52 |
| Louisiana | $1,095.69 | $115.72 | $1,211.41 | $490.53 | $153.63 | $644.16 |
| Maine | $1,095.99 | $115.15 | $1,211.14 | $491.42 | $153.06 | $644.48 |
| Maryland | $1,160.29 | $119.58 | $1,279.87 | $535.79 | $158.84 | $694.63 |
| Massachusetts | $1,286.75 | $127.26 | $1,414.01 | $624.27 | $169.18 | $793.45 |
| Michigan | $1,166.28 | $121.95 | $1,288.22 | $538.66 | $161.65 | $700.31 |
| Minnesota | $1,115.07 | $115.89 | $1,230.96 | $505.09 | $154.15 | $659.24 |
| Mississippi | $1,026.97 | $111.63 | $1,138.60 | $442.93 | $148.25 | $591.18 |
| Missouri | $1,077.90 | $117.63 | $1,195.53 | $478.13 | $152.31 | $630.43 |
| Montana | $1,027.04 | $111.66 | $1,138.70 | $442.95 | $148.28 | $591.23 |
| Nebraska | $1,035.74 | $111.57 | $1,147.31 | $449.67 | $148.35 | $598.02 |
| Nevada | $1,174.28 | $120.24 | $1,294.52 | $545.33 | $159.67 | $705.00 |
| New Hampshire | $1,158.17 | $118.73 | $1,276.90 | $534.64 | $157.80 | $692.44 |
| New Jersey | $1,272.26 | $129.18 | $1,401.44 | $613.79 | $171.61 | $785.40 |
| New Mexico | $1,065.30 | $114.02 | $1,179.32 | $469.35 | $151.37 | $620.72 |
| New York | $1,268.27 | $127.06 | $1,395.32 | $609.15 | $168.72 | $777.87 |
| North Carolina | $1,076.34 | $114.17 | $1,190.51 | $477.59 | $151.71 | $629.30 |
| North Dakota | $1,019.96 | $110.90 | $1,130.86 | $438.44 | $147.38 | $585.82 |
| Ohio | $1,099.59 | $116.03 | $1,215.62 | $493.13 | $154.03 | $647.16 |
| Oklahoma | $1,021.19 | $110.98 | $1,132.17 | $439.27 | $147.49 | $586.76 |
| Oregon | $1,111.19 | $115.94 | $1,227.12 | $502.19 | $154.14 | $656.33 |
| Pennsylvania | $1,160.05 | $120.11 | $1,280.15 | $535.17 | $159.43 | $694.59 |
| Puerto Rico | $899.06 | $103.97 | $1,003.03 | $354.37 | $138.20 | $492.57 |
| Rhode Island | $1,172.39 | $122.03 | $1,294.42 | $544.19 | $162.09 | $706.28 |
| South Carolina | $1,050.73 | $112.48 | $1,163.21 | $460.03 | $149.55 | $609.58 |
| South Dakota | $1,028.92 | $111.26 | $1,140.18 | $444.84 | $147.91 | $592.75 |
| Tennessee | $1,047.76 | $112.58 | $1,160.34 | $457.66 | $149.59 | $607.25 |
| Texas | $1,121.93 | $117.77 | $1,239.70 | $508.62 | $156.31 | $664.94 |
| Utah | $1,083.83 | $114.84 | $1,198.67 | $482.51 | $152.53 | $635.04 |
| Vermont | $1,108.98 | $115.78 | $1,224.76 | $500.58 | $153.93 | $654.51 |
| Virgin Islands | $1,142.94 | $118.28 | $1,261.22 | $523.55 | $157.08 | $680.63 |
| Virginia | $1,088.52 | $114.81 | $1,203.33 | $486.12 | $152.58 | $638.70 |
| Washington | $1,166.55 | $119.80 | $1,286.35 | $540.40 | $159.20 | $699.60 |
| West Virginia | $1,036.32 | $113.01 | $1,149.33 | $448.45 | $149.82 | $598.27 |
| Wisconsin | $1,072.63 | $113.91 | $1,186.54 | $475.06 | $151.39 | $626.45 |
| Wyoming | $1,033.34 | $112.16 | $1,145.50 | $447.16 | $148.91 | $596.07 |
| NATIONAL AVERAGE | $1,114.58 | $117.03 | $1,231.61 | $503.99 | $155.39 | $659.39 |
Technical component for SPECT includes CPT (Current Procedure Terminology) descriptor 78465 and 2 doses of radiopharmaceutical (rest and stress imaging)
Professional component for SPECT includes CPT descriptor 78465 (myocardial perfusion imaging multiple studies) and CPT descriptors 78478 and 78480 (add on codes for gated study for wall motion and ejection fraction) as per ASNC coding guidelines.
Both technical and professional components for CMR are billed using CPT descriptor 75563.
VI. Conclusion
CMR can play an important role in the evaluation of ischemic heart disease. Although coronary MRA and dobutamine stress CMR may have a role in selected scenarios, the vast majority of CMR ischemia evaluation is generally performed using adenosine stress perfusion CMR. When combined with delayed enhancement CMR, the sensitivity, specificity, and diagnostic accuracy of the multi-component stress perfusion CMR exam rival other currently available modalities for the evaluation of myocardial ischemia. Importantly, CMR perfusion stress testing has been deemed appropriate for the evaluation of chest pain syndromes in patients with intermediate probability of coronary artery disease (CAD) and for ascertaining the physiologic significance of indeterminate coronary artery lesions. In the future, improvements in parallel imaging and pulse sequence technology, use of higher magnetic field strengths, and protocol optimizations will continue the rapid advance in image quality. Multicenter clinical trials, which are currently ongoing, will soon be available and will establish the diagnostic accuracy and prognostic value of CMR perfusion stress testing in a broad population of patients.
Acknowledgments
This work was supported in part by National Institutes of Health grant RO1-HL64726 (RJK)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
VIII. References
- 1.Hunt SA, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112(12):e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 2.Gheorghiade M, et al. Navigating the Crossroads of Coronary Artery Disease and Heart Failure. Circulation. 2006;114(11):1202–1213. doi: 10.1161/CIRCULATIONAHA.106.623199. %R 10.1161/CIRCULATIONAHA.106.623199. [DOI] [PubMed] [Google Scholar]
- 3.Weber OM, Martin AJ, Higgins CB. Whole-heart steady-state free precession coronary artery magnetic resonance angiography. Magn Reson Med. 2003;50(6):1223–8. doi: 10.1002/mrm.10653. [DOI] [PubMed] [Google Scholar]
- 4.Sakuma H, et al. Assessment of coronary arteries with total study time of less than 30 minutes by using whole-heart coronary MR angiography. Radiology. 2005;237(1):316–21. doi: 10.1148/radiol.2371040830. [DOI] [PubMed] [Google Scholar]
- 5.Hackenbroch M, et al. 3D motion adapted gating (3D MAG): a new navigator technique for accelerated acquisition of free breathing navigator gated 3D coronary MR-angiography. Eur Radiol. 2005;15(8):1598–606. doi: 10.1007/s00330-005-2731-z. [DOI] [PubMed] [Google Scholar]
- 6.Angelini P. Coronary artery anomalies: an entity in search of an identity. Circulation. 2007;115(10):1296–305. doi: 10.1161/CIRCULATIONAHA.106.618082. [DOI] [PubMed] [Google Scholar]
- 7.McConnell MV, et al. Identification of anomalous coronary arteries and their anatomic course by magnetic resonance coronary angiography. Circulation. 1995;92(11):3158–62. doi: 10.1161/01.cir.92.11.3158. [DOI] [PubMed] [Google Scholar]
- 8.Taylor AM, et al. Coronary artery imaging in grown up congenital heart disease: complementary role of magnetic resonance and x-ray coronary angiography. Circulation. 2000;101(14):1670–8. doi: 10.1161/01.cir.101.14.1670. [DOI] [PubMed] [Google Scholar]
- 9.Post JC, et al. Magnetic resonance angiography of anomalous coronary arteries. A new gold standard for delineating the proximal course? Circulation. 1995;92(11):3163–71. doi: 10.1161/01.cir.92.11.3163. [DOI] [PubMed] [Google Scholar]
- 10.Vliegen HW, et al. Value of fast gradient echo magnetic resonance angiography as an adjunct to coronary arteriography in detecting and confirming the course of clinically significant coronary artery anomalies. Am J Cardiol. 1997;79(6):773–6. doi: 10.1016/s0002-9149(96)00866-1. [DOI] [PubMed] [Google Scholar]
- 11.Bluemke DA, et al. Noninvasive Coronary Artery Imaging: Magnetic Resonance Angiography and Multidetector Computed Tomography Angiography: A Scientific Statement From the American Heart Association Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention, and the Councils on Clinical Cardiology and Cardiovascular Disease in the Young. Circulation. 2008;118(5):586–606. doi: 10.1161/CIRCULATIONAHA.108.189695. %R 10.1161/CIRCULATIONAHA.108.189695. [DOI] [PubMed] [Google Scholar]
- 12.Kim WY, et al. Coronary magnetic resonance angiography for the detection of coronary stenoses. N Engl J Med. 2001;345(26):1863–9. doi: 10.1056/NEJMoa010866. [DOI] [PubMed] [Google Scholar]
- 13.Manning WJ, et al. Coronary magnetic resonance imaging. Cardiol Clin. 2007;25(1):141–70. vi. doi: 10.1016/j.ccl.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Nagel E, et al. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation. 1999;99(6):763–70. doi: 10.1161/01.cir.99.6.763. [DOI] [PubMed] [Google Scholar]
- 15.Hundley WG, et al. Utility of fast cine magnetic resonance imaging and display for the detection of myocardial ischemia in patients not well suited for second harmonic stress echocardiography. Circulation. 1999;100(16):1697–702. doi: 10.1161/01.cir.100.16.1697. [DOI] [PubMed] [Google Scholar]
- 16.Hendel RC, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group. J Am Coll Cardiol. 2006;48(7):1475–97. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Rehwald WG, et al. In: Clinical CMR Imaging Techniques, in Cardiovascular Magnetic Resonance. Manning WJ, Pennell DJ, editors. Churchill Livingstone; New York: In press. [Google Scholar]
- 18.Jerosch-Herold M, et al. Analysis of myocardial perfusion MRI. J Magn Reson Imaging. 2004;19(6):758–70. doi: 10.1002/jmri.20065. [DOI] [PubMed] [Google Scholar]
- 19.Beller GA, Holzgrefe HH, Watson DD. Effects of dipyridamole-induced vasodilation on myocardial uptake and clearance kinetics of thallium-201. Circulation. 1983;68(6):1328–38. doi: 10.1161/01.cir.68.6.1328. [DOI] [PubMed] [Google Scholar]
- 20.Glover DK, Okada RD. Myocardial kinetics of Tc-MIBI in canine myocardium after dipyridamole. Circulation. 1990;81(2):628–37. doi: 10.1161/01.cir.81.2.628. [DOI] [PubMed] [Google Scholar]
- 21.Wilke N, et al. Myocardial perfusion reserve: assessment with multisection, quantitative, first-pass MR imaging. Radiology. 1997;204(2):373–84. doi: 10.1148/radiology.204.2.9240523. [DOI] [PubMed] [Google Scholar]
- 22.Epstein FH, et al. Multislice first-pass cardiac perfusion MRI: validation in a model of myocardial infarction. Magn Reson Med. 2002;47(3):482–91. doi: 10.1002/mrm.10085. [DOI] [PubMed] [Google Scholar]
- 23.Klocke FJ, et al. Limits of detection of regional differences in vasodilated flow in viable myocardium by first-pass magnetic resonance perfusion imaging. Circulation. 2001;104(20):2412–6. doi: 10.1161/hc4501.099306. [DOI] [PubMed] [Google Scholar]
- 24.Lee DC, et al. Magnetic resonance versus radionuclide pharmacological stress perfusion imaging for flow-limiting stenoses of varying severity. Circulation. 2004;110(1):58–65. doi: 10.1161/01.CIR.0000133389.48487.B6. [DOI] [PubMed] [Google Scholar]
- 25.Christian TF, et al. Absolute myocardial perfusion in canines measured by using dual-bolus first-pass MR imaging. Radiology. 2004;232(3):677–84. doi: 10.1148/radiol.2323030573. [DOI] [PubMed] [Google Scholar]
- 26.Plein S, et al. Assessment of non-ST-segment elevation acute coronary syndromes with cardiac magnetic resonance imaging. J Am Coll Cardiol. 2004;44(11):2173–81. doi: 10.1016/j.jacc.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 27.Plein S, et al. Coronary artery disease: myocardial perfusion MR imaging with sensitivity encoding versus conventional angiography. Radiology. 2005;235(2):423–30. doi: 10.1148/radiol.2352040454. [DOI] [PubMed] [Google Scholar]
- 28.Klein MA, et al. Detection of chronic coronary artery disease: value of pharmacologically stressed, dynamically enhanced turbo-fast low-angle shot MR images. AJR Am J Roentgenol. 1993;161(2):257–63. doi: 10.2214/ajr.161.2.8333357. [DOI] [PubMed] [Google Scholar]
- 29.Hartnell G, et al. Detection of myocardial ischemia: value of combined myocardial perfusion and cineangiographic MR imaging. AJR Am J Roentgenol. 1994;163(5):1061–7. doi: 10.2214/ajr.163.5.7976875. [DOI] [PubMed] [Google Scholar]
- 30.Al-Saadi N, et al. Noninvasive detection of myocardial ischemia from perfusion reserve based on cardiovascular magnetic resonance. Circulation. 2000;101(12):1379–83. doi: 10.1161/01.cir.101.12.1379. [DOI] [PubMed] [Google Scholar]
- 31.Eichenberger AC, et al. Ischemic heart disease: assessment with gadolinium-enhanced ultrafast MR imaging and dipyridamole stress. J Magn Reson Imaging. 1994;4(3):425–31. doi: 10.1002/jmri.1880040331. [DOI] [PubMed] [Google Scholar]
- 32.Bertschinger KM, et al. Magnetic resonance myocardial first-pass perfusion imaging: parameter optimization for signal response and cardiac coverage. J Magn Reson Imaging. 2001;14(5):556–62. doi: 10.1002/jmri.1219. [DOI] [PubMed] [Google Scholar]
- 33.Schwitter J, et al. Assessment of myocardial perfusion in coronary artery disease by magnetic resonance: a comparison with positron emission tomography and coronary angiography. Circulation. 2001;103(18):2230–5. doi: 10.1161/01.cir.103.18.2230. [DOI] [PubMed] [Google Scholar]
- 34.Panting JR, et al. Echo-planar magnetic resonance myocardial perfusion imaging: parametric map analysis and comparison with thallium SPECT. J Magn Reson Imaging. 2001;13(2):192–200. doi: 10.1002/1522-2586(200102)13:2<192::aid-jmri1029>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 35.Sensky PR, et al. Magnetic resonance perfusion imaging in patients with coronary artery disease: a qualitative approach. Int J Cardiovasc Imaging. 2002;18(5):373–83. doi: 10.1023/a:1016057821005. [DOI] [PubMed] [Google Scholar]
- 36.Ibrahim T, et al. Assessment of coronary flow reserve: comparison between contrast-enhanced magnetic resonance imaging and positron emission tomography. J Am Coll Cardiol. 2002;39(5):864–70. doi: 10.1016/s0735-1097(01)01829-0. [DOI] [PubMed] [Google Scholar]
- 37.Chiu CW, et al. Combined first-pass perfusion and viability study at MR imaging in patients with non-ST segment-elevation acute coronary syndromes: feasibility study. Radiology. 2003;226(3):717–22. doi: 10.1148/radiol.2263011902. [DOI] [PubMed] [Google Scholar]
- 38.Ishida N, et al. Noninfarcted myocardium: correlation between dynamic first-pass contrast-enhanced myocardial MR imaging and quantitative coronary angiography. Radiology. 2003;229(1):209–16. doi: 10.1148/radiol.2291021118. [DOI] [PubMed] [Google Scholar]
- 39.Nagel E, et al. Magnetic resonance perfusion measurements for the noninvasive detection of coronary artery disease. Circulation. 2003;108(4):432–7. doi: 10.1161/01.CIR.0000080915.35024.A9. [DOI] [PubMed] [Google Scholar]
- 40.Doyle M, et al. The impact of myocardial flow reserve on the detection of coronary artery disease by perfusion imaging methods: an NHLBI WISE study. J Cardiovasc Magn Reson. 2003;5(3):475–85. doi: 10.1081/jcmr-120022263. [DOI] [PubMed] [Google Scholar]
- 41.Wolff SD, et al. Myocardial first-pass perfusion magnetic resonance imaging: a multicenter dose-ranging study. Circulation. 2004;110(6):732–7. doi: 10.1161/01.CIR.0000138106.84335.62. [DOI] [PubMed] [Google Scholar]
- 42.Giang TH, et al. Detection of coronary artery disease by magnetic resonance myocardial perfusion imaging with various contrast medium doses: first European multi-centre experience. Eur Heart J. 2004;25(18):1657–65. doi: 10.1016/j.ehj.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 43.Paetsch I, et al. Comparison of dobutamine stress magnetic resonance, adenosine stress magnetic resonance, and adenosine stress magnetic resonance perfusion. Circulation. 2004;110(7):835–42. doi: 10.1161/01.CIR.0000138927.00357.FB. [DOI] [PubMed] [Google Scholar]
- 44.Klem I, et al. Improved detection of coronary artery disease by stress perfusion cardiovascular magnetic resonance with the use of delayed enhancement infarction imaging. J Am Coll Cardiol. 2006;47(8):1630–8. doi: 10.1016/j.jacc.2005.10.074. [DOI] [PubMed] [Google Scholar]
- 45.Pennell DJ, et al. Clinical indications for cardiovascular magnetic resonance (CMR): Consensus Panel report. Eur Heart J. 2004;25(21):1940–65. doi: 10.1016/j.ehj.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 46.Cecil MP, et al. The importance of work-up (verification) bias correction in assessing the accuracy of SPECT thallium-201 testing for the diagnosis of coronary artery disease. J Clin Epidemiol. 1996;49(7):735–42. doi: 10.1016/0895-4356(96)00014-5. [DOI] [PubMed] [Google Scholar]
- 47.Detrano R, et al. Factors affecting sensitivity and specificity of a diagnostic test: the exercise thallium scintigram. Am J Med. 1988;84(4):699–710. doi: 10.1016/0002-9343(88)90107-6. [DOI] [PubMed] [Google Scholar]
- 48.Cury RC, et al. Diagnostic performance of stress perfusion and delayed-enhancement MR imaging in patients with coronary artery disease. Radiology. 2006;240(1):39–45. doi: 10.1148/radiol.2401051161. [DOI] [PubMed] [Google Scholar]
- 49.Ingkanisorn WP, et al. Prognosis of negative adenosine stress magnetic resonance in patients presenting to an emergency department with chest pain. J Am Coll Cardiol. 2006;47(7):1427–32. doi: 10.1016/j.jacc.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 50.Jahnke C, et al. Prognostic value of cardiac magnetic resonance stress tests: adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation. 2007;115(13):1769–76. doi: 10.1161/CIRCULATIONAHA.106.652016. [DOI] [PubMed] [Google Scholar]
- 51.Bodi V, et al. Prognostic value of dipyridamole stress cardiovascular magnetic resonance imaging in patients with known or suspected coronary artery disease. J Am Coll Cardiol. 2007;50(12):1174–9. doi: 10.1016/j.jacc.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 52.Weinmann HJ, et al. Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent. AJR Am J Roentgenol. 1984;142(3):619–24. doi: 10.2214/ajr.142.3.619. [DOI] [PubMed] [Google Scholar]
- 53.Cerqueira MD, et al. Safety profile of adenosine stress perfusion imaging: results from the Adenoscan Multicenter Trial Registry. J Am Coll Cardiol. 1994;23(2):384–9. doi: 10.1016/0735-1097(94)90424-3. [DOI] [PubMed] [Google Scholar]
- 54.Rossen JD, et al. Comparison of coronary vasodilation with intravenous dipyridamole and adenosine. J Am Coll Cardiol. 1991;18(2):485–91. doi: 10.1016/0735-1097(91)90604-8. [DOI] [PubMed] [Google Scholar]
- 55.Fuster V, Kim RJ. Frontiers in cardiovascular magnetic resonance. Circulation. 2005;112(1):135–44. doi: 10.1161/01.CIR.0000155618.37779.A0. [DOI] [PubMed] [Google Scholar]
- 56.Di Bella EV, Parker DL, Sinusas AJ. On the dark rim artifact in dynamic contrast-enhanced MRI myocardial perfusion studies. Magn Reson Med. 2005;54(5):1295–9. doi: 10.1002/mrm.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shah DJ, Judd RM, Kim RJ. Technology insight: MRI of the myocardium. Nat Clin Pract Cardiovasc Med. 2005;2(11):597–605. doi: 10.1038/ncpcardio0352. [DOI] [PubMed] [Google Scholar]
- 58.Panting JR, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346(25):1948–53. doi: 10.1056/NEJMoa012369. [DOI] [PubMed] [Google Scholar]
- 59.Vermeltfoort IA, et al. Is subendocardial ischaemia present in patients with chest pain and normal coronary angiograms? A cardiovascular MR study. Eur Heart J. 2007;28(13):1554–8. doi: 10.1093/eurheartj/ehm088. [DOI] [PubMed] [Google Scholar]
- 60.Cerqueira MD, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 61.Kim RJ, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343(20):1445–53. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 62.Mahrholdt H, et al. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26(15):1461–74. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 63.Choudhury L, et al. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40(12):2156–64. doi: 10.1016/s0735-1097(02)02602-5. [DOI] [PubMed] [Google Scholar]
- 64.McCrohon JA, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108(1):54–9. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 65.Mieres JH, et al. Noninvasive cardiac imaging. Am Fam Physician. 2007;75(8):1219–28. [PubMed] [Google Scholar]
- 66.Gani F, Jain D, Lahiri A. The role of cardiovascular imaging techniques in the assessment of patients with acute chest pain. Nucl Med Commun. 2007;28(6):441–9. doi: 10.1097/MNM.0b013e3281744491. [DOI] [PubMed] [Google Scholar]