Abstract
A new analytical method suitable for high throughput measurements of LTE4 in human urine is described. The methodology utilizes on-line enrichment and liquid chromatography/ tandem mass spectrometry (LC/MS/MS). The novel LC/MS/MS method is rapid, linear from 5 to 500 pg/mL in spiked urine samples of both healthy and asthmatic subjects and more accurate and precise than enzyme immunoassay (EIA) and previous LC/MS/MS methods. Results from sample integrity experiments and preliminary values of urinary LTE4 from healthy adults and children are reported.
Keywords: LTE4, leukotriene, asthma, liquid chromatography, mass spectrometry
Introduction
Lipid mediators have emerged as key players in asthmatic inflammation and are specific targets for asthma therapy to improve symptoms, lung function, and disease control [1]. The best characterized in the context of asthma, the cysteinyl leukotrienes (i.e., LTC4, LTD4, LTE4: “cysLT”), are potent mediators of bronchoconstriction and inflammation in asthma and have become targets for asthma therapy. Our data, and that of others, has shown that the measurement of leukotriene E4 (LTE4) in human urine can potentially be used to predict asthma worsening and to identify likely responders to leukotriene modifying medications [1-8]. The widely used LTE4 measurement assay is an antibody-based method that is time-consuming and subject to high variation thereby limiting its efficacy as a clinical tool. Developing an assay that is quick, reliable and precise is a crucial step in determining the clinical efficacy of this biomarker.
In general, two basic methods of measuring LTE4 are currently employed; antibody-based, and mass spectrometry-based. Although both techniques have merit, neither appears to have been accepted as a standardized approach and it has yet to be established which method provides the highest degrees of sensitivity, accuracy and precision. Critics of LTE4 immunoassays cite concerns over sample loss during the sample preparation phase and cross-reactivity of the antibody resulting in overestimation of LTE4 levels [9]. Conversely, mass spectrometry-based assays may result in significant sample loss during sample preparation [9].
Measurement of LTE4 using immunoassay
The LTE4 immunoassay is based on the competition between free LTE4 in the sample and an LTE4-acetylcholinesterase (AChE) conjugate for a limited amount of LTE4 antiserum. Urine contains impurities which can interfere with the assay, in effect competing for LTE4 binding sites. To eliminate contaminants from the urine, it is often subjected to an affinity purification step prior to its use in the immunoassay. Not only is the accuracy of these methods dependent on the specificity of the chosen antibody, extraction methods and efficiencies can also vary widely, making it difficult to compare values between clinical research studies. One such method utilizes crude urine [10] and another HPLC for separation of various leukotrienes [11], both followed by enzyme immunoassay (EIA). Another strategy employs a peptidoleukotriene immunoaffinity resin to reportedly simplify extraction [12,13]. An EIA kit is distributed by Cayman Chemical Company (Ann Arbor, MI) and has been utilized in clinical studies of children with asthma [13].
Measurement of LTE4 using mass spectrometry
A number of mass spectrometry based methods to measure LTE4 levels in urine have been previously employed, many of which employ multiple reaction monitoring (MRM) with a triple quadrupole (QQQ) mass spectrometer. When a calibration curve and labeled internal standards are used, this method is often thought to be more precise than antibody-based assays. However, to our knowledge a direct comparison of the two methods for quantitating LTE4 has never been published. In addition, previously published methods rely on often-complex upfront enrichment strategies that make them less suitable for adaptation to a clinical assay. For example, Wu et al [14] extracted LTE4 and LTE4-d3 with Empore membrane disks (3M, St. Paul, MN) followed by analysis using a MRM strategy. Hardy, et al [15] used solid phase extraction (SPE) followed by MRM. Kishi et al [9] sought to improve the efficiency and speed of these methods through the use of on-line extraction of LTE4 followed by MRM. Specifically, a column switching/enrichment column strategy was employed which resulted in the concentration of the analyte on the column and removed salts and contaminants that could interfere with ionization. The assay displayed good linearity from 10 pg/ml to 3 ng/ml and inter-assay precision relative standard deviation (RSD) and intra-assay precision (DEV) were both under 6%. We sought to develop a LC/MS/MS assay that could be readily adopted by clinical laboratories based in part on these previous methods.
Experimental
Reagents
LTE4 and LTE4-d3 standards were purchased from Cayman Chemical (Ann Arbor, Michigan) or Biomol (Plymouth meeting, PA). LTE4 EIA and affinity sorbent and cysteinyl leukotriene purification kits were also purchased from Cayman Chemical. Oasis HLB (Hydrophilic-Lipophilic Balanced) solid phase extraction cartridges were obtained from Waters (Milford Massachussets). Water (HPLC grade) used for HPLC mobile phases was obtained from Burdick and Jackson (Morristown, New Jersey). Acetic acid, methanol (LC/MS grade), acetonitrile (LC/MS grade) and ammonium hydroxide were obtained from Fisher Scientific (Fair Lawn, New Jersey). Protease inhibitor tablets (Product S8820) were purchased from Sigma-Aldrich and contain 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF), E-64, bestatin, leupeptin, aprotinin, and EDTA.
Standards and calibration curve preparation for LC/MS/MS analysis
An internal standard spike solution was prepared daily at 1 ng/ml LTE4-d3 in 50:50 methanol:water in 0.1% acetic acid and 0.036% ammonium hydroxide. Calibration solutions of LTE4 were prepared from stock solutions in methanol at concentrations of 4, 8, 20, 40, 80, 200 and 400 pg/ml in 1 ml of 90:10 water:methanol and 0.02% acetic acid and 0.007% ammonium hydroxide. This is equivalent to 5-500pg/ml final concentration in 800ul of urine before addition of 200 uL of internal standard. Reported physiological concentrations of LTE4 are 10 to 60 pg/ml in normal subjects and up to several hundred pg/ml in some asthmatic patients [1,16].
Sample preparation
Human urine from healthy and asthmatic volunteers was collected in 120 ml urine collection vesicles. Immediately after sample collection urine was placed in a 15-45 ml falcon tube and centrifuged at 3,000 × g, for 10 minutes at 4°C. The supernatant was collected and placed in a new falcon tube or subaliquoted into microfuge tubes and frozen at -80°C. Alternatively, samples were analyzed prior to freezing or were kept at 4°C, room temperature, or 30°C degrees for stability assessment experiments. For sample integrity comparisons, urine was pooled from three asthmatic subjects. Pooled urine from asthmatics was also used for accuracy and precision experiments. The Institutional Review Board at National Jewish Health approved this study. Parents/guardians provided informed consent for children less than 7 years of age and written consent was obtained from older children and their parents.
Purification prior to EIA analysis
Afinity sorbent purification
Samples were processed according to the protocol provided by Cayman Chemical. Briefly, sorbent is provided in a microfuge tube as a loose material with storage buffer. The sorbent is activated and sample added. Following washing, methanol is added as an eluant. The sample is centrifuged and the supernatant used for EIA. Methanol eluants were stored at -80° C prior to vacuum drying and analysis by EIA.
Cysteinyl leukotriene purification kit (purification columns)
Sorbent is also available pre-packed in 5 or 20 ml SPE columns. Current samples were processed using the 20 ml columns according to the protocol provided by Cayman Chemical. Methanol eluants were stored at -80° C prior to vacuum drying and analysis by EIA.
Waters HLB solid phase extraction cartridges
A 10CC syringe barrel was attached to an HLB cartridge and then attached to a vacuum SPE manifold. The cartridge was conditioned with 2 mls of methanol followed by 2 mls of H20 at 5 mls/min. A 2 ml aliquot of urine was adjusted to pH 4 using 10% acetic acid, and then drawn through the extraction cartridge at 1 ml/min, followed by a wash with 2 mls of 5% methanol at 5 mls/min. LTE4 was then eluted from the column with 2 mls of 100% methanol, and then stored in a 12 × 75 mm polypropylene snap cap tube at -80° C prior to vacuum drying and analysis by EIA.
Off-Line reverse phase HPLC
A 900 ul aliquot of urine was injected on an Agilent 1200 series autosampler with an isocratic pump (pump “A”), a quaternary pump (pump “B”), a variable wavelength detector and analytical scale fraction collector. The loading/wash buffer on pump A was 1:1 Methanol:H2O with 10 mM ammonium acetate with a flow rate of 1 ml/min. For Pump B buffer A was 10 mM ammonium acetate in H2O and buffer B was 10mM ammonium acetate in methanol. Gradient conditions for pump B were as follows: isocratic at 70% B from 0 - 6 minutes, 100% B from 6.01 to 10 minutes, 70% B from 10.01 to 15 minutes. The column switching valve was switched to redirect the flow from pump B to backflush the enrichment column onto the analytical column from 3.01 to 10 minutes. The fraction collector was set to collect from 5.51 minutes to 6.5 minutes. The sample was stored in a 12 × 75 mm polypropylene snap cap tube at -80° C prior to vacuum drying and analysis by EIA. The enrichment column was an Agilent SB-C18 4.6 × 15 mm 3.5 um, and the analytical column was an Agilent Eclipse XDB-C18 4.6 × 50 mm 1.8 um.
Sample preparation prior to LC/MS/MS analysis
For LC/MS/MS, 800 uL of supernatant from centrifuged urine was placed in an autosampler vial and 200 uL of internal standard spike solution was added, giving a final concentration of 200 pg/ml of LTE4-d3. The sample was vortexed for 5 seconds and loaded directly on to the LC/MS/MS system using the HPLC method outlined below.
Enzyme Immunosorbent Assay (EIA)
Prior to EIA analysis, the methanol eluants were dried in a vacuum centrifuge. EIA was performed by Cayman Chemical Company according to the product insert. Urine samples were also sent to Cayman Chemical (Ann Arbor, Michigan) for analysis, which was conducted using an EIA for LTE4.
High Performance Liquid Chromatography
Liquid chromatography was carried out using an Agilent 1200 series HPLC equipped with a quaternary pump (pump “A”), a binary pump (pump “B”), an autosampler with thermostat, and a column heating compartment with switching valve (Agilent Technologies, Palo Alto, CA). Buffer A was 0.02% acetic acid in HPLC water adjusted to pH 5.6 with ammonium hydroxide (about 0.007% ammonium hydroxide), and Buffer B was 0.02% acetic acid and 0.007% ammonium hydroxide in 100% acetonitrile. Gradient conditions for pump A were as follows: 5% B from 0 - 1 minute, 30% B from 1.01 - 4.5 minutes, 100% B from 4.51 – 7 minutes, 5% B from 7.01 – 14 minutes. Gradient conditions for pump B were as follows: 30% B from 0 - 3 minutes, 35% B from 3.01 – 4.5 minutes, 35-38% B from 4.5 – 11 minutes, and 38% B from 11-14 minutes. 900uL of the prepared urine was injected onto the enrichment column for concentration/purification followed by backflushing and separation on the analytical column using the gradient conditions described above. The column switching valve was switched to redirect the flow from pump B to backflush the enrichment column onto the analytical column from 3 to 4.5 minutes. Flow rates were 1 ml/min for pump A and 0.15 ml/min for pump B. The enrichment column was an Extend C18 4.6 × 12.5 mm 5 uM guard cartridge (Agilent Technologies, Palo Alto, CA). The analytical column was an Agilent Eclipse C8 column (2.1 × 50 mm) with a 1.8 uM particle size fitted with an Eclipse C8 2.1 × 12.5 mm 5 uM guard column operated at 40°C.
Tandem Mass Spectrometry and MRM
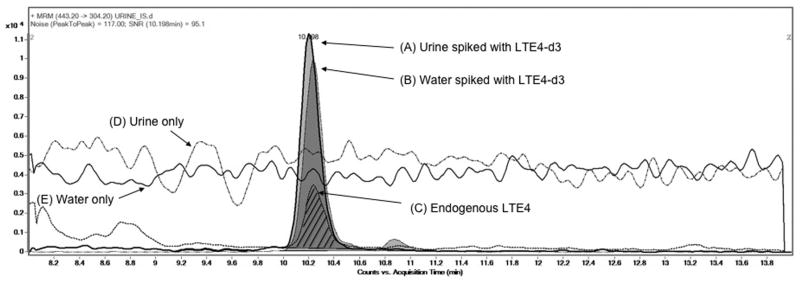
Detection of LTE4 was accomplished using an Agilent 6410 triple quadrupole mass spectrometer (QQQ) coupled to a positive electrospray ionization source. The HPLC column effluent was diverted to waste until 10 minutes, and then diverted to the electrospray source during the elution of LTE4 from 10.01-13 minutes. Heated (300°C) drying gas flowing at 10 L/min, with a nebulizer pressure of 15 PSIG, was used for droplet desolvation. Spray was induced with a capillary voltage of 4000V. The optimal fragmentor voltage of 80V and collision energy of 8V was determined by flow injection analysis. The QQQ mass spectrometer was tuned and calibrated using Agilent G1969-85000 calibration and tuning mix (Agilent Technologies). The transition of LTE4 440.2→301.2 m/z and LTE4-d3 443.2→304.2 m/z (Figure 1) was measured as described by Kishi et al [9]. Both transitions were monitored for 500 ms resulting in 1 scan/sec.
Figure 1. Evaluation of ion suppression on response of LTE4 and LTE4-d3 in urine and water.
Injection Experiments: Extracted ion chromatogram (EIC) of MRM transitions for LTE4-d3 (m/z 443.2>304.3) spiked at 200 pg/ml in a human urine sample (A) and water (B), and MRM transition of endogenous LTE4 (m/z 440.2>301.3) in a human urine sample (C, hatched lines). Data demonstrate the response of internal standard is not affected by ion suppression. Data was collected in positive electrospray ionization mode. The peak area for urine and water spiked with LTE4-d3 was 115754 and 107672 respectively. Post- Column Infusion experiments: LTE4 was infused and the 440.2 > 301.2 transition was monitored during injection of urine (D) and water (E) samples. Data demonstrate the absence of ion suppression in a urine matrix.
LTE4 and LTE4-d3 were monitored for quantitation by extracting ion chromatograms for the transitions 440.2→301.2 m/z and 443.2→304.2 m/z using Mass Hunter Quantitative Analysis Software (Agilent Technologies). In some experiments the transitions of LTE4 440.2→189.2 m/z and LTE4-d3 443.2→192.2 m/z were monitored as qualifiers. Calibration curves were calculated using linear regression with a 1/× weighting. The calibration curves were not forced through the origin.
Statistics
Intraday variation was monitored by spiking LTE4 into pooled urine at concentrations of 0, 25, 50, 100, 250, 500 pg/ml and analyzing sample replicates (n=6) on the same day. Interday variation was evaluated over 6 days. Spike recovery was calculated as [(measured spike sample concentration) – (measured unspiked sample concentration)/(theoretical spike concentration] × 100 (%). Coefficient of variation was calculated as [(standard deviation n)/(average n)] × 100. Linearity was determined using linear regression analysis as described [17]. Comparison of normal adult versus child data was conducted in JMP 7.0.02 (SAS Institute Inc).
Results/Discussion
LTE4 quantitation using off-line purification methods followed by EIA
Results from the 4 purification methods prior to EIA were compared for accuracy and precision (Table 1). Using the software provided by Cayman, linear regression analysis produced a calibration curve of y=-0.79× + 4.055, r2 = 0.953 with 8 calibration points. Of the off-line sorbent/SPE based methods, the best results were obtained using the affinity sorbent. Overall the best accuracy and precision for purification prior to EIA were obtained by off-line HPLC.
Table 1. Analysis of spiked pooled urine samples from healthy adults by enzyme immunoassay.
| Method | 1- Loose Sorbent | 2- Sorbent Column | 3- HLB SPE cartridge | 4- Off-line HPLC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spike Amount (pg/mL) |
Ave Conc | CV | %R | Ave Conc | CV | %R | Ave Conc | CV | %R | Ave Conc | CV | %R |
| 0 | 17.5 | 75 | NA | 133.6 | * | NA | 147.9 | 13.8 | NA | 27.5 | 10 | NA |
| 15.6 | 36.7 | 4.2 | 123.4 | 198.3 | * | 414.7 | 148.2 | 25.1 | 2.2 | 36.7 | 13.1 | 59.3 |
| 62.5 | 38.8 | 8 | 34.2 | 224.7 | * | 145.8 | 262.1 | 7.9 | 182.7 | 107.3 | 8.8 | 127.7 |
| 250 | 197.1 | 11.4 | 71.9 | 69.3 | * | -25.7 | 489.4 | 6.3 | 136.6 | 216.1 | 5 | 75.5 |
Pooled urine samples (n=2) were spiked with 0, 15.6, 62.5, or 250 pg/ml of LTE4 and then purified using the indicated method, followed by EIA analysis.
Method 1=Cayman loose sorbent
Method 2=Cayman sorbent columns
Method 3=HLB SPE cartridge
Method 4=Off-line HPLC
Precision data was not obtained
LTE4 quantitation using LC/MS/MS
Method development focused on rapid, on-line extraction of unprocessed urine. Similar to Kishi et al [9], we utilized a column-switching strategy to both concentrate the analyte and to remove contaminants. Significant ion suppression was observed before optimization of the HPLC method. Ion suppression was reduced by washing the trapping column with 30% Buffer B (0.02% acetic acid and 0.007% ammonium hydroxide). The gradient was further optimized to separate closely eluting endogenous contaminants from LTE4. Several combinations of enrichment column and analytical column were compared and it was determined that the Extend C18 4.6 × 12.5 mm 5 uM guard cartridge for the enrichment column and the Agilent Eclipse C8 column 2.1 × 50 mm 1.8uM analytical column resulted in optimal separation and elimination of ion suppression (data not shown). The elimination of ion suppression was confirmed by comparing the response of equivalent amounts of LTE4-d3 spiked into reagent water and pooled urine, in addition to a continuous post column infusion of LTE4-d3 with unspiked water and urine (Figure 1). Greater than 90% recovery of LTE4-d3 in pooled urine was obtained using these methods. Although operating the instrument in negative mode resulted in less noise than positive mode, an adequate signal could not be generated at lower concentrations even after optimization (data not shown). The instrument was operated in positive mode as a result. A typical standard curve was linear in the range 5-500 pg/ml with an equation of y=0.0033× (SE=2.11E-05)-1.43E-005 (SE=1.28E-03, and a correlation coefficient of 0.9985 for 7 calibration levels. Typical response values (ie. Intensities) ranged from ∼1000 for 5 pg/ml samples to ∼150,000 for 500 pg/ml samples (data not shown). Response values are arbitrary and have no units of measurement. Standards from two independent sources (Cayman and Biomol) were compared, with the product from Cayman Chemical producing a slightly lower response (-9.9%) than the product from Biomol International (Plymouth meeting, PA) (Data not shown).
LC/MS/MS method is precise and accurate over a physiological range
Intra- and inter-day analyses were conducted over a six-day period using both calibration standards and spiked, pooled urine from asthmatic children (Table 2). With the exception of one out of thirteen measurements, CV's were under 10% and the average CV was 5.76% for all intra-day experiments. Similarly, the average CV for inter-day measurements was 7.20%.
Table 2. Intra-day and inter-day accuracy and precision of LTE4 assay using calibration standards or pooled spiked urine from asthmatic children.
Intra- and inter-day analyses were conducted over a six-day period using either calibration standards or spiked, pooled urine from asthmatic children. Calibration spike standards were prepared in 30:70 Water:Methanol in 0.08% acetic acid and 0.04% ammonium hydroxide. Standards were then spiked into HPLC water and pooled urine from asthmatics at the indicated concentrations before analysis. Individual results were averaged and coefficient of variation (CV) and percent recovery (%R) were determined. Abbreviations: Average (AVG), Percent recovery (%R).
| Calibration standards | Intra-day Accuracy and Precision (n=6) | Inter-day Accuracy and Precision (n=6) | ||||
|---|---|---|---|---|---|---|
| (pg/mL) | AVG | CV | %R | AVG | CV | %R |
| 5 | 5.35 | 15.90 | 107.07 | 5.25 | 11.52 | 104.92 |
| 10 | 10.11 | 9.82 | 101.07 | 9.77 | 11.28 | 97.74 |
| 25 | 24.13 | 2.59 | 96.52 | 24.77 | 11.69 | 99.09 |
| 50 | 49.26 | 7.01 | 98.52 | 50.32 | 5.84 | 100.64 |
| 100 | 98.66 | 1.69 | 98.66 | 99.35 | 4.43 | 99.35 |
| 250 | 238.32 | 4.00 | 95.33 | 244.26 | 2.18 | 97.71 |
| 500 | 514.17 | 3.11 | 102.83 | 506.66 | 1.38 | 101.33 |
| Spiked urine | Intra-day Accuracy and Precision (n=6) | Inter-day Accuracy and Precision (n=6) | ||||
| (pg/mL) | AVG | CV | %R | AVG | CV | %R |
| +0 | 81.09 | 4.59 | NA | 82.62 | 10.20 | NA |
| +25 | 111.31 | 6.84 | 120.87 | 106.16 | 9.53 | 94.15 |
| +50 | 132.11 | 6.08 | 102.04 | 131.67 | 5.30 | 98.09 |
| +100 | 184.98 | 4.34 | 103.90 | 180.31 | 4.52 | 97.69 |
| +250 | 322.11 | 6.72 | 96.41 | 317.71 | 8.43 | 94.04 |
| +500 | 571.85 | 2.17 | 98.15 | 584.47 | 7.31 | 100.37 |
Recoveries ranged from 95.33 to 120.87% for the intra-day experiments, and 94.15 to 104.92% in the inter-day experiments.
Urinary LTE4 appears to be stable under typical laboratory handling conditions
Sample integrity was measured using the LC/MS/MS method following various storage procedures: 1) samples analyzed on the day of collection, 2) following a single freeze/thaw cycle, 3) following up to 7 days at 4° C, 4) following 24 hours at room temperature, or 5) following 24 hours at 30°C. As shown in Table 3, storing the samples at 4° C for 7 days had the least affect (-11%) while samples stored at 30° C for 24 h underwent the most loss of LTE4 (-26%). Analysis was also performed on samples undergoing up to 5 freeze/thaw cycles. Perhaps surprisingly, single and multiple (5) freeze/thaw cycles had an equivalent effect on samples, with losses of 18.30% and 10.79% respectively (Table 4). These results may be used to determine shipping and storage of urine samples for the purpose of LTE4 analysis.
Table 3. Integrity of LTE4 in urine following various handling procedures.
The integrity of LTE4 in urine was assessed following various storage procedures. Human urine from 3 asthmatic individuals was collected, pooled, and handled under five different conditions: 1) Immediate centrifugation and analysis within 1 hour of collection (Fresh), 2) Immediate centrifugation and storage at − 80°C prior to analysis, 3) Remaining at 4°C for 7 days prior to centrifugation and analysis (no freezing), 4) Remaining at room temperature (RT) for 24 hours prior to centrifugation and storage at -80° C, and 5) after remaining at 30° C for 24 hours prior to centrifugation and storage at -80° C. Five samples were processed and analyzed in duplicate for a total n=10 injections per condition. The average LTE4 value, average CV, and average percent difference (%D) from fresh levels was assessed for the 5 samples. Abbreviations: Average (AVG), Percent Difference from fresh (%D), and coefficient of variation (CV).
| Procedure | Freeze/thaw | AVG | CV | %D from fresh | |
|---|---|---|---|---|---|
| 1 | Analyzed fresh | 0 | 71.27 | 2.33 | 0 |
| 2 | Immediately centrifuged and frozen after collection | 1 | 61.04 | 3.29 | -15.47 |
| 3 | Remained at 4°C for 7 days prior to centrifuging and analysis | 1 | 63.56 | 3.94 | -11.43 |
| 4 | Remained at room temp for 24 h before centrifuging and freezing | 1 | 55.15 | 3.1 | -25.51 |
| 5 | Remained at 30°C for 24 h before centrifuging and freezing | 1 | 54.75 | 1.99 | -26.21 |
Table 4. Effect of multiple freeze/thaws on LTE4 levels in urine.
Human urine from 3 asthmatic individuals was collected, pooled, and subjected to multiple freeze/thaw cycles. Samples were analyzed in duplicate. The average LTE4 value, CV, and percent difference (%D) from fresh levels was assessed. Abbreviations: Average (AVG), Percent Difference from fresh (%D), and coefficient of variation (CV).
| No. of Freeze/Thaws | Multiple Freeze/Thaws | ||
|---|---|---|---|
| AVG | CV | %D from fresh | |
| 0 | 71.27 | 2.33 | 0 |
| 1 | 59.32 | 3.51 | -18.30 |
| 2 | 62.77 | 7.19 | -12.69 |
| 3 | 60.51 | 7.57 | -16.33 |
| 4 | 65.98 | 5.27 | -7.71 |
| 5 | 63.97 | 6.20 | -10.79 |
| AVG | 62.51 | 5.95 | -13.16 |
Because initial steps can vary greatly between laboratories, sample handling studies also included a comparison of samples that were centrifuged prior to freezing versus samples that were centrifuged after freezing and the addition of protease inhibitors. In general there was no significant difference between samples regardless of time of centrifugation (Table 5). Similarly, the addition of protease inhibitors in clinical samples had no effect on the recovery as demonstrated by comparison of LTE4-d3 internal standard areas from samples with protease inhibitors and those without (data not shown).
Table 5. Effects of sample handling on LTE4 in urine.
The effects of centrifugation (spin) of urine prior to storage and analysis were determined for several levels of LTE4 spiked into normal human urine. Urine was spiked with 0, 25, 100, or 250 pg of LTE4 and samples were either centrifuged followed by freezing at -80°C or were immediately frozen and centrifuged upon thawing prior to analysis. Samples were prepared and injected in duplicate and the average, CV, and percent recovery (%R) were determined. Results were subjected to a t-test and no significant difference (p-values ≥ 0.05) was found between centrifuged and non-centrifuged samples at any spike level.
| Std (p-value) | Spin | AVG | CV | %R |
|---|---|---|---|---|
| 0 pg (0.46) | Y | 21.72 | 10.10 | NC |
| N | 22.85 | 4.33 | NC | |
| 25 pg (0.05) | Y | 40.72 | 1.46 | 76.02 |
| N | 45.41 | 6.15 | 90.24 | |
| 100 pg (0.57) | Y | 108.93 | 1.32 | 87.21 |
| N | 111.90 | 7.36 | 89.04 | |
| 250 pg (0.21) | Y | 253.89 | 3.73 | 92.87 |
| N | 265.20 | 3.45 | 96.94 |
Comparison of EIA and LC/MS/MS using Clinical Samples
Urine was obtained from 10 asthmatic subjects and compared using EIA and LC/MS/MS. One aliquot was analyzed using LC/MS/MS on the day of collection and the remaining sample was aliquoted and stored at -80° C. Frozen aliquots were either sent for EIA analysis or were analyzed in-house using LC/MS/MS. Calibration curves were run prior to LC/MS/MS (r2 = 0.9995). Creatinine values were obtained through Cayman or using an in-house procedure. No r2 values were provided with the EIA results.
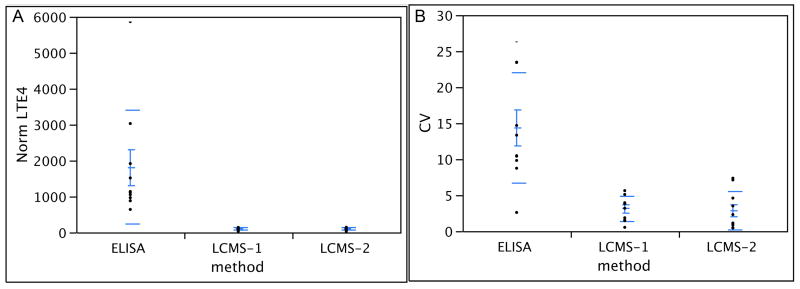
Following normalization to creatinine, the EIA resulted in values ranging from 639 to 5685 pg/mg creatinine with CV's ranging from 2.60% to 26.42% (average 14.34%). The LC/MS/MS method resulted in values ranging from 29 to 143 pg/mg creatinine with CV's ranging from 0.44% to 7.36% (average 2.99%). Overall, LC/MS/MS analysis of fresh (LCMS1) versus frozen (LCMS2) samples had excellent correlation (0.9897) and no significant correlation was apparent between LCMS versus EIA (-0.1906 and -0.1182 respectively). Results are summarized in Figure 2. It should be noted that although requested, affinity purification prior to EIA was not conducted at the commercial laboratory. To the best of our knowledge this was due to the unavailability of the affinity sorbent at the time of sample submission, which was discovered when attempts were made to purchase the sorbent directly. Table 1 illustrates the wide range of CVs (4.2 -75%) and percent recoveries (2.2 - 182.7%) that occur even when various purification methods are used prior to EIA. A comparison with Table 2 demonstrates the excellent CV's (2.1 - 10.2%) and recovery (94.04 – 120.87%) when the LC/MS/MS method is used. Note that pooled, normal urine samples were used for in-house EIA (Table 1) while pooled urine from asthmatic children was used for LC/MS/MS (Table 2).
Figure 2. Comparison of ELISA and LC/MS/MS analysis of LTE4 in urine from asthmatics.
Urine from 10 asthmatic subjects was obtained, centrifuged within 15 hours, and processed as follows: One aliquot was analyzed in-house using LC/MS/MS on the day of collection (LCMS-1) and several aliquots were frozen at -80°C immediately after collection. One frozen aliquot was analyzed by Cayman using ELISA (ELISA) according to the manufacturer's protocol and a frozen aliquot was analyzed in-house using LC/MS/MS (LCMS-2). Creatinine levels were obtained from Cayman and used to normalize all data. For LC/MS/MS samples were injected in duplicate and their average and CV were determined. CV values for ELISA were obtained from Cayman. Methods used are on the x-axis and Panel A shows normalized LTE4 values in pg/mg creatinine (y-axis) and Panel B shows CVs (y-axis).
Levels of LTE4 in urine from non-asthmatic adults and children
Control subjects did not have physician-diagnosed asthma and were not currently taking allergy or asthma medication. Urine was obtained from 39 control children (ages 3 – 17 years; 17 female, 21 male) and 10 control adults (ages 23 – 46 years; 4 female, 6 male), and analyzed as described using LC/MS/MS. Calibration curves were run prior to LC/MS/MS (r2 = 0.9982). Creatinine values were obtained through the National Jewish Clinical Laboratory. Following normalization to creatinine, normal adult LTE4 values ranged from <17.2 to 63.0 pg/mg creatinine and averaged 36.7 pg/mg creatinine with an average CV of 9.20%. Normal LTE4 values for children ranged from 9.0 to 115.1 pg/mg creatinine and averaged 50.7 pg/mg creatinine with an average CV of 5.06%. No significant gender differences were detected in either age group. There was no significant difference between adult and children values (p < 0.12); however, this may be misleading as only 10 adults were sampled compared to 39 children. In addition, only limited exclusion criteria were used. LTE4 levels for normal children and adults have been previously reported at 103 pg/ml and 80 pg/ml, respectively [12]. Normal adult ranges appear consistent with previously reported values when LC/MS/MS is used. For example, Kishi et al reported an LTE4 value in a single healthy male subject at 155.5 pg/ml using LC/MS/MS with no normalization to creatinine [9]. Mizugaki reported values of 63.1 +/- 18.7 pg/mg creatinine in 10 healthy human samples when an Empore disk was used prior to LC/MS/MS analysis [16].
Conclusion
We have demonstrated the sensitivity, precision, and accuracy for an LC/MS/MS-based assay for the measurement of LTE4 in urine. The method is linear along a physiological range, is fairly rapid (15 minutes), requires no sample preparation beyond a simple centrifugation, and is suitable for adaptation to clinical samples. In addition to minor sample loss following freeze/thaw cycles, samples were relatively stable when stored at 4°C for up to 7 days. Results from sample integrity studies may be used to determine shipping and storage conditions.
A comparison of EIA and LC/MS/MS methods shows a higher concentration when EIA is used. In the current study, the EIA assay was conducted at an off-site laboratory according to their protocol and no affinity-purification was used. However, samples analyzed via EIA using various affinity purification methods in our laboratory showed highly variable values and high %CV when different purification methods are used prior to EIA. A possible explanation for variable and/or high EIA values is interference from contaminants for the antibody binding sites. For EIA assays that incorporate purification, although somewhat lower values are reported, high variance can still potentially confound data analysis. The lower variance and easily reproducible LC/MS/MS methodology reported in this study should enable investigators to better assess the capacity for LTE4 levels to differentiate between patient phenotypes and predict asthma control and susceptibility to anti-leukotriene medications.
Acknowledgments
The authors would like to thank the children of Kunsberg School and Trails West Elementary and the adult volunteers who participated in this study. This study was funded in part through NIH/NHLBI U01 HL-81335 and the National Jewish Health GCRC and Mass Spectrometry Facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rabinovitch N. Immunol Allergy Clin North Am. 2007;27:651. doi: 10.1016/j.iac.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Rabinovitch N, Strand M, Stuhlman K, Gelfand EW. J Allergy Clin Immunol. 2008;121:1365. doi: 10.1016/j.jaci.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Cai C, Yang J, Hu S, Zhou M, Guo W. Lung. 2007;185:105. doi: 10.1007/s00408-006-0001-8. [DOI] [PubMed] [Google Scholar]
- 4.Rabinovitch N, Zhang L, Gelfand EW. J Allergy Clin Immunol. 2006;118:635. doi: 10.1016/j.jaci.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Lazarus SC, Chinchilli VM, Rollings NJ, Boushey HA, Cherniack R, Craig TJ, Deykin A, DiMango E, Fish JE, Ford JG, Israel E, Kiley J, Kraft M, Lemanske RF, Jr, Leone FT, Martin RJ, Pesola GR, Peters SP, Sorkness CA, Szefler SJ, Wechsler ME, Fahy JV. Am J Respir Crit Care Med. 2007;175:783. doi: 10.1164/rccm.200511-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, Zeiger RS, Larsen G, Spahn JD, Bacharier LB, Bloomberg GR, Guilbert TW, Heldt G, Morgan WJ, Moss MH, Sorkness CA, Taussig LM. J Allergy Clin Immunol. 2005;115:233. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Yu C, Holgate ST, Reiss TF. Eur Respir J. 2002;20:1102. doi: 10.1183/09031936.02.02402001. [DOI] [PubMed] [Google Scholar]
- 8.Malmstrom K, Rodriguez-Gomez G, Guerra J, Villaran C, Pineiro A, Wei LX, Seidenberg BC, Reiss TF. Ann Intern Med. 1999;130:487. doi: 10.7326/0003-4819-130-6-199903160-00005. [DOI] [PubMed] [Google Scholar]
- 9.Kishi N, Mano N, Asakawa N. Anal Sci. 2001;17:709. doi: 10.2116/analsci.17.709. [DOI] [PubMed] [Google Scholar]
- 10.Kumlin M, Stensvad F, Larsson L, Dahlen B, Dahlen SE. Clin Exp Allergy. 1995;25:467. doi: 10.1111/j.1365-2222.1995.tb01079.x. [DOI] [PubMed] [Google Scholar]
- 11.Westcott JY, Johnston K, Batt RA, Wenzel SE, Voelkel NF. J Appl Physiol. 1990;68:2640. doi: 10.1152/jappl.1990.68.6.2640. [DOI] [PubMed] [Google Scholar]
- 12.Westcott JY, Maxey KM, MacDonald J, Wenzel SE. Prostaglandins Other Lipid Mediat. 1998;55:301. doi: 10.1016/s0090-6980(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 13.Mai XM, Bottcher MF, Bruhammar M, Nilsson L, Zetterstrom O. Allergy. 2005;60:60. doi: 10.1111/j.1398-9995.2004.00623.x. [DOI] [PubMed] [Google Scholar]
- 14.Wu Y, Li LY, Henion JD, Krol GJ. J Mass Spectrom. 1996;31:987. doi: 10.1002/(SICI)1096-9888(199609)31:9<987::AID-JMS382>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 15.Hardy G, Boizel R, Bessard J, Cracowski JL, Bessard G, Halimi S, Stanke-Labesque F. Prostaglandins Other Lipid Mediat. 2005;78:291. doi: 10.1016/j.prostaglandins.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Mizugaki M, Hishinuma T, Suzuki N. J Chromatogr B Biomed Sci Appl. 1999;729:279. doi: 10.1016/s0378-4347(99)00174-7. [DOI] [PubMed] [Google Scholar]
- 17.Westcott JY, Sloan S, Wenzel SE. Anal Biochem. 1997;248:202. doi: 10.1006/abio.1997.2132. [DOI] [PubMed] [Google Scholar]