Abstract
Recent studies in experimental autoimmune encephalomyelitis (EAE) have found that CNS injury in Daf1-/- mice is much greater than in wild types (WTs), suggesting that upregulating DAF levels in vivo might ameliorate disease. To test this, we generated a Daf1 transgenic (Tg) mouse which had elevated DAF levels on its cell surfaces. In bystand C3b uptake assays, Daf1 Tg mouse erythrocytes took up less C3b on their surfaces than WT erythrocytes. When co-cultured with OT-II CD4+ T cells together with OVA323-339 peptide, Daf1 Tg mouse bone marrow derived dendritic cells (BM-DCs) produced less C5a and C3a than WT BM-DCs and stimulated a lesser T cell response. In MOG35-55 immunization induced EAE model, Daf1 Tg mice exhibited delayed disease onset and decreased clinical scores compared to WTs. Histological analyses showed that there were less inflammation and demyelination in spinal cords in Daf1 Tg mice than those in WTs. In accordance with these results, Daf1 Tg mice had decreased MOG35-55 specific Th1 and Th17 responses. These data provide further evidence that DAF suppresses autoreactive T cell responses in EAE, and indicate that augmenting its expression levels could be effective therapeutically in treating multiple sclerosis as well as other T cell mediated diseases.
Introduction
Decay accelerating factor (DAF, CD55) is a cell surface complement regulator that dissociates C3/C5 convertases and thereby prevents the generation of C3a and C5a (Medof et al., 1984). In previous studies using mice targeted in Daf1, the murine homologue of human DAF, we (Liu et al., 2008) and others (Liu et al., 2005) showed that Daf1-/- mice exhibited greater central nervous system (CNS) inflammation and demyelination in experimental autoimmune encephalomyelitis (EAE), a myelin-specific T cell mediated disease model for multiple sclerosis (MS). We also found that during APC: T cell interactions, both partners locally synthesize C3, factor B, factor D and C5, components required for C5a and C3a generation via the alternative pathway complement activation (Heeger et al., 2005). The absence of DAF lowers restraint on junctional C3/C5 convertases formation which results in increased levels of local C5a and C3a, both of which promote Th1/Th17 cell generation (Liu et al., 2008; Strainic et al., 2008) and sustain effector T cell viabilities after binding to their respective receptors (C5aR or C3aR) on APCs and/or T cells (Lalli et al., 2007; Lalli et al., 2008).
Although much new information on T cell responses has been gained through the above and other studies on Daf1-/- mice, until now it has not been possible to study the effects of DAF overexpression in vivo. To address this issue, we generated a Daf1 transgenic (Tg) mouse under the constitutive ROSA26 promotor (Zambrowicz et al., 1997) on the C57BL/6 background. To better characterize the distribution of the transgenic Daf1 gene expression, we crossed Daf1 Tg mice with Daf1-/- mice to generate the Daf1 Tg/KO mice that only express the transgenic Daf1 gene. Using these Daf1 Tg/KO mice, we verified that the transgenic Daf1 gene was expressed on all tissues and cells examined. By studying the Daf1 Tg mice on C57BL/6 background, we found that they expressed elevated levels of DAF protein on all cells examined and their bone marrow derived dendritic cells (BM-DCs) generated 2 fold less C5a and C3a during antigen specific APC: T cell interactions and stimulated lesser T cell responses in vitro. In in vivo studies on EAE, Daf1 Tg mice exhibited lowered clinical scores, diminished MOG35-55 specific Th1/Th17 responses, decreased central nervous system (CNS) inflammation and reduced demyelination. These data indicate that upregulating DAF levels in vivo suppresses T cell autoimmunity and ameliorates disease severity in EAE.
Material and Methods
Peptides and antibodies
Mouse MOG35-55 peptide (MEVGWYRSPFSRVVHLYRNGK) was purchase from Anaspec (San Jose, CA), Complete Frunds' adjuvant (CFA) was from Sigma (St. Louis, MO), supplemental Mycobacterium tuberculosis was from BD Biosciences (Franklin Lakes, NJ). Rat anti mouse IFN-γ mAbs were purchased from BD Biosciences. Rat anti-mouse DAF mAb 2C6 (Spiller et al., 1999) was kindly provided by Dr. B.P. Morgan, Cliff University, U.K.
Generation of Daf1 Tg mice
The cDNA coding for mouse DAF1 protein was amplified by RT-PCR from mouse liver total RNA (Ambion, TX) using proof-reading Vent DNA Polymerase (NEB, MA) and cloned into the expression vector pBroad3 under the ROSA26 promotor (Invivogen, CA). After sequencing, the expression construct was further verified by transfecting into CHO cells and transient DAF expression assayed by flow cytometry with 2C6 mAb. Linearized DAF expression cassette was injected into fertilized C57BL/6 mouse oocytes. Transgenic founders were identified by PCR (P1: 5′-GTC TCG TCG CTG ATT GGC TTC TTT-3′, P2: 5′-TGT ACC CTG GGT TGC ATG AGA AGT-3′) and bred with C57BL/6 mice. To generate the Daf1 Tg mice without endogenous DAF expression (Daf1 Tg/KO mice) for identifying the transgenic DAF expression pattern in vivo, Daf1 Tg mice were bred with Daf1-/- mice, and the resulted Daf1 Tg/KO mice were identified by PCR and verified by flow cytometry. All mice were housed in pathogen-free microisolator cages in the animal facility at Case Western Reserve University, all breeding and experiments were performed in accordance with the guidelines of the IACUC of Case Western Reserve University.
Reverse transcription PCR for transgenic Daf1 gene expression detection
Total RNAs were isolated from Daf1 Tg/KO mice using Trizol (Invitrogen, CA). 2 μg total RNAs purified from brain, spinal cord, muscle, heart, kidney, stomach, liver, spleen were treated with 2 units of RNase-free DNase I at 37°C for 30 min before the reverse transcription to remove possible genomic DNA contamination. 1μl of the reverse transcripted cDNA was used as template to amplify Daf1 cDNA by PCR using primers on a thermal cycler (PTC-200, MJ Research, MA) using the following condition: 94°C 30 sec, 60°C 30 sec and 72°C 60 sec, 35 cycles.
Flow cytometry analysis of transgenic Daf1 gene expression on cell surfaces
To verify transgenic Daf1 gene expression on cell surfaces at the protein level, erythrocytes and leukocytes were isolated from WT, Daf1-/- or Daf1 Tg/KO mice and stained with 2 μg/ml rat anti-mouse DAF mAb 2C6 or non-relevant rat IgG as control followed by analysis on a flow cytometer (LSR I, BD Biosciences, CA). For leukocytes isolation, 50 μl of blood were collected through tail vein from WT, Daf1-/- or Daf1 Tg/KO mice into 1 ml of FACS buffer (1% BSA in PBS), then erythrocytes were lysed by incubating with RBC lysis buffer (eBioscience, CA) for 10 min. The lymphocytes, monocytes and neutrophils populations were gated by their characteristic distributions in a forward scatter-side scatter plot.
Mouse erythrocytes C3b uptake assay
To determine whether the Daf1 transgene products are functional in inhibiting by-stand complement activation, C3b uptake assays using erythrocytes from WT or Daf1 Tg mice were preformed. To do this, 2×106 erythrocytes were incubated with 1:10 dilution of normal mouse serum together with 400 μg/ml zymosan (Sigma, MO) in the GVB-EGTA Mg++ buffer for 20 min. After incubation, erythrocytes were washed and stained with 5 μg/ml FITC conjugated rat anti-mouse C3 IgG or nonspecific IgG as a control. The samples were analyzed on a flow cytometer (LSR I). C3b deposition on erythrocyte surfaces were expressed as mean fluorescence intensity.
Western Blot of locally produced C5a/C3a during APC: T cell interactions
BM-DCs from WT, Daf1-/- or Daf1 Tg mice were generated by incubating BM cells with GM-CSF and IL-4 as described before (Heeger et al., 2005) and CD4+ T cells were isolated by magnetic beads using negative selection following manufacturer provided protocol (Stemcell Tech., Vancouver, Canada). The BM-DCs were incubated with purified OT-II CD4+ T cells together with 5 μg/ml OVA 323-339 peptide in serum-free HL-1 medium. Supernatants (equal amounts from each culture well) were collected after 3 days of culture and concentrated 10-fold using Microcon YM-10 centrifugal concentrators (Millipore, cutoff M.W. 3000). Equal volumes of supernatants were loaded and separated by SDS-PAGE and blotted on polyvinylidene difluoride membranes. Blots were probed with monoclonal Rat anti-mouse C5a Ab or anti-mouse C3a Ab (BD Biosciences, CA) which only reacts with the C5a or C3a neoatigen and anti-rat HRP (Sigma, MO) Ab and visualized with ECL Western blotting detection kit (GE Healthcare, CA). The densities of the bands were semi-quantified using Metamorph Imaging software package (Molecular Devices, CA)
EAE induction and evaluation
8-10 weeks old female Daf1 Tg and WT mice were immunized at base of tail and both thighs with 300 μg of mouse MOG35-55 emulsified in CFA that had been supplemented with M. tuberculosis strain H37RA to 4 mg/ml. 0.4 μg of Pertussis toxin (List Biolab, CA) was injected i.p. right after immunization and the following day. Clinical severity was assessed daily with a 0 to 5 scoring system (0, no signs; 1, flaccid tail; 2, impaired righting reflex and/or gait; 3, partial hind limb paralysis; 4, total hind limb paralysis; 5, moribund or dead).
Histology and histochemical staining of spinal cords
At time of sacrifice, spinal cords were removed and fixed in 4% paraformaldehyde for 24 h, then embedded in paraffin. Sections were cut at 5 μm on a microtome and stained by haematoxylin and eosin (H&E) to reveal CNS inflammatory infiltrates and Luxol Fast Blue (LFB) to reveal demyelinated areas using standard protocols.
IFN-γ ELISA
IFN-γ levels in culture supernatants were measured by ELISA (eBioscience, CA) following the manufacturer provided protocol.
IFN-γ and IL-17 ELISPOT assays
23 days after immunization, mice were sacrificed and splenocytes were used for IFN-γ and IL-17 ELISPOT assays as has been done before in the lab (Heeger et al., 2005). Briefly, after lysis of the red blood cells with ACK lysis buffer (Cambrex BioScience, MA), 600,000 splenocytes were resuspended in 200 ul HL-1 media and added into the ELISPOT plate (Millipore, MA) pre-coated with rat anti mouse IFN-γ mAb (clone 34, BD Pharmingen, CA) or rat anti mouse IL-17 mAb (clone 50104, R&D systems, MN). 5 μg/ml of MOG35-55 peptide was added and incubated at 37°C, 5% CO2 overnight. After developing, spots were counted using an ELISpot anaylsis system (CTL, OH)
Statistical analysis
All experiments were repeated at least twice. To determine whether groups were statistically different, the clinical scores were analyzed by the ANOVA test while other results were compared using the Student t test. A p value < 0.05 was considered significant.
Results
Generation of Daf1 Tg mice and Daf1 Tg/KO mice
For preparing the Daf1 Tg mice, we cloned the murine Daf1 cDNA into pBroad3 vector under the control of ROSA26 promoter which constitutively drives high levels of transgene expression in vivo (Farley et al., 2000; Kisseberth et al., 1999). Preliminary studies showed that this construct expressed high level of mDAF protein on the surface of CHO cells after transient transfection (data not shown). Following pronucleic injection of fertilized C57BL/6 oocytes, we screened 60 pups by PCR and identified 5 founders carrying the transgenic Daf1 gene. After breeding with C57BL/6 mice, we selected the founder with the highest DAF expression, bred it with Daf1-/- mice and generated Daf1 Tg/KO mice which lack the endogenous Daf1 gene expression.
Tissue distribution of transgenic Daf1 expression
We employed the Daf1 Tg/KO mice to determine the tissue distribution of the transgenic Daf1 expression without the interference from the endogenous Daf1 expression products. Briefly, we isolated total RNA from different tissues of Daf1 Tg/KO mice and treated them with DNase I to remove any possible genomic DNA contamination. After this, we amplified Daf1 cDNA transcripts by RT-PCR. This analysis showed that the transgenic Daf1 gene transcript was expressed in all tissues that were examined (Fig. 1A).
Figure 1.
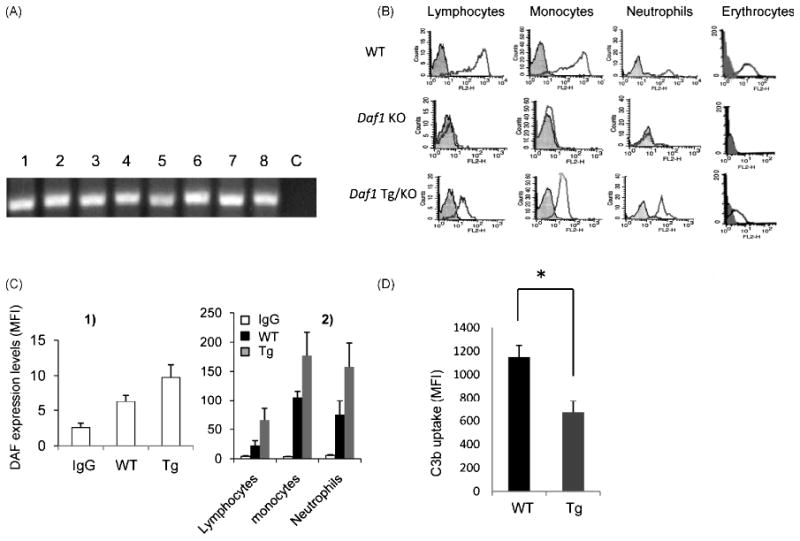
Characterization of Daf1 Tg mice. A. RT-PCR of transgenic Daf1 transcripts in Daf1 Tg/KO mouse tissues. Brain (1), spinal cord (2), muscle (3), heart (4), kidney (5), stomach (6), liver (7) and spleen (8) were homogenized to isolate total RNA by Trizol. After Dnase I treatment to remove possible genomic DNA contamination, reserve transcripted RNA samples were used as PCR template to amplify a 200 bp fragment of Daf1 cDNA. c, control; B. DAF expression on leukocytes and erythrocytes of WT, Daf1-/- and Daf1 Tg/KO mice. Shaded area, rat IgG control; open area, rat anti-mDAF mAb 2C6 staining; C. DAF protein levels on Daf1 Tg and WT mice cell surfaces. Daf1 Tg and WT mice (n=10) erythrocytes (1) and leukocytes (2) were stained with anti-mouse DAF mAb 2C6 and analyzed by flow cytometry. Averaged mean fluorescence intensities (MFI) were presented; D. By-stand C3 uptake assay using Daf1 Tg and WT erythrocytes. Complement activation was initiated by zymosan with 10% mouse serum in GVB Mg++-EGTA buffer and activation product C3b deposited on erythrocytes was detected by FITC-label anti mouse C3 mAb and analyzed by flow cytometry. Averaged MFIs were presented (*p<0.05).
To determine the Daf1 transgene expression at the protein level on cell surfaces, we stained erythrocytes and leukocytes from WT, Daf1-/- and Daf1 Tg/KO mice using rat anti mDAF mAb 2C6. Consistent with the RT-PCR results, flow cytometry analyses showed that transgenic DAF protein was present on surfaces of all tested cells (Fig. 1B).
Daf1 Tg mice possess higher level of DAF protein
Having verified that the Daf1 transgene is expressed on all cells and tissues examined using the Daf1 Tg/KO mice, we performed all our following studies using the original Daf1 Tg mice on C57BL/6 background. We first assessed DAF levels on the Daf1 Tg mouse erythrocytes and leukocytes by flow cytometry. These assays showed that these Daf1 Tg mice possessed ∼ 40% higher levels of DAF protein than WT mice on erythrocytes (Fig.1C1), lymphocytes, monocytes and neutrophils (Fig.1C2)
Daf1 Tg mouse erythrocytes have stronger complement regulatory activity
The originally established role of DAF is to protect self cells from autologous complement mediated injury by inhibiting C3/C5 convertases formation on their surfaces (Medof et al., 1984). To test whether Daf1 Tg mice possess stronger complement regulatory activity because of higher DAF protein levels, we performed a C3b uptake assay on erythrocytes from Daf1 Tg mice and WT mice using mouse serum as a complement source and zymosan as a complement activator. These assays showed that after incubation, Daf1 Tg erythrocytes accumulated ∼ half as much C3b on their surfaces as WT erythrocytes (Fig.1D).
Daf1 Tg BM-DCs produce less C5a/C3a during T activation
We have previously shown that during antigen-specific APC: T cell interactions, Daf1-/- APCs generate more C5a/C3a than WT APCs which drives more robust T cell activation (Lalli et al., 2007; Strainic et al., 2008). To test whether the higher levels of DAF protein on Daf1 Tg APCs inhibits local C5a/C3a generation, we mixed BM-DCs from WT, Daf1-/- or Daf1 Tg mice with OT-II CD4+ T cells together with 5 μg/ml OVA323-339. After incubation for 72 hr at 37°C, 5% CO2, we assayed supernatants for locally produced C5a/C3a by western blots and semi-quantitated their levels by densitometry. These assays (Fig. 2) showed that while Daf1-/- DCs generated higher levels of C5a/C3a than WT DCs, Daf1 Tg DCs produced 2 fold less C5a/C3a.
Figure 2.
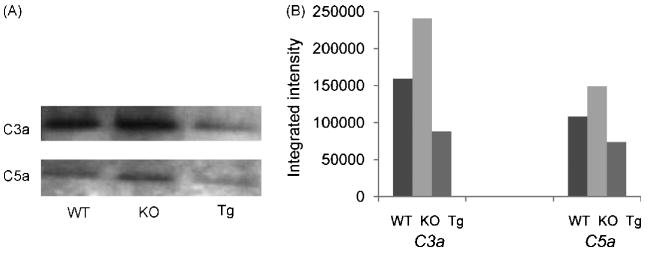
Daf1 Tg BM-DCs locally produced decreased levels of C5a and C3a when interacting with T cells. WT, Daf1-/- and Daf1 Tg BM-DCs were incubated with purified OT-II CD4+ T cells together with 5 μg/ml OVA323-339 peptide for 3 days and supernatants were concentrated and blotted with anti mouse C5a and C3a mAbs. The bands intensities were semi-quantitated by densitometry. Representative results from two individual experiments were shown.
Daf1 Tg BM-DCs stimulate less T cell activation in vitro
Having verified that Daf1 Tg BM-DCs locally generate less C5a/C3a during antigen specific T cell activation, we next repeated the above experiment with Daf1 Tg BM-DCs and OT-II CD4+ T cells together with 5 μg/ml OVA323-339 peptide. After 48 hr incubation, we assessed T cell activation by measuring IFN-γ released into the supernatants by ELISA. These assays (Fig. 3) showed that while OT-II CD4+ T cells stimulated by WT DCs produced 160±19 pg/ml of IFN-γ, OT-II CD4+ T cells stimulated by Daf1 Tg DCs produced 63 ± 24 pg/ml.
Figure 3.
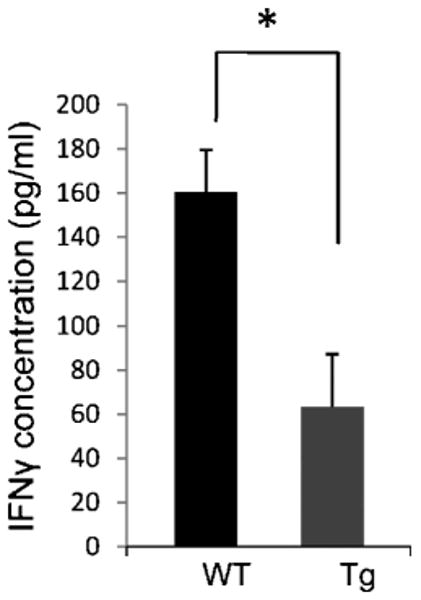
Daf1 Tg BM-DCs stimulated lesser T cell responses in vitro. Daf1 Tg and WT BM-DCs were incubated with OT-II CD4+ T cells together with 5 μg/ml OVA323-339 peptide for 2 days and IFN-γ levels were measured in the supernatants by ELISA. (*p<0.05). Results are representative of three individual experiments.
Daf1 Tg mice are protected against EAE
As indicated (Introduction), we (Liu et al., 2008) and others (Liu et al., 2005) previously found that DAF deficiency leads to markedly more severe EAE. To study the effect of Daf1 overexpression on EAE, we immunized both Daf1 Tg and WT mice with MOG35-55 in CFA plus pertussis toxin. Daily evaluation showed that compared to WTs, Daf1 Tg mice had delayed disease onset and decreased clinical scores. The average clinical score at the disease flare was 1.5 in the Daf1 Tg mice as compared to 3.0 in the WT controls (Fig. 4).
Figure 4.
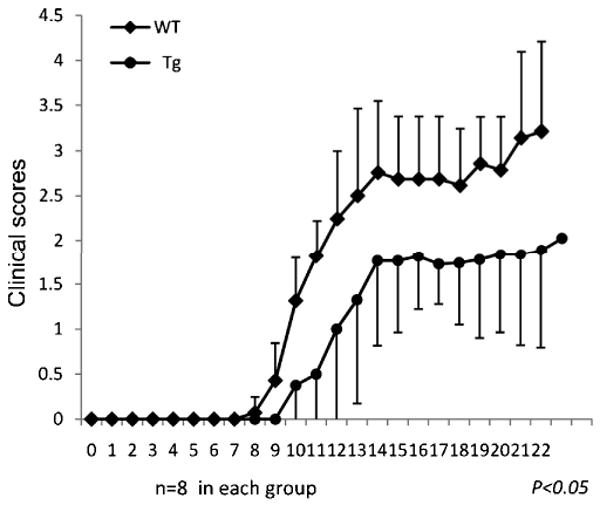
Daf1 Tg mice exhibited decreased disease severity in EAE. Both Daf1 Tg and WT mice (n=8 in each group) were immunized s.c. with 300 μg MOG35-55 in CFA together with 400 ng of pertussis toxin to induce EAE. Clinical scores were recorded on daily basis. (*p<0.05) Data were representative of three independent experiments.
Daf1 Tg mice have diminished T cell responses against MOG35-55
To assess MOG35-55 specific T cells responses in Daf1 Tg and WT mice with EAE, we collected splenocytes and performed IFN-γ and IL-17 ELISPOT assays. These studies showed that consistent with the reduced clinical scores, Daf1 Tg mice had ∼ 3 fold less MOG35-55 specific Th1 and Th17 responses than WT controls (Fig. 5).
Figure 5.
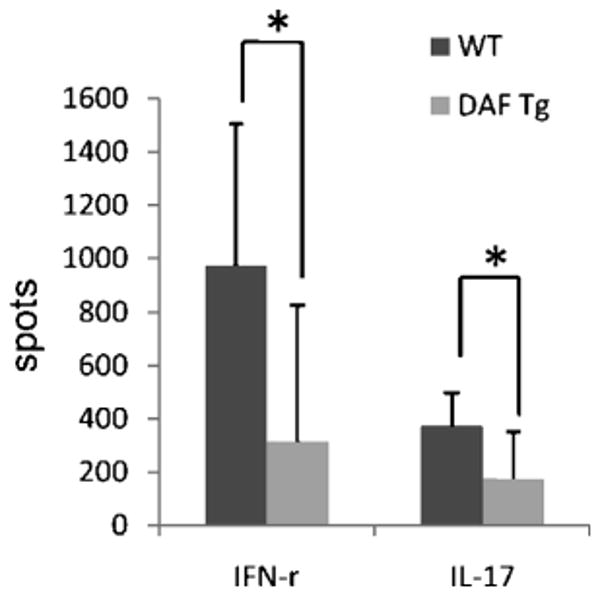
Daf1 Tg mice exhibited decreased MOG35-55 specific Th1 and Th17 responses in EAE. 6×105 Spleen cells were collected from each WT and Daf1 Tg mice with EAE on day 22 and assayed for the numbers of IFN-γ and IL-17 producing T cells with the existence of different concentrations of MOG35-55 by ELISPOT assays (means plus SD, n=4-8 in each group). (*p<0.05)
Daf1 Tg mice exhibit decreased CNS inflammation and reduced demyelination
To compare CNS inflammation and demyelination, we collected spinal cords from Daf1 Tg and WT mice with EAE 23 days after immunization, processed them for H&E and myelin staining. These analyses (Fig. 6) showed that in accordance with lowered clinical scores and diminished MOG specific Th1/Th17 responses, Daf1 Tg mice exhibited decreased leukocytes infiltration and reduced demyelination in spinal cords.
Figure 6.
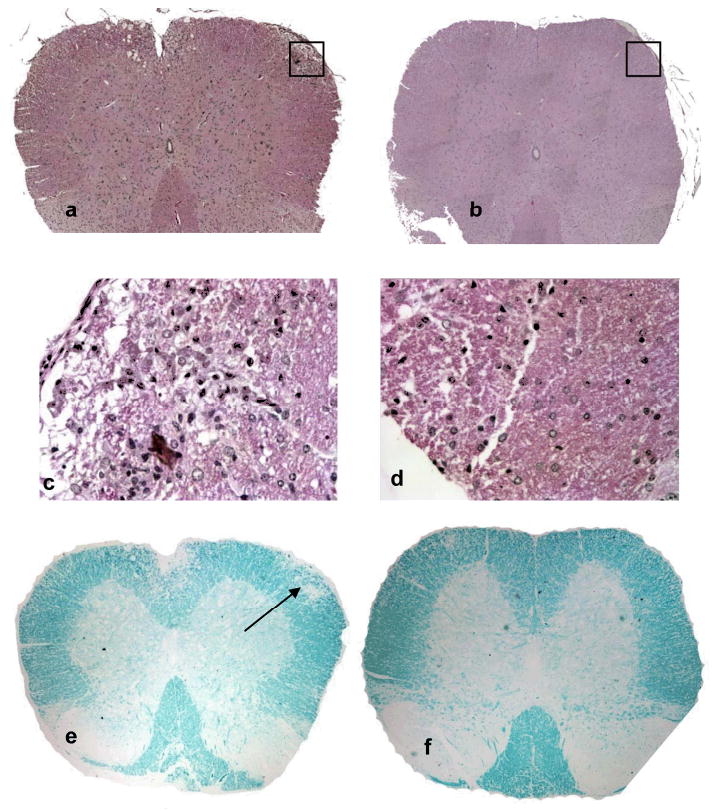
Daf1 Tg mice exhibited decreased inflammatory cell infiltration and demyelination in spinal cords. Spinal cord sections from WT and Daf1 Tg mice with EAE were stained with H&E to reveal inflammatory cell infiltration and Luxol Fast Blue to show demyelination. Representative pictures were shown here. a, c WT H&E staining (20×)(a) and (400×) (c), showing infiltrated leukocytes; b, d, Daf1 Tg H&E staining (20×)(b) and (400×) (d), little if any infiltrated cell was seen; e, WT luxol fast blue staining, showing demyelination in the infiltrated area (arrow); d. Daf1 Tg luxol fast blue staining, showing intact myelin sheath.
Discussion
In this report, we described the generation of a murine Daf1 Tg mouse on C57BL/6 background and its derivative Daf1 Tg/KO mice which only expressed the transgenic Daf1 gene. Using the Daf1 Tg/KO mice, we found that the transgenic Daf1 gene is ubiquitously expressed. Consequently, Daf1 Tg mice had ∼ 40% higher DAF protein levels on examined cell surfaces and exhibited stronger complement regulatory activity against by-stand complement activation. During antigen specific APC: T cell interactions, Daf1 Tg BM-DCs produced less C5a/C3a than WTs and stimulated less T cell activation. Contrary to Daf1-/- mice which exhibited more severe CNS injury in EAE, Daf1 Tg mice were protected. They had lower clinical scores, less CNS inflammation/demyelination and fewer MOG35-55 specific IFN-γ and IL-17-producing effector T cells than WT mice.
Accumulating studies in recent years have shown that in addition to their conventional roles of protecting self tissues from autologous complement mediated attack, cell surface complement regulators moderate T cell activity through different mechanisms (Kemper and Atkinson, 2007). Cross-linking membrane cofactor protein (MCP, CD46), a cofactor of factor I to inactivate C3b fragments during complement activation, induces the generation of IL-10 producing regulatory T cells which inhibits bystander T cell responses (Barchet et al., 2006; Kemper et al., 2003). Knocking out genes of CD59, whose conventional role is to inhibit the formation of membrane attack complex (MAC) in the terminal complement activation pathway, appears to augment T cell activity in a complement independent manner (Longhi et al., 2005). In addition, cross-linking Crry, a rodent specific cell surface complement regulator which possesses activities similar to DAF and MCP, triggers co-stimulating signals that induce T cell proliferation and IL-4 production (Jimenez-Perianez et al., 2005).
By studying Daf1-/- mice independently developed using different strategies (Lin et al., 2001; Sun et al., 1999), we and others have shown that DAF deficiency leads to markedly more severe tissue injury mediated by systemic complement activation in different disease models including experimental autoimmune myasthenia gravis (Kaminski et al., 2006; Lin et al., 2002b), acute and chronic nephrotoxic serum induced nephritis (Lin et al., 2002a; Sogabe et al., 2001), documenting the important roles of DAF in protecting self tissue from autologous complement mediated injury. Recently we and others also found that in addition to its traditional role of protecting self cells from autologous complement mediated attack, DAF protects mice from T cell mediated injury by inhibiting local C5a/C3a productions during antigen specific APC: T cell interactions (Heeger et al., 2005; Liu et al., 2005). Daf1-/- mice exhibit heightened central nervous system injury in EAE (Liu et al., 2008) and worsened retinal damage in experimental autoimmune uveitis (EAU) (An et al., 2009), a model for human autoimmune posterior uveitis, both of which are mediated by augmented autoantigen specific Th1 and Th17 responses. In addition, Daf1-/- mice also develop exacerbated lymphadenopathy, splenomegaly and dermatitis when bred onto the MRL/lpr background (Miwa et al., 2002; Miwa et al., 2007). All these data strongly argue that upregulating DAF levels in vivo could protect mice from T cell mediated autoimmunity.
Although systemic administration of soluble recombinant DAF protein could partially test the above hypothesis, because DAF is a cell surface bound protein, it is more appropriate to use the DAF overexpressing Tg mice to study the long term effects of DAF upregulation in vivo. It is unclear why the DAF transgenic construct delivered high levels of DAF expression in vitro but only moderate levels of DAF expression in vivo in the transgenic mice. One explanation could be that no generic intron was included within the construct (Choi et al., 1991). Nevertheless, in our newly generated Daf1 Tg mice, although expression levels of the Daf1 transgene are not as high as we expected, the effects of DAF upregulation on T cell responses are significant in our experimental settings. The upregualted DAF levels effectively inhibit local C5a/C3a generations during antigen specific T cell activations which result in less intense T cell responses both in vitro and in vivo, thereby protect Daf1 Tg mice against CNS injury in EAE. This report not only provide further evidence that DAF is important in modulating T cell responses in EAE, but also suggest that upregulating DAF levels by pharmaceutics or gene therapy could suppress myelin specific T cell responses and ameliorate disease severity in multiple sclerosis.
Acknowledgments
We thank Dr. Edward Medof for discussion. We also thank Dr. Scott Howell at the Vision Science Research Center (VSRC) imaging core for digital imaging analysis and Catherine Doller at the histology core for excellent histology services. This work was supported by National Institute of Health grant NS052471 (FL) and National Multiple Sclerosis Society grant RG3664 (FL). Qing Li and Hong Bu were supported in part by Natural Science Foundation of China grant 30671988. The VSRC is supported by NIH grant EY11373.
References
- An F, Li Q, Tu Z, Bu H, Chan CC, Caspi RR, Lin F. DAF Protects Against T Cell Autoreactivity That Leads To Experimental Autoimmune Uveitis. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.08-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchet W, Price JD, Cella M, Colonna M, MacMillan SK, Cobb JP, Thompson PA, Murphy KM, Atkinson JP, Kemper C. Complement-induced regulatory T cells suppress T-cell responses but allow for dendritic-cell maturation. Blood. 2006;107:1497–504. doi: 10.1182/blood-2005-07-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi T, Huang M, Gorman C, Jaenisch R. A generic intron increases gene expression in transgenic mice. Mol Cell Biol. 1991;11:3070–4. doi: 10.1128/mcb.11.6.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley FW, Soriano P, Steffen LS, Dymecki SM. Widespread recombinase expression using FLPeR (flipper) mice. Genesis. 2000;28:106–10. [PubMed] [Google Scholar]
- Heeger PS, Lalli PN, Lin F, Valujskikh A, Liu J, Muqim N, Xu Y, Medof ME. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201:1523–30. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Perianez A, Ojeda G, Criado G, Sanchez A, Pini E, Madrenas J, Rojo JM, Portoles P. Complement regulatory protein Crry/p65-mediated signaling in T lymphocytes: role of its cytoplasmic domain and partitioning into lipid rafts. J Leukoc Biol. 2005;78:1386–96. doi: 10.1189/jlb.1104642. [DOI] [PubMed] [Google Scholar]
- Kaminski HJ, Kusner LL, Richmonds C, Medof ME, Lin F. Deficiency of decay accelerating factor and CD59 leads to crisis in experimental myasthenia. Exp Neurol. 2006;202:287–93. doi: 10.1016/j.expneurol.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nat Rev Immunol. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–92. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- Kisseberth WC, Brettingen NT, Lohse JK, Sandgren EP. Ubiquitous expression of marker transgenes in mice and rats. Dev Biol. 1999;214:128–38. doi: 10.1006/dbio.1999.9417. [DOI] [PubMed] [Google Scholar]
- Lalli PN, Strainic MG, Lin F, Medof ME, Heeger PS. Decay accelerating factor can control T cell differentiation into IFN-gamma-producing effector cells via regulating local C5a-induced IL-12 production. J Immunol. 2007;179:5793–802. doi: 10.4049/jimmunol.179.9.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112:1759–66. doi: 10.1182/blood-2008-04-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Emancipator SN, Salant DJ, Medof ME. Decay-accelerating factor confers protection against complement-mediated podocyte injury in acute nephrotoxic nephritis. Lab Invest. 2002a;82:563–9. doi: 10.1038/labinvest.3780451. [DOI] [PubMed] [Google Scholar]
- Lin F, Fukuoka Y, Spicer A, Ohta R, Okada N, Harris CL, Emancipator SN, Medof ME. Tissue distribution of products of the mouse decay-accelerating factor (DAF) genes. Exploitation of a Daf1 knock-out mouse and site-specific monoclonal antibodies. Immunology. 2001;104:215–25. doi: 10.1046/j.0019-2805.2001.01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Kaminski HJ, Conti-Fine BM, Wang W, Richmonds C, Medof ME. Markedly enhanced susceptibility to experimental autoimmune myasthenia gravis in the absence of decay-accelerating factor protection. J Clin Invest. 2002b;110:1269–74. doi: 10.1172/JCI16086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lin F, Strainic MG, An F, Miller RH, Altuntas CZ, Heeger PS, Tuohy VK, Medof ME. IFN-gamma and IL-17 production in experimental autoimmune encephalomyelitis depends on local APC-T cell complement production. J Immunol. 2008;180:5882–9. doi: 10.4049/jimmunol.180.9.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Miwa T, Hilliard B, Chen Y, Lambris JD, Wells AD, Song WC. The complement inhibitory protein DAF (CD55) suppresses T cell immunity in vivo. J Exp Med. 2005;201:567–77. doi: 10.1084/jem.20040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhi MP, Sivasankar B, Omidvar N, Morgan BP, Gallimore A. Cutting edge: murine CD59a modulates antiviral CD4+ T cell activity in a complement-independent manner. J Immunol. 2005;175:7098–102. doi: 10.4049/jimmunol.175.11.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof ME, Kinoshita T, Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984;160:1558–78. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa T, Maldonado MA, Zhou L, Sun X, Luo HY, Cai D, Werth VP, Madaio MP, Eisenberg RA, Song WC. Deletion of decay-accelerating factor (CD55) exacerbates autoimmune disease development in MRL/lpr mice. Am J Pathol. 2002;161:1077–86. doi: 10.1016/S0002-9440(10)64268-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa T, Maldonado MA, Zhou L, Yamada K, Gilkeson GS, Eisenberg RA, Song WC. Decay-accelerating factor ameliorates systemic autoimmune disease in MRL/lpr mice via both complement-dependent and -independent mechanisms. Am J Pathol. 2007;170:1258–66. doi: 10.2353/ajpath.2007.060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogabe H, Nangaku M, Ishibashi Y, Wada T, Fujita T, Sun X, Miwa T, Madaio MP, Song WC. Increased susceptibility of decay-accelerating factor deficient mice to anti-glomerular basement membrane glomerulonephritis. J Immunol. 2001;167:2791–7. doi: 10.4049/jimmunol.167.5.2791. [DOI] [PubMed] [Google Scholar]
- Spiller OB, Harris CL, Morgan BP. Efficient generation of monoclonal antibodies against surface-expressed proteins by hyperexpression in rodent cells. J Immunol Methods. 1999;224:51–60. doi: 10.1016/s0022-1759(99)00008-3. [DOI] [PubMed] [Google Scholar]
- Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, Shapiro VS, Dubyak GR, Heeger PS, Medof ME. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–35. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Funk CD, Deng C, Sahu A, Lambris JD, Song WC. Role of decay-accelerating factor in regulating complement activation on the erythrocyte surface as revealed by gene targeting. Proc Natl Acad Sci U S A. 1999;96:628–33. doi: 10.1073/pnas.96.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG, Soriano P. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci U S A. 1997;94:3789–94. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]


