Since the original descriptions of the role of stromal cell-derived factor-1 (SDF-1) in recruiting bone marrow derived stem cells to the sites of vascular1 and myocardial injury,2 there has been increasing evidence of the broader importance of the SDF-1:CXCR4 axis in regulating myocardial repair following ischemic injury.2-6 In this issue of Circulation Research, Tang and colleagues investigate the role of the SDF-1:CXCR4 axis in the recruitment of exogenously derived cardiac stem cells.7 They further investigate how cardiac stem cell exposure to hypoxia prior to their intravenous administration alters cardiac stem cell engraftment and subsequent effects on myocardial repair.
Cardiac stem cells were derived from cardiosphere cultures and were defined by as cardiosphere derived c-Kit+ Lin- (CLK) cells. The cells were shown to have evidence of cardiac potential through the expression of green fluorescent protein under control of the Nkx2.5 promoter. The cells were administered intravenously 1 hour following permanent LAD ligation in the murine model of myocardial infarction.
In vitro studies demonstrated that CLK cells grown under normoxic conditions did not migrate significantly in response to SDF-1, nor was there significant engraftment of functional effects of these cells following the intravenous administration 1 hour after acute myocardial infarction. Conversely, culturing of the CLK cells for 6 hours prior to harvest and infusion led to robust migration of the CLK cells in response to SDF-1 in vitro. Furthermore, the hypoxia treatment led to significant migration and engraftment of CLK cells following intravenous infusion and significant functional effects including decreased infarct size, increased vascular growth and improved cardiac function.
Several lines of evidence are offered suggesting that the up-regulation of CXCR4 in response to hypoxia is responsible for the increased CLK cell migration and engraftment. Protein array analysis demonstrated that hypoxia induced CXCR4 expression; immunofluorescence and flow cytometry showed the CXCR4 expression was at the surface of the CLK cells. Up-regulation of cellular HIF-1α expression preceded the up-regulation of CXCR4, and inhibition of HIF-1a with shRNA inhibited the increase in CXCR4 expression as well as the enhanced migration of the hypoxia treated CLK cells. Finally, the co-treatment of CLK cells with hypoxia and AMD-3100, a CXCR4 antagonist, inhibited the migration of CLK cells in response to SDF-1 in vitro, as well as the engraftment of CLK cells following intravenous infusion in vivo. The inhibition of CLK cell engraftment through the inhibition of the SDF-1:CXCR4 axis completely inhibited any of the functional benefits that were observed following the administration of hypoxia treated CLK cells.
The data from this study demonstrate the importance of the SDF-1:CXCR4 axis in stem cell recruitment to the heart following myocardial infarction. Less clear is the mechanism of benefit associated with the engraftment of the hypoxia treated CLK cells, and whether hypoxia led to improved functional effects beyond just improving stem cell homing and engraftment. The exposure of CLK cells to hypoxia resulted in an approximate 3 fold increase in SDF-1 protein expression. We have previously demonstrated that constitutively increasing SDF-1 expression approximately 3 fold in mesenchymal stem cells leads to significant reduction in cardiac myocyte death, increased vascular density and improvement in cardiac function following the intravenous infusion of mesenchymal stem cells 24 h after myocardial infarction.8 Thus it is likely that the hypoxic pretreatment of CLK cells leads not only to improved CLK homing and engraftment but also may render the CLK cells more potent.
The field of cell based cardiac repair has arguably struggled with defining key molecular mechanisms that underlie myocardial repair in response to cell therapy. These data from Tang and colleague build upon the growing body of literature that suggests the following testable hypothesis: Stem cell based repair of the heart is mediated through the temporal alignment of the SDF-1:CXCR4 axis.
Within minutes to an hour after myocardial infarction SDF-1 is expression is rapidly up-regulated in heart providing a signal to which stem cells can be recruited.2 However, as shown by Tang and colleagues, stem cells have to express CXCR4 in order to home and engraftment in the heart 7. While bone marrow derived stem cells express CXCR4, the bone marrow cells need to be mobilized and apparently mature prior to having functional effects. A multivariate analysis of the REPAIR-AMI trial 9 that randomized patients to placebo or autologous bone marrow mononuclear cell infusion down the infarct related vessel showed that when magnitude of SDF-1 migration was included as a variable, treatment time following myocardial infarction was no longer a predictor of response to therapy. These data suggest that cells in the bone marrow alter their responsiveness to SDF-1 over time suggesting that cell 4-7 days after myocardial infarction may be more responsive to SDF-1 than bone marrow cells at baseline. The difficulty here, as suggested in Figure 1, is that SDF-1 expression in the heart is declining by 4-7 days after myocardial infarction.2 Thus, harvesting bone marrow mononuclear cells and infusing them into the infarct related vessel days after a myocardial infarction may be presenting CXCR4 expressing SDF-1 responsive bone marrow mononuclear cells to the heart at a time of declining in myocardial SDF-1 expression. Without this intervention the SDF-1 responsive bone marrow mononuclear stem cells might be mobilized at a time at which myocardial SDF-1 expression is not optimal to recruit them.
Figure 1.
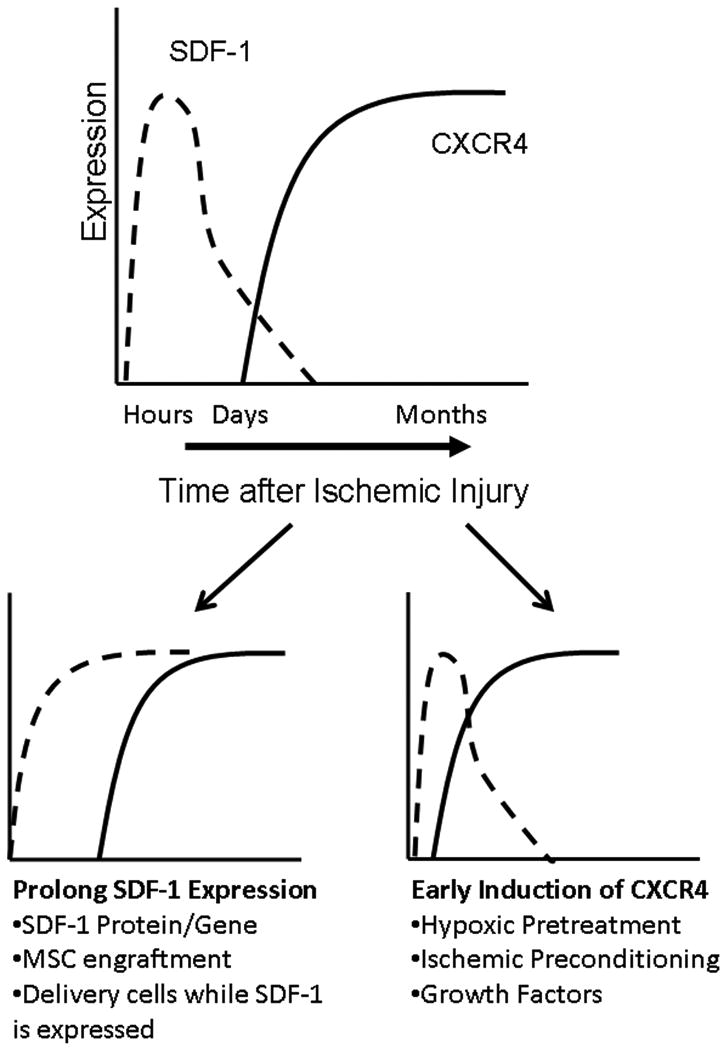
Schematic diagram of the timing of myocardial expression of SDF-1 and the myocardial and optimal functional stem cell expression of CXCR4. The lower figures list strategies for sustaining SDF-1 expression (Left) and induce early CXCR4 expression (Right).
While the effect of the SDF-1:CXCR4 axis on stem cell homing is well demonstrated by the study of Tang and colleagues, the effect of the SDF-1:CXCR4 axis on myocardial response to therapy is not addressed. There are several lines of evidence that support the hypothesis that the temporal alignment of the SDF-1:CXCR4 axis improves myocardial repair and functional response. We have previously shown that surviving cardiac myocytes in the infarct border zone begin to express CXCR4 36-48 h after myocardial infarction and that the level of expression increases through 96 h.5 However, as schematized in Figure 1, this up-regulation of cardiac myocyte CXCR4 expression occurs at a time of declining myocardial SDF-1 expression. That said, we and others have demonstrated that the sustained expression of SDF-1 following myocardial infarction leads to decreased cardiac myocyte death and improved cardiac function.8 Thus, the temporal alignment of SDF-1 and cardiac myocyte CXCR4 expression leads to improved cardiac function. How has this alignment been shown to be achievable?
Mesenchymal stem cells express SDF-1, thus their engraftment could sustain myocardial SDF-1 levels in the infarct border zone to a time at which cardiac myocytes express CXCR4.8, 10
Ischemic preconditioning leads to the early expression of CXCR4 by cardiac myocytes.11
Growth factors, like FGF-2, that signal through PI3-kinase can lead to synergistic up-regulation of CXCR4 in the setting of hypoxia.12, 13
This same hypothesis appears to be relevant in ischemic cardiomyopathy. Cardiac myocytes in the infarct border zone of explanted human hearts have been shown to express CXCR4.8 The delivery of SDF-1 in the setting of ischemic cardiomyopathy has been shown to improve cardiac function.3, 6 Similarly, the delivery of preparations of whole bone marrow mononuclear cells, but not only CD34+ cells, has been shown to improve cardiac function in patients left ventricular dysfunction at times remote from acute myocardial infarction.14 A noteworthy difference between a whole bone marrow mononuclear preparation and CD34+ cells is that the mesenchymal stem cells in the bone marrow mononuclear preparation express SDF-1, where as CD34+ cells do not.
Clearly the SDF-1:CXCR4 axis is not the only pathway active in stem cell based myocardial repair. IL-10,15 thymosin β416 and non-CXCR4 effects of a variety of growth factors have all been shown to mediate myocardial repair. That said, as demonstrated by the study of Tang and colleagues, data from multiple laboratories, using multiple cell types over multiple years have consistently shown that this axis is important in the biology of myocardial repair and that it can be exploited for therapeutic benefit.
Acknowledgments
Sources of Funding: NHLBI 1RO1-074400 and the Skirball Foundation
Footnotes
Disclosures: Dr. Penn is names as an inventor on patent applications filed by the Cleveland Clinic for the use of SDF-1 for the prevention and treatment of cardiac dysfunction. He is the Founder and Chief Scientific Officer of Juventas Therapeutics which has licensed these patent applications from the Cleveland Clinic. As such, he receives consulting fees and has the potential to receive equity and royalties.
References
- 1.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003 March 11;107(9):1322–8. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 2.Askari A, Unzek S, Popovic ZB, Goldman CK, Forudi F, kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor-1 on stem cell homing and tissue regeneration in ischemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 3.Deglurkar I, Mal N, Mills WR, Popovic ZB, McCarthy P, Blackstone EH, laurita KR, Penn MS. Mechanical and electrical effects of cell-based gene therapy for ischemic cardiomyopathy are independent. Hum Gene Ther. 2006 November;17(11):1144–51. doi: 10.1089/hum.2006.17.1144. [DOI] [PubMed] [Google Scholar]
- 4.Elmadbouh I, Haider HK, Jiang S, Idris NM, Lu G, Ashraf M. Ex vivo delivered stromal cell-derived factor-1alpha promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2007 April;42(4):792–803. doi: 10.1016/j.yjmcc.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang M, Mal N, kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007 October;21(12):3197–207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 6.Zhang G, Nakamura Y, Wang X, Hu Q, Suggs LJ, Zhang J. Controlled release of stromal cell-derived factor-1 alpha in situ increases c-kit+ cell homing to the infarcted heart. Tissue Eng. 2007 August;13(8):2063–71. doi: 10.1089/ten.2006.0013. [DOI] [PubMed] [Google Scholar]
- 7.Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor-cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:XXX–XXX. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jane-wit D, Altuntas CZ, Johnson JM, Yong S, Wickley PJ, Clark P, Wang Q, Popovic ZB, Penn MS, Damron DS, Perez DM, Tuohy VK. Beta 1-adrenergic receptor autoantibodies mediate dilated cardiomyopathy by agonistically inducing cardiomyocyte apoptosis. Circulation. 2007 July 24;116(4):399–410. doi: 10.1161/CIRCULATIONAHA.106.683193. [DOI] [PubMed] [Google Scholar]
- 9.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006 September 21;355(12):1210–21. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 10.Zhang D, Fan GC, zhou X, Zhao T, Pasha Z, Xu M, Zhu Y, Ashraf M, Wang Y. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2008 February;44(2):281–92. doi: 10.1016/j.yjmcc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu X, Dai S, Wu WJ, Tan W, Zhu X, Mu J, Guo Y, Bolli R, Rokosh G. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007 August 7;116(6):654–63. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zagzag D, Lukyanov Y, Lan L, Ali MA, Esencay M, Mendez O, Yee H, Voura EB, Newcomb EW. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab Invest. 2006 December;86(12):1221–32. doi: 10.1038/labinvest.3700482. [DOI] [PubMed] [Google Scholar]
- 13.Phillips RJ, Mestas J, Gharaee-Kermani M, Burdick MD, Sica A, Belperio JA, Keane MP, Strieter RM. Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1alpha. J Biol Chem. 2005 June 10;280(23):22473–81. doi: 10.1074/jbc.M500963200. [DOI] [PubMed] [Google Scholar]
- 14.Assmus B, Fischer-Rasokat U, Honold J, Seeger FH, Fichtlscherer S, Tonn T, Seifried E, Schachinger V, Dimmeler S, Zeiher AM. Transcoronary transplantation of functionally competent BMCs is associated with a decrease in natriuretic peptide serum levels and improved survival of patients with chronic postinfarction heart failure: results of the TOPCARE-CHD Registry. Circ Res. 2007 April 27;100(8):1234–41. doi: 10.1161/01.RES.0000264508.47717.6b. [DOI] [PubMed] [Google Scholar]
- 15.Burchfield JS, Iwasaki M, Koyanagi M, Urbich C, Rosenthal N, Zeiher AM, Dimmeler S. Interleukin-10 from transplanted bone marrow mononuclear cells contributes to cardiac protection after myocardial infarction. Circ Res. 2008 July 18;103(2):203–11. doi: 10.1161/CIRCRESAHA.108.178475. [DOI] [PubMed] [Google Scholar]
- 16.Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004 November 25;432(7016):466–72. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]


