Abstract
UV-induced p53 activation promotes cutaneous pigmentation by increasing transcriptional activity of pro-opiomelanocortin (POMC) in the skin. Induction of POMC/α-melanocyte-stimulating hormone (α-MSH) activates the melanocortin 1 receptor (MC1R), resulting in skin pigmentation. The common p53 codon 72 polymorphism alters the protein's transcriptional activity, which may influence the UV-induced tanning response. We assessed the association of the p53 codon 72 polymorphism with tanning response, and its interaction with MC1R variants on tanning response and skin cancer risk, in a nested case-control study within the Nurses' Health Study (NHS) (219 melanoma cases, 286 squamous cell carcinoma (SCC) cases, 300 basal cell carcinoma (BCC) cases and 874 controls), and among controls from four nested case-control studies within the NHS. We found that the p53 Pro allele was positively associated with childhood tanning response only among black/dark brown haired women. Compared with the Arg/Arg genotype, odds ratios (ORs) of childhood tanning tendency for Arg/Pro and Pro/Pro genotypes were 1.59 (95%CI, 0.96-2.65) and 1.56 (95%CI, 0.55-4.40), respectively. The association between MC1R variants and childhood tanning tendency was similar in both p53 Arg/Arg genotype and Pro allele carriers (Arg/Pro or Pro/Pro). The association of the p53 Pro/Pro genotype with melanoma risk was strongest among women with light pigmentation, and with MC1R variants, with the joint risk categories having the highest overall risk. We did not observe such interaction for SCC and BCC. Our study suggests the involvement of p53 codon 72 polymorphism in the skin tanning response and potential interaction with skin pigmentation on melanoma risk. Further work is needed to evaluate the association between p53 and its associated proteins and skin cancer risk.
Keywords: p53, MC1R, suntan response, skin cancer, melanoma
Introduction
Ultraviolet (UV) radiation is a cause of skin cancer 1-3. UV radiation induces DNA damage, producing cyclobutane-type pyrimidine dimmers (CPD) and pyrimidine pyrimidone or photoproducts 4-6. It has been shown that p53 is involved in cell cycle arrest and apoptosis in response to UV-induced DNA damage 7,8.
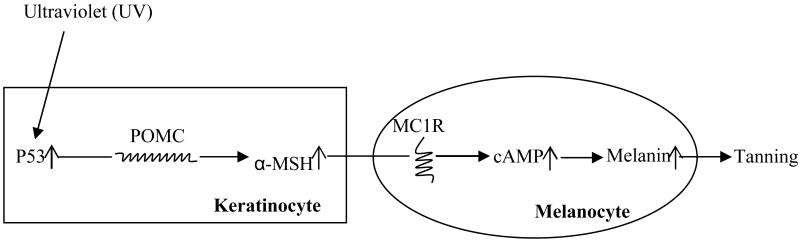
Most recently, Cui et al. reported a new genoprotective function of p53 by stimulating the suntan response. They showed that the tanning response is partially controlled by p53 9. Tanning is acquired pigmentation that results from exposure to UV 10. UV-induced pigmentation is the result of melanin synthesis by cutaneous melanocytes and transportation into adjacent keratinocytes 11. Melanin production is initiated by α-melanocyte-stimulating hormone (α-MSH), which is produced by proteolysis from a multicomponent precursor polypeptide, encoded by the pro-opiomelanocortin (POMC) gene 12. The induced POMC/α-MSH binds to MC1R, which further activates the cAMP signaling system, leading to eumelanin production. UV-induced p53 activation promotes transient pigmentation by increasing transcriptional activation of POMC in the skin (Figure 1).
Figure 1. The role of p53 in tanning response.
UV-induced DNA damage activates p53 gene in the skin keratinocyte. As a transcription factor, p53 stimulates the transcription of the POMC gene. The POMC precursor is then cleaved into α-MSH. The α-MSH-bound MC1R activates adenylate cyclase enzyme, inducing cyclic AMP (cAMP) production in melanocyte. This cAMP signaling leads to eumelanin production, resulting in darker pigmentation.
A common p53 gene polymorphism is a G/C substitution at codon 72 in exon 4, which occurs in a proline-rich domain of p53 that is essential for p53-mediated apoptosis 13,14. This polymorphism encodes the amino acids Proline (Pro) and Arginine (Arg), resulting in two structurally distinct forms of the protein 15. It has been reported that the Pro allele has higher transcriptional activity and lower capacity to induce apoptosis 16,17.
It is known that MC1R is a major determining factor for pigment phenotype 18. A genotype-phenotype relationship has been reported for certain MC1R variants and light pigmentation. We reported that the red hair color (RHC) variants are associated with red hair, fair skin color, and childhood tanning tendency 19.
We evaluated the effect of the p53 codon 72 polymorphism on tanning response and its interaction with MC1R variants on tanning response and skin cancer risk in a nested case-control study within the Nurses' Health Study (NHS).
Materials and Methods
Study population
The NHS was established in 1976, when 121,700 female registered nurses between the ages of 30 and 55, residing in 11 larger U.S. states, completed a self-administered questionnaire on their medical histories and baseline health-related exposures. Updated information has been obtained by questionnaires every 2 years. Between 1989 and 1990, blood samples were collected from 32,826 of the cohort members. The distributions of risk factors for skin cancer were very similar in the subcohort of those who donated blood samples as in the overall cohort 19. Eligible cases in this study consisted of women with incident skin cancer from the subcohort who had given a blood specimen, including SCC and BCC cases with a diagnosis anytime after blood collection up to June 1, 1998 and melanoma cases up to June 1, 2000 that had no previously diagnosed skin cancer. A common control series was randomly selected from participants who gave a blood sample and were free of diagnosed skin cancer up to and including the questionnaire cycle in which the case was diagnosed. One or two controls were matched to each case by year of birth (±1 year) and self-reported race (Caucasian/missing). Less than 5% of cases and controls had missing race/ethnicity. The participation rate of cases and controls were 92% and 89%, respectively. The nested case-control study consisted of 219 melanoma cases, 286 SCC cases, 300 BCC cases and 874 matched controls. The study protocol was approved by the Committee on Use of Human Subjects of the Brigham and Women's Hospital, Boston, MA.
Exposure data
Information regarding skin cancer risk factors was obtained from the prospective biennial questionnaires and a retrospective supplementary questionnaire. Questions on natural hair color and childhood and adolescent tanning tendency were asked in the 1982 prospective questionnaire and for the ethnic group in the 1992 questionnaire. The question for childhood and adolescent tanning tendency was “as a child or adolescent, after repeated sun exposures, e.g., a two-week vacation outdoors, what kind of tan would you get?”, and the multiple choices were practically none, light tan, average tan, and deep tan. In the skin cancer nested case-control study, natural skin color and other sun exposure-related information were collected by the retrospective supplementary questionnaire in 2002. In addition, the 11 states of residence of cohort members at baseline were grouped into 3 regions: Northeast (Connecticut, Massachusetts, Maryland, New Jersey, New York, and Pennsylvania), Northcentral (Michigan and Ohio) and West and South (California, Texas, and Florida). Estimation of past sunlight exposure for each subject was described previously 20.
Identification of MC1R variants
The distribution and frequency of MC1R variants in 179 Caucasian controls from the US were determined by Kanetsky et al. using a direct sequencing method 21. Seven nonsynonymous polymorphisms with allele frequency above 1% were identified (Val60Leu, Val92Met, Arg151Cys, Ile155Thr, Arg160Trp, Arg163Gln, and Asp294His) in the coding region and were genotyped in our case-control study. The frequency distribution of MC1R variants in our study has been previously reported 19.
Laboratory assays
The Arg160Trp polymorphism of the MC1R gene was genotyped by restriction fragment length polymorphism (RFLP). Other MC1R polymorphisms and the p53 Arg72Pro polymorphism were genotyped by the 5′ nuclease assay (TaqMan®) in 384-well format, using the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). TaqMan® primers and probes were designed using the Primer Express® Oligo Design software v2.0 (ABI PRISM). Laboratory personnel were blinded to case-control status, and blinded quality control samples were inserted to validate genotyping procedures; concordance for the blinded samples was 100%. Primers, probes, and conditions for genotyping assays are available upon request.
Statistical methods
In addition to the skin cancer nested case-control study, we had previously genotyped the p53 codon 72 polymorphism among the participants in nested case-control studies of colon cancer, colon polyps, and breast cancer within the NHS. To increase statistical power, we assessed the association between the p53 Arg72Pro polymorphism and childhood tanning tendency according to hair color among controls from all four studies. We used all controls in the skin cancer nested case-control study to evaluate the interaction between the p53 polymorphism and MC1R genotypes on childhood tanning tendency. We compared each type of skin cancer to the common control series in the skin cancer nested case-control study to increase the statistical power.
We classified the MC1R variants as follows; the Arg151Cys, Arg160Trp and Asp294His variants were categorized as “red hair color” (RHC) variants; the other 4 variants were referred to as “nonred hair color” (NRHC) variants 22-24. We also defined the chromosome without the MC1R variant at any of the 7 polymorphic sites that we genotyped as the consensus allele (WT). We used a χ2 test to assess whether the MC1R genotypes and the p53 genotype were in Hardy-Weinberg equilibrium among the controls (the distributions of two genotypes were in Hardy-Weinberg equilibrium), and the relation between the p53 genotype and childhood tanning tendency according to hair color among the controls. A test for trend was calculated across the three p53 genotypes. Unconditional logistic regression was used to estimate the association between the p53 genotype and childhood tanning tendency.
Interactions between the MC1R genotypes and the p53 polymorphism on childhood tanning tendency and skin cancer risk were evaluated in unconditional logistic regression models. Unconditional logistic regression was also employed to assess the interaction between p53 polymorphism and MC1R variants, hair color, and constitutional susceptibility score on skin cancer risk. We used cross-classified categories of the p53 polymorphism and MC1R genotypes (or hair color or a constitutional susceptibility score) compared to a common reference category. We modeled these predictors as ordinal variables to test the statistical significance of a single multiplicative interaction term. To summarize multiple constitutional phenotypic variables, we constructed a multivariate confounder score for skin cancer 25. Briefly, we applied the logistic regression coefficients from a multivariate model, including age, race, natural skin color, natural hair color, childhood or adolescent tendency to burn, and the number of palpably raised moles on arms, to each individual's values for the latter four variables and summed the values to compute a susceptibility risk score in the logit scale. We used this score to define women with low, intermediate, and high constitutional susceptibility based on tertiles among controls.
Results
Descriptive Characteristics of Cases and Controls
A detailed description of the characteristics of cases and controls in the skin cancer nested case-control study was reported elsewhere 19. In brief, at the beginning of the follow-up of this nested case-control study, the nurses were between 43 and 68 years old with a mean age of 58.7. The mean age at diagnosis of melanoma was 63.4. Those with skin cancers were more likely to have used sunlamps or attended tanning salons. A family history of skin cancer was a risk factor for melanoma. Those with skin cancers had higher cumulative sun exposure while wearing a bathing suit, more lifetime severe sunburns that blistered, and a higher constitutional susceptibility score.
Association between the p53 Arg72Pro polymorphism and childhood tanning tendency according to hair color
We evaluated the association between the p53 Arg72Pro polymorphism and childhood tanning tendency according to hair color among controls from four nested case-control studies. A borderline positive association of the Pro/Pro genotype with childhood tanning tendency was observed among women who had black/dark brown hair. Compared with Arg/Arg genotype, odds ratio (ORs) of childhood tanning tendency for Arg/Pro and Pro/Pro genotypes were 1.59 (95% confidence interval (CI), 0.96–2.65) and 1.56 (95%CI, 0.55-4.40), respectively (P, trend, 0.08) (Table 1). No association was observed among women who had light hair (P, trend, 0.79) and red/blonde hair (P, trend, 0.54) (Table 1).
Table 1. Association between the p53 Arg72Pro polymorphism and childhood tanning tendency according to hair color among controls.
| Black/dark brown hair | |||
|---|---|---|---|
| Tan (%) | Non-tan (%) | OR (95% CI) | |
| Arg/Arg | 774 (56) | 53 (67) | 1.00 |
| Arg/Pro | 511 (37) | 22 (28) | 1.59 (0.96-2.65) |
| Pro/Pro | 91 (7) | 4 (5) | 1.56 (0.55-4.40) |
| P, trend | 0.08 | ||
| Light brown hair | |||
| Tan (%) | Non-tan (%) | OR (95% CI) | |
| Arg/Arg | 668 (54) | 53 (54) | 1.00 |
| Arg/Pro | 480 (39) | 40 (41) | 0.95 (0.62-1.46) |
| Pro/Pro | 85 (7) | 5 (5) | 1.35 (0.52-3.47) |
| P, trend | 0.79 | ||
| Red/blonde hair | |||
| Tan (%) | Non-tan (%) | OR (95% CI) | |
| Arg/Arg | 231 (59) | 64 (63) | 1.00 |
| Arg/Pro | 142 (36) | 34 (33) | 1.16 (0.73-1.84) |
| Pro/Pro | 17 (4) | 4 (4) | 1.18 (0.38-3.62) |
| P, trend | 0.54 | ||
Crude ORs of childhood tanning tendency according to the p53 genotypes were calculated among the 1,455 black/dark brown hair women, among the 1,331 light brown hair women, and among the 492 red/blonde hair women (the controls in four nested studies of skin cancer, colon cancer, colon polyps, and breast cancer within the NHS). OR>1 means increased likelihood of tanning. Four categories of the responses to the question of childhood tanning tendency were grouped into tan (light tan or average tan or tan) and non-tan (practically none). The percentages may not sum to 100 due to rounding.
Interaction between the p53 Arg72Pro polymorphism and MC1R variants on childhood tanning tendency
We evaluated the interaction between the p53 Arg72Pro polymorphism and MC1R variants on childhood tanning tendency among controls in the nested case-control study of skin cancer (Table 2). As compared with women with MC1R WT/WT genotype and p53 Arg/Arg genotype, MC1R variants were significantly associated with childhood tanning tendency regardless of the p53 genotype (p, interaction, 0.55). The association between MC1R variants and childhood tanning tendency was similar in both the p53 Arg/Arg carriers and other p53 genotypes carriers. Similarly, no significant interaction was observed between p53 Arg72Pro polymorphism and MC1R variants on hair color and childhood sunburn reaction. Women carrying MC1R variants were more likely to have red hair color or childhood sunburn reactions, and p53 Arg72Pro polymorphism did not significantly modify these associations (Supplementary Table 1).
Table 2. Interaction between the p53 Arg72Pro polymorphism and MC1R variants on childhood tanning tendency among controls.
| p53\MC1R | WT/WT | WT/NRHC or NRHC/NRHC | WT/RHC | RHC/RHC or NRHC/RHC |
|---|---|---|---|---|
| Arg/Arg | ||||
| Tan/Non-tana | 124/7 | 173/20 | 65/12 | 35/15 |
| Crude OR | 1.00 | 0.49 (0.20-1.19) | 0.31 (0.12-0.81) | 0.13 (0.05-0.35) |
| Multivariate ORb | 1.00 | 0.45 (0.18-1.15) | 0.36 (0.13-1.01) | 0.26 (0.09-0.73) |
| Arg/Pro or Pro/Pro | ||||
| Tan/Non-tana | 101/6 | 105/13 | 54/10 | 21/16 |
| Crude OR | 0.95 (0.31-2.92) | 0.46 (0.18-1.19) | 0.31 (0.11-0.84) | 0.07 (0.03-0.20) |
| Multivariate ORb | 0.68 (0.21-2.19) | 0.45 (0.17-1.24) | 0.28 (0.09-0.85) | 0.14 (0.05-0.41) |
WT stands for consensus allele. NRHC stands for non-red hair color allele. RHC stands for red hair color allele.
Four categories of the responses to the question of childhood tanning tendency were grouped into tan (light tan or average tan or tan) and non-tan (practically none).
The number of participants does not add up to the total women because of missing genotypes.
Unconditional logistic regression adjusted for natural skin color and natural hair color (p, interaction, 0.55).
Interactions between the p53 Arg72Pro polymorphism and hair color, MC1R variants, and constitutional susceptibility score on melanoma risk
We evaluated the interaction between p53 Arg72Pro polymorphism and hair color on melanoma risk (Table 3). Compared to women with p53 Arg/Arg genotype and black/dark hair color, we observed that the effect of the p53 Pro/Pro genotype was strongest for melanoma risk among the women who had blonde/red hair color (OR, 10.51; 95% CI, 3.14-35.22) (p, trend, 0.04) (Table 3). The test for departure from multiplicative interaction between the p53 Arg72Pro polymorphism and blonde/red hair color was not statistically significant (p, interaction, 0.67), suggesting that these are independent risk factors and their combination gives rise to an approximately multiplicative level of risk. A similar result was found when we further evaluated the interaction between p53 Arg72Pro polymorphism and MC1R variants on melanoma risk (OR, 10.66; 95% CI, 1.93-58.92) (Table 4). This OR was attenuated after controlling for skin color and hair color, but the pattern or multiplicative risk remained (OR, 6.97; 95% CI, 1.23-39.60) (Table 4).
Table 3. Melanoma risk by p53 Arg72Pro polymorphism and hair color.
| Hair color | Arg/Arg | Arg/Pro | Pro/Pro | P, trend |
|---|---|---|---|---|
| Black or dark brown | ||||
| Case/Controlsa | 35/237 | 27/118 | 4/19 | |
| ORb | 1.00 | 1.57 (0.90-2.72) | 1.43 (0.46-4.48) | 0.15 |
| Light brown | ||||
| Case/Controlsa | 51/181 | 36/136 | 4/22 | |
| ORb | 1.90 (1.18-3.06) | 1.82 (1.09-3.04) | 1.34 (0.43-4.15) | 0.62 |
| Blonde or red | ||||
| Case/Controlsa | 23/68 | 24/53 | 7/5 | |
| ORb | 2.38 (1.31-4.32) | 3.13 (1.71-5.72) | 10.51 (3.14-35.22) | 0.04 |
p-value for trend was calculated across the three p53 genotypes.
The number of participants does not add up to the total women because of missing genotypes.
Unconditional logistic regression adjusted for the matching variables: age and race (Caucasian/missing) (p, interaction, 0.67).
Table 4. Melanoma risk by p53 Arg72Pro polymorphism and MC1R variants.
| MC1R\p53 | Arg/Arg | Arg/Pro | Pro/Pro |
|---|---|---|---|
| WT/WT | |||
| Case/Controlsa | 13/135 | 17/92 | 2/15 |
| Multivariate ORb | 1.00 | 1.98 (0.91-4.28) | 1.41 (0.29-6.93) |
| Multivariate ORc | 1.00 | 2.06 (0.94-4.49) | 1.49 (0.30-7.45) |
| WT/NRHC or NRHC/NRHC | |||
| Case/Controlsa | 36/196 | 34/102 | 6/18 |
| Multivariate ORb | 1.92 (0.98–3.76) | 3.45 (1.73-6.89) | 3.81 (1.28-11.34) |
| Multivariate ORc | 1.82 (0.93-3.60) | 3.28 (1.63-6.58) | 3.72 (1.23-11.26) |
| WT/RHC | |||
| Case/Controlsa | 24/78 | 14/55 | 4/9 |
| Multivariate ORb | 3.17 (1.52–6.60) | 2.60 (1.15-5.91) | 4.54 (1.22-16.96) |
| Multivariate ORc | 2.67 (1.27-5.61) | 2.26 (0.98-5.20) | 3.17 (0.83-12.15) |
| RHC/RHC or NRHC/RHC | |||
| Case/Controlsa | 26/50 | 16/35 | 3/3 |
| Multivariate ORb | 5.54 (2.62-11.68) | 4.78 (2.09-10.90) | 10.66 (1.93-58.92) |
| Multivariate ORc | 3.75 (1.71-8.19) | 3.02 (1.25-7.31) | 6.97 (1.23-39.60) |
p-value for trend was calculated across the three p53 genotypes.
WT stands for consensus allele. NRHC stands for non-red hair color allele. RHC stands for red hair color allele.
The number of participants does not add up to the total women because of missing genotypes.
Unconditional logistic regression adjusted for the matching variables: age and race (Caucasian/missing) (p, interaction, 0.23).
Unconditional logistic regression adjusted for the matching variables, natural skin color, natural hair color (p, interaction, 0.18).
We also evaluated the interaction between p53 Arg72Pro polymorphism and the constitutional susceptibility score on melanoma risk (Table 5). The interaction pattern was similar to those between p53 Arg72Pro polymorphism and hair color and MC1R variants on melanoma risk. The constitutional susceptibility score that we used reflects general constitutional susceptibility index for skin cancer. Compared to women with low constitutional susceptibility scores and p53 Arg/Arg genotype, the p53 Pro/Pro carriers with high constitutional susceptibility scores had significantly increased risk of melanoma (OR, 7.09; 95% CI, 2.69-18.66) (p, trend, 0.01), whereas the women who had p53 Arg/Arg genotype and high constitutional susceptibility scores showed an OR of 3.00 (95% CI, 1.69-5.33) (p, interaction, 0.15).
Table 5. Melanoma risk by p53 Arg72Pro polymorphism and constitutional susceptibility score.
| Constitutional susceptibility score | Arg/Arg | Arg/Pro | Pro/Pro | P, trend |
|---|---|---|---|---|
| Low | ||||
| Case/Controlsa | 18/150 | 12/108 | 2/17 | |
| Multivariate ORb | 1.00 | 0.95 (0.44-2.05) | 1.03 (0.22-4.91) | 0.99 |
| Medium | ||||
| Case/Controlsa | 30/171 | 19/97 | 3/17 | |
| Multivariate ORb | 1.48 (0.79-2.77) | 1.69 (0.84-3.39) | 1.51 (0.40-5.70) | 0.70 |
| High | ||||
| Case/Controlsa | 61/173 | 56/104 | 10/13 | 0.01 |
| Multivariate ORb | 3.00 (1.69-5.33) | 4.62 (2.55-8.37) | 7.09 (2.70-18.66) |
p-value for trend was calculated across the three p53 genotypes.
The number of participants does not add up to the total women because of missing genotypes.
Unconditional logistic regression adjusted for the matching variables: age and race (Caucasian/missing) (p, interaction, 0.15).
Additionally, we evaluated the interactions between p53 Arg72Pro polymorphism and hair color, MC1R variants, and constitutional susceptibility score on the risks of SCC and BCC. There were no significant interactions observed (Supplementary Tables 2, 3, and 4).
Discussion
A newly discovered important protective role of p53 was its ability to regulate the suntan response. Cui et al. found that p53 can directly modulate transcriptional activity of the POMC promoter following UV exposure 9. It is notable that the prevalence of p53 Arg72Pro polymorphism exhibits substantial latitudinal differences, with dark pigmented populations closer to the equator having increased Pro allele frequency 26,27. In this regard, it is speculated that p53 Pro allele is a more competent inducer of POMC transcription, an evolutionary selection in heavily sun-exposed areas 28. This speculation is consistent with the fact that the Pro allele was associated with increased transcriptional activity 17 . In this study, we evaluated the association between p53 Arg72Pro polymorphism and childhood tanning tendency according to different hair color. At the outset, we surmised that the effect of functional p53 polymorphism on tanning responses could only be evaluated in individuals with an intact MC1R pathway, e.g. in individuals with MC1R polymorphisms resulting in red hair, we would not be able to evaluate the effect of p53 as tanning responses are poor. As expected, only among the women with black/dark brown hair, the test for trend showed a borderline positive correlation of Pro allele with childhood tanning tendency. This finding may provide a plausible molecular explanation for different tanning response to UV among people with similar pigmentation.
The melanocortin 1 receptor (MC1R) gene is located at 16q24.3. It encodes a seven-transmembrane, G-protein-coupled receptor of 317 amino acids, and can be activated by its ligand α-MSH or adrenocorticotrophic hormone (ACTH) 29,30. Valverde et al. illustrated that variants in the coding region of the MC1R play an important role in tanning and pigmentation in humans 24. MC1R variants encode receptor proteins with decreased function to increase cAMP, resulting in decreased constitutional and transient pigmentation 31,32. We found that the women with RHC variants are less likely to tan; these associations were not modified by the p53 Arg72Pro polymorphism. The role of MC1R in skin pigmentation is predominant, and the variants of MC1R, especially RHC alleles, are consistently associated with constitutional and transient pigmentation 19. This result suggests that the propensity of the p53 Arg72Pro to increase the tanning response is mitigated by the strong influence of MC1R variants in the POMC/α-MSH-induced tanning response.
Previous studies reported that MC1R variants are associated with an increased risk of developing melanoma and are also associated with phenotypic features such as fair skin and red hair, which are strong risk factors for skin cancer 33-36. These observations are consistent with the findings of this study; i.e., the women who had MC1R RHC variants or red hair had statistically increased risk of melanoma. Similar result was also found for the women with high constitutional susceptibility score. The constitutional susceptibility score reflects the multiple constitutional phenotypic variables including hair color, skin color, childhood tendency to burn, and the number of palpably raised moles on arms, simultaneously. Interestingly, these associations were strongest among women who carried the p53 Pro/Pro genotype; namely, darker pigmentation substantially attenuated the melanoma risk associated with p53 Pro/Pro genotype. In an analysis on the same participants from this study, Han et al. observed that the Pro/Pro genotype carriers had higher melanoma risk than the Arg/Arg genotype carriers 37. In the present study, we observed that the p53 Pro allele was more strongly associated with tanning response among dark haired women, presumably those retaining substantial function of MC1R. These data suggest that the p53 Pro allele maybe a more active transcription factor of POMC than the Arg allele in tanning response, resulting in darker pigmentation. However, the Pro allele is associated with lower capacity to induce apoptosis than the Arg allele 16. Hence, even though the Pro allele is associated with tanning response, its reduced activity to induce apoptosis plays a predominant role in melanoma development among Caucasians.
The nested case-control design, high follow-up rate, and high response rate for the retrospective supplementary questionnaire are among strengths of this study. The limitations of the study include self-reported assessment of pigmentary phenotypes, which may lead to misclassification. Nevertheless, we observed that the Pro allele was associated with tanning response, probably due to enhanced induction of POMC transcription. The association of the p53 Pro/Pro genotype with cutaneous melanoma risk was strongest among the women with light pigmentation. The underlying mechanism of the p53 Arg72Pro polymorphism in the etiology of cutaneous melanoma remains to be demonstrated.
Supplementary Material
Supplementary Table 1. Interaction between the p53 Arg72Pro polymorphism and MC1R variants on hair color and childhood sunburn reaction among controls
Supplementary Table 2. SCC and BCC risks by p53 Arg72Pro polymorphism and hair color
Supplementary Table 3. SCC and BCC risks by p53 Arg72Pro polymorphism and MC1R variants
Supplementary Table 4. SCC and BCC risks by p53 Arg72Pro polymorphism and constitutional susceptibility score
Acknowledgments
Grant sponsor: NIH; Grant numbers: CA113100, CA128080, and CA087969.
We thank Dr. David E. Fisher at Dana-Farber Cancer Institute for scientific discussion, Ms. Carolyn Guo for her programming support. We are indebted to the participants in the Nurses' Health Study for their dedication and commitment.
Abbreviations
- BCC
basal cell carcinoma
- SCC
squamous cell carcinoma
- CI
confidence interval
- OR
odds ratio
- NRHC
non red hair color allele
- RHC
red hair color allele
- WT
consensus allele
- UV
ultraviolet
References
- 1.Armstrong BK, Kricker A, English DR. Sun exposure and skin cancer. Australas J Dermatol. 1997;38 1:S1–6. doi: 10.1111/j.1440-0960.1997.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 2.Brash DE. Sunlight and the onset of skin cancer. Trends Genet. 1997;13:410–4. doi: 10.1016/s0168-9525(97)01246-8. [DOI] [PubMed] [Google Scholar]
- 3.English DR, Armstrong BK, Kricker A, et al. Sunlight and cancer. Cancer Causes Control. 1997;8:271–83. doi: 10.1023/a:1018440801577. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell DL. The relative cytotoxicity of (6-4) photoproducts and cyclobutane dimers in mammalian cells. Photochem Photobiol. 1988;48:51–7. doi: 10.1111/j.1751-1097.1988.tb02785.x. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell DL, Nairn RS. The biology of the (6-4) photoproduct. Photochem Photobiol. 1989;49:805–19. doi: 10.1111/j.1751-1097.1989.tb05578.x. [DOI] [PubMed] [Google Scholar]
- 6.Setlow RB, Carrier WL. Pyrimidine dimers in ultraviolet-irradiated DNA's. J Mol Biol. 1966;17:237–54. doi: 10.1016/s0022-2836(66)80105-5. [DOI] [PubMed] [Google Scholar]
- 7.Hussain SP, Hollstein MH, Harris CC. p53 tumor suppressor gene: at the crossroads of molecular carcinogenesis, molecular epidemiology, and human risk assessment. Ann N Y Acad Sci. 2000;919:79–85. doi: 10.1111/j.1749-6632.2000.tb06870.x. [DOI] [PubMed] [Google Scholar]
- 8.Olivier M, Eeles R, Hollstein M, et al. The IARC TP53 database: new online mutation analysis and recommendations to users. Hum Mutat. 2002;19:607–14. doi: 10.1002/humu.10081. [DOI] [PubMed] [Google Scholar]
- 9.Cui R, Widlund HR, Feige E, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128:853–64. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 10.Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445:843–50. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- 11.Pathak MA, Fanselow DL. Photobiology of melanin pigmentation: dose/response of skin to sunlight and its contents. J Am Acad Dermatol. 1983;9:724–33. doi: 10.1016/s0190-9622(83)70186-6. [DOI] [PubMed] [Google Scholar]
- 12.Schauer E, Trautinger F, Kock A, et al. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J Clin Invest. 1994;93:2258–62. doi: 10.1172/JCI117224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakamuro D, Sabbatini P, White E, et al. The polyproline region of p53 is required to activate apoptosis but not growth arrest. Oncogene. 1997;15:887–98. doi: 10.1038/sj.onc.1201263. [DOI] [PubMed] [Google Scholar]
- 14.Walker KK, Levine AJ. Identification of a novel p53 functional domain that is necessary for efficient growth suppression. Proc Natl Acad Sci U S A. 1996;93:15335–40. doi: 10.1073/pnas.93.26.15335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matlashewski GJ, Tuck S, Pim D, et al. Primary structure polymorphism at amino acid residue 72 of human p53. Mol Cell Biol. 1987;7:961–3. doi: 10.1128/mcb.7.2.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dumont P, Leu JI, Della Pietra AC, 3rd, et al. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat Genet. 2003;33:357–65. doi: 10.1038/ng1093. [DOI] [PubMed] [Google Scholar]
- 17.Pim D, Banks L. p53 polymorphic variants at codon 72 exert different effects on cell cycle progression. Int J Cancer. 2004;108:196–9. doi: 10.1002/ijc.11548. [DOI] [PubMed] [Google Scholar]
- 18.Rees JL. Genetics of hair and skin color. Annu Rev Genet. 2003;37:67–90. doi: 10.1146/annurev.genet.37.110801.143233. [DOI] [PubMed] [Google Scholar]
- 19.Han J, Kraft P, Colditz GA, et al. Melanocortin 1 receptor variants and skin cancer risk. Int J Cancer. 2006;119:1976–84. doi: 10.1002/ijc.22074. [DOI] [PubMed] [Google Scholar]
- 20.Han J, Colditz GA, Hunter DJ. Risk factors for skin cancers: a nested case-control study within the Nurses' Health Study. Int J Epidemiol. 2006;35:1514–21. doi: 10.1093/ije/dyl197. [DOI] [PubMed] [Google Scholar]
- 21.Kanetsky PA, Ge F, Najarian D, et al. Assessment of polymorphic variants in the melanocortin-1 receptor gene with cutaneous pigmentation using an evolutionary approach. Cancer Epidemiol Biomarkers Prev. 2004;13:808–19. [PubMed] [Google Scholar]
- 22.Beaumont KA, Newton RA, Smit DJ, et al. Altered cell surface expression of human MC1R variant receptor alleles associated with red hair and skin cancer risk. Hum Mol Genet. 2005;14:2145–54. doi: 10.1093/hmg/ddi219. [DOI] [PubMed] [Google Scholar]
- 23.Box NF, Wyeth JR, O'Gorman LE, et al. Characterization of melanocyte stimulating hormone receptor variant alleles in twins with red hair. Hum Mol Genet. 1997;6:1891–7. doi: 10.1093/hmg/6.11.1891. [DOI] [PubMed] [Google Scholar]
- 24.Valverde P, Healy E, Jackson I, et al. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet. 1995;11:328–30. doi: 10.1038/ng1195-328. [DOI] [PubMed] [Google Scholar]
- 25.Miettinen OS. Stratification by a multivariate confounder score. Am J Epidemiol. 1976;104:609–20. doi: 10.1093/oxfordjournals.aje.a112339. [DOI] [PubMed] [Google Scholar]
- 26.Beckman G, Birgander R, Sjalander A, et al. Is p53 polymorphism maintained by natural selection? Hum Hered. 1994;44:266–70. doi: 10.1159/000154228. [DOI] [PubMed] [Google Scholar]
- 27.Sjalander A, Birgander R, Kivela A, et al. p53 polymorphisms and haplotypes in different ethnic groups. Hum Hered. 1995;45:144–9. doi: 10.1159/000154275. [DOI] [PubMed] [Google Scholar]
- 28.Oren M, Bartek J. The sunny side of p53. Cell. 2007;128:826–8. doi: 10.1016/j.cell.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 29.Gantz I, Yamada T, Tashiro T, et al. Mapping of the gene encoding the melanocortin-1 (alpha-melanocyte stimulating hormone) receptor (MC1R) to human chromosome 16q24.3 by Fluorescence in situ hybridization. Genomics. 1994;19:394–5. doi: 10.1006/geno.1994.1080. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki I, Cone RD, Im S, et al. Binding of melanotropic hormones to the melanocortin receptor MC1R on human melanocytes stimulates proliferation and melanogenesis. Endocrinology. 1996;137:1627–33. doi: 10.1210/endo.137.5.8612494. [DOI] [PubMed] [Google Scholar]
- 31.Healy E, Jordan SA, Budd PS, et al. Functional variation of MC1R alleles from red-haired individuals. Hum Mol Genet. 2001;10:2397–402. doi: 10.1093/hmg/10.21.2397. [DOI] [PubMed] [Google Scholar]
- 32.Ringholm A, Klovins J, Rudzish R, et al. Pharmacological characterization of loss of function mutations of the human melanocortin 1 receptor that are associated with red hair. J Invest Dermatol. 2004;123:917–23. doi: 10.1111/j.0022-202X.2004.23444.x. [DOI] [PubMed] [Google Scholar]
- 33.Bliss JM, Ford D, Swerdlow AJ, et al. Risk of cutaneous melanoma associated with pigmentation characteristics and freckling: systematic overview of 10 case-control studies. The International Melanoma Analysis Group (IMAGE) Int J Cancer. 1995;62:367–76. doi: 10.1002/ijc.2910620402. [DOI] [PubMed] [Google Scholar]
- 34.Healy E, Todd C, Jackson IJ, et al. Skin type, melanoma, and melanocortin 1 receptor variants. J Invest Dermatol. 1999;112:512–3. doi: 10.1046/j.1523-1747.1999.00554.x. [DOI] [PubMed] [Google Scholar]
- 35.Palmer JS, Duffy DL, Box NF, et al. Melanocortin-1 receptor polymorphisms and risk of melanoma: is the association explained solely by pigmentation phenotype? Am J Hum Genet. 2000;66:176–86. doi: 10.1086/302711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rees JL, Healy E. Melanocortin receptors, red hair, and skin cancer. J Investig Dermatol Symp Proc. 1997;2:94–8. doi: 10.1038/jidsymp.1997.18. [DOI] [PubMed] [Google Scholar]
- 37.Han J, Cox DG, Colditz GA, et al. The p53 codon 72 polymorphism, sunburns, and risk of skin cancer in US Caucasian women. Mol Carcinog. 2006;45:694–700. doi: 10.1002/mc.20190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Interaction between the p53 Arg72Pro polymorphism and MC1R variants on hair color and childhood sunburn reaction among controls
Supplementary Table 2. SCC and BCC risks by p53 Arg72Pro polymorphism and hair color
Supplementary Table 3. SCC and BCC risks by p53 Arg72Pro polymorphism and MC1R variants
Supplementary Table 4. SCC and BCC risks by p53 Arg72Pro polymorphism and constitutional susceptibility score