Abstract
Tec family non-receptor tyrosine kinases (Itk, Btk, Tec, Rlk and Bmx) are characterized by the presence of an autophosphorylation site within the non-catalytic Src homology 3 (SH3) domain. The full length Itk mutant containing a phenylalanine in place of the autophosphorylated tyrosine has been previously studied in Itk deficient primary T cells. These studies revealed that the non-phosphorylated enzyme only partially restores Itk mediated signaling. In spite of these insights, the precise role of the Tec kinase autophosphorylation site remains unclear and the mechanism of the autophosphorylation reaction within the Tec kinases is not known. Here we show both in vitro and in vivo that Itk autophosphorylation on Y180 within the SH3 domain occurs exclusively via an intramolecular, in cis mechanism. Using an in vitro kinase assay we also show that mutation of the Itk autophosphorylation site Y180 to Phe decreases kinase activity of the full-length enzyme by increasing Km for a peptide substrate. Moreover, mutation of Y180 to Glu, a residue chosen to mimic the phosphorylated tyrosine, alters the ligand binding capability of the Itk SH3 domain in a ligand dependent fashion. NMR chemical shift mapping gives residue-specific structural insight into the effect of the Y180E mutation on ligand binding. These data provide a molecular level context with which to interpret in vivo functional data and allow development of a structural model for Itk autophosphorylation.
Keywords: Tec kinases, autophosphorylation, SH3 domain, SH2 domain, proline rich ligand, Itk
INTRODUCTION
Phosphorylation cascades mediated by protein tyrosine kinases are initiated in a rapid fashion following ligand engagement of extracellular receptors such as the T cell receptor. Several distinct families of protein kinases play critical roles in the signaling processes that control T cell activation. Specifically, engagement of the T cell receptor leads to the activation of the lipid kinase phosphoinositide 3-kinase (PI3K) and the Syk family kinase, Zap-70. Activation of PI3K leads to the formation of phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3), a ligand for the PH domain of Itk. Zap-70 phosphorylates the adaptor protein, LAT (Linker for activation of T cells) creating a binding site for the Tec family kinase Itk within the LAT/SLP76 (SH2-domain-containing leukocyte protein of 76 kDa) signaling complex1; 2. Binding of PtdIns(3,4,5)P3 by the PH domain of Itk and phospho-SLP76/LAT complex by the SH2 domain of Itk recruites Itk to the membrane signaling complex. The Src family kinase, Lck, phosphorylates Itk on Y511, a residue that resides in the activation loop of the Itk kinase domain and which must be phosphorylated for subsequent Itk catalytic activity3. Itk also autophosphorylates Y180 in its own SH3 domain4. Activated Itk phosphorylates phospholipase C-γ15; the enzyme responsible for hydrolysis of phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) to diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). From here, DAG and IP3 mediate intracellular calcium flux and activation of the Ras-Raf-MEK-ERK pathway that lead to cytokine production and T cell proliferation6; 7.
Our interests have rested on understanding the molecular details of the complex regulation of Tec family kinase signaling. The Tec family of non-receptor tyrosine kinases consists of five members: Tec, Btk, Rlk/Txk, Bmx/Etk and Itk. All Tec kinases contain a Src homology 3 (SH3), Src homology 2 (SH2) and kinase domain, and (with the exception of Rlk) also contain an N-terminal pleckstrin homology (PH) and Tec homology (TH) domain. We and others have reported that the non-catalytic regulatory domains of the Tec kinases mediate self-association of these enzymes both in vitro and in cells8; 9; 10; 11; 12; 13. Intermolecular self-association might suggest that the Tec kinase autophosphorylation step occurs in trans yet to date the precise mechanism of autophosphorylation has not been elucidated. In addition to mechanistic considerations, the functional significance of Tec kinase autophosphorylation is not yet firmly established. While mutation of the Lck phosphorylation site, Y511, to phenylalanine in Itk renders Itk catalytically impaired and results in severely defective cytokine production and ERK activation in activated T cells3, disruption of the autophosphorylation site in the Itk SH3 domain (by mutation of Y180 to Phe) only partially alters downstream signaling4. Related studies in B cells found that the Btk Y223F mutant (corresponding to Itk Y180) was able to partially restore calcium mobilization and PLC-γ2 phosphorylation in a Btk deficient cell line14; 15, whereas in another study the same Y223F mutation enhanced the oncogenic ability of a gain-of-function Btk mutant16.
The effect of mutation at the autophosphorylation site in the SH3 domain has been difficult to interpret as the experimental outcome seems to be dependent on the assay system used14; 15; 16. In an effort to better understand the observed effects of the Y180F/Y223F mutations in cells, in vitro kinase assays have been carried out using immunoprecipitated Tec kinases4; 16. The results of these experiments suggest that the Itk Y180F and Btk Y223F mutant enzymes exhibit similar catalytic activity to the wild type enzyme. Complementing these functional data, binding studies of the phosphorylated Btk SH3 domain suggest that modification of Y223 alters ligand affinity17. Thus, altered ligand binding of the Itk(Y180F) mutant rather than changes in catalytic activity is currently presumed to be the explanation for the differences in the activity of the full-length wild type and Y180F Itk proteins in T cells4.
Given the complexity of the in vivo experiments and the qualitative nature of the in vitro kinase assays carried out to date, we decided to address the significance of Itk autophosphorylation using quantitative biochemical and structural approaches. In this report we show that the SH3 domain of the Itk Y180F mutant is partially ‘trapped’ in a conformation where it occupies the active site of the Itk kinase domain. Consistent with a steric block of the active site, the Itk Y180F mutant is characterized by a moderately higher Km but the same kcat for a peptide substrate compared to the wild-type enzyme. In addition to modulating this kinetic parameter of full length Itk, SH3 mediated ligand binding is also affected by mutation of Y180. Replacing Y180 within the isolated SH3 domain to Glu to mimic a phosphorylated Tyr has opposite effects on the affinity of different ligands. NMR chemical shift mapping experiments show that the SH3 Y180E mutation causes structural perturbations that extend into the ligand-binding pocket. We also show that the Itk autophosphorylation reaction occurs by an intramolecular, in cis mechanism both in vitro and in vivo. Thus, the autophosphorylated Tyr in the SH3 domain is only phosphorylated by the kinase domain in the same molecule and not by a separate Itk molecule. Together, these data provide molecular level insights into previous experiments that probed the role of Itk autophosphorylation in T cell activation. Our data point to changes in both ligand binding by the SH3 domain and kinetic parameters of the enzyme itself that likely contribute to the partial activity of the Itk Y180F mutant that has been previously studied in the context of T cells deficient in wild type Itk.
RESULTS
Mechanism of Itk autophosphorylation
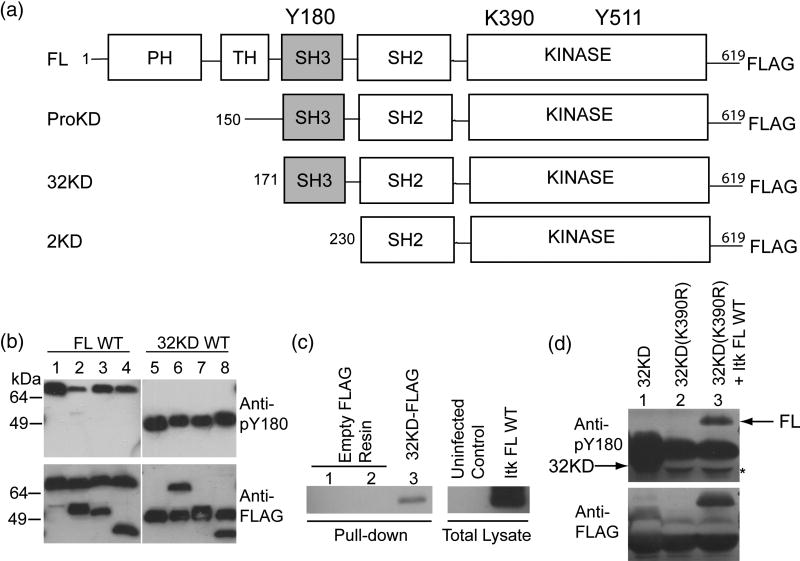
To test whether Itk autophosphorylation occurs via an intramolecular, in cis or an intermolecular, in trans mechanism, we constructed several Itk deletion variants (Fig. 1A) and measured the phosphorylation of Y180 in a series of mixing experiments. Mixing full length Itk and shorter Itk deletion fragments allows for separate visualization of kinase active (WT) and kinase inactive (K390R) enzymes by western blot.
Figure 1. Itk autophosphorylation occurs in cis.
(A) Itk deletion fragments used in this study. Y180 in the SH3 domain is the site of autophosphorylation, K390 is mutated to R to render the kinase domain inactive, Y511 is the site of trans phosphorylation by Lck. (B) 0.5 μM Itk FL WT was incubated with 0.5 μM kinase inactive (K390R) versions of the following proteins: Itk ProKD (lane 2), Itk 32KD (lane 3) or Itk 2KD (lane 4), in a kinase assay buffer. Lane 1 is 1μM Itk FL WT alone. The samples were boiled, separated by SDS-PAGE and western blotted using an anti-phospho-Y180 or anti-FLAG antibody. Lanes 5–8: Reversal of the incubation scheme: Itk 32KD WT was incubated with kinase inactive (K390R) versions of the following proteins: Itk FL (lane 6), Itk ProKD (lane 7) and Itk 2KD (lane 8). Lane 5 is 1 μM Itk 32KD WT enzyme alone. The Itk 2KD fragment that lacks the Itk SH3 domain (lanes 4 and 8) is used as a control. (C) Itk FL can associate with Itk 32KD. Empty FLAG-resin (lanes 1 and 2) or purified FLAG-tagged Itk 32KD immobilized on an anti-FLAG resin (lane 3) was incubated with uninfected Sf9 cell lysate (lane 1) or untagged Itk FL infected Sf9 cell lysate (lanes 2 and 3) in a pull-down assay. The samples were rinsed, separated by SDS-PAGE and western blotted using an anti-Itk antibody. (D) In vivo Itk autophosphorylation occurs in cis. FLAG-tagged Itk 32KD WT, Itk kinase inactive 32KD (K390R) and Itk FL WT were co-expressed with Lck and immunoprecipitated from Sf9 cells using an anti-FLAG resin and western blotted as before with anti-phospho-Y180 or anti-FLAG antibodies. Lane 1: Itk 32KD WT alone, Lane 2: Itk kinase inactive 32KD (K390R) alone and Lane 3: Itk kinase inactive 32KD (K390R) co-expressed with Itk FL WT. Arrows on the top panel indicate the positions of Y180 phosphorylated Itk FL and Itk 32KD. Itk 32KD migrates slightly below the immunoglobulin heavy chain and a mobility shift associated with the phosphorylated Itk 32KD fragment is evident in lane 1 (the band is seen both below and slightly above the heavychain). The asterisk on the top panel indicates the heavy chain that has been displaced by the presence of Itk 32KD.
We tested the extent to which a kinase inactive mutant of Itk (K390R) is phosphorylated on Y180 in trans by incubating it with catalytically active Itk. First, purified FLAG-tagged, active full-length WT Itk is incubated with three different N-terminal deletion fragments of inactive (K390R) Itk in a 1:1 ratio and subjected to in vitro kinase assay conditions (Fig. 1B). The proteins were separated by SDS-PAGE and then probed by western blotting with a Btk pY223 specific antibody (previously used to detect specific phosphorylation on Itk Y180)4. As shown in lanes 1–4 of Figure 1B, only full-length, kinase active Itk is phosphorylated on Y180, while none of the kinase inactive Itk fragments are phosphorylated on this residue. Reversal of the scheme by incubation of the kinase active 32KD fragment of Itk with either kinase inactive (K390R) full-length Itk or the kinase inactive deletion fragments of Itk shows that only the kinase active 32KD fragment is phosphorylated on Y180 and that neither the inactive full-length Itk nor the inactive Itk fragments are phosphorylated on Y180 (Fig. 1B, lanes 5–8). These data indicate that autophosphorylation of Itk occurs exclusively in an intramolecular, in cis fashion. The absence of in trans autophosphorylation is not due to the inability of the fragments of Itk to associate intermolecularly with full length Itk as shown by the pull-down assay in Figure 1C. Purified FLAG-tagged Itk 32KD immobilized on an anti-FLAG resin readily interacts with full-length untagged Itk expressed in Sf9 cells. We have also previously demonstrated that deletion of the PH domain of Itk does not significantly affect the catalytic activity (kcat/Km) of Itk18. These data indicate that in spite of self-association of Itk, in vitro autophosphorylation of Itk on Y180 occurs in cis.
To explore the mechanism of Itk autophosphorylation in vivo, we tested the extent to which kinase inactive Itk 32KD (K390R) fragment can be phosphorylated in trans by full-length wild-type Itk, by co-expressing FLAG-tagged versions of both in Sf9 cells. The extent of phosphorylation on Y180 was assessed by immunoprecipitating the proteins using an anti-FLAG resin, and western blotting with a pY180 specific antibody as before. As shown in Figure 1D, while kinase active Itk, either full length or 32KD, are efficiently phosphorylated on Y180, the kinase inactive Itk 32KD (K390R) fragment is not phosphorylated on Y180 even when co-expressed with wild type Itk. These results perfectly mirror the in vitro results already described, providing further indication that autophosphorylation of Itk on Y180 within the SH3 domain is an intramolecular reaction.
Absence of Y180 phosphorylation influences Km
Previous analysis of the full-length Itk Y180F mutant in vivo showed that this mutant has partial activity4. As well, previous in vitro analyses using immunoprecipitated enzymes suggested that the activity of the Y180F mutant does not differ from WT4. In order to quantitatively compare the in vitro kinase activity of the full length wild type Itk and the Itk Y180F mutant lacking the autophosphorylation site, we determined the kinetic parameters of wild type Itk and Itk Y180F mutant using Sf9 purified enzymes (Fig. 2A & B). The Km and kcat values for ATP are unaffected by the Y180F mutation (Fig. 2A and B). The kcat values for wild-type Itk and the corresponding Itk Y180F mutant are also identical within experimental error. In contrast, the Km value for the peptide substrate differs between wild-type Itk and the Itk Y180F mutant resulting in nearly a factor of three difference in kcat/Km (Fig. 2A and B).
Figure 2. Kinetic parameters for Itk Y180F mutant and wild type Itk.
(A & B) The in vitro kinase activities of full-length Itk wild-type (WT), Y180F mutant and Y180F mutant in the presence of 2.5 μM free Itk SH2 domain are compared. Substrate (Peptide B or ATP) curves of WT full-length Itk (filled triangles), Itk Y180F (open squares), and Y180F mutant in the presence of free Itk SH2 domain (open circles) were fit to the Michealis-Menten equation using GraphFit to obtain the kinetic parameters reported in (B). (C) Western blot of purified Itk enzymes. 250 nM purified Itk WT or Y180F mutant enzymes were separated by SDS-PAGE, transferred onto PVDF, and probed with either an anti-pY180, anti-pY511 or anti-FLAG antibody. Y511 phosphorylation levels are comparable for WT and Y180F Itk. (D) Comparison of the activities of Itk full-length WT enzyme with that of the full-length kinase inactive (K390R) mutant. One μM enzyme was used for each reaction and Peptide B was used as substrate. Data shown are the average of at least two independent experiments.
Western blot analysis of the proteins using phosphospecific antibodies confirmed that wild-type Itk and the Y180F mutant are equally phosphorylated on Y511 and that, as expected, wild type Itk alone is phosphorylated on Y180 (Fig. 2C). Full length kinase inactive (K390R) Itk showed negligible activity when compared to wild-type enzyme confirming that the activity being measured in Figure 2A corresponds to that of Itk and not to co-purifying impurities (Fig. 2D). Thus, differences in activation loop (Y511) phosphorylation levels and/or possible impurities in the Itk preparations do not account for the observed differences in Km values between wild-type and Y180F Itk.
Another possible explanation for the observed increase in Km upon mutation of Y180 to phenylalanine stems from our recently reported results regarding substrate recognition by Itk and related Tec kinases19. In that work we demonstrated that the Itk kinase domain recognizes the autophosphorylation site in the SH3 domain via direct binding to the adjacent Itk SH2 domain. The Itk kinase domain binds directly to the SH2 domains of its substrates mediating specific phosphorylation of the target tyrosine residue. Since full length Y180F Itk contains no mutations in either the SH2 or kinase domains, the SH2-mediated docking mechanism should be maintained in the Itk Y180F mutant and, as a result, the SH3 domain harboring the Y180F mutation is likely positioned within the active site of Itk regardless of its inability to be phosphorylated. Indeed, the fact that the phenylalanine side chain cannot be phosphorylated suggests that the Y180F mutant may form a dead-end intramolecular complex leading to an apparent increase in Km for the peptide substrate (Fig. 2A and B).
To test this hypothesis we followed up on our earlier observation that addition of isolated Itk SH2 domain to the in vitro kinase reaction will directly compete with docked substrate and reduce Y180 autophosphorylation19. This suggests that addition of excess SH2 domain to the kinase assay should disfavor formation of the dead-end complex and eliminate competition between the Y180F SH3 domain and the peptide substrate for the active site. The kinetic parameters of the Itk Y180F mutant were therefore measured with the addition of isolated SH2 domain to the assay conditions (Fig. 2A). The saturation curves for full-length wild type Itk alone and the Itk Y180F mutant in the presence of free SH2 domain are identical. Exogenous free Itk SH2 domain restores the Km for Peptide B of the Itk Y180F mutant to that of wild-type Itk (Fig. 2A and B). Thus, the decrease in kcat/Km for the Itk Y180F mutant likely reflects reduced peptide substrate binding to the Itk active site due to steric occlusion by the mutant SH3 domain. These measurements therefore suggest that, at least in part, the diminished activity observed for the Itk Y180F mutant in vivo4 likely arises from formation of a dead-end intramolecular complex that reduces substrate phosphorylation by increasing Km for exogenous substrates. With regard to reported similarities between in vitro activities of wildtype and Y180F mutant Itk using immunoprecipitated enzymes4, we expect that the differences in Km of the magnitude that we measure here would not be readily detected by qualitative western blot approaches used previously.
Modulation of ligand affinity by an SH3 Y180E mutation
SH3 domains are well known ligand binding modules and so we also investigated the extent to which autophosphorylation of the Itk SH3 domain might influence ligand binding affinity. Structures of the SH3 domains of three of the Tec kinases have been solved and clearly demonstrate that the autophosphorylated tyrosine is located within the conserved SH3 binding pocket11; 20; 21. The Itk SH3 domain has been shown to bind two disparate ligands, the canonical proline-rich peptide and the SH2 domain of Itk8; 22. Both ligand-binding events involve the conserved aromatic cleft of the SH3 domain that contains Tyr 180.
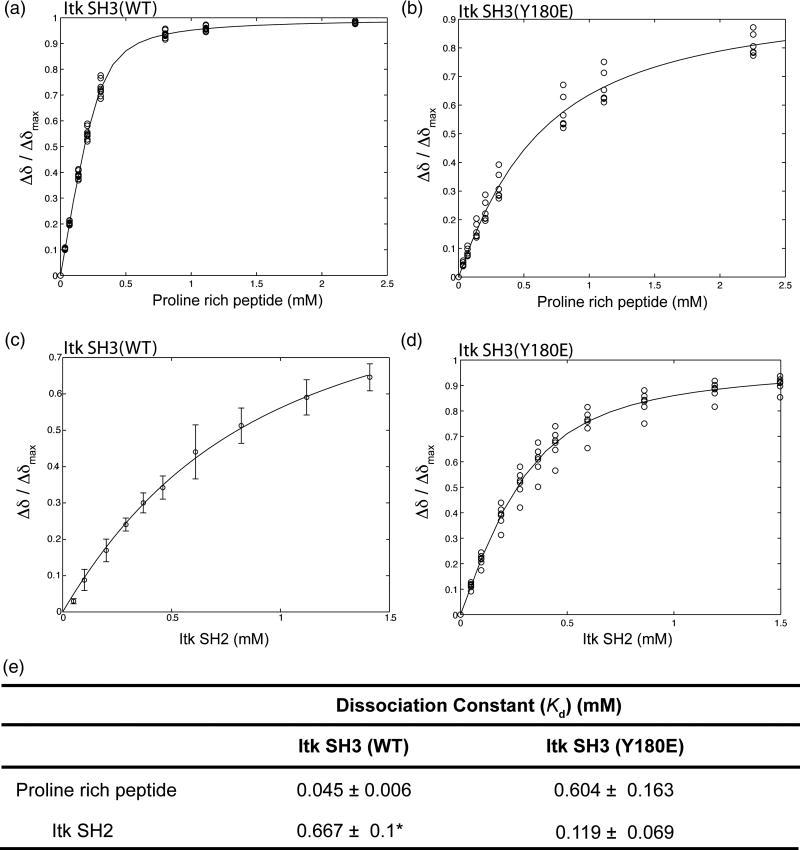
NMR spectroscopy provides a means to evaluate the molecular details of how autophosphorylation at position 180 in the SH3 domain influences ligand binding. As already described however, the SH3 domain alone is not a good substrate for the Itk kinase (presence of the SH2 domain within the substrate is required to achieve efficient phosphorylation of Y18019) and so production of sufficient quantities of phosphorylated SH3 domain in isolation for NMR analysis is impractical. We therefore opted to mutate Y180 in the isolated SH3 domain to the negatively charged amino acid glutamate, a reasonable mimic for a phosphorylated side chain23; 24; 25; 26. NMR chemical shift perturbations reveal that mutation of the Itk SH3 Tyr 180 to Glu brought about a 13-fold reduction in the affinity of the SH3 domain for a canonical proline rich peptide (Fig. 3A, B, E). Conversely, the Itk SH3 Y180E mutation leads to a 6 times increase in the affinity for the Itk SH2 domain (Fig. 3C, D, E). Thus, replacing Y180 within the Itk SH3 domain with a negatively charged side chain alters ligand binding affinity and the magnitude and nature of the effect appears to be ligand specific.
Figure 3. Mutation of Itk SH3 Y180 to Glu modulates ligand binding by the SH3 domain.
300 μM (initial concentration) of either 15N labeled wild type Itk SH3 (A) or 15N labeled SH3 Y180E mutant (B) were titrated with increasing concentration of a proline rich peptide (GWYSKPPPPIP). In a similar manner, 250 μM (initial concentration) of either 15N labeled wild type Itk SH3 (C) or 15N labeled SH3 Y180E mutant (D) were titrated with increasing concentration of unlabeled Itk SH2 domain. 1H-15N HSQC spectra were obtained upon addition of each aliquot of ligand. Binding curves were generated by plotting the normalized concentration dependence of amide 1H chemical shifts for resonances corresponding to Y182, T184, Q188, E189, L192, D202, W208, W209, R210, A221, S223 and S224. (E) Dissociation constants are derived from the binding curves shown in A–D. Asterisk indicates that the dissociation constant for the wild type Itk SH3/SH2 interaction has been reported previously along with the binding curve shown in (C)22.
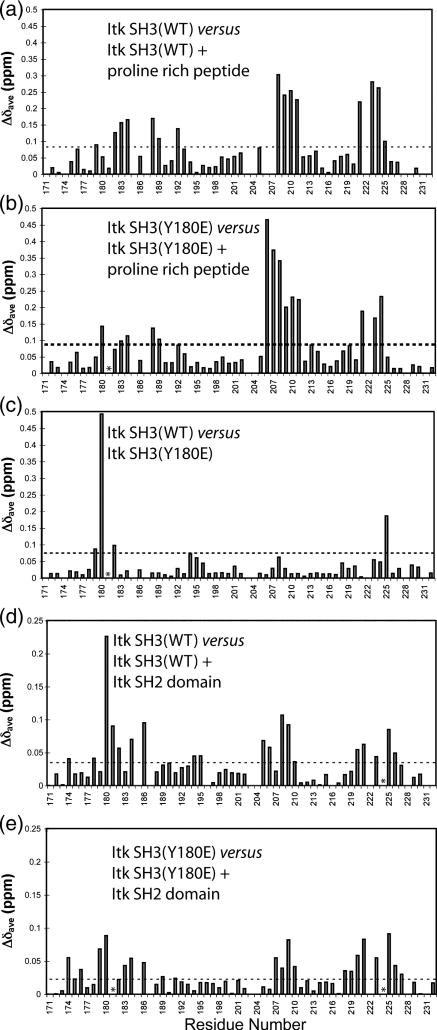
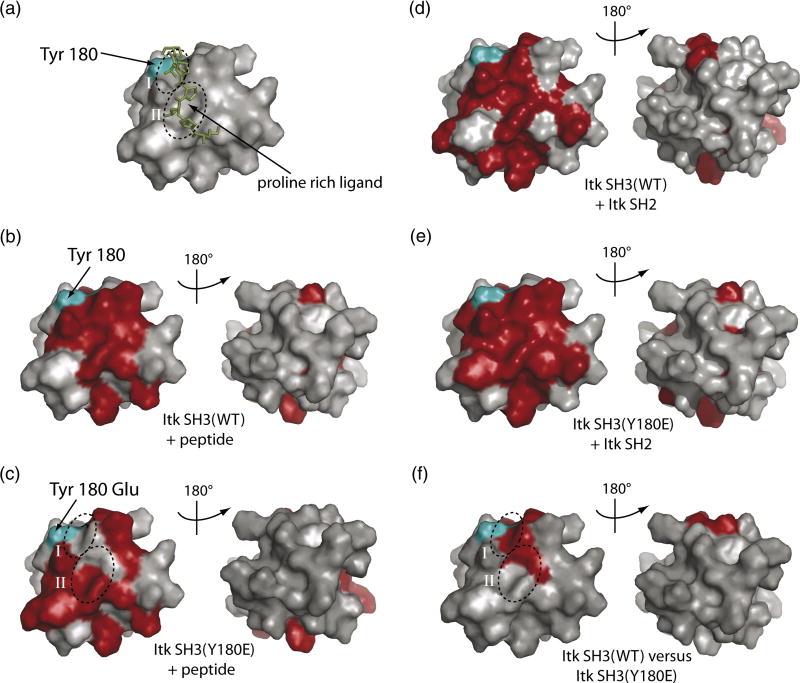
To more carefully dissect the differences in ligand binding affinity observed upon introducing a negative charge into the SH3 binding surface, we mapped the chemical shift perturbations that are induced upon canonical peptide ligand binding or Itk SH2 domain binding to either wild type Itk SH3 or the Y180E SH3 mutant (Fig. 4A–E). Chemical shift changes that occur upon ligand binding indicate SH3 surface residues that either directly contact the ligand or are indirectly affected by ligand binding (such as by conformational changes in the SH3 domain that are a result of ligand binding). Multiple structures of proline-rich peptides bound to SH3 domains are available27 and all show a conserved recognition motif consisting of two adjacent binding clefts (labeled I and II in Fig. 5) on the SH3 surface that contact the aliphatic-proline dipeptide segments of the proline-rich ligand (Fig. 5A). The binding surface that is mapped upon titration of the GWYSKPPPPIP peptide into the wild type Itk SH3 domain (Fig. 5B) is consistent with the structure of a related peptide bound to this SH3 domain (Fig. 5A).
Figure 4. Chemical shift perturbations induced by Itk SH3 Y180E mutation and ligand binding.
Purified 15N labeled Itk SH3 WT, or SH3 Y180E mutant were titrated with increasing amounts of proline rich peptide or Itk SH2 domain. The average chemical shift (Δδave) for each amide is plotted against the residue number of Itk. The horizontal dashed lines represent the mean plus one standard deviation for each titration. Residues that exhibit chemical shift changes above this line are considered significant and are mapped onto the structure of the SH3 domain in Figure 5. (A) Chemical shift changes upon binding of proline rich peptide to wild type 15N Itk SH3; (B) Binding of proline rich peptide to Y180E 15N Itk SH3 mutant; (C) Chemical shift differences between wild type Itk SH3 and the Y180E Itk SH3 mutant. (D) Chemical shift changes upon binding of Itk SH2 domain to wild type 15N Itk SH3; (E) Binding of Itk SH2 domain to the Y180E 15N Itk SH3 mutant. Asterisks indicate resonances that broaden beyond detection upon ligand binding. Such residues are included in the surface maps shown in Figure 5.
Figure 5. Surface plot of the chemical shift changes upon ligand binding.
The color scheme used for all the panels are as follows: The Itk SH3 domain (PDB ID 1AWJ) is shown in grey and is represented in identical orientations in each panel. Residues corresponding to resonances that exhibit significant chemical shift changes are shown in red and the autophosphorylation site Y180 is cyan on each structure. (A) Structure of the Itk SH3 domain (surface rendering) bound to the proline rich (KPLPPTP) ligand (represented as sticks in green). The conserved binding clefts I and II for the proline peptide on the surface of the SH3 domain are circled and labeled. (B) Proline rich ligand binding to the wild type Itk SH3 domain. (C) Proline rich ligand binding to the Y180E Itk SH3 mutant. (D) Itk SH2 domain binding to the wild type Itk SH3 domain. (E) Itk SH2 domain binding to the Y180E Itk SH3 mutant. (F) SH3 residues that exhibit changes in resonance frequencies upon mutation of Y180 to Glu.
Titration of the same peptide into the Y180E SH3 domain reveals a different pattern of chemical shift perturbations (Fig. 5C). Notably, for the Itk Y180E mutant, resonances corresponding to the key regions in the wild type SH3 domain that contact the bound peptide (conserved binding clefts I and II) either do not exhibit chemical shift changes upon peptide binding (cleft I) or only partially exhibit chemical shift perturbations (cleft II). These data suggest that the canonical peptide ligand associates with the Y180E mutant in a manner that is distinct from association with wild type SH3 and provide insight into the observed decrease in affinity that accompanies mutation of Y180 to glutamate.
The basis for the differences in peptide binding to wild type and Y180E SH3 domain becomes more apparent upon comparison of resonance frequencies for the wild type and Y180E SH3 domains in the absence of peptide ligand (Figs. 4C, 5F). In Figure 5F the SH3 residues for which significant chemical shift differences are apparent between wild type and Y180E SH3 domains are highlighted on the surface of the domain. The changes are not localized to the region immediately surrounding the site of mutation (position 180) but rather are directed into the peptide binding pocket and coincide remarkably well with the residues that do not exhibit chemical shift perturbations in the Y180E mutant upon peptide binding (Fig. 5C). The data suggest that insertion of a negative charge at position 180 modulates the SH3 binding surface in a manner that disrupts the primary sites of ligand contact (clefts I and II, Fig. 5F) causing the peptide ligand to alter its interaction with the SH3 surface. Consequently, canonical binding of the GWYSKPPPPIP peptide is disfavored and the affinity drops significantly (Fig. 3A, B and E). These data provide insight into how autophosphorylation at Y180 might alter ligand binding to the Itk SH3 domain during signaling. Indeed, given the size and charge differences between a phosphate and the carboxylic acid of glutamate, the effects on ligand binding affinity may be more pronounced for the phosphorylated SH3 domain than those observed for Y180E mimic.
In contrast to the decrease in affinity observed for the proline rich peptide ligand used in this study, the Itk SH3 Y180E mutation leads to an increase in affinity for the Itk SH2 domain (Fig. 3C, D and E). Surface mapping of the chemical shift changes induced upon binding of the Itk SH2 domain by either wild-type Itk SH3 domain or SH3 Y180E shows significant similarity (Fig. 5D and E). Consistent with the observed increase in affinity of the Itk SH2/SH3(Y180E) interaction, the surface defined by chemical shift perturbations for the Itk SH2/SH3(Y180E) interaction is more extensive than that of the wild-type Itk SH2/SH3 interaction. The Y180E mutation likely provides an additional contact to a residue or residues of the SH2 ligand leading to the observed affinity increase. At this juncture, we also measured the affinity of the Itk SH2 domain binding to the SH3(Y180F) mutant. In this case we find that the tyrosine to phenylalanine mutation alters the interaction with the SH2 domain to only a small extent (Kd = 0.4 ± 0.2 mM) providing further support that the tyrosine to glutamate mutation (or phosphorylation of Y180) likely causes a significant change in SH3 mediated ligand binding. A precise description of the molecular determinants of the observed affinity increase must await full structure determination of this non-canonical SH3 interaction. Together, the two examples of ligand binding by the Itk SH3 domain examined here demonstrate that autophosphorylation in the SH3 binding pocket can have distinct consequences in ligand recognition and binding affinity.
DISCUSSION
Each of the Tec family kinases is characterized by the presence of an autophosphorylation site within their SH3 domain28 yet the functional significance and mechanism of this autophosphorylation event in Tec kinase mediated signaling have remained unclear. The data presented here clearly demonstrate an in cis autophosphorylation mechanism for Itk in spite of earlier observations that Itk and the other Tec kinases self-associate in an intermolecular fashion. Given the similarities in the domain structures of the Tec kinases, it is reasonable to expect that like Itk, the other Tec family kinases undergo autophosphorylation in cis.
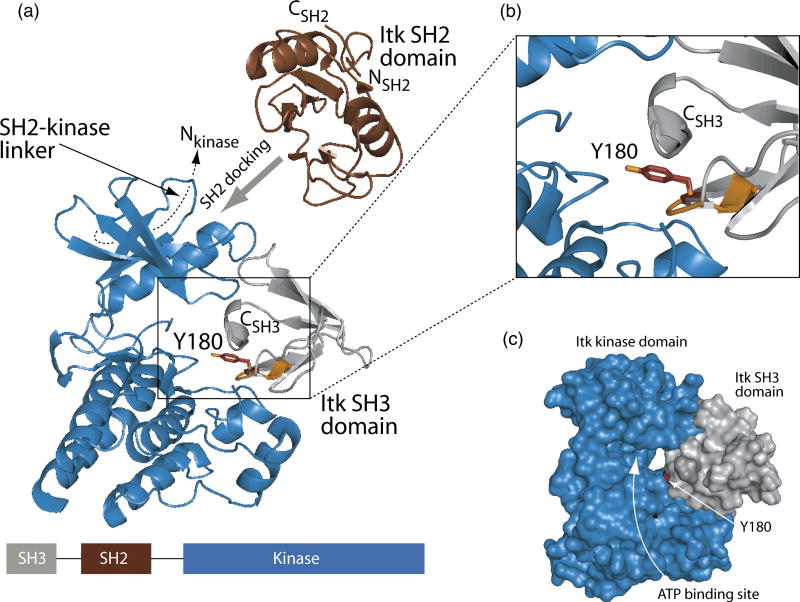
In an effort to obtain a structural model of the in cis autophosphorylation of the Itk SH3 domain we made use of the fact that structures have been solved for each of the individual domains of interest20; 29; 30. The structure of the isolated Itk kinase domain (PDB ID 1SNX) was aligned with the structure of the insulin receptor kinase domain that is bound to a tyrosine containing peptide substrate31 (PDB ID 1IR3). Tyr180 within the Itk SH3 domain was then superimposed with the tyrosine of the bound peptide substrate of the insulin receptor kinase (Fig. 6A). Simple alignment of these two target tyrosine residues (Y180 in the Itk SH3 domain and the peptide substrate tyrosine in the insulin receptor kinase structure) with no further adjustment results in a remarkable fit of the Itk SH3 domain within the Itk kinase domain active site (Fig. 6B and C). The intervening SH2 domain can also be included in the model. The amino terminus of the SH2 domain must be positioned such that it is covalently linked to the carboxy terminus of the SH3 domain. The carboxy terminus of the SH2 domain leads to the 17 amino acid linker between the SH2 and kinase domains. Biochemical evidence suggests that the 17 amino acid linker contacts the small lobe of the kinase domain in a manner that resembles the structure of Csk18; 32. Given these structural restraints and the fact that the Itk SH2 domain binds directly to the Itk kinase domain in a substrate docking role we can place the SH2 domain into this model for Itk autophosphorylation (Fig. 6A). The model is also consistent with SAXS analysis of full-length Btk33, where the SH2 domain is predicted to be located close to the N-terminal lobe of the kinase domain (Fig. 6A). Thus, the in cis autophosphorylation mechanism predicts that, during the course of the autophosphorylation reaction, the position of the SH3 and SH2 regulatory domains of Itk with respect to the catalytic kinase domain is distinct from related enzymes such as Src34, Abl35 and Csk32. Furthermore, this regulatory region must be flexible enough to position the SH3 domain within the kinase active site in an intramolecular manner.
Figure 6. Model of in cis Itk autophosphorylation.
(A) The Itk kinase domain (shown in blue, PDB ID 1SNX) was aligned with the structure of the substrate bound insulin receptor kinase domain (PDB ID 1IR3). The insulin receptor kinase domain itself is not shown for clarity while the tyrosine containing peptide substrate in that structure is indicated in orange. Itk Y180 (represented by sticks in red) within the Itk SH3 domain (grey ribbons) was placed into the active site of the kinase domain by superimposing Y180 (red) with the tyrosine residue of the insulin receptor kinase substrate (orange). To achieve efficient phosphorylation of Y180 in the SH3 domain, the SH2 domain must dock onto the Itk kinase domain outside of the active site19. Additionally, the 17-amino acid SH2-kinase linker contacts the ‘backside’ of the small lobe of the kinase domain (indicated with dashed line)18. Thus, restraints imposed by the amino and carboxy termini of the SH2 domain and its neighboring domains (SH3 and kinase) suggest that SH2 docking likely occurs on the small lobe of the kinase domain (indicated with wide grey arrow). The linear domain structure of the SH3-SH2-kinase portion of Itk is shown at the bottom of the figure. The enlarged view shown in (B) more clearly shows the superposition of Y180 with the peptide substrate tyrosine of the insulin receptor kinase structure. (C) Surface rendering of the Itk kinase domain (blue) and the Itk SH3 domain (grey). Y180 is red and the ATP binding cleft of the Itk kinase domain is labeled. Figures 5 and 6 were generated using PyMol40.
Previous in vivo work analyzing the effect of mutation at the site of autophosphorylation suggested that the unphosphorylated Itk Y180F mutant exhibits partial activity4. The quantitative in vitro kinase assays reported here show that the kcat values for both the purified wild-type enzyme and Itk Y180F full-length mutant are indistinguishable (Fig. 2A and B). However, the Itk Y180F mutant is characterized by a modestly higher Km for peptide B when compared to the wild-type enzyme. This apparent increased Km for peptide B phosphorylation by the Itk Y180F mutant is restored to that of wild type enzyme in the presence of excess free Itk SH2 domain. This result suggests that the Itk Y180F mutant enzyme forms a dead end complex that is disrupted by excess SH2 domain that competes for the docking site on the Itk kinase domain. Thus, the decreased activity of the Itk Y180F mutant reported in vivo is likely due in part to decreased substrate binding affinity; physiological substrates must compete with this dead end complex for binding to the active site of Itk. We conclude that Tec kinase autophosphorylation per se plays no role in regulating the catalytic activity of Itk since loss of the autophosphorylation site in the Itk SH3 domain has no measurable effect on the catalytic activity (either Km or kcat) of full length Itk provided these measurements are made under conditions that disfavor formation of the dead end complex. Since the primary function of the SH3 domain is ligand binding, the predominant role of Tec family autophosphorylation is therefore likely related to this function. This has been put forth previously4; 16; 17 and we now provide molecular level insight into how phosphorylation triggers changes in ligand recognition.
Structures of the SH3 domains of Btk, Tec and Itk all show that the autophosphorylated Tyr is located within the conserved ligand-binding pocket11; 20; 21. In a previously reported study, pull-down experiments using phosphorylated Btk SH3 domain indicated that only a subset of the SH3 mediated interactions are disrupted by tyrosine phosphorylation in the SH3 binding pocket17. In that work, the unphosphorylated SH3 domain of Btk interacts with proline-rich regions of c-Cbl and Wiskott-Aldrich syndrome protein (WASP), and the phosphorylated Btk SH3 domain interacts with c-Cbl but not WASP. Here NMR data suggest that the ligand binding affinity of the Itk SH3 domain can also be modulated by autophosphorylation in a ligand dependent manner and that the decrease in binding affinity of the canonical proline-rich ligand for the SH3 domain can be directly attributed to structural perturbations in the SH3 ligand binding clefts. Specifically, chemical shift perturbation analysis indicates that addition of a negatively charged residue at position 180 in the Itk SH3 domain leads to very directed changes on the SH3 surface (Fig. 5F) that are localized to the conserved binding clefts I and II. The apparent reduction of affinity for the proline-rich peptide ligand for this region, as evidenced by lack of chemical shift perturbations in the corresponding region upon titration of peptide into the Y180E mutant, suggests that autophosphorylation re-tunes the SH3 surface in a very precise and specific manner.
Quantitative kinetic analysis combined with NMR spectroscopic approaches now allow us to provide a refined interpretation of the factors leading to defects in Itk function observed by disrupting Itk autophosphorylation in T cells4. First, a decrease in substrate affinity (increased Km) due to formation of a dead-end complex mediated by SH2 domain docking is observed for full length Itk Y180F. This suggests that this same Y180F mutant in primary T cells likely exhibits decreased kinase activity. Second, quantitative ligand binding affinity measurements for the Y180E SH3 domain show that binding of one ligand is increased and another decreased upon introduction of a negatively charged residue in the SH3 binding pocket. Thus, the inability of the full length Y180F mutant in Itk-deficient primary T cells to fully restore signaling arises from the balance of changes in kinase function and ligand dependent changes in the ligand binding affinity of the Itk SH3 domain.
MATERIALS AND METHODS
Materials
The proline rich peptide (GWYSKPPPPIP) with high affinity for Itk SH3 domain36 was obtained from Genscript. The anti-pY223 Btk antibody (corresponding to Itk pY180), and anti-pY551 Btk antibody (corresponding to Itk pY511) were obtained from Dr. Owen Witte.
Constructs
Full-length and N-terminal deletion mutants of wild-type (WT) and kinase dead (K390R) Itk (mouse sequence) were PCR amplified using a reverse primer which encoded a FLAG epitope tag. The PCR products were cloned into the pENTR/D-TOPO vector (Invitrogen) by TOPO cloning. Point mutations in the full-length Itk construct were created by using the site-directed mutagenesis (SDM) kit (Stratagene). Bacterial expression constructs for Itk SH2 domain and SH3 domain have been described earlier8. The Y180E and Y180F point mutations in the Itk SH3 domain were introduced by SDM as before. All constructs were verified by sequencing at the Iowa State University DNA synthesis and sequencing facility.
Baculovirus production
The pENTR vectors with various inserts were recombined in vitro with BaculoDirect C-Term Linear DNA (Invitrogen) using LR Clonase II enzyme according to the manufacturers instructions (Invitrogen). The DNA was then transfected into Sf9 cells using Effectene (Qiagen) according to the manufacturers instructions. Three rounds of viral selection and amplifications were carried out as described in the instruction manual (Invitrogen). For protein production, the cells were infected with a 1:1 ratio of Itk: Lck baculovirus to ensure uniform phosphorylation of Y511 in Itk. The cells were harvested 72 hrs post-infection, rinsed once with phosphate buffered saline (PBS) and stored at −80°C.
Protein purification
Bacterially expressed proteins were purified as described earlier8. Sf9 protein purification was carried as previously described with minor modifications37. Briefly, the cell pellets were resuspended in lysis buffer (50 mM Tris pH 8.0, 500 mM NaCl, 2 mM EDTA, 1 mM PMSF) and lysed by dounce homogenization. The homogenate was spun at 16K for 1hr at 4°C. Glycerol was added to the supernatant to a final volume of 10%, and then incubated with anti-FLAG M2 affinity resin (Sigma) for 5hrs at 4°C. The resin was rinsed five times in wash buffer (50 mM Tris pH 8.0, 500 mM NaCl, 1 mM PMSF, 10% glycerol), and the protein was eluted in elution buffer (wash buffer with 200 μg/ml FLAG peptide) and stored at −80°C. The purified protein was quantified by measuring absorbance at 280 nM. All proteins were greater than 95% pure by Coomassie staining.
Kinase assay
Kinase assays were carried out as described previously18. Km determinations for Peptide B [(Aminohexanoyl biotin-EQEDEPEGIYGVLF-NH2) (Anaspec Inc.)] or ATP were carried out by incubating purified enzyme in reaction buffer (50 mM Hepes pH 7.0, 10 mM MgCl2, 1 mM DTT, 1 mg/ml BSA, and 1 mM Pefabloc) and 5 μCi [32P] ATP (Amersham Biosciences) at room temperature. Peptide B concentration was varied between 0 and 400 μM. ATP concentration was varied between 0 and 320 μM. The enzyme concentration used was 250nM. Each assay was done in duplicate. The data obtained was fit onto the Michealis-Menten equation using GraphFit 5 software, and the kinetic parameters were obtained.
In vitro kinase assay and western blotting
Full-length Itk WT or Itk 32Kinase domain WT fragment was incubated in a 1:1 ratio with various kinase inactive (K390R) deletion fragments in kinase assay buffer (50 mM Hepes pH 7.0, 10 mM MgCl2, 1 mM DTT, 1 mg/ml BSA, 1 mM Pefabloc and 200 μM ATP) for one hour at RT. The samples were boiled, separated by SDS-PAGE and transferred onto a PVDF membrane. The membranes were then blotted with either an anti-pY223 Btk antibody, or anti-pY551 Btk antibody or anti-FLAG antibody (Sigma) and developed using a chemiluminescent substrate (Pierce).
NMR Titrations
NMR Spectra were acquired using a Bruker DRX500 spectrometer operating at 1H frequency of 499.867 MHz. All spectra were obtained at 298K. 1H and 15N chemical shifts were externally referenced to DSS in identical buffer. NMR titrations were carried out as described previously9; 10; 22. Briefly, all protein samples were prepared in 50 mM sodium phosphate, 75 mM NaCl, 2 mM DTT, 0.02% NaN3 (pH 7.2). Unlabeled protein or peptide ligand was added stepwise to 15N labeled protein and each step was monitored by acquiring a 2D 1H-15N HSQC spectrum38. The changes in 1H and 15N chemical shifts were quantified using the formula39: Δδave = {[½[(ΔδH)2 + (0.2ΔδN)2]}1/2. Residues with chemical shift changes above the mean plus one standard deviation were considered significant. The dissociation constants were derived from binding curves generated using the Matlab (version 5.3.1, The Mathworks Inc.) suite of programs by plotting Δδave versus ligand concentration.
Acknowledgments
The authors would like to thank Dr. Leslie Berg for kindly providing the Lck baculovirus. We would also like to thank Dr. Owen Witte for providing the phospho-specific (pY223 and pY551) Btk antibodies. This work is supported by a grant from the National Institutes of Health (National Institute of Allergy and Infectious Diseases, AI43957) to A.H.A.
ABBREVIATIONS
- Itk
Interleukin-2 tyrosine kinase
- Btk
Bruton’s tyrosine kinase
- SH2
Src homology 2
- SH3
Src homology 3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shan X, Wange RL. Itk/Emt/Tsk activation in response to CD3 cross-linking in Jurkat T cells requires ZAP-70 and Lat and is independent of membrane recruitment. J Biol Chem. 1999;274:29323–30. doi: 10.1074/jbc.274.41.29323. [DOI] [PubMed] [Google Scholar]
- 2.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 3.Heyeck SD, Wilcox HM, Bunnell SC, Berg LJ. Lck phosphorylates the activation loop tyrosine of the Itk kinase domain and activates Itk kinase activity. J Biol Chem. 1997;272:25401–8. doi: 10.1074/jbc.272.40.25401. [DOI] [PubMed] [Google Scholar]
- 4.Wilcox HM, Berg LJ. Itk phosphorylation sites are required for functional activity in primary T cells. J Biol Chem. 2003;278:37112–21. doi: 10.1074/jbc.M304811200. [DOI] [PubMed] [Google Scholar]
- 5.Liu KQ, Bunnell SC, Gurniak CB, Berg LJ. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J Exp Med. 1998;187:1721–7. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller AT, Berg LJ. New insights into the regulation and functions of Tec family tyrosine kinases in the immune system. Curr Opin Immunol. 2002;14:331–40. doi: 10.1016/s0952-7915(02)00345-x. [DOI] [PubMed] [Google Scholar]
- 7.Miller AT, Berg LJ. Defective Fas ligand expression and activation-induced cell death in the absence of IL-2-inducible T cell kinase. J Immunol. 2002;168:2163–72. doi: 10.4049/jimmunol.168.5.2163. [DOI] [PubMed] [Google Scholar]
- 8.Brazin KN, Fulton DB, Andreotti AH. A specific intermolecular association between the regulatory domains of a Tec family kinase. J Mol Biol. 2000;302:607–23. doi: 10.1006/jmbi.2000.4091. [DOI] [PubMed] [Google Scholar]
- 9.Laederach A, Cradic KW, Brazin KN, Zamoon J, Fulton DB, Huang XY, Andreotti AH. Competing modes of self-association in the regulatory domains of Bruton’s tyrosine kinase: intramolecular contact versus asymmetric homodimerization. Protein Sci. 2002;11:36–45. doi: 10.1110/ps.26702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laederach A, Cradic KW, Fulton DB, Andreotti AH. Determinants of intra versus intermolecular self-association within the regulatory domains of Rlk and Itk. J Mol Biol. 2003;329:1011–20. doi: 10.1016/s0022-2836(03)00531-x. [DOI] [PubMed] [Google Scholar]
- 11.Pursglove SE, Mulhern TD, Mackay JP, Hinds MG, Booker GW. The solution structure and intramolecular associations of the Tec kinase SRC homology 3 domain. J Biol Chem. 2002;277:755–62. doi: 10.1074/jbc.M108318200. [DOI] [PubMed] [Google Scholar]
- 12.Hansson H, Okoh MP, Smith CI, Vihinen M, Hard T. Intermolecular interactions between the SH3 domain and the proline-rich TH region of Bruton’s tyrosine kinase. FEBS Lett. 2001;489:67–70. doi: 10.1016/s0014-5793(00)02438-8. [DOI] [PubMed] [Google Scholar]
- 13.Qi Q, Sahu N, August A. Tec kinase Itk forms membrane clusters specifically in the vicinity of recruiting receptors. J Biol Chem. 2006;281:38529–34. doi: 10.1074/jbc.M609180200. [DOI] [PubMed] [Google Scholar]
- 14.Kurosaki T, Kurosaki M. Transphosphorylation of Bruton’s tyrosine kinase on tyrosine 551 is critical for B cell antigen receptor function. J Biol Chem. 1997;272:15595–8. doi: 10.1074/jbc.272.25.15595. [DOI] [PubMed] [Google Scholar]
- 15.Yang W, Desiderio S. BAP-135, a target for Bruton’s tyrosine kinase in response to B cell receptor engagement. Proc Natl Acad Sci U S A. 1997;94:604–9. doi: 10.1073/pnas.94.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park H, Wahl MI, Afar DE, Turck CW, Rawlings DJ, Tam C, Scharenberg AM, Kinet JP, Witte ON. Regulation of Btk function by a major autophosphorylation site within the SH3 domain. Immunity. 1996;4:515–25. doi: 10.1016/s1074-7613(00)80417-3. [DOI] [PubMed] [Google Scholar]
- 17.Morrogh LM, Hinshelwood S, Costello P, Cory GO, Kinnon C. The SH3 domain of Bruton’s tyrosine kinase displays altered ligand binding properties when auto-phosphorylated in vitro. Eur J Immunol. 1999;29:2269–79. doi: 10.1002/(SICI)1521-4141(199907)29:07<2269::AID-IMMU2269>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Joseph RE, Min L, Andreotti AH. The Linker between SH2 and Kinase Domains Positively Regulates Catalysis of the Tec Family Kinases. Biochemistry. 2007;46:5455–62. doi: 10.1021/bi602512e. [DOI] [PubMed] [Google Scholar]
- 19.Joseph RE, Min L, Xu R, Musselman ED, Andreotti AH. A remote substrate docking mechanism for the tec family tyrosine kinases. Biochemistry. 2007;46:5595–603. doi: 10.1021/bi700127c. [DOI] [PubMed] [Google Scholar]
- 20.Andreotti AH, Bunnell SC, Feng S, Berg LJ, Schreiber SL. Regulatory intramolecular association in a tyrosine kinase of the Tec family. Nature. 1997;385:93–7. doi: 10.1038/385093a0. [DOI] [PubMed] [Google Scholar]
- 21.Hansson H, Mattsson PT, Allard P, Haapaniemi P, Vihinen M, Smith CI, Hard T. Solution structure of the SH3 domain from Bruton’s tyrosine kinase. Biochemistry. 1998;37:2912–24. doi: 10.1021/bi972409f. [DOI] [PubMed] [Google Scholar]
- 22.Breheny PJ, Laederach A, Fulton DB, Andreotti AH. Ligand Specificity Modulated by Prolyl Imide Bond Cis/Trans Isomerization in the Itk SH2 Domain: A Quantitative NMR Study. Journal of the American Chemical Society. 2003;125:15706 – 15707. doi: 10.1021/ja0375380. [DOI] [PubMed] [Google Scholar]
- 23.Charbon G, Breunig KD, Wattiez R, Vandenhaute J, Noel-Georis I. Key role of Ser562/661 in Snf1-dependent regulation of Cat8p in Saccharomyces cerevisiae and Kluyveromyces lactis. Mol Cell Biol. 2004;24:4083–91. doi: 10.1128/MCB.24.10.4083-4091.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kassenbrock CK, Anderson SM. Regulation of ubiquitin protein ligase activity in c-Cbl by phosphorylation-induced conformational change and constitutive activation by tyrosine to glutamate point mutations. J Biol Chem. 2004;279:28017–27. doi: 10.1074/jbc.M404114200. [DOI] [PubMed] [Google Scholar]
- 25.Samejima I, Mackie S, Fantes PA. Multiple modes of activation of the stress-responsive MAP kinase pathway in fission yeast. Embo J. 1997;16:6162–70. doi: 10.1093/emboj/16.20.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seta K, Sadoshima J. Phosphorylation of tyrosine 319 of the angiotensin II type 1 receptor mediates angiotensin II-induced trans-activation of the epidermal growth factor receptor. J Biol Chem. 2003;278:9019–26. doi: 10.1074/jbc.M208017200. [DOI] [PubMed] [Google Scholar]
- 27.Dalgarno DC, Botfield MC, Rickles RJ. SH3 domains and drug design: ligands, structure, and biological function. Biopolymers. 1997;43:383–400. doi: 10.1002/(SICI)1097-0282(1997)43:5<383::AID-BIP4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 28.Mano H. Tec family of protein-tyrosine kinases: an overview of their structure and function. Cytokine Growth Factor Rev. 1999;10:267–80. doi: 10.1016/s1359-6101(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 29.Brown K, Long JM, Vial SC, Dedi N, Dunster NJ, Renwick SB, Tanner AJ, Frantz JD, Fleming MA, Cheetham GM. Crystal structures of interleukin-2 tyrosine kinase and their implications for the design of selective inhibitors. J Biol Chem. 2004;279:18727–18732. doi: 10.1074/jbc.M400031200. [DOI] [PubMed] [Google Scholar]
- 30.Mallis RJ, Brazin KN, Fulton DB, Andreotti AH. Structural characterization of a proline-driven conformational switch within the Itk SH2 domain. Nat Struct Biol. 2002;9:900–5. doi: 10.1038/nsb864. [DOI] [PubMed] [Google Scholar]
- 31.Hubbard SR. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. Embo J. 1997;16:5572–81. doi: 10.1093/emboj/16.18.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogawa A, Takayama Y, Sakai H, Chong KT, Takeuchi S, Nakagawa A, Nada S, Okada M, Tsukihara T. Structure of the carboxyl-terminal Src kinase, Csk. J Biol Chem. 2002;277:14351–4. doi: 10.1074/jbc.C200086200. [DOI] [PubMed] [Google Scholar]
- 33.Marquez JA, Smith CI, Petoukhov MV, Lo Surdo P, Mattsson PT, Knekt M, Westlund A, Scheffzek K, Saraste M, Svergun DI. Conformation of full-length Bruton tyrosine kinase (Btk) from synchrotron X-ray solution scattering. Embo J. 2003;22:4616–24. doi: 10.1093/emboj/cdg448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu W, Harrison SC, Eck MJ. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- 35.Nagar B, Hantschel O, Seeliger M, Davies JM, Weis WI, Superti-Furga G, Kuriyan J. Organization of the SH3-SH2 unit in active and inactive forms of the c-Abl tyrosine kinase. Mol Cell. 2006;21:787–98. doi: 10.1016/j.molcel.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 36.Bunnell SC, Diehn M, Yaffe MB, Findell PR, Cantley LC, Berg LJ. Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J Biol Chem. 2000;275:2219–30. doi: 10.1074/jbc.275.3.2219. [DOI] [PubMed] [Google Scholar]
- 37.Hawkins J, Marcy A. Characterization of Itk tyrosine kinase: contribution of noncatalytic domains to enzymatic activity. Protein Expr Purif. 2001;22:211–9. doi: 10.1006/prep.2001.1447. [DOI] [PubMed] [Google Scholar]
- 38.Mori S, Abeygunawardana C, Johnson MO, van Zijl PC. Improved sensitivity of HSQC spectra of exchanging protons at short interscan delays using a new fast HSQC (FHSQC) detection scheme that avoids water saturation. J Magn Reson B. 1995;108:94–8. doi: 10.1006/jmrb.1995.1109. [DOI] [PubMed] [Google Scholar]
- 39.Chang YG, Song AX, Gao YG, Shi YH, Lin XJ, Cao XT, Lin DH, Hu HY. Solution structure of the ubiquitin-associated domain of human BMSC-UbP and its complex with ubiquitin. Protein Sci. 2006;15:1248–59. doi: 10.1110/ps.051995006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeLano WL. The PyMOL Molecular Graphics System. DeLano Scientific; San Carlos, CA, USA: 2002. http://www.pymol.org. [Google Scholar]