Abstract
Investigations of the neural pathways associated with responses to predators have implicated the medial amygdala (MeA) as an important region involved in defensive behaviors. To our knowledge, however, the involvement of the MeA in neuroendocrine responses to predator odor exposure has not been investigated. Therefore, the present study examined the effects of MeA disruption in rats exposed to ferret or control odor on hypothalamo-pituitary-adrenocortical (HPA) axis activation. Bilateral lesions of the MeA were made in Sprague- Dawley rats with the neurotoxin ibotenic acid (10 µg/µl; 0.3 µl /side). As a control for regional specificity, additional groups of rats were given lesions in the central amygdala (CeA). One week after recovery, the rats were exposed to ferret or strawberry control towels in small cages to examine HPA axis responses as determined by plasma corticosterone and adrenocorticotropin hormone (ACTH) levels. Rats with complete bilateral MeA but not CeA lesions displayed significantly less corticosterone and ACTH release compared to sham-operated control rats only in the ferret odor conditions. These results suggest that the MeA is an important structure involved in the HPA axis responses to predator odors, in support of previous studies investigating behavioral responses under similar conditions.
Keywords: ACTH, central amygdala, corticosterone, medial amygdala, predator odor, stress
1. Introduction
Predators, and more specifically predator odors, provide a unique opportunity to study the neural circuitry of unconditioned stress responses. Predators and their odors elicit increases in the stress-related hormones, corticosterone and adrenocorticotropin hormone (ACTH), defensive behavioral responses, and autonomic nervous system activation (Blanchard et al., 1998, Day et al., 2004, Dielenberg et al., 1999, Dielenberg et al., 2001, Dielenberg et al., 2004, Dielenberg and McGregor, 2001, Dielenberg and McGregor, 1999, File et al., 1993, Masini et al., 2005, Masini et al., 2006, Masini et al, 2006, Masini, Srinidhi, et al., 2006, McGregor et al., 2002, Perrot-Sinal et al., 1999) when presented to laboratory rats. Predator odors offer the ability to study the multidimensional nature of stress responses in a relatively simple model that has the advantages of involving one sensory modality, no learning, and no physical pain to the organism.
Research on the neuronal circuitry of the predator-evoked stress response in recent years has mainly been focused on defensive behavioral responses. The most common behaviors examined are immobility or freezing behavior and risk assessment postures. Research has focused on areas in the brain that are considered part of the accessory olfactory bulb projection system. These areas include nuclei of the amygdala (especially the medial amygdala; MeA), hippocampus, bed nucleus of the stria terminalis (BNST), dorsal premammillary nucleus (PMd), and the periaqueductal gray (PAG). Lesions of the medial amygdala, basolateral amygdala, PMd, and ventral hippocampus decreased freezing behavior in rats exposed to cat odor (Blanchard et al., 2005; Blanchard et al., 2003; Li et al., 2004, Pentkowski et al., 2006, Takahashi et al, 2007, Takahashi et al., 2005, Vazdarjanova et al., 2001). Lesions of the PMd, hippocampus, medial amygdala, and ventrolateral PAG also decreased defensive behavior in response to a live cat exposure (Blanchard et al., 1972, Blanchard et al., 2005, Blanchard et al., 2003, Canteras et al., 1997, Cezario et al., 2008, Farook et al., 2003). Temporary inactivation of the BNST, medial and basolateral amygdala have been shown to decrease freezing to TMT, a predator odor that is a component of fox feces (Fendt et al., 2003, Fendt et al., 2005, Muller et al., 2006).
Whereas the majority of investigations have focused on the brain regions involved in the behavioral defensive responses of a prey to a predator or predator odor, little attention has been given to the regions that mediate HPA axis activation upon exposure to predators or their associated cues (but see Kobayakawa et al., 2007). Several studies have suggested that both the long-term and short-term effects of predator exposure have similarities to post-traumatic stress disorder (PTSD) in humans (Adamec, 1997; Adamec et al.,1998; Adamec et al., 2007). Predator stress may model aspects of PTSD including disturbances in HPA axis function found in humans (see reviews: De Kloet et al., 2006; Meewisse et al., 2007). Activation of the HPA axis leads to the release of ACTH and its downstream target, corticosterone, in rats. These hormones are routinely used as a primary indicator of an acute stress response (Endroczi, 1983, Levine, 2000, Selye, 1956). Plasma corticosterone and ACTH levels are elevated after exposures to predators and their odors (Blanchard et al, 1998, Day et al., 2004, Masini et al., 2005, Perrot-Sinal et al., 1999, Roseboom et al., 2007), but the neural circuitry implicated in these responses has not been assessed in rats. The present study examined these neuroendocrine responses to predator odor.
The medial amygdala has been implicated in both the behavioral and neuroendocrine responses to psychological stressors in general (Dayas et al., 1999, Feldman et al., 1994). And more specifically, it has been implicated in responses to predators and their odors. The dorsal division of the MeA has been posited to be related to neuroendocrine and autonomic functions, while the ventral division has been associated with reproductive and agonistic behaviors (Canteras et al., 1995, Dielenberg et al., 2001, Halpern, 1987, Martinez-Marcos et al., 1999, Risold et al., 1997). The MeA receives projections from both the accessory and main olfactory bulbs (Canteras et al., 1995, Coolen et al., 1998, Gomez et al., 1992, Martinez-Marcos et al., 1999, Meredith, 1998, Pro-Sistiaga et al., 2007) and therefore may play roles in predator odor-evoked responses. The MeA has dense projections to the hippocampus, BNST, anteroventral periventricular nucleus of the hypothalamus, medial preoptic nucleus, ventromedial nucleus of the hypothalamus, and the ventral premammillary nucleus (Canteras et al., 1995). Because many of these regions also project to the paraventricular nucleus of the hypothalamus, the MeA is in an excellent position to be a relay for HPA axis activation by predator odor.
In laboratory settings, both live ferrets and ferret skin/fur odor are effective at eliciting predator-prey responses, increasing plasma corticosterone and ACTH and eliciting behavioral changes in rats that are indicative of a state of fear (Anisman et al., 1997, Masini et al., 2005, Masini et al., 2006, McIntyre et al., 1999, Pro-Sistiaga et al., 2007, Roseboom et al., 2007). In our laboratory we have demonstrated that the fur/skin odor from a ferret is a highly potent stimulus that has both acute and long-lasting effects on behavior, HPA axis, and autonomic responses (see review Campeau et al., 2008). MeA lesions disrupt defensive behavioral responses to acute ferret odor exposure (Campeau et al., 2008). The current study, therefore, examined neuroendocrine (corticosterone and ACTH) responses to acute ferret odor exposure following neurotoxic lesions of the MeA. In addition, the effect of lesioning the CeA on the neuroendocrine responses to ferret odor was determined in view of its prior associations with learned fear responses, but its apparent lack of association with predator odor-evoked defensive responses. Some of these data have been presented in abstract form (Masini et al., 2007) and preliminary medial amygdala lesion data shown in review form (Campeau et al., 2008).
2. Results
A total of 15 out of 30 MeA and 13 out of 30 CeA lesions attempted were assessed as complete and selective/specific lesions and used in the data analysis. Typically, lesions were excluded because they were too large or only unilaterally specific. Good MeA lesions were specific to anterior and posterior portions of the MeA and did not encroach on the basolateral amygdala (BLA) or CeA. Good CeA lesions did not encroach the MeA and had only minimal BLA or lateral amygdala encroachment. See Figure 1 for examples of complete lesions and Figure 2 and Figure 3 for representations of large and small lesions at levels of the Paxinos and Watson’s The Rat Brain In Stereotaxic Coordinates 5th Edition. Data (means +/− SEM) from the excluded lesions are displayed in Table 1 (missed MeA lesions) and Table 2 (missed CeA lesions). Means (+/−SEMs) from missed MeA lesions that were considered “small” lesions show that the increased hormone responses (corticosterone and ACTH) to ferret odor were not prevented with small lesions that do not completely destroy the MeA. However, the one subject that was found to sustain mostly a unilateral MeA lesion and was exposed to ferret odor displayed a plasma corticosterone response that was similar to the six subjects with unilateral MeA lesions that were exposed to strawberry odor, suggesting a prevention of the normal increased ferret odor response. Means (+/−SEM) from missed CeA lesions that were considered “unilateral” and “small” suggest that the increased hormone responses (corticosterone and ACTH) to ferret odor that are normally found were not affected by these missed lesions. But “large” lesions that also infringed upon the basolateral, lateral, and some medial amygdala led to decreased corticosterone and ACTH responses to ferret odor, suggesting that either these areas may also play a role in the ferret odor neuroendocrine responses, or that there was enough medial amygdala damage to induce a deficit.
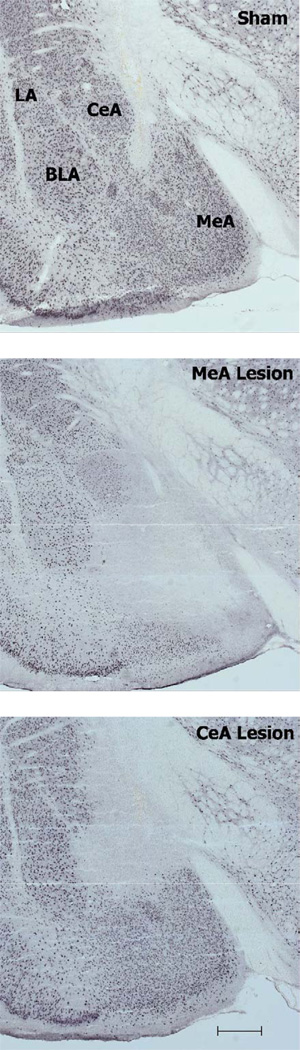
Figure 1.
Examples of NeuN immunohistochemistry verification of sham and complete MeA and CeA lesions. MeA = medial amygdala, CeA = central amygdala, LA = lateral amygdala, BLA = basolateral amygdala. Scale bar: 500 µm.
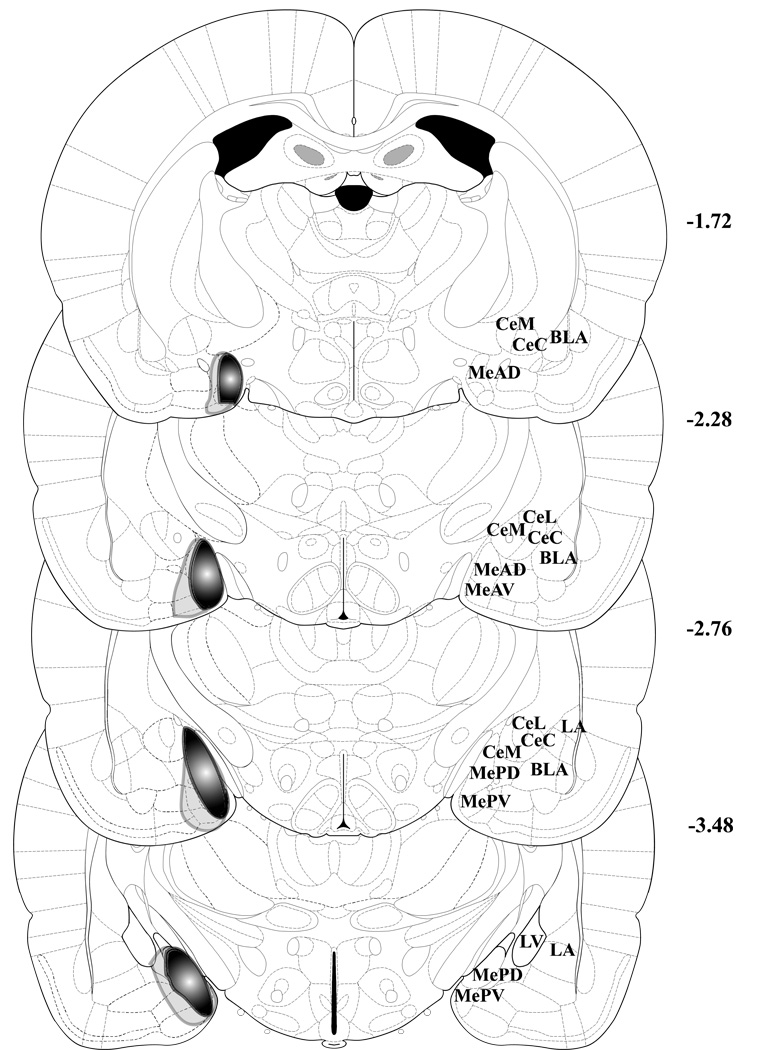
Figure 2.
Depiction of the largest (light gray opaque outline) and smallest (darker filled outline) MeA lesions. Though actual lesions were bilateral, the lesions are shown unilaterally to show anatomy on the other side. Figures 47 (bregma –1.72 mm), 52 (bregma –2.28 mm), 56 (bregma –2.76), and 62 (bregma –3.48) from Paxinos and Watson’s The Rat Brain In Stereotaxic Coordinates, 5th Edition are shown to depict the extent of the MeA lesions. MeAD = anterodorsal MeA, MeAV = anteroventral MeA, MePD = posterodorsal MeA, MePV = posteroventral MeA, CeM = medial CeA, CeC = capsular part of CeA, CeL = lateral CeA, BLA = basolateral amygdala, LA = lateral amygdala, LV = lateral ventricle.
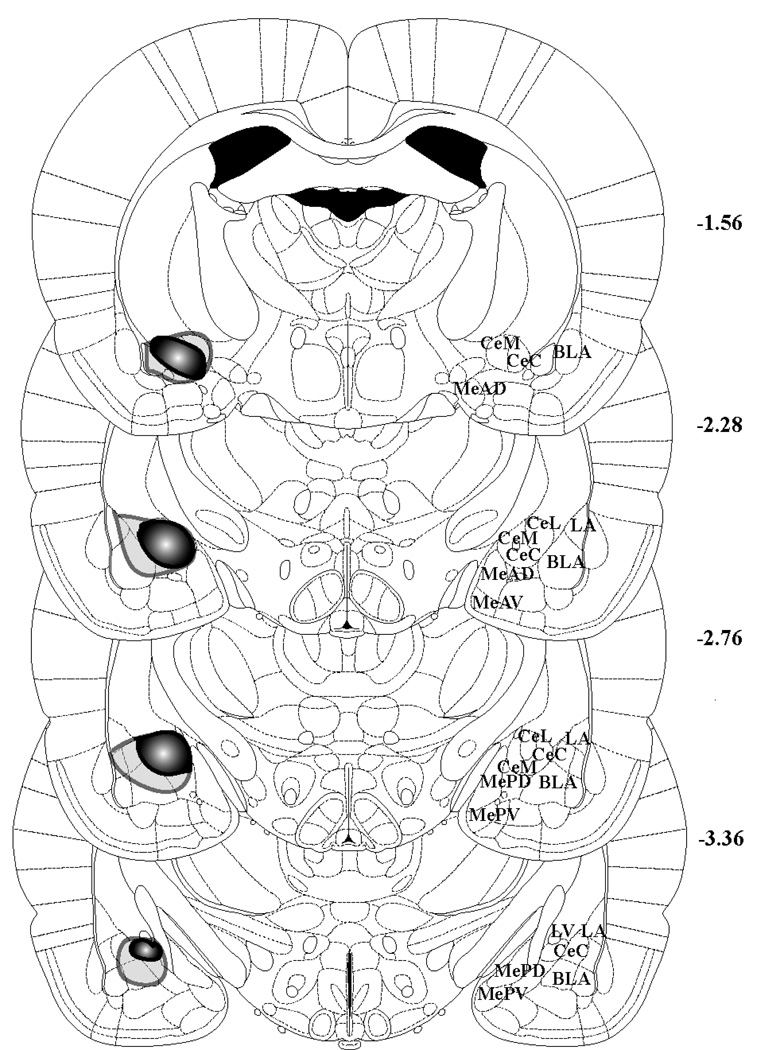
Figure 3.
Depiction of the largest (light gray opaque outline) and smallest (darker filled outline) CeA lesions. Though actual lesions were bilateral, the lesions are shown unilaterally to show anatomy on the other side. Figures 46 (bregma –1.56 mm), 52 (bregma –2.28 mm), 56 (bregma –2.76), and 61 (bregma –3.36) from Paxinos and Watson’s The Rat Brain In Stereotaxic Coordinates, 5th Edition are shown to depict the extent of the CeA lesions. MeAD = anterodorsal MeA, MeAV = anteroventral MeA, MePD = posterodorsal MeA, MePV = posteroventral MeA, CeM = medial CeA, CeC = capsular part of CeA, CeL = lateral CeA, BLA = basolateral amygdala, LA = lateral amygdala, LV = lateral ventricle.
Table 1. Missed MeA lesion data.
Mean plasma corticosterone (CORT) and ACTH (+/− SEMs) responses of MeA-lesioned rats exposed to strawberry or ferret odor for 30 min in small cages and later evaluated as unilateral or small “missed” lesions.
| Unilateral | Small | ||||
|---|---|---|---|---|---|
| CORT: | Strawberry | 3.298 (+/−1.146) | n=6 | 3.376 (+/−2.224) | n=4 |
| Ferret | 2.699 | n=1 | 22.990 (+/−4.046) | n=4 | |
| ACTH: | Strawberry | 29.479 (+/−9.503) | n=6 | 31.748 (+/−10.540) | n=4 |
| Ferret | 58.565 | n=1 | 163.021 (+/−56.917) | n=4 | |
Table 2. Missed CeA lesion data.
Mean plasma corticosterone (CORT) and ACTH (+/− SEMs) responses of CeA-lesioned rats exposed to strawberry or ferret odor for 30 min in small cages and later evaluated as unilateral, small, or large “missed” lesions.
| Unilateral | Small | Large | |||||
|---|---|---|---|---|---|---|---|
| CORT: | Strawberry | 0.988 (+/−0.497) | n=3 | 2.970 (+/−2.970) | n=2 | 0.662 (+/−0.406) | n=2 |
| Ferret | 11.058 (+/−3.149) | n=5 | 15.462 (+/−0.519) | n=2 | 4.699 (+/−1.127) | n=3 | |
| ACTH: | Strawberry | 10.263 (+/−5.958) | n=3 | 32.140 (+/−3.085) | n=2 | 52.001 (+/−11.170) | n=2 |
| Ferret | 144.047 (+/−47.866) | n=5 | 275.642 (+/−37.774) | n=2 | 81.108 (+/−8.789) | n=3 | |
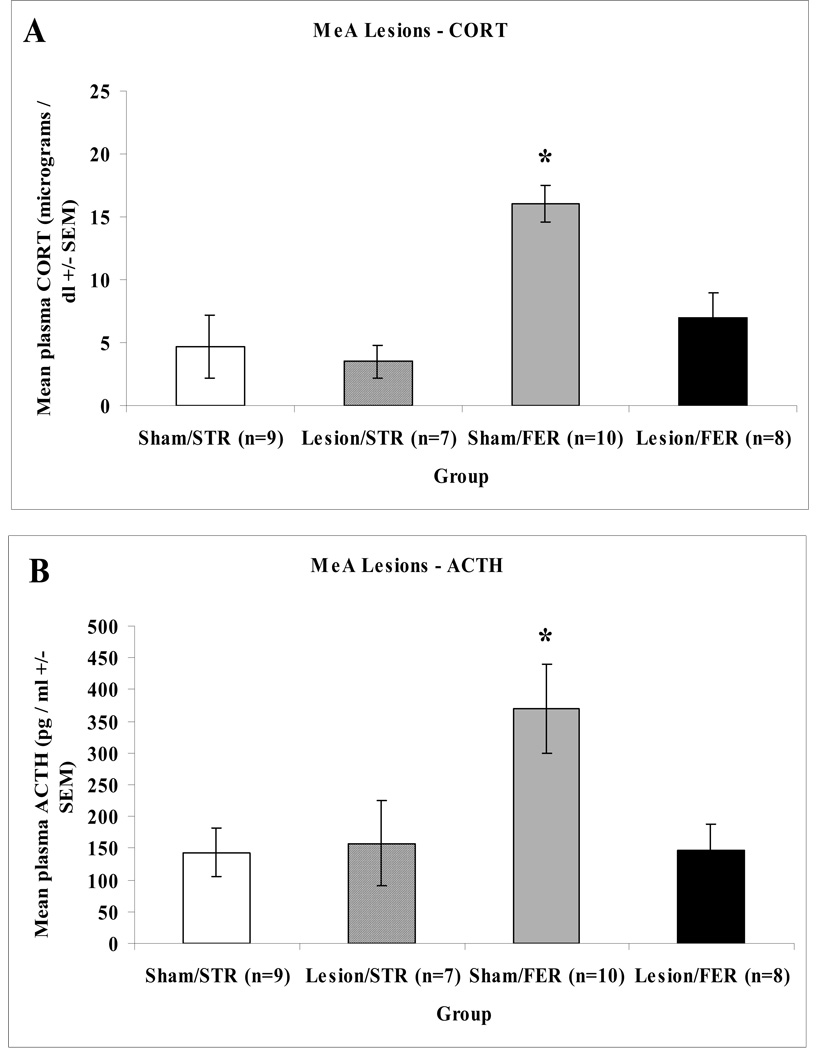
Plasma corticosterone and ACTH responses to ferret or strawberry towels were assessed in a small cage exposure paradigm. For complete bilateral MeA lesions or sham-operated control rats, an overall ANOVA on the corticosterone data revealed significant main effects of surgery: F(1,30) = 7.056, p = 0.013, odor: F(1,30) = 14.743, p = 0.001, and interaction, F(1,30) = 4.162, p = 0.050. An ANOVA on the ACTH data uncovered significant main effects of surgery: F(1,30) = 4.210, p = 0.049, and odor: F(1,30) = 4.473, p = 0.043, but no interaction, F(1,30) = 3.074, p = 0.090. Post-hoc analyses showed that MeA lesioned rats had significantly lower corticosterone and ACTH responses to ferret odor, as compared to sham-operated animals (as shown in Figure 4).
Figure 4.
Mean hormonal responses of sham-operated control or MeA-lesioned rats exposed to strawberry (STR) or ferret (FER) odor for 30 min in small cages. Panel A depicts mean corticosterone (+/−SEM) responses in sham-operated control (Sham) or MeA lesioned (Lesion) rats. Panel B depicts mean ACTH (+/−SEM) responses in sham-operated control (Sham) or MeA lesioned (Lesion) rats. Asterisks indicate a significant difference from the Lesion/FER group (p < 0.05).
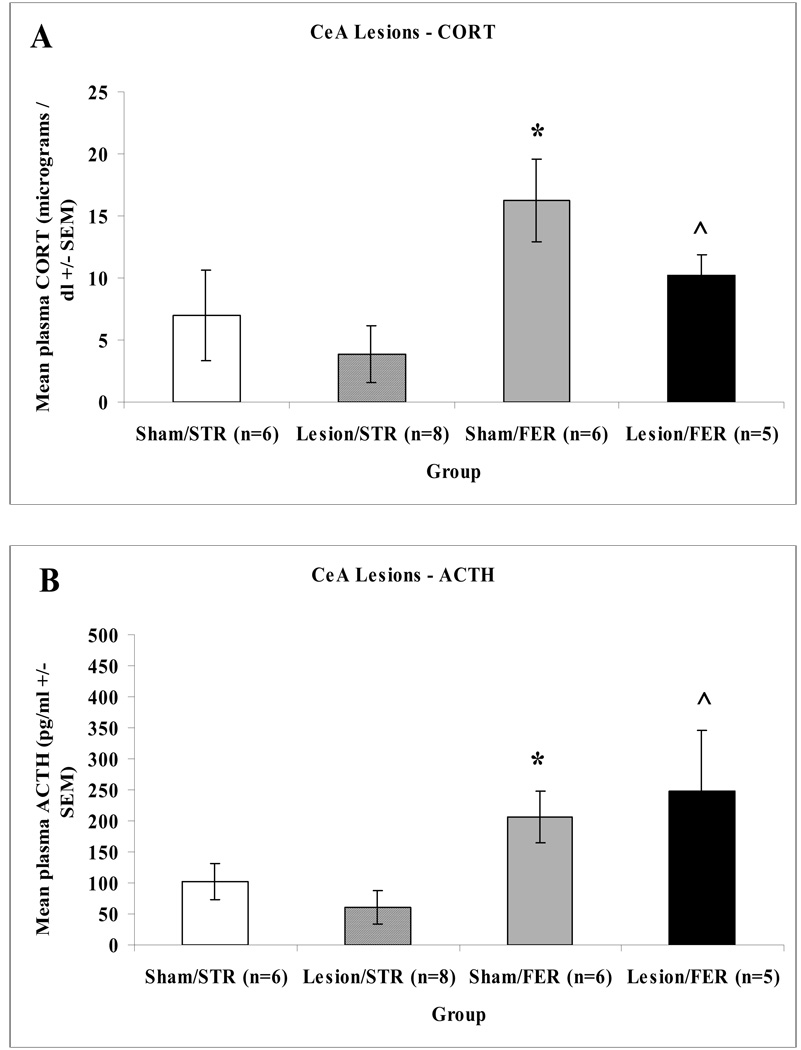
An overall ANOVA on plasma corticosterone and ACTH responses following CeA lesions uncovered a significant main effect of odor: F(1,20) = 6.365, p = 0.020 and F(1,20) = 8.802, p = 0.007, but not surgery, F(1,20) = 2.097, p = 0.163 and F(1,20) = 0.001, p = 0.997, or interaction effect, F(1,20) = 0.319, p = 0.578 and F(1,20) = 0.732, p = 0.402 for corticosterone and ACTH data, respectively. As shown in Figure 5 no significant differences between complete bilateral CeA lesioned and sham-operated control rats exposed to ferret odor for either plasma corticosterone or ACTH responses were found.
Figure 5.
Mean hormonal responses of sham-operated control or CeA-lesioned rats exposed to strawberry (STR) or ferret (FER) odor for 30 min in small cages. Panel A depicts mean corticosterone (+/−SEM) responses in sham-operated control (Sham) or CeA lesioned (Lesion) rats. Panel B depicts mean ACTH (+/−SEM) responses in sham-operated control (Sham) or CeA lesioned (Lesion) rats. Asterisks indicate a significant difference from the Sham/STR group (p < 0.05). ^ indicates a significant difference from the Lesion/STR group (p < 0.05).
3. Discussion
This study examined the role of the medial amygdala (MeA) in the neuroendocrine stress responses induced by ferret odor exposure in male rats. Neuroendocrine stress responses were assessed following exposure of rats to ferret or strawberry odor on towels hung in small cages. Plasma corticosterone and ACTH were examined as indices of a stress response. Sham operated rats displayed low levels of corticosterone and ACTH in response to strawberry odor towel exposures. In response to ferret odor towels, the sham operated control rats exhibited elevated levels of both hormones, as has been previously found (Campeau et al., 2008, Masini et al., 2005, Masini et al., 2006). Both MeA and CeA lesions led to similar low levels of plasma corticosterone and ACTH, as did the sham rats, when exposed to strawberry odor for 30 minutes. MeA lesioned rats exposed to ferret odor had significantly attenuated levels of both corticosterone and ACTH compared to sham-operated control rats. In contrast, CeA lesioned rats exhibited elevated levels of these hormones when exposed to ferret odor compared to strawberry odor, suggesting these lesions do not prevent the predator odor-evoked neuroendocrine responses. It is possible CeA lesioned rats also had damage to the stria terminalis fiber tract but damage to this bundle was not specifically evaluated given that the CeA lesion and sham-operated control animals did not exhibit difference in their neuroendocrine responses.
Prior reports have suggested that there may be a regional dissociation between the behavioral and endocrine responses to predator odor exposure. For example, File and colleagues (1993) found that rats repeatedly exposed to cat odor continued to behaviorally avoid the odor stimulus after five presentations while the corticosterone response declined. They suggested that the behavioral and endocrine responses reflect two separate components of a phobic or fear response (File et al., 1993). In addition, Perrot-Sinal and colleagues (1999) concluded that predator odor activates the HPA axis independently from behavior after finding significant corticosterone and ACTH responses to weasel odor exposure but no locomotor changes (Pro-Sistiaga et al., 2007). And, recent evidence in our laboratory suggests that the HPA axis responses to ferret odor may habituate after repeated presentations, unlike the behavioral responses, which remained stable across repeated exposures (Campeau et al., 2008). It should be noted that most of the evidence supporting distinct pathways for different responses was obtained from repeated predator odor exposure studies, and therefore may indicate differential susceptibility of these distinct responses to adaptation (i.e. habituation and /or sensitization) rather than regionally distinct pathways. With these studies in mind, we had originally hypothesized that bilateral MeA lesions would not block HPA axis responses, since there are no direct projections from the MeA to the paraventricular hypothalamus (Canteras et al., 1995). The present results however, do not support this hypothesis; MeA lesions specifically eliminated the HPA axis responses to predator odors.
Medial amygdala lesions have been found to have disruptive effects on HPA axis responses to stressors that are psychological / emotional categorically. Some studies indicated that MeA lesions reduced the corticosterone and ACTH responses to noise and light stressors but had no effect on responses to ether stress, which is considered a systemic stressor (Feldman et al., 1994). Neuroendocrine responses to restraint stress, another stressor that is considered to be psychological, were also reduced by MeA but not CeA lesions (Dayas et al., 1999). In light of these previous findings, it is not surprising that MeA but not CeA lesions disrupted the corticosterone and ACTH responses to predator odor, which is also categorically psychological. The possibility that predator odors may be mediated unilaterally was suggested from the finding that a rat with unilateral MeA damage also had a reduced hormonal response to ferret odor; this possibility could be explored further in additional animals.
Together with prior results indicating reductions in defensive behaviors to predator odors following medial amygdala lesions (Blanchard et al., 2005, Campeau et al., 2008, Takahashi et al., 2007), the present results indicate that the MeA also mediates the neuroendocrine responses to a predator odor. The MeA therefore appears to integrate information, from predators and their odors, and additional situations (Dayas et al., 1999), and controls multidimensional responses. It is conceivable that downstream outputs from the MeA (Canteras et al., 1995) control specific responses to predators and other stressors. Using predator odor exposure as a model may lead to a better understanding of the neural circuitry involved in multidimensional and integrated stress responses.
4. Experimental Procedure
4.1. Subjects
Ninety Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 200 – 250 g at the time of arrival to the colony were used. They were group-housed (4/cage) in a room kept on a controlled light-dark cycle (lights on 7:00 a.m. and off 7:00 p.m.) under constant humidity and temperature conditions. The rats were acclimated to the animal colony for a period of 7 days after arrival from the supplier before any experimental manipulation. Rats were provided with food (rat chow) and water ad libitum. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Colorado and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
4.2. Surgeries
Rats were anesthetized with halothane and placed in a Kopf stereotaxic apparatus. The skin overlying the skull was disinfected (Betadine), an incision made, and small burr holes drilled through the skull bone to allow penetration of the injector (Hamilton 1 µl syringe). Bilateral excitotoxic lesions were produced by injections of 0.3 µl (for MeA lesions) or 0.1 µl (for CeA lesions) of ibotenic acid (10 µg/µl in 0.1 M sodium phosphate buffer, pH: 7.4; Tocris Bioscience, Ellisville, MO). The injector was lowered in the brain and left in place 5 min before and 5 min after each injection. The flat skull coordinate system of Paxinos and Watson (2005) was used to determine coordinates for MeA lesions: AP −3.0, ML +/− 3.3, DV −8.3 and CeA lesions: AP −2.9, ML +/− 3.9, DV −8.2. The rate of infusion was 0.05 µl/min. Following the injections, the scalp incision was closed with surgical stainless steel wound clips, and rats kept warm until recovery from anesthesia. The same procedure was followed for sham-operated rats except that only the vehicle solution (0.1 M sodium phosphate buffer) was injected. All rats were kept under close observation for any indications of weight loss or other debilitating signs post-surgery and allowed 7 days to recover before testing.
4.3. Odor Stimuli
Ferret odor was collected by placing a small hand towel in a cage with breeding adult ferrets for approximately 1 month. The towel was cut into 5 × 5 cm squares and kept in an −80° C freezer until use. The towels were then thawed in a glass bell jar for 30 min before use. Strawberry odor was used as a novel control odor (Masini et al., 2005, Masini et al., 2006). Towels were scented with strawberry odor by pipetting 100 µl of strawberry extract (McCormick & Co., Inc., Hunt Valley, MD) onto clean 5 × 5 cm towels.
4.4. Small cage odor exposures
One week after recovery from surgery, the rats were placed in small individual cages (28 × 18 × 14 cm), which were then placed in wood sound-attenuating chambers over night (kept on the same light cycle) to avoid manipulation and transport of the rats immediately prior to odor exposure. The next morning (rats’ light/inactive phase), 2 pieces of towel (ferret or strawberry odor) were carefully placed at each end of the cages without disturbing the rats by hooking the towels to the wire cage lid with paper clips, so the towels hung inside the cage. Immediately following the 30 min towel exposure, the rats were taken to an adjacent room, decapitated, trunk blood collected, and the brains rapidly removed and frozen in −30° C isopentane, and kept in a − 80° C freezer until sectioned.
4.5. Corticosterone ELISA
Blood was collected into ice-chilled tubes containing EDTA (20 mg/ml). Blood samples were then centrifuged at 2000 rpm for 10 min at 4° C, the plasma pipetted into 0.5 ml Ependorf microcentrifuge tubes, and stored at −80° C until assayed. The corticosterone assay was performed according to the manufacturer’s instructions (kit #901-097 – AssayDesigns, Ann Arbor, MI). Levels were then quantified on a BioTek Elx808 microplate reader and calculated against a standard curve generated concurrently.
4.6. ACTH radioimmunoassay
The ACTH assay was performed according to the manufacturer’s instructions (ACTH IRMA Ref27130 - Diasorin, Stillwater, MN). Two hundred µl of plasma was used for this assay, and ACTH values were quantified and calculated against a standard curve generated concurrently. The sensitivity of the assay ranged from 1.5 to 1400 pg/ml.
4.7. NeuN Immunohistochemistry
To determine the areas of neuronal loss after the ibotenic acid lesions, neuronal nuclei (NeuN) immmunoreactivity was examined. Frozen brains were sectioned through the amygdala with a cyrostat (Leica 1850) at a thickness of 35 µm in the coronal plane, and thaw mounted onto microfrost plus glass slides (Fisher, cat #12-550-19). All of the incubations were carried out with gentle agitation at room temperature and the sections were washed in 0.1 M phosphate buffer saline (PBS) between incubations in different solutions. The sections were first fixed in 4% paraformaldehyde for 60 minutes. Sections were washed and incubated in a 0.1 M PBS solution containing 0.3% hydrogen peroxide for 20 minutes. Sections were then blocked for 20 minutes each in avidin and biotin blocking solutions (blocking kit #SP-2001, Vector Laboratories, Burlingame, CA). After a 1-hr incubation in the immunohistochemical diluent (0.1 M PBS containing 0.25% carageenan lambda and 0.5% Triton X-100), sections were incubated in the immunohistochemical diluent containing a mouse anti-NeuN (1:10,000; Chemicon International, Temecula, CA) 40 – 50 hours at 4° C. Sections were then washed and incubated in a solution containing biotinylated anti-mouse raised in horse secondary antibody (1:500) for 2 hours, then washed and incubated for an additional 2 hours in the ABC complex (Vectastain Elite ABC peroxidase kit; Vector Laboratories, Burlingame, CA). Sections were again washed before a peroxidase reaction was performed using the chromagen 3,3’-diaminobenzidene (DAB) enhanced with nickel chloride and hydrogen peroxide. The slides were then dehydrated, coverslipped, and examined blindly for complete neuronal loss in the MeA or CeA.
4.8. Data analysis
Corticosterone and ACTH data were analyzed separately using univariate analyses of variance (ANOVA) with surgery (lesion or sham) and odor (ferret or strawberry) as the between subject factors. Post-hoc comparisons were made using Tukey’s honestly significant difference (HSD) multiple means comparisons (p < 0.05). Medial amygdala and central amygdala lesion data sets were assayed separately and therefore analyzed separately.
Acknowledgements
We would like to thank the Mile High Ferret Club for providing the ferret towels. These studies were supported by a grant from the National Institute of Mental Health, R01 MH065327 (SC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamec R. Transmitter systems involved in neuroplasticity underlying increased anxiety and defense following traumatic stress. Neurosci Biobehav Rev. 1997;21:755–765. doi: 10.1016/s0149-7634(96)00055-3. [DOI] [PubMed] [Google Scholar]
- Adamec R, Kent P, Anisman H, Shallow T, Merali Z. Neural plasticity, neuropeptides and anxiety in animals: implications for understanding and treating affective disorder following traumatic stress in humans. Neurosci Biobehav Rev. 1998;23:301–318. doi: 10.1016/s0149-7634(98)00032-3. [DOI] [PubMed] [Google Scholar]
- Adamec R, Muir C, Grimes M, Pearcey K. Involvement of noradrenergic and corticoid receptors in the consolidation of the lasting anxiogenic effects of predator stress. Behav Brain Res. 2007;179:192–207. doi: 10.1016/j.bbr.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Anisman H, Lu ZW, Song C, Kent P, McIntyre DC, Merali Z. Influence of psychogenic and neurogenic stressors on endocrine and immune activity: differential effects in fast and slow seizing rat strains. Brain Behav Immun. 1997;11:63–74. doi: 10.1006/brbi.1997.0482. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Effects of hippocampal lesions on the rat’s reaction to a cat. J Comp Physiol Psychol. 1972;78:77–82. doi: 10.1037/h0032176. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing Fos expression to cat presentation: Effect on responsivity to a cat,cat odor, and nonpredator threat. Neurosci Biobeh Rev. 2005;29:1243–1253. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Li CI, Hubbard D, Markham CM, Yang M, Takahashi LK, Blanchard RJ. Dorsal premammillary nucleus differentially modulates defensive behaviors induced by different threat stimuli in rats. Neurosci Lett. 2003;345:145–148. doi: 10.1016/s0304-3940(03)00415-4. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Nikulina JN, Sakai RR, McKittrick C, McEwen B, Blanchard DC. Behavioral and endocrine change following chronic predatory stress. Physiol Behav. 1998;63:561–569. doi: 10.1016/s0031-9384(97)00508-8. [DOI] [PubMed] [Google Scholar]
- Campeau S, Nyhuis TJ, Sasse SK, Day HE, Masini CV. Acute and chronic effects of ferret odor exposure in Sprague-Dawley rats. Neuroscience and Biobehav Rev. 2008;32:1277–1286. doi: 10.1016/j.neubiorev.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Chiavegatto S, Valle LE, Swanson LW. Severe reduction of rat defensive behavior to a predator by discrete hypothalamic chemical lesions. Brain Res Bull. 1997;44:297–305. doi: 10.1016/s0361-9230(97)00141-x. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1995;360:213–245. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- Cezario AF, Ribeiro-Barbosa ER, Baldo MVC, Canteras NS. Hypothalamic sites responding to predator threats – the role of the dorsal premammillary nucleus in unconditioned and conditioned antipredatory defensive behavior. Euro J Neurosci. 2008;28:1003–1015. doi: 10.1111/j.1460-9568.2008.06392.x. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Day HEW, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5,-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025:139–151. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor:evidence for involvement of the medial but not the central amygdala. Eur J Neurosci. 1999;11:2312–2322. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- De Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HGM. Assessment of HPA-axis function in posttraumatic stress disorder: Pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res. 2006;40:550–567. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Arnold JC, McGregor IS. Low-dose midazolam attenuates predatory odor avoidance in rats. Pharmacol Biochem Behav. 1999;62:197–201. doi: 10.1016/s0091-3057(98)00064-1. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Carrive P, McGregor IS. The cardiovascular and behavioral response to cat odor in rats: unconditioned and conditioned effects. Brain Res. 2001;897:228–237. doi: 10.1016/s0006-8993(01)02227-2. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Hunt GE, McGregor IS. "When a rat smells a cat": the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neurosci. 2001;104:1085–1097. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Leman S, Carrive P. Effect of dorsal periaqueductal gray lesions on cardiovascular and behavioral responses to cat odor exposures in rats. Behav Brain Res. 2004;153:487–496. doi: 10.1016/j.bbr.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, McGregor IS. Defensive behavior in rats towards predatory odors: a review. Neurosci Biobehav Rev. 2001;25:597–609. doi: 10.1016/s0149-7634(01)00044-6. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, McGregor IS. Habituation of the hiding response to cat odor in rats (Rattus norvegicus) J Comp Psychol. 1999;113:376–387. doi: 10.1037/0735-7036.113.4.376. [DOI] [PubMed] [Google Scholar]
- Endroczi E. Limbic system, pituitary-adrenal axis, and adaptive behavior. In: Selye H, editor. Selye's Guide to Stress Research. Vol. 2. Scarborough, Ontario: Van Nostrand Reinhold Co. Inc.; 1983. pp. 249–270. [Google Scholar]
- Farook JM, Wang Q, Moochhala SM, Zhu ZY, Lee L, Wong PTH. Distinct regions of periaqueductal gray (PAG) are involved in freezing behavior in hooded PVG rats on the cat-freezing test apparatus. Neurosci Lett. 2003;354:139–142. doi: 10.1016/j.neulet.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Feldman S, Conforti N, Itzik A, Weidenfeld J. Differential effect of amygdaloid lesions on CRF-41, ACTH and corticosterone responses following neural stimuli. Brain Res. 1994;658:21–26. doi: 10.1016/s0006-8993(09)90005-1. [DOI] [PubMed] [Google Scholar]
- Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23:23–28. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Siegl S, Steiniger-Brach B. Noradrenaline transmission within the ventral bed nucleus of the stria terminalis is critical for fear behavior induced by trimethylthiazoline, a component of fox odor. J Neurosci. 2005;25:5998–6004. doi: 10.1523/JNEUROSCI.1028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Zangrossi H, Sanders FL, Mabbutt PS. Dissociation between behavioral and corticosterone responses on repeated exposures to cat odor. Physiol Behav. 1993;54:1109–1111. doi: 10.1016/0031-9384(93)90333-b. [DOI] [PubMed] [Google Scholar]
- Gomez DM, Newman SW. Differential projections of the anterior and posterior regions of the medial amygdaloid nucleus in the Syrian hamster. J Comp Neurol. 1992;317:195–218. doi: 10.1002/cne.903170208. [DOI] [PubMed] [Google Scholar]
- Halpern M. The organization and function of the vomeronasal system. Annu Rev Neurosci. 1987;10:325–362. doi: 10.1146/annurev.ne.10.030187.001545. [DOI] [PubMed] [Google Scholar]
- Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, Mori K, Sakano H. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–510. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- Levine S. Influence of psychological variables on the activity of the hypothalamicpituitary-adrenal axis. Eur J Pharmacol. 2000;405:149–160. doi: 10.1016/s0014-2999(00)00548-3. [DOI] [PubMed] [Google Scholar]
- Li C, Maglinao TL, Takahashi LK. Medial amygdala modulation of predator odorinduced unconditioned fear in the rat. Behav Neurosci. 2004;118:324–332. doi: 10.1037/0735-7044.118.2.324. [DOI] [PubMed] [Google Scholar]
- Martinez-Marcos A, Halpern M. Differential projections from the anterior and posterior divisions of the accessory olfactory bulb to the medial amygdala in the opossum, Monodelphis domestica. Eur J Neurosci. 1999;11:3789–3799. doi: 10.1046/j.1460-9568.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- Masini CV, Nyhuis TJ, Day HEW, Campeau S. Program No. 732.10. 2007 Abstract Viewer/Itinerary Planner. San Diego, CA: Society for Neuroscience; 2007. Ibotenic acid lesions of the medial amygdala reduce corticosterone and behavioral responses to acute ferret odor exposures in rats. Online. [Google Scholar]
- Masini CV, Sauer S, Campeau S. Ferret odor as a processive stress model in rats:neurochemical, behavioral, and endocrine evidence. Behav Neurosci. 2005;119:280–292. doi: 10.1037/0735-7044.119.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini CV, Sauer S, White J, Day HEW, Campeau S. Non-associative defensive responses of rats to ferret odor. Physiol Behav. 2006;87:72–78. doi: 10.1016/j.physbeh.2005.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini CV, Srinidhi SK, Day HEW, Campeau S. Program No. 59.19 2006 Abstract Viewer/Itinerary Planner. Atlanta, GA: Society for Neuroscience; 2006. Exposure to predator odor increases heart rate, temperature, and activity measures in rats. Online. [Google Scholar]
- McGregor IS, Schrama L, Ambermoon P, Dielenberg RA. Not all 'predator odours' are equal: cat odour but not 2,4,5 trimethylthiazoline (TMT; fox odour) elicits specific defensive behaviours in rats. Behav Brain Res. 2002;129:1–16. doi: 10.1016/s0166-4328(01)00324-2. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Kent P, Hayley S, Merali Z, Anisman H. Influence of psychogenic and neurogenic stressors on neuroendocrine and central monoamine activity in fast and slow kindling rats. Brain Res. 1999;840:65–74. doi: 10.1016/s0006-8993(99)01771-0. [DOI] [PubMed] [Google Scholar]
- Meewisse M, Reitsma JB, De Vries G, Bersons BPR, Olff M. Cortisol and post-traumatic stress disorder. Systematic review and meta-analysis. Br J Psychiat. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Meredith M. Vomeronasal, olfactory, hormonal convergence in the brain: cooperation or coincidence. Ann NY Acad Sci. 1998;855:349–361. doi: 10.1111/j.1749-6632.1998.tb10593.x. [DOI] [PubMed] [Google Scholar]
- Mohedano-Moriano A, Pro-Sistiaga P, Ubeda-Banon I, Crespo C, Insausti R, Martinez-Marcos A. Segregated pathways to the vomeronasal amygdala: differential projections from anterior and posterior divisions of the accessory olfactory bulb. Eur J Neurosci. 2007;25:2065–2080. doi: 10.1111/j.1460-9568.2007.05472.x. [DOI] [PubMed] [Google Scholar]
- Muller M, Fendt M. Temporary inactivation of the medial and basolateral amygdale differentially affects TMT-induced fear behavior in rats. Behav Brain Res. 2006;167:57–62. doi: 10.1016/j.bbr.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5th Edition. San Diego, CA: Academic Press; 2005. [Google Scholar]
- Pentkowski NS, Blanchard DC, Lever C, Litvin Y, Blanchard RJ. Effects of lesions to the dorsal and ventral hippocampus on defensive behaviors in rats. Eur J Neurosci. 2006;23:2185–2196. doi: 10.1111/j.1460-9568.2006.04754.x. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Ossenkopp KP, Kavaliers M. Brief predator odour exposure activates the HPA axis independent of locomotor changes. Neuroreport. 1999;10:775–780. doi: 10.1097/00001756-199903170-00021. [DOI] [PubMed] [Google Scholar]
- Pro-Sistiaga P, Mohedano-Moriano A, Ubeda-Banon I, Arroyo-Jimenez M, Marcos P, Artacho-Perula E, Crespo C, Insaustix R, Martinez-Marcos A. Convergence of olfactory and vomeronasal projections in the rat basal telencephalon. J Comp Neurol. 2007;504:346–362. doi: 10.1002/cne.21455. [DOI] [PubMed] [Google Scholar]
- Risold PY, Thompson RH, Swanson LW. The structural organization of connections between hypothalamus and cerebral cortex. Brain Res Rev. 1997;24:197–254. doi: 10.1016/s0165-0173(97)00007-6. [DOI] [PubMed] [Google Scholar]
- Roseboom PH, Nanda SA, Bakshi VP, Trentani A, Newman SM, Kalin NH. Predator threat induces behavioral inhibition, pituitary-adrenal activation and changes in amygdale CRF-binding protein gene expression. Psychoneuroendocrinol. 2007;32:44–55. doi: 10.1016/j.psyneuen.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H. The Stress of Life. New York: McGraw-Hill Book Company, Inc.; 1956. [Google Scholar]
- Takahashi LK, Hubbard DT, Lee I, Dar Y, Sipes SM. Predator odor-induced conditioned fear involves the basolateral and medial amygdala. Behav Neurosci. 2007;121:100–110. doi: 10.1037/0735-7044.121.1.100. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Nakashima BR, Hong H, Watanabe K. The smell of danger: A behavioral and neural analysis of predator odor-induced fear. Neurosci Biobehav Rev. 2005;29:1157–1167. doi: 10.1016/j.neubiorev.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Vazdarjanovax A, Cahill L, McGaugh JL. Disrupting basolateral amygdala function impairs unconditioned freezing and avoidance in rats. Eur J Neurosci. 2001;14:709–718. doi: 10.1046/j.0953-816x.2001.01696.x. [DOI] [PubMed] [Google Scholar]