Abstract
Background
Acute wheezing illnesses in preschoolers need better management strategies to reduce morbidity.
Objectives
To examine the effectiveness of episodic use of an inhaled corticosteroid and a leukotriene receptor antagonist in preschoolers with intermittent wheezing.
Methods
In a randomized, double-blind placebo-controlled twelve-month trial, 238 children aged 12-59 months with moderate-severe intermittent wheezing received 7-days of either budesonide inhalation suspension (1mg twice daily), montelukast (4mg daily), or placebos in addition to albuterol with each identified respiratory tract illness. Proportion of episode-free days (EFDs) during the 12-month trial was the primary outcome.
Results
The three treatment groups did not differ in proportions of EFDs, with adjusted mean (95% CI) EFDs of 76% (70%, 81%) for budesonide, 73% (66%, 79%) for montelukast, and 74% (65%, 81%) for conventional therapy (p=0.66). The three groups did not differ in oral corticosteroid use, health care utilization, quality of life, or linear growth. However, during respiratory tract illnesses, budesonide and montelukast therapy led to modest reductions in trouble breathing [(38% (p=0.003) and 37% (p=0.003)] and interference with activity scores [32% (p=0.01) and 40% (p=0.001)], most evident in those with positive asthma predictive indices.
Conclusions
In preschool children with moderate-to-severe intermittent wheezing, episodic use of either budesonide or montelukast early in respiratory tract illnesses, when added to albuterol, did not increase the proportion of EFDs or decrease oral corticosteroid use over a twelve-month period. However, indicators of severity of acute illnesses were reduced, particularly in children with positive asthma predictive indices.
Capsule Summary
The episodic use of budesonide or montelukast in preschool children with moderate-to-severe intermittent wheezing does not increase the proportion of episode free days, but decreases symptom severity during acute respiratory tract illnesses.
Keywords: Wheezing, preschool children, montelukast, budesonide
Introduction
Wheezing illnesses are frequent occurrences in preschool children, and many young children who wheeze repeatedly in the context of lower respiratory tract illnesses (RTI) have severe exacerbations, even though these exacerbations are separated by extended periods of wellness. Rates for wheezing related emergency department visits1 and hospitalizations 1-5 are highest among children under five years of age, reflecting not only the significant morbidity associated with these exacerbations, but also the difficulty in treating wheezing illness in a way that might prevent progression of illness severity. Evidence for management strategies in this population is not consistent. The NAEPP Guidelines, recognizing the lack of convincing data on this subject, proposes the consideration of episodic use of oral corticosteroids at the first sign of RTI as a treatment option in patients with histories of severe exacerbations 6, based on clinical experience and a study that was not a randomized controlled trial 7. However, three randomized controlled trials of early use of oral corticosteroids demonstrated no effect on symptom scores 8-11, although the largest of these trials had low levels of adherence to the protocol. In addition, for those children in this age group who have several RTI during a single respiratory viral season, parents are often reluctant to use oral corticosteroids for each of the episodes, and repeated courses of oral corticosteroids may be associated with significant side-effects 12-15. Three studies suggested that initiating ICS therapy at the early signs of RTI does not result in reduction in oral corticosteroid use16-18, but these studies were small (22-55 subjects each) and used different time points for intervening with medication, thus limiting interpretation of results. An alternative treatment strategy, utilizing montelukast episodically in children 2-14 years of age with intermittent asthma, was recently reported to lead to a reduction in unscheduled health care utilization 19, along with modest reductions in symptom scores and nocturnal awakenings, without a reduction in oral corticosteroid use.
Based on these previously reported disparate results using different medications and treatment approaches, as well as parental and clinician reluctance to use frequent courses of oral corticosteroids, a large, double-blind, randomized controlled clinical trial was designed that would permit a comparison of three episodic treatment strategies initiated at the early signs of acute RTI on the course of moderate-to-severe intermittent wheezing over a twelve-month period.
Methods
Patients
Patients were recruited between February and October 2004 at five clinical centers. The protocol was reviewed and approved by the Childhood Asthma Research and Education (CARE) Network Protocol Review Committee and then by the institutional review boards at each center. Written informed consent was obtained from parents of each participant. The trial was monitored by the CARE Network Data and Safety Monitoring Board.
Inclusion criteria were age 12-59 months and having experienced at least two episodes of wheezing in the context of RTI within the past year. One episode must have occurred within the past six months and one documented by a health care provider. In an effort to include children with prior moderate-to-severe wheezing episodes, children were required to have experienced either: two urgent care visits for acute wheezing within the past year, or two wheezing episodes for which oral corticosteroids were prescribed, or one episode requiring urgent care and one episode requiring oral corticosteroids. Children were excluded if, over the past year, they had received >6 courses of oral corticosteroids, were hospitalized more than twice for wheezing, or had used asthma controller medications (including inhaled corticosteroids (ICS), leukotriene receptor antagonists (LRTA), cromolyn/nedocromil, or theophylline) for four or more months cumulative or within the preceding two weeks. Other exclusion criteria included: birth before 36 weeks gestational age, presence of other significant lung or other medical conditions, gastroesophageal reflux under medical therapy, current antibiotic use for sinusitis, or a history of life threatening wheezing episode.
Patients meeting all of the eligibility criteria were followed for two weeks during which parents completed diary cards twice daily. Diary cards incorporated the validated Pediatric Asthma Caregiver Diary 20 and included five symptom categories (nocturnal cough, daytime cough, wheezing, difficulty breathing, and symptoms interfering with activities), each scored on a zero through five scale (Electronic Supplement Table E3). Children were excluded if, during the two week observation period parents completed diary cards on <80% of days, if asthma controller medications were used, or if the score for albuterol use, wheezing, difficulty breathing, nighttime cough, or asthma symptoms interfering with activities was ≥1 , or if daytime cough score was >2, on an average of four or more days/week.
Protocol
After completing the two week run-in/observation period, participants were randomly assigned to one of three parallel treatment groups. Participants received one of the following regimens for seven days at the first-sign of RTI-associated symptoms: (1) budesonide group [budesonide inhalation suspension (Pulmicort Respules® 1.0 mg twice daily, donated by AstraZeneca) and placebo LTRA once daily], or (2) montelukast group [montelukast (Singulair® 4 mg once daily, donated by Merck) and placebo ICS twice daily], or (3) conventional therapy group [placebo ICS twice daily and placebo LTRA once daily]. Placebos were identical to active drugs in terms of appearance and taste and were also donated by the makers of the active agents. Nebulized medications were administered using a PariLC Plus® nebulizer and a tight-fitting face mask or mouth-piece depending upon the age of the child, as both delivery methods have been demonstrated to be comparably effective in improving clinical parameters in infants and young children 21, 22. All participants received albuterol inhalation treatments (Proventil HFA® via AeroChamber with Mask 180 mcg/treatment (Monhagan Medical Corp, Plattsburgh, NY) or Proventil® nebulization solution 2.5 mg/treatment, donated by ScheringPlough) four times daily while awake (plus as needed) for the first 48 hours followed by albuterol use on an as needed basis. The same intervention treatment was repeated with each subsequent illness characterized by RTI-associated symptoms over the twelve-month study period without a pre-specified limit on the number of treatment courses. Oral corticosteroids (prednisolone) were available for all children at home and were started based upon a specific algorithm (Electronic Supplement and Table E1) 23. Other asthma medications were not permitted during RTI, but use of non-asthma medications was not restricted.
Based upon the variability in the signs and symptoms of RTI which precede the development of significant wheezing, the individualized timing for starting study medications was derived according to an educational protocol designed and evaluated in a pilot study preceding the main trial (See Electronic Supplement). Parents were instructed to begin a 7-day course of the study medication at onset of the individualized set of symptoms identified as the child’s starting point. Parents received extensive education at all study visits regarding close attention to development of symptoms that were likely to represent an RTI followed by extension to chest symptoms.
The schedule of study procedures is detailed in the electronic supplement. Clinic visits were scheduled four weeks following randomization, and then every eight weeks, while telephone calls were scheduled two weeks following randomization followed by calls four weeks after each scheduled clinic visit.
Outcome Measures
The primary outcome measure was the proportion of episode-free days (EFD) over the study period as recorded on diary cards twice daily for the twelve-month trial. An episode-free day was defined as a day during which the child was free from: cough, wheeze, trouble breathing, asthma associated interference with daily activities or awakening from sleep, health care utilization due to wheezing (unscheduled contact, urgent visit, ED visit, or hospitalization), and use of asthma-related non-study medications (including inhaled beta-agonists, controller asthma medications other than study medications, and systemic corticosteroids) 24-26. Use of masked study medications was not used in determining episode-free days. Secondary outcome measures included the severity of lower respiratory tract symptoms as reflected by the area under the curve (AUC) for symptom scores in the 14-day intervals following initiation of study medication. Other secondary outcomes included time to initiation of the first course of oral corticosteroids, the total number of oral corticosteroid courses, number of wheezing episodes, days missed from daycare and parental work, caregiver quality of life, number of unscheduled visits for acute wheezing episodes (primary care office, urgent care, and ED/hospitalization), and linear growth. The a priori analysis plan included examination of the effects of study interventions stratified by asthma predictive index (API) status as determined at the randomization visit. Treatment failure was defined as the occurrence of: four oral corticosteroid courses, hospitalization or intubation for wheezing, hypoxic seizure, or serious adverse event related to a study medication. Participants meeting treatment failure status were prescribed open label budesonide 0.5mg once daily for 6 weeks and returned to their primary care physician for further management.
An unequal allocation ratio was implemented, in which the budesonide and montelukast groups each were allocated twice as many randomized subjects as the conventional therapy group. Allowing for a 10% drop-out rate, the targeted sample size of 225 randomized participants (90 per active therapy, 45 for conventional therapy) provided 90% statistical power for detecting an absolute effect size of 0.15 in each of the two primary comparisons of proportion of EFDs for the active therapy versus conventional therapy using a Bonferroni-corrected, two-tailed 0.025 significance level, and 80% statistical power for detecting an effect size of 0.10 in the secondary comparison of the active therapies at the 0.05 significance level, two-tailed. A previous study comparing oral steroids to control displayed mean proportions of 0.94 and 0.77, respectively, and served as the basis of the power calculation 7. For the secondary outcomes, the targeted sample size provided 90% statistical power for detecting effect sizes of 0.5 standard deviation units (active treatment versus conventional therapy) and 80% power to detect a difference of 30 EFDs per year between the two active treatment arms.
The randomization sequence was stratified according to center, age (12-23 months or 24-59 months), and asthma predictive index status (positive or negative 23) in blocks of five to maintain balanced treatment allocation within strata. Criteria for determination of Asthma Predictive Index status are provided in the electronic supplement (Table E3).
Statistical analyses
Baseline characteristics were summarized using descriptive statistics and compared across treatments using ANOVA for continuous measures and chi-square test for categorical measures.
The primary analysis of a child’s proportion of EFDs was performed using binomial regression, accounting for overdispersion with a quasi-likelihood function, along with pairwise comparisons of the group proportions. Dropouts and treatment failures were included, treating days between treatment failure and scheduled study completion date as episode days, as specified a priori. Additional post hoc analyses were performed with 3 additional approaches to data related to treatment failure: 1) all days between treatment failure and termination as episode free days, 2) carrying the proportion of episode free days observed before treatment failure through to the termination date, and 3) using only available data prior to the treatment failure date. These 3 approaches provided qualitatively similar findings to the primary analysis.
Secondary outcomes of number of oral corticosteroid courses, days of oral corticosteroid use, number of urgent care and ED visits, and days missed from school or daycare, were analyzed using Poisson regression analysis. Time to first prednisone course and time to treatment failure outcomes were analyzed using proportional hazards regression. Growth (defined as change in height or length from baseline to study end) and quality of life outcomes were analyzed using ANCOVA.
Area under the curve was calculated via trapezoidal method for the 14 days following initiation of study medication (Day 1) for symptoms scores, excluding those who never used study medication. This value was analyzed as a difference from ‘baseline’ symptom levels, defining baseline as twice the AUC from Days −13 to −7, which preceded onset of symptoms to avoid any subtle increase in symptoms during the seven days immediately preceding initiation of study medications. Group comparisons were made using a mixed-effects linear model to account for repeated illnesses within children.
All analyses were performed using SAS Version 9.1 statistical software and adjusted for the randomization strata. Reported p-values for active versus conventional therapy comparisons for all outcomes were considered statistically significant if they were lower than 0.025 (Bonferroni correction for two primary comparisons), while p-values for active therapy comparisons were compared to 0.05.
RESULTS
Subject Characteristics
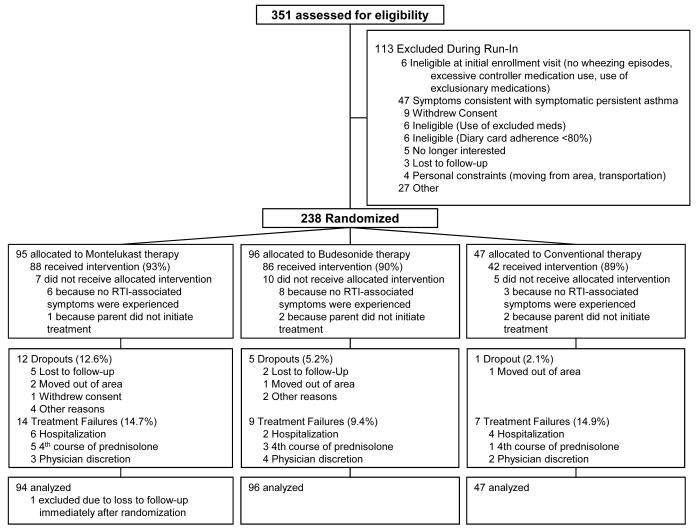
Of 351 patients enrolled, 238 were randomized, and 220 completed the trial or reached criteria for treatment failure (Figure 1). The three treatment groups were well matched for demographic features, pre-trial morbidity including health care utilization, atopic features, and baseline quality of life, with the exception of higher proportions of male children in the budesonide and montelukast groups (Table 1). Further characteristics of this cohort have been described previously 27. Children with positive asthma predictive indices exhibited features of greater morbidity in the preceding year, including greater numbers of wheezing illnesses, acute care visits, and number of courses of oral corticosteroids (Electronic supplement Table 2). The three groups were significantly different with respect to drop-out rate (overall p = 0.04, montelukast greater than conventional therapy p=0.04), but were not significantly different with respect to the rate or time to treatment failure (p=0.48 and p=0.4, respectively), although the use of the intent-to-treat analysis strategy, along with use of all available data on drop-outs, results in a minimal impact of these drop-outs on the findings described herein.
Figure 1. Enrollment and outcome.
Treatment failure rates were comparable across treatment groups (p=0.5). Dropouts were more frequent in the montelukast group (12.6%) compared to budesonide (5.2%) and the conventional therapy group (2.1%) (p=0.04 across groups) and were predominantly due to loss to follow-up.
Table 1.
Participant Characteristics
| Montelukast (N=95) |
Budesonide (N=96) |
Conventional Therapy (N=47) |
|
|---|---|---|---|
| Demographics/Asthma History | |||
| Age (months) | 35.4 ± 12.4 | 36.7 ± 13.5 | 35.7 ± 13.7 |
| Male (%) * | 65.3 | 72.9 | 48.9 |
| Minority (%) | 23.2 | 25.0 | 25.5 |
| Height (cm) | 95.2 ± 8.0 | 95.2 ± 9.6 | 94.4 ± 10.2 |
| Age at MD diagnosis of asthma (yrs)** |
1.4 ± 0.9 (N=56) | 1.6 ± 1.1 (N=62) | 1.5 ± 1.1 (N=31) |
| Age of onset of asthma (yrs)** | 1.0 ± 0.8 (N=56) | 1.1 ± 1.0 (N=62) | 1.0 ± 0.9 (N=30) |
| ED visits for wheezing in past year | |||
| Number per year | 1.1 ± 2.9 | 0.9 ± 1.4 | 1.1 ± 1.5 |
| Percentage of participants | 36.8 | 40.6 | 46.8 |
| MD visits for wheezing in past year |
|||
| Number per year | 4.3 ± 3.4 | 3.7 ± 2.5 | 4.7 ± 3.4 |
| Percentage of participants | 9.5 | 5.2 | 10.6 |
| Missed school/daycare days in past year |
5.5 ± 12.4 (N=58) |
4.8 ± 6.2 (N=65) | 3.7 ± 5.6 (N=34) |
| Exposed to tobacco smoke at home or daycare (%) |
4.2 | 4.2 | 1.7 |
| Medication use in previous year | |||
| Any controller(%) | 36.8 | 36.5 | 27.7 |
| Inhaled corticosteroid (%) | 34.7 | 32.3 | 19.2 |
| Leukotriene modifier (%) | 6.3 | 7.3 | 8.5 |
| Long-acting β-agonist (%) | 1.1 | 0 | 0 |
| Number of oral corticosteroid courses n (%) |
0 = 35 (36.8%) 1 = 25 (26.3%) 2 = 17(17.9%) 3 = 6 (6.3%) 4+ = 12 (12.6%) |
0 = 39 (40.6%) 1 = 21 (21.9%) 2 = 25 (26.0%) 3 = 8 (8.3%) 4+ = 3 (3.1%) |
0 = 22 (46.8%) 1 = 13 (27.7%) 2 = 8 (17.0%) 3 = 2 (4.3%) 4+ = 2 (4.3%) |
| Atopic characteristics | |||
| Positive aeroallergen ST (%) | 50.0 | 44.8 | 44.7 |
| Positive aeroallergen ST (#) | 1.0 ± 1.3 | 0.8 ± 1.3 | 0.8 ± 1.1 |
| IgE (IU/mL) — Geo Mean ± CV | 35.4 ± 5.5 | 39.8 ± 4.5 | 47.5 ± 6.0 |
| Eosinophils (%) | 4.0 ± 2.7 | 4.4 ± 3.0 | 4.6 ± 3.4 |
| Eczema (%) | 28.4 | 34.4 | 42.6 |
| Parental asthma (%) | 43.6 | 41.7 | 53.2 |
| API Positive (%) | 60.0 | 58.3 | 66.0 |
| Quality of Life | |||
| PACQLQ overall score † | 6.6 ± 0.6 | 6.5 ± 0.9 | 6.5 ± 0.8 |
| PedsQL total scale score ‡ | 89.8 ± 8.8 | 88.3 ± 12.6 | 90.6 ± 7.9 |
Data are expressed as mean ± SD except at noted.
p=0.019 across treatment groups.
Highest possible score = 7
Highest possible score = 100
Among those participants with an asthma diagnosis (number of participants noted in parentheses).
Differences not significant for characteristic across treatment groups except as noted.
Adherence
Adherence to the study medication regimens was estimated based upon diary card recordings of illness kits used and counting returned medications. Children in the three treatment arms experienced comparable numbers of RTI per child with means (95% CIs) of 3.4 (2.9, 3.9), 3.7 (3.2, 4.2), and 3.6 (3.0, 4.3) for the montelukast, budesonide, and conventional therapy groups, respectively (total of 840 illnesses). Study medication kits were used for 95% of RTI during the trial and use did not differ by treatment arm. Study medication kits were not used for one of the RTI by 23 participants, for two RTI by seven participants, and for three RTI by one participant. Lack of use did not differ by treatment group. Diary cards were completed on a median of 89.5% of days (Lower quartile 67.1%, upper quartile 96.4%).
Twelve-month global outcomes
The primary outcome measure, EFDs, did not differ significantly among the three treatment groups, reaching an adjusted mean (95% CI) of 76% (70%, 81%) EFDs in the budesonide group, 73% (66%, 79%) EFDs in the montelukast group, and 74% (65%, 81%) EFDs in the conventional therapy group (p=0.66) (Table 2). There were no significant interactions between treatment group and either asthma predictive index status (positive or negative; p=0.71) or oral corticosteroid use in the preceding year (p=0.49) with respect to the proportion of EFDs. During the fourteen days following initiation of study medications for RTI, 45±20% of days were episode free and did not differ by treatment group. In contrast, during times when the child was not experiencing RTI or using study medication, 82±25% of days were episode free and did not differ by treatment group.
Table 2.
Study Outcomes
| Montelukast (N=94) |
Budesonide (N=96) |
Conventional Therapy (N=47) |
|
|---|---|---|---|
| Primary Outcome | |||
| Proportion of episode free days* |
0.73 (0.66, 0.79) | 0.76 (0.70, 0.81) | 0.74 (0.65, 0.81) |
| Secondary Outcomes | |||
| Number of RTIs/participant |
3.4 (2.9, 3.9) | 3.7 (3.2, 4.2) | 3.6 (3.0, 4.3) |
| Oral corticosteroid use | |||
| Time to first oral corticosteroid course (days) (Median; Lower quartile, upper quartile) |
292 (85, 364) | 354 (137, 365) | 292 (127, 359) |
| Number of oral corticosteroid courses/participant* |
1.0 (0.7, 1.3) | 0.7 (0.5, 1.0) | 0.9 (0.6, 1.4) |
| % of participants receiving ≥1 course |
46.8 (36.4, 57.4) | 38.5 (28.8, 49.0) | 55.3 (40.1, 69.8) |
| Days of oral corticosteroid use/participant* |
4.3 (3.7, 5.8) | 2.9 (2.1, 4.1) | 3.0 (1.9, 4.8) |
| Health care utilization | |||
| % with at least 1 urgent care or ED visit |
54.8 (44.7, 65.6) | 53.7 (43.7, 64.4) | 55.6 (40.1, 69.8) |
| Hospitalization (%) | 6.4 (2.4, 13.4) | 2.1 (0.25, 7.3) | 8.5 (2.4, 20.4) |
| Number of urgent care and ED visits/participant* |
1.5 (1.1, 2.0) | 1.1 (0.8, 1.5) | 1.6 (1.1, 2.3) |
| Days missed from school or daycare/participant (# who attended school or daycare)* |
2.9 (2.0, 4.3) (N=61) |
2.1 (1.4, 3.1) (N=69) |
2.6 (1.7, 4.1) (N=35) |
| Quality of Life | |||
| PACQLQ total score (change)* † |
−0.11 (−0.33, 0.11) | −0.04 (−0.24, 0.17) | −0.03 (−0.25, 0.31) |
| PedsQL total scale score (change)* † |
0.88 (−2.31, 4.08) | 0.49 (−3.07, 4.04) | −2.79 (−7.02, 1.45) |
| Growth (cm) | 7.9 (7.4, 8.3) | 7.8 (7.4, 8.1) | 7.5 (7.0, 8.1) |
Data are expressed as mean (95% CI) except at noted.
Differences not significant for characteristic across treatment groups.
Values adjusted for age group (12-23 mos, 24-59 mos), asthma predictive index status (positive, negative), and clinical center
Increases in PACQLQ total score and PedsQL total scale score indicate improvements in quality of life
The three groups did not differ significantly in several other outcomes assessed over the one-year trial (Table 2), including oral corticosteroid use (p=0.15), health care utilization (p=0.98), linear growth (p=0.59), quality of life (p>0.16), and frequencies of adverse events. There were 6 (6.3%), 2 (2.1%), and 4 (8.5%) hospitalizations in the montelukast, budesonide, and conventional therapy groups, respectively (p = 0.22).
Acute respiratory tract illness outcomes
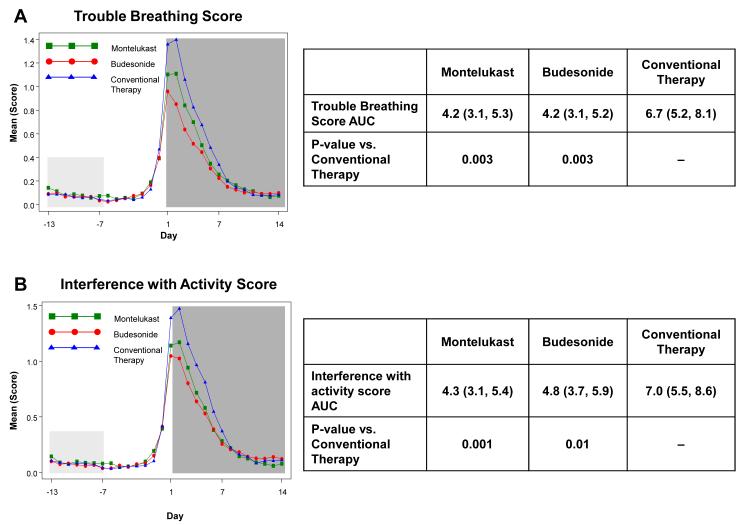
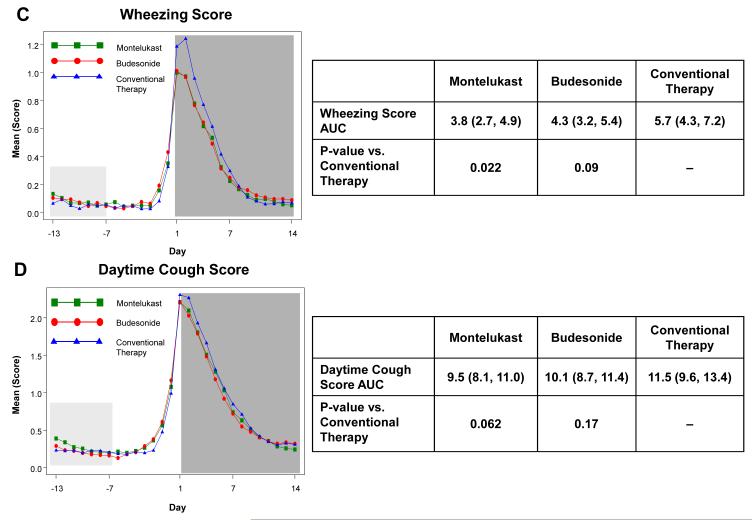
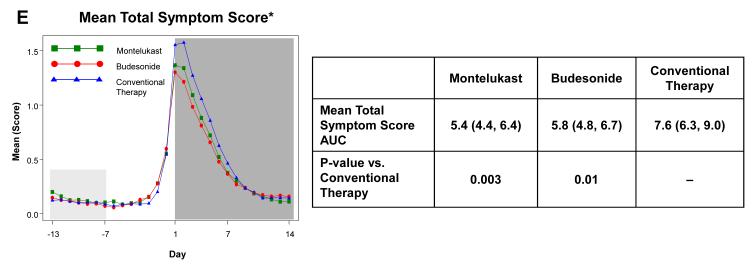
The a priori analysis plan included examination of the effects of study interventions during the fourteen days following initiation of study medications for RTI to determine the effects of treatment during RTI in terms of illness severity and duration. Relative to conventional therapy, there were statistically significant reductions in trouble breathing score AUC (37.5% reduction for budesonide, p=0.003; 36.8% reduction for montelukast, p=0.003) and interference with activity AUC (31.9% reduction for budesonide, p=0.01; 39.6% reduction for montelukast, p=0.001) (Figure 2). Wheezing score AUC was significantly reduced with montelukast therapy (33.5% reduction, p=0.02), but not with budesonide (24.6% reduction, p=0.09). Cough score AUCs did not differ by treatment group (p≥0.12, Electronic Supplement Table E5). Total symptom score (wheeze + cough + interference with activity + trouble breathing) AUC was significantly reduced with montelukast (29.6% reduction, p=0.006) and budesonide therapy (24.6% reduction, p=0.02). There were no significant differences between budesonide and montelukast for any of these symptom measures (p>0.4). Similar results were obtained when the AUCs were calculated over just the seven days during which study medications were administered (data not shown).
Figure 2. Area Under the Curve (AUC) during respiratory tract illnesses.
Area under the curve was calculated for the 14 days following initiation of study medication (shaded in dark grey) for symptoms scores of trouble breathing score (Panel A), interference with activity score (Panel B), wheezing (Panel C), daytime cough (Panel D), and mean total symptom score (Panel E). This value was analyzed as a difference from ‘baseline’ symptom levels, defining baseline as twice the AUC from Days −13 to −7, which preceded onset of symptoms (shaded in light grey). The tables present the AUCs (95% CIs) and p-values comparing each active therapy to conventional therapy. There were no significant differences between montelukast and budesonide for any of these symptom measures (p>0.4).
* Mean of Daytime Cough + Wheezing + Trouble Breathing + Interference with Activity
Examination of the effect of the API status stratification factor on episode severity revealed that, among participants with positive APIs, both budesonide and montelukast significantly reduced AUC for trouble breathing scores (48.0% reduction for budesonide, p=0.001; 40.3% reduction for montelukast, p=0.007) and interference with activity scores (43.6% reduction for budesonide, p=0.001; 53.7% reduction for montelukast, p<0.001), while only montelukast significantly reduced wheezing score AUC (p=0.049) (Table 3). Among participants with negative APIs, neither active treatment led to significant reductions in AUCs compared to conventional therapy for any of the symptom scores. The interaction between treatment group and API status reached significance only for the montelukast group in terms of interference with activity score AUC (p=0.03). In a post hoc analysis, similar findings were obtained when the cohort was stratified by oral corticosteroid use (0 vs. ≥1 course) during the year preceding participation in the trial (Electronic Supplement Table E6).
Table 3.
Area Under The Curve for Symptom Measures During 14 Days Following Initiation of Study Medications by Asthma Predictive Index Status
| Area Under the Curve | |||
|---|---|---|---|
| Montelukast | Budesonide | Conventional Therapy | |
| Wheezing Score | |||
| API Positive | 4.45 # (2.75, 6.14) n = 58 |
4.88 (3.33, 6.44) n = 54 |
6.64 (4.61, 8.67) n = 30 |
| API Negative | 3.97 (2.35, 5.59) n = 36 |
4.05 (2.47, 5.63) n = 42 |
5.97 (3.82, 8.13) n = 17 |
| Trouble Breathing Score | |||
| API Positive | 4.57 † (2.85, 6.30) n = 58 |
3.98 ‡ (2.40, 5.57) n = 54 |
7.65 (5.59, 9.72) n = 30 |
| API Negative | 4.14 (2.54, 5.73) n = 36 |
4.26 (2.71, 5.80) n = 42 |
5.94 (3.83, 8.04) n = 17 |
| Interference with Activity Score | |||
| API Positive | 3.84 * (2.09, 5.59) n = 58 |
4.69 ‡ (3.08, 6.29) n = 54 |
8.30 (6.21, 10.40) n = 30 |
| API Negative | 5.35 (3.49, 7.21) n = 36 |
5.27 (3.48, 7.05) n = 42 |
6.01 (3.57, 8.44) n = 17 |
p=0.049 vs. Conventional therapy
p=0.007 vs. Conventional therapy
p=0.001 vs. Conventional therapy
p=0.025 API positive vs. API negative
Data are expressed as adjusted means (95% CI)
Discussion
We have demonstrated that, in preschool children with moderate-to-severe intermittent wheezing, neither budesonide nor montelukast initiated at early signs of RTI increase the proportion of EFDs over a twelve-month period relative to conventional therapy, nor was there an effect on oral corticosteroid rescue, asthma health care utilization (urgent care visits, emergency department or hospitalizations), or quality of life. However, budesonide or montelukast initiated at early signs of RTI significantly reduce episode severity relative to conventional therapy, with montelukast reducing wheezing, trouble breathing and activity limitation and budesonide reducing trouble breathing and activity limitation, despite the use of four-times daily albuterol during the peak symptom period of the first 48 hours of illnesses.
Our findings are consistent with a recent trial of episodic montelukast treatment in children 2-14 years of age with intermittent asthma, which noted modest reductions in symptom scores (14%) and nocturnal awakenings (8.6%), a 28.5% reduction in unscheduled health care utilization 19, but no effect on use of oral corticosteroids or beta-agonists. Conversely, another recent trial found no effect of ICS initiated after 3 days of wheezing on episode severity in young children aged one month to two years 28, although the lack of effect observed may have been due to the relatively late initiation of therapy relative to the onset of symptoms.
Our results extend these observations in at least two ways: first, we directly compared the effects of intervention with both ICS and montelukast within the same trial and second, we demonstrated a differential response during RTI to both episodic ICS and LTRA therapy based upon two indicators of heterogeneity in terms of baseline disease severity among the enrolled population - API status (a priori analysis) and prior oral corticosteroid use (post hoc analysis). Children with positive APIs or prior oral corticosteroid use derived significantly greater benefit from study medications than children with negative APIs or lack of prior oral corticosteroid use in terms of 40-54% reductions in episode severity as reflected by trouble breathing and interference with activity scores AUC. The absence of detectable effect in the API negative group may be due, in part, to the smaller sample and effect sizes, and thus lower power, in this subgroup relative to the API positive group (94 vs. 144 participants, respectively).
During the study, eighty two percent of days outside of RTI, corresponding to a mean of 5.74 EFD per week, were considered EFDs, confirming the low frequency of asthma-like symptoms outside of episodes that were severe. These findings corroborate clinical experience for the existence of a “severe intermittent wheezing” 27 phenotype in early childhood, that is, children with low impairment but high risk. The new findings of more clinical benefit being demonstrable in a subgroup of these children (those with a positive API) raises an important clinical question. Should these positive API children be treated episodically, given the evidence for some benefits to be gained during the wheezing episode, or should they be treated as if they had persistent asthma -- that is, with daily long term control medication?
The primary outcome, EFDs, is a frequently used measure for asthma control and reflects the multiple components of asthma disease burden. While often informative in comparing the effects of long-term controller medications for asthma in patients with chronic symptoms, it could be argued that this measure may not have been sufficiently sensitive to detect treatment effects among children with an episodic disorder such as severe intermittent asthma. However, there were no differences in prednisolone use between treatment groups, and thus our results would have been comparable had oral corticosteroid use, rather than EFDs, served as the primary outcome measure.
Regarding progression of the illness to the point of prednisolone use, it is possible that the initiation of high dose budesonide or montelukast therapy after symptom onset and presumably following stimulation of the immune response usually triggered by an acute viral infection, was incapable of changing the natural course of each such episode. Initiation of therapy too late into the development of an RTI may also have contributed to these findings. However, while it is possible that earlier initiation may have improved the treatment effects, most parents were not confident that some very early (and likely nonspecific) symptoms would be followed by wheezing, and thus were directed to not start study medication for what appeared to be trivial symptoms. Alternatively, despite having 90% statistical power to detect a 0.5 standard deviation unit difference in effect sizes for secondary outcomes, the lack of effect of study therapies on oral corticosteroid use may have been the result of inadequate statistical power for this secondary outcome.
The use of long term control medication has been examined for the outcome of attenuating either the frequency and/or severity of lower respiratory tract symptoms initiated by RTI with inconsistent findings. Some studies suggest that continuous use of ICS for four to six months in young children with episodic wheezing does not reduce oral corticosteroid use or episode severity 29-31. On the other hand, in preschool children with intermittent wheezing and a positive modified API, continuous use of ICS for two years led to significant improvements in illness burden including increasing EFDs and decreasing oral corticosteroid use, although it was accompanied by a statistically significant, but mild and apparently transient reduction in linear growth velocity 25. Daily administration of montelukast has been shown to reduce the rate of protocol-defined exacerbations, but not oral corticosteroid use, among 2-5 year old children with intermittent asthma symptoms 32. Our results suggest that the episodic use of an inhaled corticosteroid (such as budesonide) or a leukotriene receptor antagonist (such as montelukast) can decrease an important source of respiratory morbidity, namely symptom burden during acute RTI, in these children, particularly those with high risk to develop subsequent asthma (e.g. positive asthma predictive index). Comparisons between intermittent and continuous therapy (or both) with these two controllers are needed, particularly among children at greatest risk for the persistence of asthma symptoms, to determine which of these two approaches is associated with greater efficacy, less parental and child burden, and fewer undesirable side effects.
This clinical trial was conducted in order to address a very important clinical question — is the episodic use of an inhaled corticosteroid or a leukotriene modifier effective in decreasing the morbidity associated with severe intermittent wheezing in preschool children? The clinical strategies examined in this trial are commonly used in clinical practice today. Our findings provide new insights into whether this treatment approach is rational in three important ways. First, this study demonstrates that, while there was no significant effect of these therapies on episode free days over a one year period, there was statistically significant, albeit modest, reduction in symptom burden during respiratory tract illnesses. Second, we were able to demonstrate that there was also variability in the response to these interventions, with children possessing risk factors for asthma at school entry (i.e. positive asthma predictive indices) or greater illness severity (i.e. use of oral corticosteroids in the preceding year) having a greater likelihood of experiencing a clinical benefit with these therapeutic strategies during respiratory tract illnesses. Finally, we have demonstrated that the two strategies, high dose inhaled corticosteroids and leukotriene receptor antagonists, provided very similar effects.
Supplementary Material
Acknowledgments
The role of commercial sponsors was limited to providing drug and matched placebo, which they did after reviewing the drafted protocol. The text of the manuscript was made available to all commercial sponsors two weeks prior to finalization for comments. However, all final decisions regarding the study design and interpretation of data were made exclusively by the NHLBI CARE Network Steering Committee.
Supported by: Grants 5U10HL064287, 5U10HL064288, 5U10HL064295, 5U10HL064307, 5U10HL064305, 5U10HL064313 from the National Heart, Lung, and Blood Institute. This study was carried out in part in the General Clinical Research Centers at Washington University School of Medicine (M01 RR00036) and National Jewish Medical and Research Center (M01 RR00051).
Abbreviations
- AIMS
Acute Intervention Management Strategies
- API
Asthma Predictive Index
- AUC
Area under the curve
- CARE
Childhood Asthma Research and Education Network
- EFD
Episode free day
- ICS
Inhaled corticosteroid
- LTRA
Leukotriene receptor antagonist
- NHLBI
National Heart, Lung and Blood Institute
- PACQLQ
Pediatric Asthma Caregiver’s Quality of Life Questionnaire
- RTI
Respiratory tract illnesses
Footnotes
Clinical Implications Episodic use of budesonide or montelukast in preschool children with moderate-to-severe intermittent wheezing does not increase the proportion of EFDs, but decreases symptom severity during acute respiratory tract illnesses.
This trial is registered at ClinicalTrials.gov as NCT00000622.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mannino D, Homa D, Akinbami L, Moorman J, Gwynn C, Redd S. Surveillance for Asthma - United States, 1980-1999. CDC Surveillance Summaries, March 29, 2002. MMWR. 2002;51:1–15. [PubMed] [Google Scholar]
- 2.Getahun D, Demissie K, Rhoads GG. Recent trends in asthma hospitalization and mortality in the United States. J Asthma. 2005;42:373–8. doi: 10.1081/JAS-62995. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Childhood asthma hospitalizations - King County, Washington, 1987-1998. MMWR. 2000;49:929–33. [PubMed] [Google Scholar]
- 4.Akinbami LJ, Schoendorf KC. Trends in Childhood Asthma: Prevalence, Health Care Utilization, and Mortality. Pediatrics. 2002;110:315–22. doi: 10.1542/peds.110.2.315. [DOI] [PubMed] [Google Scholar]
- 5.Bloomberg GR, Trinkaus KM, Fisher EB, Jr., Musick JR, Strunk RC. Hospital readmissions for childhood asthma: a 10-year metropolitan study. Am J Respir Crit Care Med. 2003;167:1068–76. doi: 10.1164/rccm.2201015. [DOI] [PubMed] [Google Scholar]
- 6.National Asthma Education and Prevention Program US Department of Health and Human Services; Bethesda, MD: Expert Panel Report II: Guidelines for the diagnosis and management of asthma. 1997
- 7.Brunette M, Lands L, Thibodeau L-P. Childhood asthma: Prevention of attacks with short-term corticosteroid treatment of upper respiratory tract infection. Pediatrics. 1988;81:624–9. [PubMed] [Google Scholar]
- 8.Oommen A, Lambert PC, Grigg J. Efficacy of a short course of parent-initiated oral prednisolone for viral wheeze in children aged 1-5 years: randomised controlled trial. Lancet. 2003;362:1433–8. doi: 10.1016/S0140-6736(03)14685-5. [DOI] [PubMed] [Google Scholar]
- 9.Webb M, Henry R, Milner A. Oral corticosteroids for wheezing attacks under 18 months. Arch Dis Child. 1986;61:15–9. doi: 10.1136/adc.61.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox GF, Marsh MJ, Milner AD. Treatment of recurrent acute wheezing episodes in infancy with oral salbutamol and prednisolone. Eur J Pediatr. 1996;155:512–6. doi: 10.1007/BF01955192. [DOI] [PubMed] [Google Scholar]
- 11.Grant C, Duggan A, DeAngelis C. Independent parental administration of prednisone in acute asthma: A double-blind, placebo-controlled, crossover study. Pediatrics. 1995;96:224–9. [PubMed] [Google Scholar]
- 12.Dolan L, Kesarwala H, Holroyde J, Fischer T. Short-term, high-dose, systemic steroids in children with asthma: The effect on the hypothalamic-pituitary-adrenal axis. J Allergy Clin Immunol. 1987;80:81–7. doi: 10.1016/s0091-6749(87)80195-1. [DOI] [PubMed] [Google Scholar]
- 13.van Staa TP, Cooper C, Leufkens HG, Bishop N. Children and the risk of fractures caused by oral corticosteroids. J Bone Miner Res. 2003;18:913–8. doi: 10.1359/jbmr.2003.18.5.913. [DOI] [PubMed] [Google Scholar]
- 14.Dawson KL, Carter ER. A steroid-induced acute psychosis in a child with asthma. Pediatr Pulmonol. 1998;26:362–4. doi: 10.1002/(sici)1099-0496(199811)26:5<362::aid-ppul10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 15.Kayani S, Shannon DC. Adverse behavioral effects of treatment for acute exacerbation of asthma in children: a comparison of two doses of oral steroids. Chest. 2002;122:624–8. doi: 10.1378/chest.122.2.624. [DOI] [PubMed] [Google Scholar]
- 16.Wilson N, Silverman M. Treatment of acute, episodic asthma in preschool children using intermittent high dose inhaled steroids at home. Arch Dis Child. 1990;65:407–10. doi: 10.1136/adc.65.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connett G, Lenney W. Prevention of viral induced asthma attacks using inhaled budesonide. Arch Dis Child. 1993;68:85–7. doi: 10.1136/adc.68.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svedmyr J, Nyberg E, Thunqvist P, Asbrink-Nilsson E, Hedlin G. Prophylactic intermittent treatment with inhaled corticosteroids of asthma exacerbations due to airway infections in toddlers. Acta Paediatr. 1999;88:42–7. doi: 10.1080/08035259950170583. [DOI] [PubMed] [Google Scholar]
- 19.Robertson CF, Price D, Henry R, Mellis C, Glasgow N, Fitzgerald D, et al. Short Course Montelukast for Intermittent Asthma in Children: a Randomised Controlled Trial. Am. J. Respir. Crit. Care Med. 2007;175:323–9. doi: 10.1164/rccm.200510-1546OC. [DOI] [PubMed] [Google Scholar]
- 20.Santanello N, DeMuro-Mercon C, Davies G, Ostrom N, Noonan M, Rooklin A, et al. Validation of a pediatric asthma caregiver diary. J Allergy Clin Immunol. 2000;106:861–6. doi: 10.1067/mai.2000.110478. [DOI] [PubMed] [Google Scholar]
- 21.Georgitis J, McWilliams B, Cruz-Rivera M, Fitzpatrick S, Smith J. Effective once-daily administration of budesonide inhalation suspension by nebulizer with facemasks or mouthpieces for persistent asthma in infants and young children. Pediatr Asthma Allergy Immunol. 2001;15:3–13. [Google Scholar]
- 22.Mellon M, Leflein J, Walton-Bowen K, Cruz-Rivera M, Fitzpatrick S, Smith JA. Comparable efficacy of administration with face mask or mouthpiece of nebulized budesonide inhalation suspension for infants and young children with persistent asthma. Am J Respir Crit Care Med. 2000;162:593–8. doi: 10.1164/ajrccm.162.2.9909030. [DOI] [PubMed] [Google Scholar]
- 23.Guilbert TW, Morgan WJ, Krawiec M, Lemanske RF, Jr., Sorkness C, Szefler SJ, et al. The Prevention of Early Asthma in Kids study: design, rationale and methods for the Childhood Asthma Research and Education network. Control Clin Trials. 2004;25:286–310. doi: 10.1016/j.cct.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Sorkness CA, Lemanske RF, Jr., Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: The Pediatric Asthma Controller Trial. J Allergy Clin Immunol. 2007;119:64–72. doi: 10.1016/j.jaci.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 25.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–97. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 26.Knorr B, Franchi LM, Bisgaard H, Vermeulen JH, LeSouef P, Santanello N, et al. Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years. Pediatrics. 2001;108:E48. doi: 10.1542/peds.108.3.e48. [DOI] [PubMed] [Google Scholar]
- 27.Bacharier LB, Phillips BR, Bloomberg GR, Zeiger RS, Paul IM, Krawiec M, et al. Severe intermittent wheezing in preschool children: A distinct phenotype. J Allergy Clin Immunol. 2007;119:604–10. doi: 10.1016/j.jaci.2006.12.607. [DOI] [PubMed] [Google Scholar]
- 28.Bisgaard H, Hermansen MN, Loland L, Halkjaer LB, Buchvald F. Intermittent inhaled corticosteroids in infants with episodic wheezing. N Engl J Med. 2006;354:1998–2005. doi: 10.1056/NEJMoa054692. [DOI] [PubMed] [Google Scholar]
- 29.Wilson N, Sloper K, Silverman M. Effect of continuous treatment with topical corticosteroid on episodic viral wheeze in preschool children. Arch Dis Child. 1995;72:317–20. doi: 10.1136/adc.72.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doull IJM, Lampe FC, Smith S, Schreiber J, Freezer NJ, Holgate ST. Effect of inhaled corticosteroids on episodes of wheezing associated with viral infection in school age children: randomised double blind placebo controlled trial. BMJ. 1997;315:858–62. doi: 10.1136/bmj.315.7112.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKean M, Ducharme F. The Cochrane Library. Update Software; Oxford: 2001. Inhaled steroids for episodic viral wheeze (Cochrane Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bisgaard H, Zielen S, Garcia-Garcia ML, Johnston SL, Gilles L, Menten J, et al. Montelukast reduces asthma exacerbations in 2- to 5-year-old children with intermittent asthma. Am J Respir Crit Care Med. 2005;171:315–22. doi: 10.1164/rccm.200407-894OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.