Abstract
Olanzapine, an atypical antipsychotic drug, was previously shown to protect neuronal cells against nutrient deprivation and to enhance neurite outgrowth. In an effort to identify small molecules with greater potency, the structure of olanzapine was used as a template to search commercially-available chemical inventories for compounds with similar features. These compounds were evaluated for their ability to protect cells against glutamine deprivation and low serum conditions. Positive compounds, “hits” from initial screening, were then tested for stimulation of neurite outgrowth, alone and in combination with suboptimum concentrations of nerve growth factor (NGF). Numerous neuroprotective compounds (m.w. < 550 Daltons) were identified that significantly stimulated neurite outgrowth in PC12 cells. These included 4′, 6′-diamidino-2-phenylindole (DAPI), a nuclear stain, staurosporine, an antibiotic and kinase inhibitor, and 2-phenylamino-adenosine, an adenosine analogue. The small molecules were comparable to NGF, and in fact, replaced NGF in outgrowth assays. Pharmacophore analysis of the hits led to the design and synthesis of an active compound, LSU-D84, which represents an initial lead for drug discovery efforts
Keywords: neurite outgrowth, neuroprotection, olanzapine, structure-based discovery
Introduction
The nervous system functions by relaying signals between neighboring neurons, sometimes over long distances and in complex circuits. The “wiring” of the brain consists of axons, neurites, dendrites, and specialized synaptic endings that ensure proper communication between cells. Neurite and dendrite outgrowth is a dynamic and energy-dependent process that, if altered, may lead to a loss of synaptic function and various disease states. Schizophrenia, for example, is a neurodevelopmental disorder characterized by aberrant cerebral development, seen as alterations in cortical cytoarchitecture and functional circuitry (Lewis and Levitt 2002). Other neurodevelopmental disorders and neurodegenerative diseases, including Rett Syndrome, autism, Alzheimer's, Parkinson's, and Huntington's disease, also appear to involve defects in neurite outgrowth and synaptic connectivity (Zhan et al. 1993; Zoghbi 2003; LeBlanc et al. 2005).
One strategy for ameliorating defective neurite/dendrite outgrowth would be to use neurotrophins to promote neuronal survival and function. Neurotrophins such as nerve growth factor (NGF), brain-derived growth factor (BDNF), and neurotrophin-3 (NT-3), were discovered on the basis of their effects on neural growth, differentiation, and plasticity. Neurotrophins have also been reported to protect against aging and neuronal injury (for review see Sofroniew et al. 2001). Because of their trophic activity, these growth factors represent a promising therapeutic approach for neurodegenerative diseases and neurodevelopmental disorders. However, neurotrophic factors show limited penetration of the blood brain barrier (BBB) due to their large size. To overcome this challenge, various strategies have been used, such as disruption of the BBB, or the use of liposomes, fusion proteins, viral vectors, and receptor-mediated transcytosis (Pan and Kastin 2004). An alternative approach to treating neuronal deficit disorders would be to administer compounds that are functionally similar to neurotrophins, but readily penetrate the BBB. Thus, the goal of this research effort is to identify low molecular weight compounds that act as neurotrophins and promote neurite outgrowth.
Small molecules have previously been reported to stimulate neurite outgrowth in neuronal cells, or enhance the activity of NGF (Paul 1990; Dago et al. 2002a, b; Nowaza et al. 2002; Lu and Dwyer 2005; Kamata et al. 2007). Staurosporine, an antimicrobial and potent kinase inhibitor, has been shown to promote rapid outgrowth of neurites in various PC12 cell lines (Hashimoto and Hagino 1989; Rasouly et al. 1992; Yao et al. 1997). However, lack of understanding of the mechanism of action of these compounds, and limited structure-activity relationship (SAR) information has slowed the development of lead compounds for clinical trials.
The present studies were inspired by the observation that olanzapine, an atypical antipsychotic drug, stimulated neurite outgrowth in PC12.XL cells, a variant of the PC12 cell line. By itself, olanzapine weakly stimulated neurite outgrowth, whereas in combination with suboptimum concentrations of NGF, it significantly enhanced neurite outgrowth and increased the number of cells bearing processes (Lu and Dwyer 2005). This prompted us to screen chemical libraries for compounds with more potent effects on neurite outgrowth. We reasoned that compounds with similar structures to olanzapine might also produce the same biological effects as this template ligand. Ligand-based virtual screening is increasingly being applied in drug discovery efforts to identify initial lead compounds (Guido et al. 2008). The findings reported here demonstrate that structure-based screening can be used to identify small molecules that stimulate neurite outgrowth and mimic the actions of NGF. Finally, an emerging SAR guided the synthesis of an active compound, which may represent an initial lead for drug discovery.
Materials and Methods
Compound screening
Olanzapine provided the prototype for structure-based searches of commercial chemical inventories. Compounds with structural features found in olanzapine (e.g., heterocyclic ring with nitrogen, thiophene and piperazine) were identified by visual inspection and automated searches of several chemical databases. For the initial screening, structural similarity was based on the presence of a central heterocyclic ring system containing at least one nitrogen atom, and a substituent ring structure with nitrogen (to mimic the piperazine group in olanzapine). In a secondary search, the parameters were revised to focus on compounds with a central benzimidazole or benzothiazole structure with flanking aromatic rings. The compounds identified in these searches were obtained from Sigma-Aldrich (St. Louis, MO), RBI (now part of Sigma), Tocris (Ellisville, MO), Calbiochem (Gibbstown, NJ), and Alfa-Aesar (Ward Hill, MD). For initial screening, the compounds were dissolved in DMSO or ethanol and diluted in phosphate-buffered saline (PBS) or dilute acetic acid for addition to cell protection assays. Over 200 compounds have been screened to date. Compounds that were positive in the protection assay (“hits”) were then evaluated for their effects on neurite outgrowth, which is the focus of this report.
Cell culture
PC12 cells were obtained from American Type Culture Collection (ATCC) and maintained in Dulbecco's Modified Eagle Medium (DMEM) (Life Technolgies, Grand Island, NY), containing 5% fetal bovine serum (FBS), 10% heat inactivated horse serum (HS), and 1% penicillin/streptomycin, in 5% CO2 at 37°C.
Cell protection assay
All compounds obtained from the chemical libraries were tested for enhancement of cell survival in low serum, glutamine-free medium. PC12 cells (5 × 105/ml) were cultured as described previously (Dwyer and Dickson 2006) using glutamine-free medium containing 0.5% FBS, and 1% HS. On day 3, MTS reagent [3-(4, 5-dimethylthiazol-2-yl)-5-(3 carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] was added and the amount of formazan dye was measured in an automated plate reader at 492 nm to determine cell viability.
Neurite outgrowth
For these studies, we used the PC12.XL cell line, which was derived in our laboratory and is a variant of the ATCC PC12 line (Dwyer and Dickson 2006). These cells show much greater adherence to plastic surfaces than the parental line and do not require adhesion substrata to undergo neuronal differentiation. In the first set of experiments, neuroprotective compounds were tested in combination with NGF to determine whether they acted like olanzapine. Four of the initial compounds, LSU-31 (Hoechst 33258), LSU-40 (Netropsin), LSU-42 (Distamycin A), and LSU-44 (4′, 6′-diamidino-2-phenylindole [DAPI]), were added to PC12.XL cells simultaneously with a concentration of NGF (20 ng/ml, or approximately 0.8 nM), which produces suboptimum neurite outgrowth. Neurite outgrowth was quantified after 4-7 days as described previously (Lu and Dwyer 2005). Briefly, morphological changes were analyzed with a digital Nikon phase-contrast inverted microscope with a CCD camera. For all experiments shown here, three random areas were selected per well and photographed, measurements were performed on duplicate wells (N=6), and each experiment was repeated at least twice. Only cells containing processes longer than two cell body diameters were counted as positive for neurite outgrowth. In addition, the length of the longest neurite was determined with measurement software from Image J (NIH).
For the second set of experiments, neuroprotective compounds were evaluated for their ability to stimulate neurite outgrowth on their own. The following compounds were used in this experiment: LSU-31, LSU-40, LSU-42, LSU-44, LSU-65 (β-carboline-3-carboxylic acid N-methylamide), LSU-109 (Harmalol), LSU-D84 (a proprietary compound), LSU-165 (2-phenylamino-adenosine), and chlorophenylthio-cAMP (CPT-cAMP). PC12.XL cells (~ 5 × 103/ml) were plated in 96-well plates, and then incubated with various compounds for 4-7 days, over a range of concentrations, typically 5-160 μM, prior to quantifying neurite outgrowth. The dose range and time required to elicit a response were also determined.
NGF replacement assay
To compare the activity of these compounds to NGF, we examined whether they could substitute for NGF in promoting neurite outgrowth. Original PC12 cells were incubated with NGF (100 ng/ml, ~ 4 nM) for 5 days. NGF was then removed and the cells were washed twice with DMEM. NGF was replaced with feeding medium containing solvent (control), NGF (100 ng/ml), or compounds (LSU-31, LSU-44, and LSU-204 [staurosporine]). The cells were photographed and scored for neurite outgrowth after an additional 4-7 days.
Structure-based design of LSU-D84
Molecular structures for olanzapine and the hits from screening were created with the Builder module of Insight II software (Accelrys; San Diego, CA). The three-dimensional structures of the compounds were derived from quantum mechanics minimization with the AMPAC/MOPAC module. The PM3 semiempirical method was used to obtain the minimized structures, which were then initially aligned by automatically superimposing key atoms in the major heterocyclic rings. Further alignment was performed manually to refine the positions of pharmacophores, which included potential hydrogen bond donors/acceptors, aromatic rings, and hydrophobic groups. A general pharmacophore model was generated from this analysis and was used to design a novel compound for synthesis, LSU-D84. Benzothiazole was selected as the central heterocyclic ring structure and substituent groups commonly found in hits from screening were added at both ends. LSU-D84 was synthesized (> 95 % purity) by iQsynthesis (St. Louis, MO); its synthesis and structure will be presented elsewhere.
Results
Protection against glutamine deprivation and low serum culture conditions
Several second generation antipsychotics have been shown to enhance proliferation and protect neuronal cells against insults (Bai, et al. 2002; Dwyer et al. 2003; He et al. 2004; Lu et al. 2004). In an effort to find more potent neuroprotective agents, we evaluated the effects on cell viability of a series of compounds that shared one or more structural features with olanzapine (e.g., heterocyclic structures with nitrogen atoms and flanking six-member rings). PC12 cells were grown in low serum, glutamine-free medium for 3 days and cell viability was measured. Several compounds including LSU-31 (Hoechst 33258), LSU-40 (Netropsin), LSU-42 (Distamycin A), and LSU-44 (DAPI), were found to have weak to moderate activities (Table 1). LSU-31 significantly enhanced cell viability over a range of concentrations, whereas LSU-40 produced clear concentration-dependent effects. The efficacy of LSU-42 and LSU-44 also increased with concentration up to 40 μM. Thus, compounds selected for testing based on similarities to olanzapine protect neuronal cells against nutrient deprivation as predicted from earlier studies of this drug (Lu et al. 2004).
Table 1.
Neuroprotection by compounds structurally similar to olanzapine*
| LSU-31 | LSU-40 | LSU-42 | LSU-44 | |
|---|---|---|---|---|
| Cell viability (% Control ± SEM) | ||||
| 5 μM | 152.8 ± 10.9* | 91.7 ± 3.2 | 103.3 ± 2.9 | 111.3 ± 2.5* |
| 10 μM | 133.3 ± 5.3* | 96.3 ± 4.1 | 120 ± 6.4* | 119 ± 1.9* |
| 20 μM | 128.3 ± 4.7* | 112.7 ± 3.6* | 135 ± 10.4* | 124 ± 0.7* |
| 40 μM | 140.8 ± 10.2* | 127.3 ± 9.6* | 135.7 ± 3.8* | 126 ± 6.4* |
| 80 μM | 127 ± 5.1* | 157.7 ± 13.2* | 118 ± 4.3* | 96± 5.1 |
PC12.XL cells were incubated in glutamine-free, low serum medium with various concentrations of compounds. On day 3, MTS reagent was added and cell viability measured as a function of dye conversion. Results from 4 experiments have been averaged and are expressed as percentage of control (DMSO solvent) ± the standard error of the mean (SEM). Significant differences from the control as determined with Student's t-test are indicated by asterisks (*p<0.05).
Neuroprotective compounds stimulate neurite outgrowth
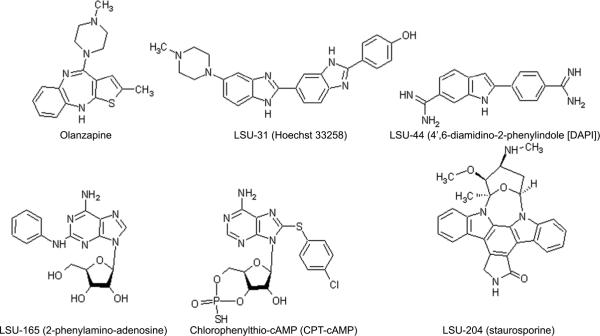
Based on the initial collection of hits, we revised the search parameters and re-explored chemical libraries for structurally related compounds. This revised search identified additional compounds with positive effects in cell viability assays. The structures of some of the hits from the initial screening (e.g., LSU-31 and LSU-44) and additional molecules identified from the secondary screen (e.g., LSU-165 and LSU-204) are shown in Fig. 1. The structure of olanzapine is also included here for comparison.
Fig. 1.
Chemical structures of olanzapine and compounds from screening.
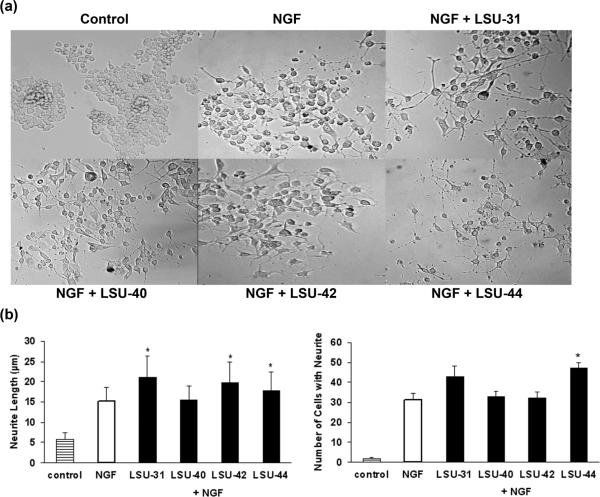
While testing the various compounds for protection against nutrient deprivation, we noticed that some stimulated modest neurite outgrowth from PC12 cells. To compare the response induced by these compounds to that previously observed with olanzapine, we examined whether they affected neurite outgrowth in combination with suboptimum concentrations of NGF (20 ng/ml, or ~ 0.8 nM). For these studies we used the PC12.XL cell line. Many variants of the original PC12 cell line (e.g. PC12-N1, PC12.4, PC12D, and PC12-E2) have been derived to facilitate studies of neuronal differentiation (Sano et al. 1995; Wu and Bradshaw 1995; Dwyer et al. 1999; Xiao et al. 2002). In general, the variant cell lines adhere better to plastic surfaces and undergo rapid and robust process elongation. PC12.XL cells behave similar to the “primed” PC12D cells of Sano et al. (1995) in terms of response time and degree of neurite extension, and are used here to study neurite outgrowth. Results in Fig. 2 show that LSU-44 significantly increased both neurite length and the number of responding cells, in combination with NGF, whereas LSU-31 and LSU-42 significantly increased neurite length compared to NGF alone. Use of the PC12.XL cell line allowed us to detect effects of the compounds on neurite extension that may have otherwise been missed.
Fig. 2.
Neurite outgrowth induced by NGF ± compounds from screening. LSU-31 (Hoechst 33258), LSU-40 (Netropsin), LSU-42 (Distamycin A), and LSU-44 (DAPI) were discovered during our initial screening and tested for their effects on neurite outgrowth in comparison to olanzapine. The compounds were tested at 10 μM, 40 μM, 20 μM, and 5 μM, respectively. (a) Photographs show changes in cellular morphology and neurite extension enhanced by the combination of NGF (20 ng/ml, ~ 0.8 nM) and compounds. (b) Quantification of the effects of NGF and NGF + compounds on neurite length and number of responding cells. Results are shown as average neurite length or cell number ± the standard error (S.E.). Significant differences from NGF alone, as determined by Student's t-test, are indicated with asterisks (*p<0.05). Actual P values for significant effects (*) are listed in parentheses as follows: for neurite length, LSU-31 (< 0.001), LSU-42 (< 0.001), LSU-44 (0.004); and for cell number, LSU-44 (0.006).
Neurite outgrowth is stimulated by small molecules independent of NGF
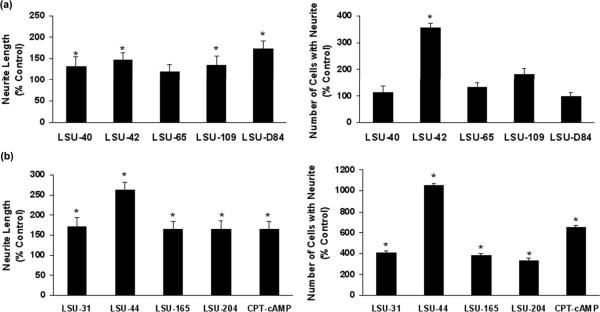
Small molecules have previously been reported to promote neurite outgrowth in PC12 cells. To determine whether the compounds stimulate neurite extension by themselves, we incubated PC12.XL cells with various compounds for 4 to 7 days, and then assessed morphological changes in the cells. With the exception of LSU-65, the compounds significantly increase neurite outgrowth compared to control independently of NGF (Fig. 3). Because the magnitude of response differed between compounds, we grouped them according to their efficacy. Fig. 3a depicts the results of the weaker compounds, as judged by the shorter neurite length and lower number of cells bearing processes. LSU-D84, a proprietary compound, has a more pronounced effect on neurite length comparable to the stronger compounds, but becomes toxic at higher concentrations. LSU-42 increased cell number and length, but its response fluctuates with concentration; whereas LSU-40 only affects neurite length.
Fig. 3.
Protective compounds stimulate neurite outgrowth in PC12.XL cells. (a) Neuroprotective compounds that show weaker biological activity in neurite outgrowth assays. These compounds include LSU-40 (80 μM), LSU-42 (80 μM), LSU-65 (40 μM), LSU-109 (80 μM), and LSU-D84 (5 μM). (b) More potent compounds show significant (*p< 0.05) effects for each measurement. These compounds include LSU-31 (10 μM), LSU-44 (2.5 μM), LSU-165 (625 nM), LSU-204 (10 nM), and CPT-cAMP (40 μM). Responses are expressed as the percentage of control and were evaluated for significance using Student's t-test. The P values for significant effects (*) are listed in parentheses as follows: for neurite length, LSU-40 (0.007), LSU-42 (< 0.001), LSU-109 (0.009), LSU-D84 (0.01), and all < 0.001 for LSU-31, LSU-44, LSU-165, LSU-204, and CPT-cAMP; for cell number, LSU-42 (< 0.001), LSU-31 (< 0.001), LSU-44 (< 0.001), LSU-165 (0.003), LSU-204 (0.004), and CPT-cAMP (0.003).
The more potent compounds produced significant increases in both neurite length and the number of cells extending processes, as shown in Fig. 3b. These compounds increase neurite length by at least 50% and cell number by 200%, in comparison to control. Thus, compounds selected on the basis of a structural resemblance to olanzapine are neuroprotective and stimulate neurite outgrowth to an even greater extent than the prototype (data not shown; and see Lu and Dwyer 2005 for comparison). Moreover, the number of hits obtained from this limited screen attests to the validity of the structural analysis.
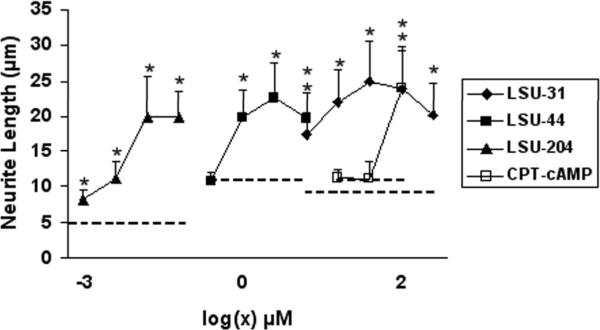
Concentration-dependent increase in neurite length
To determine the relative potencies for stimulating neurite outgrowth, several of the most efficacious compounds were tested over a range of concentrations, as illustrated in Fig. 4. The increase in neurite length induced by each compound was compared to the control for that experiment, which varies due to spontaneous extension of short neurites by these cells. LSU-204 is the most potent compound, showing activity at 2.5 nM, whereas LSU-44, LSU-31, and CPT-cAMP stimulated significant increases in neurite length at 2.5 μM, 10 μM, and 80 μM, respectively. LSU-31 showed maximal efficacy at 40 μM, increasing neurite length to 25 μm.
Fig. 4.
Concentration-response curves for LSU-31, LSU-44, LSU-204 (staurosporine), and CPT-cAMP. Individual compounds were tested over an 8-fold range to determine the concentrations required to elicit a significant response, as compared to control. Data are presented as average neurite length (μm) ± S.E. Significant differences are indicated as before by asterisks (*p<0.05). Dotted lines represent the corresponding control value for each compound. The P values were as follows: LSU-31 (all < 0.001), LSU-44 (0.01 at 2.5 μM, and all higher concentrations < 0.001), LSU-204 (0.01 at 2.5 nM, and all higher concentrations < 0.001), CPT-cAMP (< 0.001 at 80 μM).
Compound-induced neurite outgrowth persists for several days after induction
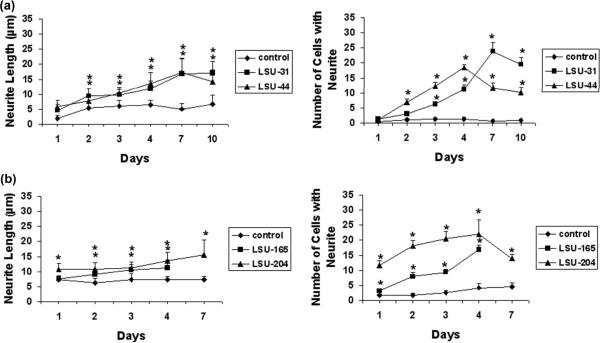
To compare these compounds to NGF, which induces stable neurite outgrowth for up to 2-3 weeks (Greene and Tischler 1976), a time course was performed with four of the most potent compounds. PC12.XL cells were incubated with each compound and photographed until a decline in the response was observed. All of the compounds tested promoted neurite extension compared to control for up to 4 to 10 days, as shown in Fig. 5. LSU-44 and LSU-204 increase neurite outgrowth for 4 days, but then decrease cell viability. On the other hand, the response induced by LSU-31 persists for more than 10 days. LSU-165 also stimulates cell proliferation, which interferes with the measurement of neurite outgrowth after 4 days. Although the neuritogenic effect of these compounds varied over time, several seemed to be comparable to NGF, and we wondered whether they might replace it in neurite outgrowth assays.
Fig. 5.
Time-course analysis of neurite outgrowth induced by compounds from screening. (a) LSU-31 (40 μM), LSU-44 (10 μM), and (b) LSU-165 (40 μM), and LSU-204 (40 nM) were added to PC12.XL cells for up to 7-10 days. Average neurite length or the number of cells bearing neurites ± S.E. were plotted here. Significant differences (*p<0.05) are indicated as before.
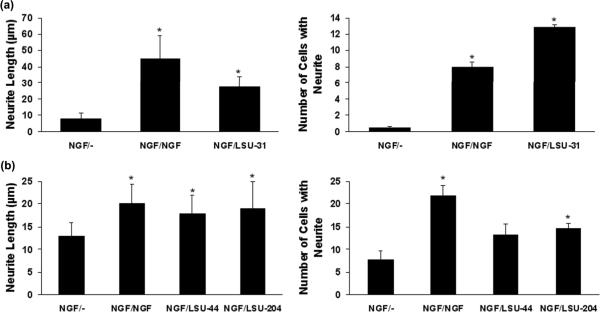
Small molecules substitute for NGF after its withdrawal
One goal of this work is to identify small molecules that mimic the neurotrophic activity of NGF on neuronal cells. To determine if the compounds could substitute for NGF, original PC12 cells were incubated with NGF for 5 days, the cells were then washed with DMEM, and NGF was replaced with media (NGF/-), NGF (NGF/NGF), LSU-31 (NGF/LSU-31), LSU-44 (NGF/LSU-44), or LSU-204 (NGF/LSU-204). Removal of NGF normally leads to a retraction of neurites and loss of some cells. Fig. 6 shows that the compounds effectively replaced NGF and maintained both neurite length and the number of cells with neurites. LSU-44 (5 μM) did not significantly increase cell number, but there was a trend (p=0.1). While there is variability in the response to NGF withdrawal (NGF/-) between experiments, these general observations have been reproduced in 2-3 experiments per compound. These data clearly demonstrate that small molecules can mimic and replace NGF as a trophic factor.
Fig. 6.
Compounds substitute for NGF in the induction of neurite outgrowth. PC12 cells were incubated with NGF (100 ng/ml, ~ 4 nM), followed by its removal (NGF/-), and then addition of either NGF at 100 ng/ml (NGF/NGF), or compound. LSU-31, 40 μM (NGF/LSU-31) was added in (a), and LSU-44, 5 μM (NGF/LSU-44) or LSU-204, 5 nM (NGF/LSU-204) were added in (b). Each compound effectively replaced NGF and maintained neurite length and cell number with neurites after NGF withdrawal. The data represent the averages of neurite length or cell number. Significant differences from the NGF withdrawal group (NGF/-) are indicated with asterisks, *p<0.05. The actual P values are listed here as before: in (a) all significant differences (*) were < 0.001, whereas in (b) NGF/NGF (< 0.001), NGF/LSU-44 (0.006), NGF/LSU-204 (0.007) for neurite length, and NGF/NGF (< 0.001), NGF/LSU-204 (0.01).
Discussion
Identification of compounds that promote neurite outgrowth is usually based on prior knowledge of a target molecule like a cell surface receptor. Combinatorial screening has been used in cases where the target is unknown (Ronn et al. 1999; Ditlevsen et al. 2003; Ralets et al. 2004). In the present study, commercially-available libraries have been screened to find low molecular weight compounds that stimulate neurite outgrowth in PC12.XL cells, a variant of the established PC12 cell line.
Starting with the structure of olanzapine as a template, we successfully identified a small subset of similar molecules (~ 200), which upon screening yielded neuroprotective compounds that also stimulated neurite outgrowth from PC12 cells. This structure-based screening resulted in a much higher hit rate (11/207) than would be expected by chance. In this paper, we report on those compounds that induced significant neurite outgrowth on their own, and compare this activity to that of NGF.
The observation that olanzapine, an antipsychotic drug, enhances NGF-induced neurite outgrowth provided the rationale for the strategy reported in this study. Here, we showed that compounds identified using olanzapine as a template enhanced neuronal differentiation in combination with NGF, which was consistent with our predictions. In contrast to olanzapine, the compounds also stimulated significant neurite outgrowth on their own. LSU-31 (Hoechst 33258), LSU-44 (DAPI), LSU-165 (2-phenylamino-adenosine), LSU-204 (staurosporine), and CPT-cAMP were the most efficacious compounds, and increased neurite length by at least 50% and the number of cells with neurites by 3-fold.
The two most potent compounds, staurosporine and DAPI, stimulated neuronal differentiation in the nanomolar range. Although staurosporine has previously been reported to induce neurite outgrowth from PC12 cells (Hashimoto and Hagino 1989; Rasouly et al. 1992; Yao et al. 1997), we discovered this compound based on its structural resemblance to other hits from screening, namely LSU-65 (β-carboline-3-carboxylic acid N-methylamide) and LSU-109 (Harmalol). In addition, we are the first to show that it substitutes for NGF with respect to maintaining neurite outgrowth in PC12 cells.
NGF is a neurotrophic factor that is important for neuronal connectivity and neuroprotection. NGF, along with other growth factors, is released by damaged nerves to promote survival and regeneration of the injured neuron (for review see Huang and Reichardt 2001). Small molecules have previously been reported to be neuroprotective in different settings (Dago et al. 2002a; Lu, Bradely and Dwyer 2004; Hirata et al. 2005). For example, T-817MA protected against amyloid-β (Aβ) accumulation and oxidative stress-induced neurotoxicity in a coculture of rat cortical neurons and glial cells, whereas olanzapine protected PC12 cells against serum deprivation (Lu, Bradley and Dwyer 2004). Four compounds found during our initial screening, (LSU-31, LSU-40, LSU-42, and LSU-44), were tested and found to be protective against glutamine deprivation and low serum in vitro. These compounds also stimulated neurite outgrowth, increasing both neurite length and the number of cells bearing neurites. Although the mechanisms involved in the induction of neurite outgrowth are not known, the neuroprotective properties of the compounds may be mediated by activation of the Akt signaling pathway as discussed elsewhere (Dwyer and Dickson, 2006). The effects of olanzapine on Akt signaling and neurite outgrowth are not mediated by antagonism of dopamine and serotonin receptors (Lu and Dwyer 2005), which suggests a novel mechanism of action. Moreover, preliminary studies suggest that LSU-165 (an adenosine analogue), and CPT-cAMP affect additional targets other than the adenosine receptor or protein kinase A, respectively.
Small molecules can regenerate retracted neurites and restore cell function after NGF withdrawal. NS 1231 rescued NGF-differentiated PC12 cells from death induced by withdrawal of trophic factors and stimulated neurite outgrowth in undifferentiated cells (Dago et al. 2002a). Small molecules like compound 1H5 have been shown to protect PC12 cells from NGF withdrawal (Lin et al. 2007). In the present study, LSU-31, LSU-44, and LSU-204 were tested for their ability to mimic NGF after its withdrawal from PC12.XL cells. Each of these compounds maintained neurite length and cell differentiation, and were thus neurotrophic, similar to NGF. Hoechst 33258 (LSU-31) and DAPI (LSU-44) are widely used as nuclear stains, however their effects on neurite outgrowth have not been previously reported. It is possible that these compounds produce direct effects on DNA to promote neuronal differentiation, and this is currently being investigated.
These findings demonstrate the utility of screening chemical libraries for compounds that are structurally similar to drugs such as olanzapine, which have intriguing trophic and neuroprotective properties (Lu, Bradley and Dwyer 2004; Lieberman et al. 2008). Small-molecule drugs that induce neurite outgrowth may be useful in the treatment of brain deficit disorders characterized by neurodegeneration, neuronal cell loss, and deficiencies in synaptic connectivity (Dwyer and Dickson 2006). Based on common features in the collection of biologically-active compounds, we have derived a current pharmacophore hypothesis (depicted in Fig. 7). Activity appears to be mediated by a central heterocyclic ring (c) (such as benzimidazole or benzodiazepine), flanked by a hydrophobic moiety (a) and a potential hydrogen-bond donor on one side (b) and an aromatic ring (e) on the other. LSU-D84 was designed to conform to this hypothesis and was found to be neuroprotective, and to stimulate neurite outgrowth, albeit less potently than some of the hits from screening. Nevertheless, its structure can guide the synthesis of more active compounds for future analysis. This line of research may ultimately lead to the discovery of small molecule mimics of NGF that could be used to treat brain disorders caused by insufficient trophic activity.
Fig. 7.
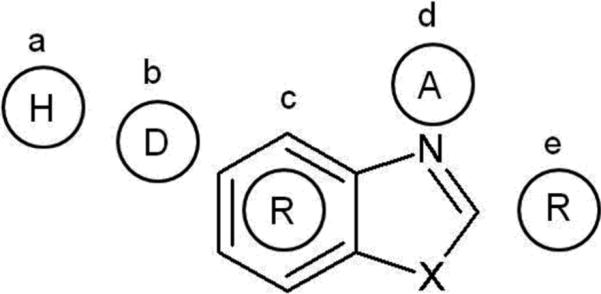
Pharmacophore hypothesis based on active compounds from screening. Coumpounds that promote neurite outgrowth have a central hetrocyclic ring structure (R) as depicted here. This central ring is typically flanked by a hydrophbic group (H) and a hydrogen-bond donor (D) to the left, and an aromatic ring is commonly substituted at the right side as shown. A hydrogen-bond acceptor group (A) is found either in the central ring or located near this position.
Acknowledgements
The authors thank Addie Dickson for initial screening of compounds for neuroprotection. This work was supported by a grant (MH 68385) from NIH.
References
- Bai O, Wei Z, Lu W, Bowen R, Keegan D, Li XM. Protective effects of typical antipsychotic drugs on PC12 cells after serum withdrawal. J. Neurosci. Res. 2002;69:278–283. doi: 10.1002/jnr.10290. [DOI] [PubMed] [Google Scholar]
- Dago L, Bonde C, Peters D, Moller A, Bomholt SF, Hartz JB, Meyer M, Drejer J, Gronborg M. NS 1231, a novel compound with neurotrophic-like effects in vitro and in vivo. J. Neurochem. 2002a;81:17–24. doi: 10.1046/j.1471-4159.2002.00803.x. [DOI] [PubMed] [Google Scholar]
- Dago L, Peters D, Meyer M, Hartz B, Kruse V, Drejer J, Gronborg M. NS-417, a novel compound with neurotrophic-like effects. Neurochem. Res. 2002b;27:207–211. doi: 10.1023/a:1014810824056. [DOI] [PubMed] [Google Scholar]
- Ditlevsen D, Kohler L, Pedersen MV, Risell M, Kolkova K, Meyer M, Berezin V, Bock E. The role of phosphatidylinositol 3-kinase in neural cell adhesion molecule-mediated neuronal differentiation and survival. J. Neurochem. 2003;84:546–556. doi: 10.1046/j.1471-4159.2003.01538.x. [DOI] [PubMed] [Google Scholar]
- Dwyer DS, Dickson A. Neuroprotection and enhancement of neurite outgrowth with small molecular weight compounds from screens of chemical libraries. Int. Rev. Neurobiol. 2006;77:245–287. doi: 10.1016/S0074-7742(06)77008-8. [DOI] [PubMed] [Google Scholar]
- Dwyer DS, Liu Y, Bradley R. An ethanol-sensitive variant of the PC12 neuronal cell line: sensitivity to alcohol is associated with increased cell adhesion and decreased glucose accumulation. J. Cell. Phys. 1999;178:93–101. doi: 10.1002/(SICI)1097-4652(199901)178:1<93::AID-JCP12>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Dwyer DS, Lu XH, Bradley R. Cytotoxicity of conventional and atypical antipsychotic drugs in relation to glucose metabolism. Brain Res. 2003;971:31–39. doi: 10.1016/s0006-8993(03)02351-5. [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler A. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido RV, Oliva G, Andricopulo AD. Virtual screening and its integration with modern drug design technologies. Curr. Med. Chem. 2008;15:37–46. doi: 10.2174/092986708783330683. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Hagino A. Staurosporine-induced neurite outgrowth in PC12h cells. Exp. Cell Res. 1989;184:351–359. doi: 10.1016/0014-4827(89)90334-0. [DOI] [PubMed] [Google Scholar]
- He J, Xu H, Yang Y, Zhang X, Li XM. Neuroprotective effects of olanzapine on methamphetamine-induced neurotoxicity are associated with an inhibition of hyperthermia and prevention of Bcl-2 decrease in rats. Brain Res. 2004;1018:186–192. doi: 10.1016/j.brainres.2004.05.060. [DOI] [PubMed] [Google Scholar]
- Hirata K, Yamaguchi H, Takamura Y, Takagi A, Fukushima T, Iwakami N, Saitoh A, Nakagawa M, Yamada T. A novel neurotrophic agent, T-817MA [1-{3-[2-(1-Benzothiophen-5-yl)Ethoxy]Propyl]3-azetidinol Maleate], attenuates amyloid-β-induced neurotoxicity and promotes neurite outgrowth in rat cultured central nervous system neurons. J. Pharmacol. Exp. Ther. 2005;314:252–259. doi: 10.1124/jpet.105.083543. [DOI] [PubMed] [Google Scholar]
- Huang E, Reichardt L. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata Y, Shiraga H, Tai A, Kawamoto Y, Gohda E. Induction of neurite outgrowth in PC12 cells by the medium-chain fatty acid octanoic acid. Neuroscience. 2007;146:1073–1081. doi: 10.1016/j.neuroscience.2007.03.001. [DOI] [PubMed] [Google Scholar]
- LeBlanc AC. The role of apoptotic pathways in Alzheimer's disease neurodegeneration and cell death. Curr. Alzheimer Res. 2005;2:389–402. doi: 10.2174/156720505774330573. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu. Rev. Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Bymaster FP, Meltzer HY, et al. Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation and neuroprotection. Pharmacol. Rev. 2008;60:357–403. doi: 10.1124/pr.107.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Pirrung M, Deng L, Li Z, Liu Y, Webster NJ. Neuroprotection by small molecule activators of the nerve growth factor receptor. J. Pharmacol. Exp. Ther. 2007;322:59–69. doi: 10.1124/jpet.106.118034. [DOI] [PubMed] [Google Scholar]
- Lu XH, Bradley RJ, Dwyer DS. Olanzapine produces trophic effects in vitro and stimulates phosphorylation of Akt/PKB, ERK1/2, and the mitogen-activated protein kinase p38. Brain Res. 2004;1011:58–68. doi: 10.1016/j.brainres.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Lu XH, Dwyer DS. Second-generation antipsychotic drugs, olanzapine, quetiapine, and clozapine enhance neurite outgrowth in PC12 cells via PI3K/Akt, ERK, and pertussis toxin-sensitive pathways. J. Mol. Neurosci. 2005;27:43–64. doi: 10.1385/jmn:27:1:043. [DOI] [PubMed] [Google Scholar]
- Nozawa Y, Sakai N, Matsumoto K, Mizoue K. A novel neuritogenic compound, NGA0187. J. Antibiot. 2002;55:629–634. doi: 10.7164/antibiotics.55.629. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin A. Polypeptide delivery across the blood-brain barrier. Curr. Drug Targets: CNS Neurol. Disord. 2004;3:131–136. doi: 10.2174/1568007043482525. [DOI] [PubMed] [Google Scholar]
- Paul JW. 1,1,3 tricyano-2-amino-2-propene (Triap): a small molecule which mimics or potentiates nerve growth factor. Brain Res. Dev. Brain Res. 1990;55:21–27. doi: 10.1016/0165-3806(90)90101-4. [DOI] [PubMed] [Google Scholar]
- Ralets I, Ostergaard S, Holm A, Kohler L, Bock E, Berezin V. Identification of neurite extension inducing peptides by means of soluble combinatorial peptide libraries. J. Neurosci. Methods. 2004;137:61–69. doi: 10.1016/j.jneumeth.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Rasouly D, Rahamim E, Lester D, Matsuda Y, Lazarovici P. Staurosporine-induced neurite outgrowth in PC12 cells is independent of protein kinase C inhibition. Mol. Pharmacol. 1992;42:35–43. [PubMed] [Google Scholar]
- Ronn LC, Olsen M, Ostergaard S, et al. Identification of a neuritogenic ligand of the neural cell adhesion molecule using a combinatorial library of synthetic peptides. Nat. Biotech. 1999;17:1000–1005. doi: 10.1038/13697. [DOI] [PubMed] [Google Scholar]
- Sano M, Kohno M, Iwanaga M. The activation and nuclear translocation of extracellular signal-regulated kinases (ERK-1 and -2) appear not to be required for elongation of neurites in PC12D cells. Brain Res. 1995;688:213–218. doi: 10.1016/0006-8993(95)00558-8. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection and neural repair. Annu. Rev. Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- Wu Y, Bradshaw R. PC12-E2 cells: a stable variant with altered responses to growth factor stimulation. J. Cell. Phys. 1995;164:522–532. doi: 10.1002/jcp.1041640310. [DOI] [PubMed] [Google Scholar]
- Xiao J, Zhou Q, Liu Y. Variant PC12 cell line that spontaneously differentiates and extends neuritic processes. J. Neurosci. Res. 2002;69:104–109. doi: 10.1002/jnr.10260. [DOI] [PubMed] [Google Scholar]
- Yao R, Yoshihara M, Osada H. Specific activation of a c-Jun NH2-terminal kinase isoform and induction of neurite outgrowth in PC-12 cells by staurosporine. J. Biol. Chem. 1997;272:18261–18266. doi: 10.1074/jbc.272.29.18261. [DOI] [PubMed] [Google Scholar]
- Zhan SS, Bayreuther K, Schmitt HP. Quantitative assessment of the synaptophysin immuno-reactivity of the cortical neuropil in various neurodegenerative disorders with dementia. Dementia. 1993;4:66–74. doi: 10.1159/000107299. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY. Postnatal neurodevelopmental disorders: meeting at the synapse? Science. 2003;302:826–830. doi: 10.1126/science.1089071. [DOI] [PubMed] [Google Scholar]