Abstract
We aimed to optimize non-viral transfection of human stromal cell derived factor (SDF-1α) gene into skeletal myoblasts (SkM) and, transplant these cells to establish transient SDF-1α gradient to favor extra-cardiac stem cell translocation into infarcted heart. Optimized conditions for transfection of SDF-1α gene into syngenic SkM were achieved using FuGene™6/phSDF-1α (3:2v/w, 4h transfection) with 125μM ZnCl2 (p<0.001). After characterization for transgene overexpression by immunostaining, ELISA, and PCR, the cells were transplanted in female rat model of myocardial infarction. Thirty-six rats were grouped (n=12/group) to receive 70μl DMEM without cells (group-1) or containing 1.5×106 non-transfected (group-2) or SDF-1α transfected SkM (group-3). On day-4 post-transplantation (in 4 animals/group), marked expression of SDF-1α/sry-gene (p=0.003), total Akt, phospho-Akt and Bcl2 was observed in group-3. The number of CD31+, C-kit+ and CD34+ cells was highest in group-3 hearts (p<0.01). Blood vessel density in group-3 was higher in both scar and peri-scar regions (p<0.001) as compared with other groups. Echocardiography showed improved indices of left ventricle contractile function and remodeling in group-3 (p<0.05) as compared with groups-1 and -2. We conclude that ex-vivo SDF-1α transgene delivery promotes stem and progenitor cell migration to the heart, activates cell survival signaling and enhances angiomyogenesis in the infarcted heart.
Keywords: Angiogenesis, Apoptosis, Myoblast, Myocardial infarction, Myogenesis, Chemokine
Introduction
SDF-1α protein and gene delivery to the ischemic heart promotes endothelial progenitor cells recruitment into the ischemic myocardium to promote angiogenesis [1,2,3]. SDF-1α specifically interacts with the CXCR4 receptor and orchestrates the mobilization and homing of hematopoietic stem cells from bone marrow (BM) to the ischemic heart [3-6]. Physiologically, BM harbors a heterogenous population of cells positive for CXCR4 receptor. BM has the highest hSDF-1α concentration gradient as compared with other tissues, and promotes homing of circulating hematopoietic stem cells into BM stromal adult heart, SDF-1α is expressed constitutively. In the early phase after MI however, elevated SDF-1α levels have been reported in the infarct zone [7-8]. This provides the required stimulus for mobilization of stem cells from BM niches to the damaged site as a part of a natural repair process [9]. The other key players involved in tissue ischemia- induced mobilization of BM progenitors to the circulation include vascular endothelial growth factor, placenta growth factor, stem cell factor, and granulocyte colony stimulating factor [10-13]. The effect of intrinsic SDF-1α up-regulated expression is, however, transient and insufficient for cardiac repair [4, 6]. In order to overcome this deficiency, SDF-1α concentration gradient in favor of myocardial scar has been achieved by the delivery of SDF-1α protein or gene encoding for hSDF-1α. The delivery strategies include intramyocardial injection of SDF-1α protein [2], naked plasmid encoding for SDF-1α [3, 5, 6], transplantation of ex vivo genetically manipulated cardiac fibroblasts overexpressing SDF-1α [4] and mesothelial cell transplantation which intrinsically express copious amounts of SDF-1α [1, 14]. Moreover, intramuscular injections of GCSF (Filgrastim)4, the sulfated polysaccharide (Fucoidan) [15-16] or “3 hydroxy-3-methylglutaryl-CoA reductase inhibitor” (Atorvastatin) [17-18] have been reported to increase the plasma concentration of hSDF-1α to a level sufficient enough to induce mobilization of stem cells from BM and their homing into the injured tissue.
The study is aimed to explore the use of synthetic vectors (3 cationic lipid vectors; Lipofectamine™2000, FuGene™6 and Polyethyleneimine-JetPEI™ wih the addition of ZnCl2 for optimal transfection of hSDF-1α into SkM. We anticipate that overexpression of hSDF-1α is cytoprotective and enhances cell survival during the initial phase after transplantation. The donor SkM overexpressing hSDF-1α established a transient and localized hSDF-1α gradient in the heart that favored stem cell translocation into ischemic myocardium, and enhanced angiogenesis.
Materials and methods
SkM purification and culture in vitro
Skeletal muscle biopsies from syngenic male Fischer rats were dissected and roughly cut into small fragments in Dulbecco's modified Eagle's medium (DMEM, Gibco BRL). The tissue fragments were enzymatically dissociated using collagenase-IA (2 mg/ml, Gibco BRL) and dispase (2 mg/ml, Gibco BRL) for 1h followed by trypsin-EDTA (0.25%, Gibco BRL) for 20 min at 37°C. The cells were filtered through a 100-μm sieve (Cell Strainer Nylon) and collected by sedimentation at 1500 rpm for 5 min. The enzyme reaction was arrested by adding 10% FBS. The cells were re-suspended in DMEM with 20% FBS and 5μg/ml basic fibroblast growth factor (Sigma) and pre-plated at 1, 2, 12 and 24h to remove cell debris and contaminant populations of cells. The cells obtained from pre-plate 4 were propagated and identified by anti-desmin immunostaining prior to use in all experimental studies.
Preparation of hSDF-1α plasmid
We used an expression vector containing hSDF-1α gene (pORF-hSDF-1α) purchased from InvivoGen, USA. Preparation of the plasmid was performed per suppliers instructions. Briefly, the disk of lyophylized GT100 E. coli bacteria transformed by pORF-hHSDF-1α was dissolved in E. coli FastMedia™ agar medium containing ampicillin. The hSDF-1α gene, was cut out by using SgrA-I and Nhe-I enzymes, followed by Simian virus 40 late polyadenylation (SV40 pAn) and a minimal E. coli origin of replication pMB1-Ori that is necessary for the transcription process. The expression vector had ampicillin resistance gene that allowed the selection of bacteria carrying the pORF plasmid. Plasmid DNA-hSDF-1α was purified with Maxi-prep protocol (Qiagen) as per supplier's instructions. The purified plasmid was stored at -20°C until used for transfection of cells.
Transfection of SkM
SkM at 80-90% confluence were transfected with phSDF-1α using commercially available cationic vectors; Lipofectamine™2000 (Invitrogen), FuGene™6 (Roche) and Polyethyleneimine-JetPEI™ (Polyplus Transfection). For these vectors, transfection conditions were optimized at various DNA:vector ratios, in the presence of 0-100μM ZnCl2 (Merck, Germany) at 37 °C or room temperature. Cell viability was assessed with a modified MTT reduction test [14]. As maximum transfection efficiency with highest cell viability was achieved using FuGene™6/phSDF-1α (v/w) 3:2 ratio in basal DMEM containing 125μM ZnCl2 (for 4h at 37 °C), these transfection conditions were used throughout the study.
ELISA for hSDF-1α
The amount of hSDF-1α secreted by the transfected SkM in vitro and in the rat heart after transplantation was assessed by hSDF-1α ELISA kit (R&D systems) per supplier's instructions. For in vitro hSDF-1α expression, conditioned medium and cell lysate from transfected and non-transfected SkM were collected at regular time intervals after transfection and used for ELISA.
For in vivo hSDF-1α expression, whole left ventricle (LV) tissue samples from groups-2 and -3 were homogenized in 0.1M Tris, 4mM EDTA, 0.1% Triton X-100, pH 7.6 buffer, sonicated and centrifuged (11000g, 15min at 4°C) to extract hSDF-1α for quantification by ELISA. The results were expressed as amount of hSDF-1α/mg protein.
Western immunoblotting
Protein samples were fractionated by 12% SDS-polyacrylamide gel electrophoresis (ISC BioExpress) and electro-transferred onto a Nitrocellulose membrane. The membrane was blocked for 1h with 1×TBS blocking buffer (Cell Signaling Technology) and 5% nonfat dry milk, followed by incubation with gentle shaking at 4°C with anti-hSDF-1α (1:200, R&D System), Akt, and phospho-Akt (1:1000, Cell Signaling Technplogy) diluted in blocking buffer. The primary antibody reaction was detected by incubating for 1h with horseradish peroxidase (HRP)-conjugated secondary antibody (1:2000) and HRP-conjugated anti-biotin antidody (1:1000, Cell Signaling Technology). The membrane was washed and developed using LumiGLO® Peroxide reagent and exposed to X-ray for detection of the expressing bands.
Cell Labeling
For identification and tracking the fate of SkM post-transplantation, the cells were labeled with PKH-67 cell tracker dye (Sigma) as per manufacturer's instruction.
Rat heart model of MI and SkM transplantation
All experimental procedures were performed in accordance with the standard human care guidelines of the “Guide for the Care and Use of Laboratory Animals” and Institutional Animal Care and Use of Committee of University of Cincinnati, which conforms to National Institutes of Health guideline.
Acute MI model was developed in young female Fischer-344 rats by permanent occlusion of left anterior descending coronary artery (LAD) as described earlier [19]. The animals were grouped (n=12/group) to receive multiple intramyocardial injections of 70μl basal DMEM without SkM (group-1) or containing 1.5×106 non-transfected SkM (group-2) or hSDF-1α transfected SkM (group-3). The chest was closed and the animals were allowed to recover. Buprenex (0.1mg/kg b.i.d) was adiministered for 24h to alleviate pain. For post-mortem studies the animals were sacrificed using an overdose of sodium pentobarbitol. The heart was excised and sectioned into 5mm blocks. Cryosections of 6-8μm thickness were cut and stained with hematoxylin and eosin for visualization of muscle architecture. Masson trichome staining was carried out to delineate fibrous tissue from the normal tissue.
PCR for gene expression
Isolation of total RNA from the different treatment groups of rSkM, and their subsequent first strand cDNA synthesis was performed using RNeasy mini kit (QIAGEN) and Omniscript Reverse Transcription (QIAGEN) kit respectively as per manufacturer's instruction. Following primer sequences were used for PCR;
| hSDF-1α (270bp) | forward-5′CATGAACGCCAAGGTCGTG′3 |
| reverse-5′TCCAGGTACTCCTGAATCC′3 | |
| rat CXCR4 (229bp) | forward-5′GGTCATCAAGCAAGGATGTGA′3 |
| reverse-5′TGACTCTGTGGAGACGGAAGA′3 | |
| rat Akt (381bp) | forward-5′CCACGCACACTCGGGCCG′3 |
| reverse-5′CAATGCAGAGGGGTGCAGG′3 | |
| rat sry (115bp) | forward-5′GAGGCACAAGTTGGCTCAACA′3 |
| reverse-5′CTCCTGCAAAAAGGGCCTTT′3 | |
| ratGAPDH (738bp) | forward-5′TTCTTGTGCAGTGCCAGCCTCGTC3′ |
| reverse-5′TAGGAACACGGAAGGCCATGCCAG′3 |
MPCR kit for rat apoptotic genes set-2 (Maxim Biotech Inc.) was used as per manufacturer's instruction.
Immunohistochemical studies
Immunostaining was performed as earlier described [19]. The primary antibodies included mouse anti-hSDF-1α (10μg/ml, R&D Systems), and pre-diluted fast skeletal myosin heavy chain (fast isoform) (Abcam). The detection system was based on immunoperoxidase (Chemicon) followed by nuclear counterstaining by hematoxylin, or using fluorescence-labelled secondary antibodies conjugated with FITC, TRIRC or Alexa Fluor-594, goat anti-mouse IgG (H+L) (2 mg/ml) highly cross absorbed (Molecular Probe, USA).
For detection of the translocated stem cells, histological sections were immunostained with primary antibodies including anti-rat PECAM-1 (1:50, Chemicon), rat anti-mouse c-kit (1:30, Chemicon), CD34 (1:50, Santa Cruz), and rabbit anti-human von Willibrand Factor-VIII (vWF-VIII) (1:50, Chemicon). The total number of stem cells that migrated into the infarct and peri-infarct areas of the heart was determined by counting the cells positive for the respective surface marker in at least 3 slides from each heart.
For blood vessel density analysis, 4 animals per group were assessed after 4 weeks as previously described [19]. Briefly, tissue samples from the respective hearts (6μm thickness) were used for fluorescent immunostaining using vWF-VIII specific antibodies. Counting of blood vessels was carried out in the infarct and peri-infarct areas in the three animal groups. At least 2 microscopic fields each in the infarct and peri-infarct regions were randomly selected and counted in at least 2 tissue sections from each animal. Blood vessel density was expressed as the number of vessels at 200× high power magnification fields (0.74 mm2).
Heart function studies
LV function and dimension measurements were carried out at 4 weeks after cell transplantation. Transthoracic echocardiography was performed using Compact Linear Array probe CL10-5 on HDI/5000 SONOS CT (HP-Company). Each animal was anesthetized, the chest of the animal was shaven and a layer of acoustic coupling gel was applied to the thorax. The animal was placed in supine position. Echocardiograms were recorded through parasternal long-axis and short-axis views. From the correct image obtained with well-defined continuous interfaces of septum and posterior wall at higher frame rate, numeric acquisitions were obtained. Anterior and posterior end-diastolic and end-systolic wall thickness, LV end-systolic (LVESD) and end-diastolic LVEDD diameters were measured from at least three consecutives cardiac cycles. Indices of LV systolic functions including fractional shortening (LVFS) and ejection fraction (LVEF) were calculated using (LVEDD-LVESD)/LVEDD×100 and LVEF={(LVEDD3-LVESD3)/LVEDD3}×100 relations respectively and the results were expressed as percentage.
Statistical analysis
Statistical analysis was performed with Statview 5.0 software. All values were expressed as means± standard errors using the Student t-test, a one-way ANOVA or a two-way factorial ANOVA for repeated measures. p<0.05 was considered significant.
Results
1. In vitro Studies
a) Transfection of SDF-1α into SkM
SkM during passage 2-4 were >90% pure for desmin expression. The efficiency of phSDF-1α transfection into SkM was markedly influenced by vector/plasmid ratio and optimal efficiency was obtained with FuGene™6/phSDF-1α (v/w 3:2), Lipofectamine™2000/phSDF-1α (v/w 2.5:1) and JetPEI™/phSDF-1α (N/P=5). Short-term ZnCl2 exposure displayed dose-dependent cytotoxicity on SkM. However, cell viability observed with concentrations of <125μM ZnCl2 was significantly higher as compared with concentrations of >250μM (p<0.001). Similarly, ZnCl2 concentration of >125μM also caused abrupt reduction in hSDF-1α expression. Consequently, we chose 125μM ZnCl2 to optimize transfection procedures with FuGene™6, Lipofectamine™2000 and JetPEI™. We observed that cell viability insignificantly changed in the presence or absence of 125μM ZnCl2 (p<0.05) (data not shown).
The number of hSDF-1α positive SkM was markedly increased in the presence of 125μM ZnCl2 with FuGene™6, and JetPEI™ (FuGene™6, p=0.007; JetPEI™, p=0.004) as compared with the absence of ZnCl2 at 4 days after transfection (Figure 1A-C). We observed that transfection efficiency with FuGene™6 was higher than Lipofectamine™2000 and JetPEI™ from day-1 after transfection until 7 days of observation (p<0.001) (Figure 1D). Optimal transfection of SkM was obtained with FuGene™6/phSDF-1α at 3:2 (v/w) for 4h in the presence of 125μM ZnCl2 as assessed by ELISA for SDF-1α in conditioned medium and cell lysate. ELISA revealed that peak level hSDF-1α expression was observed between days 3-4 after transfection (30.1±6.5 ng/mg protein in cell lysate and 15.3±0.07ng/mg protein in supernatant) followed by a gradual decline until 12 days of observation in vitro (data not shown). The non-transfected SkM secreted negligible amounts of hSDF-1α (1.8±0.1 ng/mg protein in cell lysate vs 0.67±0.1 ng/mg protein in the supernatant) (Figure 2A). RT-PCR showed that hSDF-1α expression in SkM transfected in the presence of ZnCl2 was significantly higher as compared with the ones transfected in the absence of ZnCl2 (p<0.001) using non-transfected SkM as control (p<0.01) (Figure 2B-C).
Figure-1.
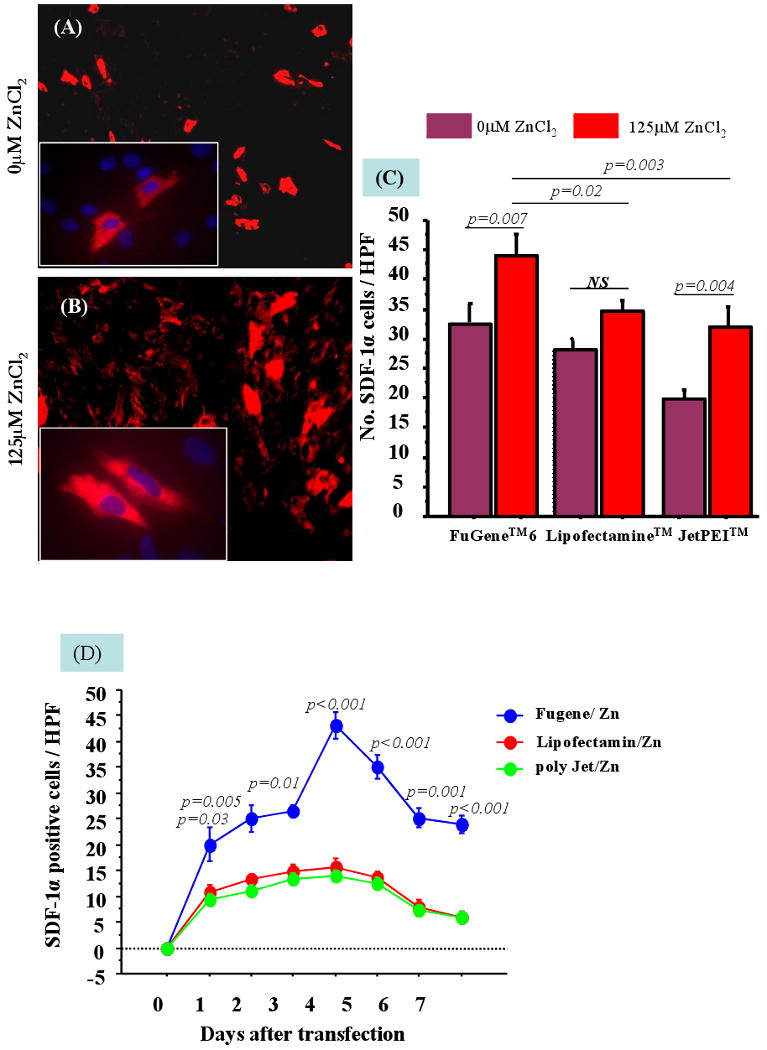
Immunostaining of SkM for hSDF-1α overexpression in vitro after transfection with FuGene™6/phSDF-1α (A) in the absence of 125μM ZnCl2 or (B) in the presence of 125μM ZnCl2. (C) The addition of 125μM ZnCl2 significantly improved the transfection efficiency of FuGene™6, Lipofectamine™2000 and JetPEI™. (D) The highest transfection and expression of SDF-1α in the presence of 125μM ZnCl2 was achieved with FuGene™6 until 7 days of observation.
Figure-2.
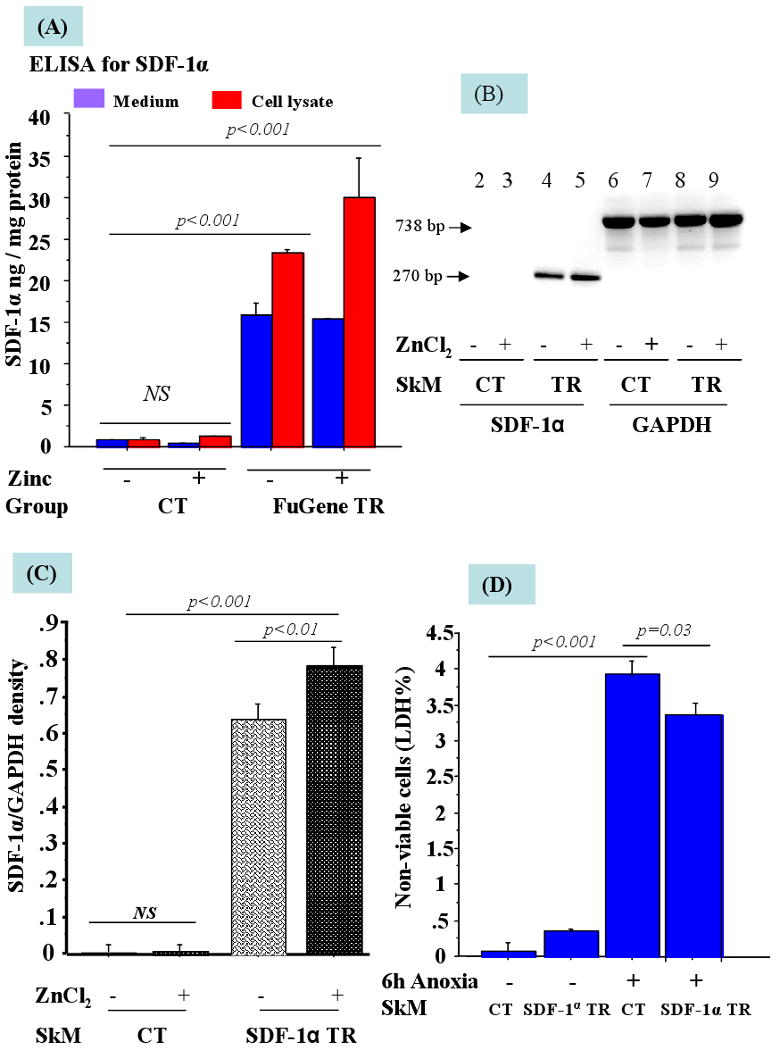
(A) ELISA for SDF-1α in the supernatant and cell lysate from SkM tranfected with FuGene™6/phSDF-1α in the presence and absence of ZnCl2. The level of hSDF-1α (ng of hSDF-1α /mg of total protein) was significantly higher in the cell lysate as compared with the cell supernatant. (B) Expression of hSDF-1α in non-transfected and transfected SkM without (-) or with (+) 125μM ZnCl2. Lanes 2-5 show PCR product of hSDF-1α and Lanes (6-9) show GAPDH. (C) Densitometry for hSDF-1α to GAPDH ratio from 3 separate experiments is shown as arbitrary units. (D) Overexpression of hSDF-1α in SkM enhanced their resistance to anoxia when SkM were exposed to 6h anoxia as shown by significantly improved cell viability by LDH assay. (CT= Control; TR= Transfected).
b) Cytoprotective effects of SDF-1 against anxoia
LDH release as a marker of cellular injuury, was significantly higher in the non-transfected SkM upon exposure of cells to 6h anoxia as compared with hSDF-1α transfected SkM (p=0.03) (Figure 2D).
2. In vivo Studies
a) Donor cell survival and in vivo gene and protein expression
PCR for sry-gene in the female heart revealed extensive survival of the donor male SkM. Densitometry revealed 5-folds higher sry-gene expression in group-3 as compared with group-2 at 4 days after cell transplantation (Figure 3A). Group-1 (control) did not show sry-gene. In agreement with improved survival of transfected SkM, expression of mRNA-hSDF-1α was also increased significantly in the LV tissue in group-3 as compared with group-2 on day-4 of observation. When normalized with the sry-gene expression, there was 4-fold (p<0.003) increase in the expression of SDF-1α in group-3 as compared to group-2 (Figure 3A-B). These observations vividly imply that the transplanted SkM continued to overexpress hSDF-1α after transplantation at the site of the cell graft. ELISA specific for hSDF-1α was perfomed on the LV tissue to show the level of hSDF-1α secretion. The hSDF-1α protein level was 251±3.5 pg/mg protein in group-3 which was significantly higher as compared with group-2 (107±6.5 pg/mg protein) (p<0.001) on day-4 after cell transplantation (Figure 3C). These results were confirmed by Western-blotting which showed 1.5-fold elevated level of hSDF-1α protein in group-3 as compared with group-2 (Figure 3D).
Figure-3.

The effect of SDF-1α overexpression in the rat heart. (A) Significantly higher sry-gene in group-3 was observed as compared with group-2. (B) The ratio of hSDF-1α to sry-gene was significanly higher in group-3 as compared with group-2 thus indicating a relationship between hSDF-1α expression with the survival of SkM in the rat heart. (C) ELISA showed significantly higher hSDF-1α (pg/mg of total protein) in group-3 as compared with group-2 at day 4 after SkM transplantation. (D) Western-blotting of rat heart ptrotein extract showed that besides elevated hSDF-1α expression, higher total Akt and phospho-Akt was observed in group-3 as compared with group-2 on day 4 posttransplantation. (E-F) Akt and Bcl2 gene expression normalized with GAPDH was also higher in group-3.
Another important observation was the up-regulated expression of total Akt and phospho-Akt in group-3 (Figure 3D). Similar results were obtained for Akt gene expression by RT-PCR (Figure 3E). Multiplex PCR for apoptotic gene cascade showed that Bcl2 expression was higher in group-3 as compared with group-2 thus indicating a possible role for Akt signaling in hSDF-1α mediated survival of the donor SkM after transplantation (Figure 3F).
b) Stem and progenitor cell homing into myocardium
The heart tissue sections were immunostained for the presence of ckit+ (CD117), CD34+, CD31+ and vWF-VIII positive cells in different groups. The number of cells expressing these surface markers was significantly higher in the infarct and peri-infarct areas as compared with the contra-lateral areas in the heart of transfected SkM transplanted animal group-3 as compared with non-transfected SkM transplanted animal group-2 (p<0.001) (Figure 4A-B).
Figure-4.
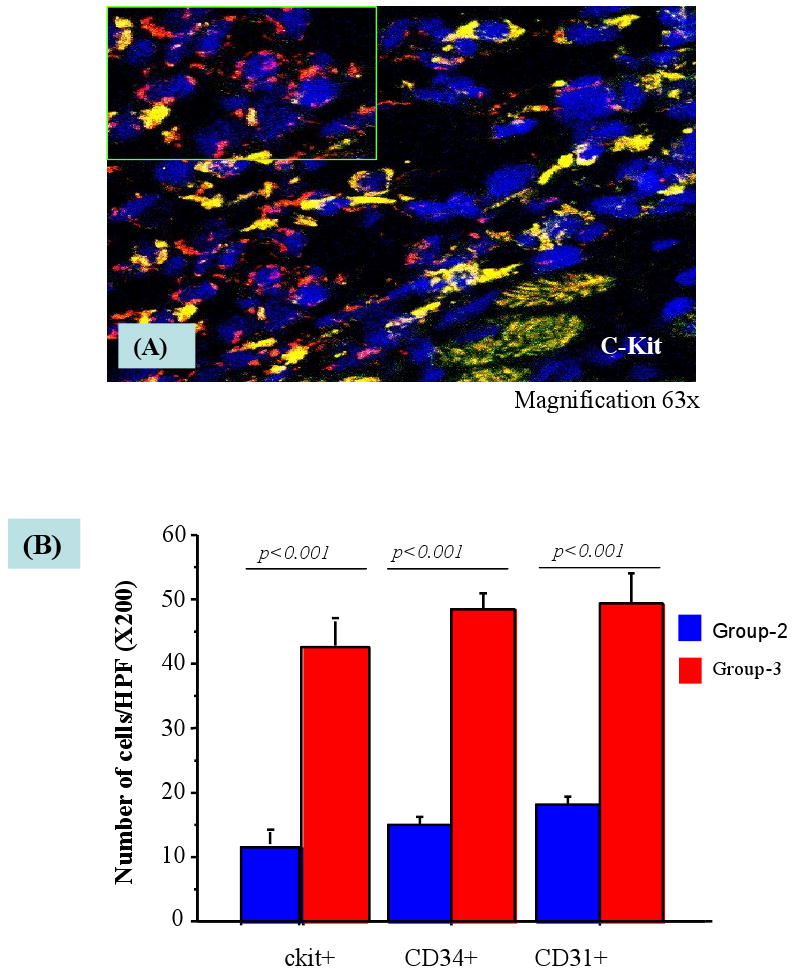
Confocal image after immunostaining of rat heart tissue grafted with SkM (PKH-67 labeled, green) and enriched with ckit+ cells (red fluorescence 63× oil immersion) with their nucleus stained with DAPI (blue) in group-3. The number of ckit+, CD34+ and CD31+ were counted per high power field.
c) Effect of SkM transfection on myogenesis and angiogenesis in the heart
The PKH-67 labeled SkM were seen extensively engrafted in the infarct and peri-infarct regions after implantation in both groups-2 and -3 (Figure 5A-B). Immunostaining for skeletal myosin heavy chain (fast-isoform) showed increased DAB-immunoperoxidase staining in infarct as well as peri-infarct regions in group-3 as well as group-2 (Figure 5C-E), indicating their myogenic differentiation.
Figure-5.

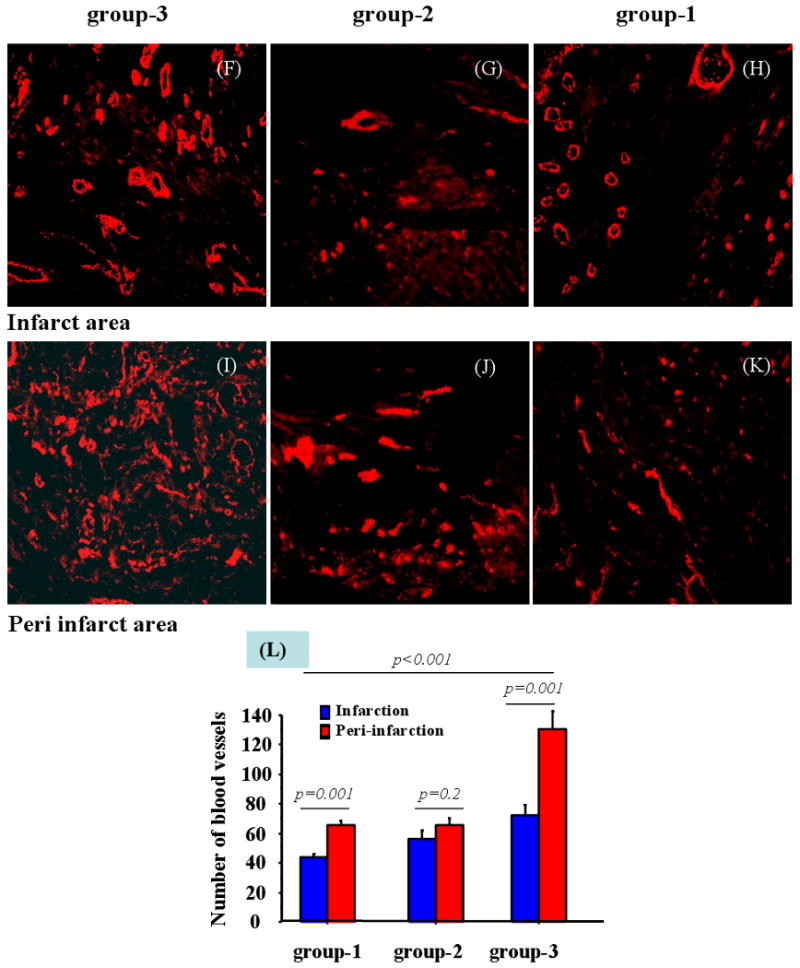
Assessment of survival and angiomyogenesis in the infarcted myocardium. (A-B) Extensive survival of PKH-67 labeled SkM was observed in group-3 in the infarcted myocardium. (C-E) Immunostaining of rat heart tissue for fast skeletal myosin heavy chain expression showed extensive myogenesis in the infarct and peri-infarct areas in both (D) group-2 and (E) group-3 using (C) group-1 as control. Blood vessel density was assessed in the infarct (F-H) and peri-infarct areas (I-K) in all the groups which showed that (L) blood vessel density was significantly higher in group-3 as compared with groups-1 and -2.
The angiogenic effect of hSDF-1α overexpression was measured in terms of blood vessel density in the center and peri-infarct regions after immunostaining for vWF-VIII (Figure 5F-L). Blood vessel counts per area (0.74 mm2) was highest in peri-infarct (130±7.1) and infarct (71.9±3.7) regions in group-3. This was significantly higher (p<0.001) as compared with groups-1 and -2. Although the number of blood vessels observed in group-2 was higher as compared with group-1, no significant difference was observed between these groups either in the infarct or peri-infarct regions. Moreover, infarct size was significantly reduced in group-3 as compared with group-1 (p=0.001) and group-2 (p=0.036) (Figure 6A-D).
Figure 6.
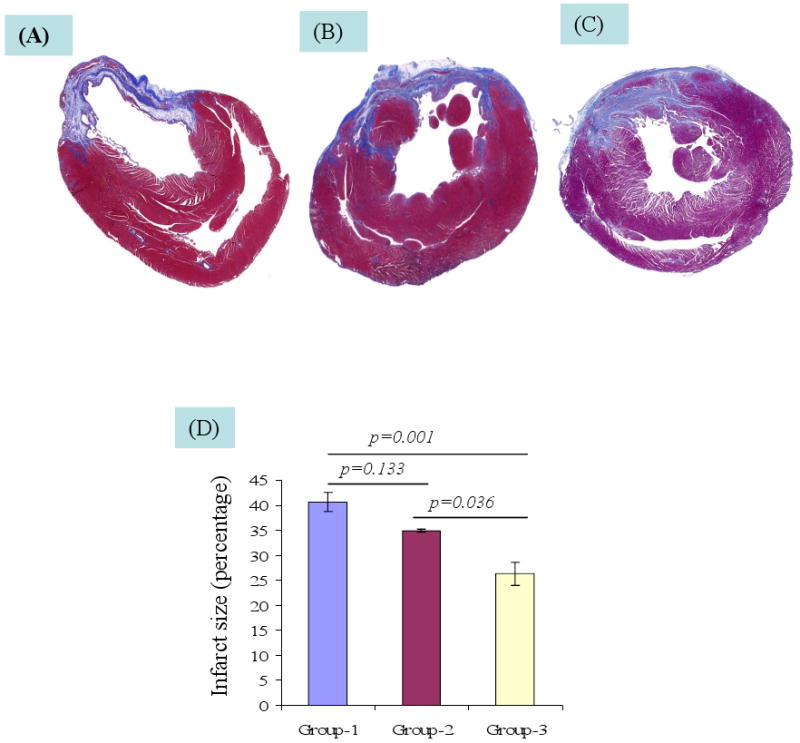
Infarction size was measured after Masson trichome staining of the rat heart tissue sections (A) DMEM injected group-1 (B) non-transfected rSkM transplanted group-2 (C) SDF-1α transfected group-3. (D)Whereas the infarct size changed insignificantly in group-2 as compared with group-1 (p=0.133), significantly reduced infarct size was observed in group-3 as compared with group-1 (p=0.001) and group-2 (p=0.036).
d) Heart function studies
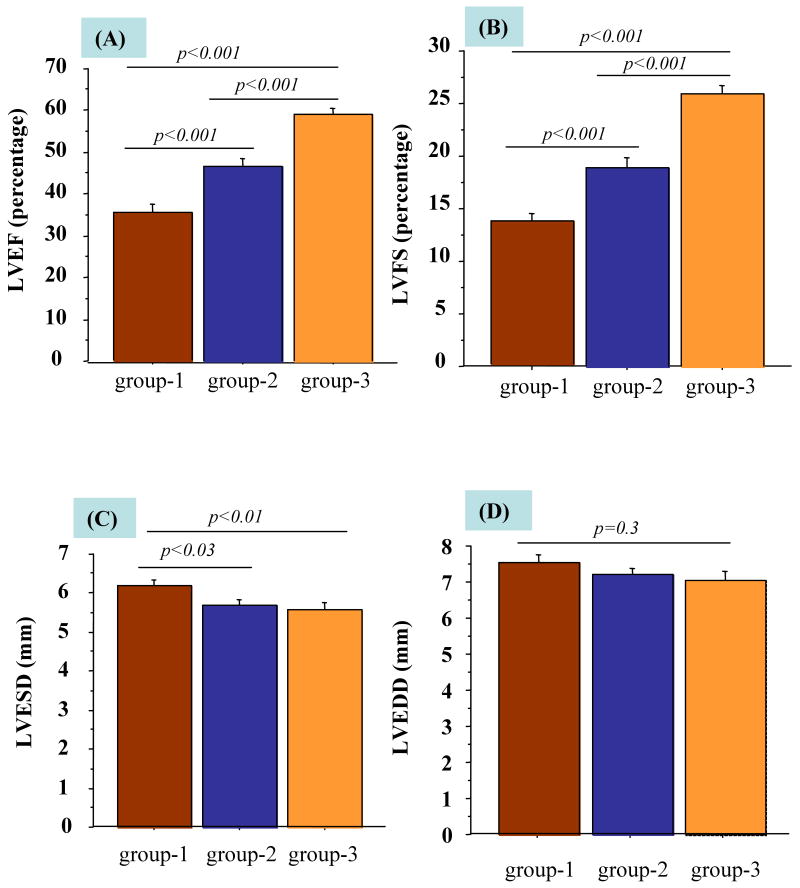
Sonography for LV function revealed significant differences amongst all the groups at 4-weeks. LVEF and LVFS in transfected SkM transplanted group-3 were significantly improved (59.2±3.6% and 25.9±2.2) as compared with non-transfected SkM transplanted group-2 (46.7±4.3% and 18.0±2.2%) and DMEM injected group-1 (35.3±5.9% and 13.6±2.7%) respectively (Figure 7A-B). Similarly, significant difference in LVEF and LVFS was observed between group-2 and group-1. Moreover, LVESD, a marker of LV remodeling, was significantly decreased in group-3 (5.5±0.2 vs 6.2±0.1mm in group-1, p<0.01) (Figure-7C). However, LVEDD changed insignificantly between the groups (p=0.3) (Figure 7D). Posterior wall and septum thickness were significantly increased in group-3 versus group-1 and -2 (p<0.001).
Figure 7.
Echocardiographic assessment of heart function. (A) LVEF (B) LVFS (C) LVESD and (D) LVEDD were compared in all the groups after 4 weeks of respective treatment.
Discussion
The main findings of our study were that (1) presence of ZnCl2 significantly enhanced the transfection of phSDF-1α into SkM (2) Overexpresion of hSDF-1α rendered the cells resistant to anoxia in vitro and promoted their survival after transplantation (3) higher gradient of hSDF-1α at the site of the cell graft triggered homing of stem and progenitor cells into the infarcted myocardium and (4) transplantation of hSDF-1α overexpressing SkM promoted angiogenesis in the infarcted region and improved indices of LV-function and reverse-remodeling.
Mobilization and homing of BM derived and resident cardiac stem cells into the infarcted myocardium is the first step in the naturally occurring repair process. These mobilized stem cells subsequently differentiate to adopt cardiac phenotype [20, 21]. There are reports defining a possible role for SDF-1α in post MI recruitment of extra-cardiac stem cells [6, 22]. Nevertheless, the intrinsic up-regulated expression of SDF-1α in response to MI is insufficient and short-lived. Hence, forced overexpression of SDF-1α can result in pronounced stem cell homing to the infarcted heart and to ensure their participation in the repair process. Such findings may have significant clinical implications.
SDF-1α binds to CXCR4 receptor and modulates several biological functions through signal transduction pathways. These include increased cell growth, proliferation, cell survival and anti-apoptosis, emigrational and transcriptional activation [23-24]. Furthermore, SDF-1α is co-participant in angiogenesis that is regulated at the receptor level by VEGF and bFGF [25]. Mobilization of angio-competent cells from BM has been achieved by various growth factors and cytokines, either alone or in combination. However, for effective participation in the repair process, their retention at the site of injury is imperative. A recent study by Graunewald et al. (2006) has defined a novel role for SDF-1α as a “retention factor” for BM cells [26]. This ensures that the recruited BM cells stay at the site of recruitment for the duration which is long enough to pledge their participation in the on-going angiogenic cascade. Taken together, SDF-1α participates in the cardiac repair process either directly or via secondary cytokine expression. We observed that forced overexpression of hSDF-1α led to elevated levels of angiogenic factors together with activation of cell survival signaling. There were elevated levels of total Akt and phospho-Akt together with Bcl2 in the hearts transplanted with SDF-1α transfected SkM. Thus cell survival signaling by Akt/Bcl2 led to enhanced cell survival under anoxic conditions in vitro as well as in vivo after transplantation.
Various strategies have been adopted to achieve overexpression of SDF-1α in the heart. Direct gene therapy necessarily encounters a delay between gene delivery and expression which renders it less attractive as an acute-phase therapeutic option. We posit that acute phase delivery of SDF-1α gene will mitigate the ongoing intrinsic molecular cascade involved in the cell death and fibrous scar formation in the ischemic myocardium. In this regard, viral vectors may show some efficiency in gene transfer to the necrotic myocardium which otherwise is resistant to direct gene transfer [27-28]. However, the strategy is not without untoward effects. Ex vivo genetic manipulation of donor cells using non-viral vector is a safe alternative and provides a tissue specific repository of the transgene product [29]. Additionally, the donor cells carrying the transgene will undergo milieu dependent differentiation for de novo myocardial regeneration and preservation of LV-function. SkM in this regard are excellent carriers of transgenes and have been extensively used for targeted delivery of therapeutic genes to different tissues and organs including the heart [30-32]. We have previously shown that SkM based delivery of VEGF and angiopoietin-1 transgenes to infarcted heart, either alone or in combination, gave improved neovascularization [33]. Drifting away from the use of viral vectors to alleviate safety and immunological concerns; we have optimized conditions for gene modulation of cells using cationic lipids in the presence of ZnCl2 [14]. Our optimized procedure significantly enhanced the efficiency of hSDF-1α transfection into otherwise transfection resistant cells. The present study showed that hSDF-1α transfection and expression was consistently increased with FuGene™6 in the presence of ZnCl2. However, the mechanism by which ZnCl2 increases cell transfection efficiency is not clearly established. ZnCl2 may act synergistically with cationic polymers to compact the plasmid DNA, or may facilitate DNA entry into cytosol via fusion of the negatively charged DNA with cell membrane. It also inhibits endonuclease activity and prevents pDNA degradation in the cytoplasm thus increasing the probability of pDNA reaching to the nucleus for more stable expression [34].
The elevated levels of hSDF-1α in and around the area of infarct elicited strong signals for homing of the extra-cardiac stem and progenitor cells into the heart including CD31, CD34 and CD117. We have recently shown a significant role of SDF-1α in the accumulation of stem cells in the heart after MI. The population of extra-cardiac cells homing into the injured myocardium was identified by their surface markers [22]. In another recently published work from our lab, we have shown that the mobilized cells exerted paracrine effects via expression of various growth factors and cytokines. This promoted cell growth, proliferation, survival and anti-apoptosis, migration and transcriptional factor activation [35]. Together with the factors from cell survival signaling cascade including Akt and Bcl2, these cellular and biochemical changes in the infarcted heart promoted better cell survival via anti-apoptotic effects, myogenesis and improved neovascularization.
Finally, we have shown significant improvement of cardiac function after hSDF-1α/SKM therapy, with preservation of LVEF%, LVFS% and a decrease in LVESD in the hearts transplanted with transfected cells. An ideal therapeutic intervention for MI requires improved reperfusion of the ischemic myocardium by restoration of regional blood flow together with compensation for cardiomyocytes loss in order to restore myocardial contractility. There is evidence in literature that the cells overexpressing angiogenic growth factors show better survival and neo-vessel formation after transplantation and improve cardiac function by limiting the remodeling process in the scar and/or decreasing apoptosis of hypertrophied myocytes in the peri-infarct region.
In conclusion, our results depict successful and efficient non-viral vector transfection of SkM with hSDF-1α using FuGene™6 in the presence of ZnCl2 and show that transplantation of these transfected SkM in vivo is a useful procedure in the development of techniques of indirect gene therapy after MI. In line with the results of a previous study which showed that combined intramyocardial delivery of SkM and BM cells gave superior prognosis as compared with either of the two [36], our approach indirectly harnesses the beneficial effects of SkM and BM cells, the two potential candidate cell types for heart cell therapy, together with cytokine therapy. Moreover, we demonstrate that the combination of therapeutic gene transfer with cell therapy may represent a promising new avenue of investigation in the treatment of patients with ischemic LV dysfunction.
Acknowledgments
This work was supported by National Institutes of Health grants # R37-HL074272; HL-23597 and HL-080686 [to M.A].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Elmadbouh I, Chen Y, Louedec L, Silberman S, Pouzet B, Meilhac O, et al. Mesothelial cell transplantation in the infarct scar induces neovascularization and improves heart function. Cardiovasc Res. 2005;68:307–17. doi: 10.1016/j.cardiores.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–8. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 3.Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S, et al. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109:2454–61. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- 4.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. 2003. [DOI] [PubMed] [Google Scholar]
- 5.Tang YL, Qian K, Zhang YC, Shen L, Phillips MI. Mobilizing of haematopoietic stem cells to ischemic myocardium by plasmid mediated stromal-cell-derived factor-1alpha (SDF-1alpha) treatment. Regul Pept. 2005;125:1–8. doi: 10.1016/j.regpep.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–5. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 7.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–8. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 8.Pillarisetti K, Gupta SK. Cloning and relative expression analysis of rat stromal cell derived factor-1 (SDF-1)1: SDF-1 alpha mRNA is selectively induced in rat model of myocardial infarction. Inflammation. 2001;25:293–300. doi: 10.1023/a:1012808525370. [DOI] [PubMed] [Google Scholar]
- 9.De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of BM progenitor cells. Blood. 2004;104:3472–82. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 10.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. Embo J. 1999;18:3964–72. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 12.Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002;8:831–40. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 13.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–9. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elmadbouh I, Rossignol P, Meilhac O, Vranckx R, Pichon C, Pouzet B, et al. Optimization of in vitro vascular cell transfection with non-viral vectors for in vivo applications. J Gene Med. 2004;6:1112–24. doi: 10.1002/jgm.604. [DOI] [PubMed] [Google Scholar]
- 15.Luyt CE, Meddahi-Pelle A, Ho-Tin-Noe B, Colliec-Jouault S, Guezennec J, Louedec L, et al. Low-molecular-weight fucoidan promotes therapeutic revascularization in a rat model of critical hindlimb ischemia. J Pharmacol Exp Ther. 2003;305:24–30. doi: 10.1124/jpet.102.046144. [DOI] [PubMed] [Google Scholar]
- 16.Sweeney EA, Lortat-Jacob H, Priestley GV, Nakamoto B, Papayannopoulou T. Sulfated polysaccharides increase plasma levels of SDF-1 in monkeys and mice: involvement in mobilization of stem/progenitor cells. Blood. 2002;99:44–51. doi: 10.1182/blood.v99.1.44. [DOI] [PubMed] [Google Scholar]
- 17.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–90. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 18.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–7. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang S, Haider HKh, Niagara MI, Salim A, Ashraf M. Supportive interaction between cell survival signaling and angio-competent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Cir Res. 2006;99:776–784. doi: 10.1161/01.RES.0000244687.97719.4f. [DOI] [PubMed] [Google Scholar]
- 20.Linke A, Müller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. PNAS. 2005;102:8966–71. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Haider HKh, Ahmad N, Zhang D, Ashraf M. Evidence for ischemia induced host-derived bone marrow cell mobilization into cardiac allografts. J Mol Cell Cardiol. 2006;41:478–87. doi: 10.1016/j.yjmcc.2006.06.074. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Haider HK, Ahmad N, Xu M, Ge R, Ashraf M. Combining pharmacological mobilization with intramyocardial delivery of bone marrow cells over-expressing VEGF is more effective for cardiac repair. J Mol Cell Cardiol. 2006;40:736–45. doi: 10.1016/j.yjmcc.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, Qin S, et al. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem. 1998;273:23169–75. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- 24.Oonakahara K, Matsuyama W, Higashimoto I, Kawabata M, Arimura K, Osame M. Stromal-derived factor-1alpha/CXCL12-CXCR 4 axis is involved in the dissemination of NSCLC cells into pleural space. Am J Respir Cell Mol Biol. 2004;30:671–7. doi: 10.1165/rcmb.2003-0340OC. [DOI] [PubMed] [Google Scholar]
- 25.Mirshahi F, Pourtau J, Li H, Muraine M, Trochon V, Legrand E, et al. SDF-1 activity on microvascular endothelial cells: consequences on angiogenesis in in vitro and in vivo models. Thromb Res. 2000;99:587–94. doi: 10.1016/s0049-3848(00)00292-9. [DOI] [PubMed] [Google Scholar]
- 26.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Yung S, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–89. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 27.Murry CE, Kay MA, Bartosek T, Hauschka SD, Schwartz SM. Muscle differentiation during repair of myocardial necrosis in rats via gene transfer with MyoD. J Clin Invest. 1996;98:2209–17. doi: 10.1172/JCI119030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Etzion S, Battler A, Barbash IM, Cagnano E, Zarin P, Granot Y, et al. Influence of embryonic cardiomyocyte transplantation on the progression of heart failure in a rat model of extensive myocardial infarction. J Mol Cell Cardiol. 2001;33:1321–30. doi: 10.1006/jmcc.2000.1391. [DOI] [PubMed] [Google Scholar]
- 29.Armeanu S, Pelisek J, Krausz E, Fuchs A, Groth D, Curth R, et al. Optimization of nonviral gene transfer of vascular smooth muscle cells in vitro and in vivo. Mol Ther. 2000;1:366–75. doi: 10.1006/mthe.2000.0053. [DOI] [PubMed] [Google Scholar]
- 30.Askari A, Unzek S, Goldman CK, Ellis SG, Thomas JD, et al. Cellular, but not direct, adenoviral delivery of vascular endothelial growth factor results in improved left ventricular function and neovascularization in dilated ischemic cardiomyopathy. J Am Coll Cardiol. 2004;43:1908–14. doi: 10.1016/j.jacc.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 31.Yau TM, Li G, Weisel RD, Reheman A, Jia ZQ, Mickle DA, et al. Vascular endothelial growth factor transgene expression in cell-transplanted hearts. J Thorac Cardiovasc Surg. 2004;127:1180–7. doi: 10.1016/j.jtcvs.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki K, Murtuza B, Smolenski RT, Sammut IA, Suzuki N, Kaneda Y, et al. Cell transplantation for the treatment of acute myocardial infarction using vascular endothelial growth factor-expressing skeletal myoblasts. Circulation. 2001;104:I207–12. doi: 10.1161/hc37t1.094524. [DOI] [PubMed] [Google Scholar]
- 33.Lei Y, Haider HK, Jiang S, Tan RS, Ge R, Law PK, et al. Improved angiogenic response in pig heart following ischaemic injury using human skeletal myoblast simultaneously expressing VEGF(165) and angiopoietin-1. Eur J Heart Fail. 2006 doi: 10.1016/j.ejheart.2006.04.008. available online. [DOI] [PubMed] [Google Scholar]
- 34.Pichon C, Guerin B, Refregiers M, Goncalves C, Vigny P, Midoux P. Zinc improves gene transfer mediated by DNA/cationic polymer complexes. J Gene Med. 2002;4:548–59. doi: 10.1002/jgm.303. [DOI] [PubMed] [Google Scholar]
- 35.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–21. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 36.Memon IA, Sawa Y, Miyagawa S, Taketani S, Matsuda H. Combined autologous cellular cardiomyoplasty with skeletal myoblasts and bone marrow cells in canine hearts for ischemic cardiomyopathy. J Thorac Cardiovasc Surg. 2005;130:646–53. doi: 10.1016/j.jtcvs.2005.02.024. [DOI] [PubMed] [Google Scholar]