Abstract
This unit presents two basic protocols that offer rapid assessments of anosmia (the absence of a sense of smell) in mice. The buried food test is used to check for the ability to smell volatile odors. The olfactory habituation/dishabituation test is used to test whether the animal can detect and differentiate different odors, including both non-social odors and social odors. A non-contact method of odor presentations, along with a general method of collecting urine samples, is given as the alternate protocol. The tests described in this unit can be readily adapted and require only the most basic equipment.
Keywords: olfaction, mouse, anosmia, buried food test, habituation/dishabituation test
Unit Introduction
Olfactory information is essential for a wide range of mouse behaviors, including navigating, foraging, avoiding predators, kin recognition, bond formation, mate selection, sexual and parental behaviors (Doty, 1986; Schellinck et al., 1993; Brennan, 2004; Keverne, 2004; Restrepo et al., 2004; Kavaliers et al., 2005). Many behavioral tasks designed for mice depend on olfactory cues, including social choice tests and some learning and memory tests. In many other mouse behavioral tasks, olfactory deficits would interfere with performance and produce false positive results. Accurate assessment of olfaction is critical for proper interpretation of various mouse behaviors, especially within the social domain. This unit presents two protocols that offer rapid assessments of anosmia (lack of the sense of smell) in mice.
The buried food test (see BASIC PROTOCOL 1), which relies on the animal's natural tendency to use olfactory cues for foraging, is used to confirm ability to smell volatile odors. The main parameter is the latency to uncover a small piece of chow, cookie, or other palatable food, hidden beneath a layer of cage bedding, within a limited amount of time. The olfactory habituation/dishabituation test (see BASIC PROTOCOL 2), which relies on the animal's tendency to investigate novel smells, is used to test whether the animal can detect and differentiate different odors. Both non-social odors and social odors can be used as stimuli. An Alternate Protocol, which describes methods of non-contact odor presentations and urine collection, is provided for the BASIC PROTOCOL 2.
NOTE: All protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) and must follow officially approved procedures for the care and use of laboratory animals.
Basic Protocol 1
Buried Food Test
The buried food test measures how quickly an overnight-fasted animal can find a small piece of familiar palatable food, such as cookies, cereal, chocolate chips, food pellets, that is hidden underneath a layer of bedding. The assumption is that food-restricted mice which fail to use odor cues to locate the food within a 15 minute period are likely to have deficits in olfactory abilities. The majority of mice with normal olfaction can find the hidden food piece within a few minutes.
Materials
-
Mice: at least 6 weeks of age
Juveniles may respond differently to food deprivation.
Clean mouse cages (standard plastic cages, 46 cm L × 23.5 cm W × 20 cm H, or similar).
Cage lids. The stainless steel top which holds food and water interferes with observation and should not be included in the setup.
-
Fresh cage bedding to create a 3 cm layer in each cage.
For each subject, a clean cage (washed and dried by the animal care cage washing facility) and clean bedding should be used. DO NOT re-use cages or bedding. The cage lid can be reused.
Food stimulus. Teddy Grahams (Nabisco, Hanover, New Jersey) or another mini cookie of a uniform size is preferred. Since this kind of cookie does not have to be broken into small pieces, there is minimal scatter of cookie crumbs, which could mislead the subject. Other choices for the food stimulus include sweetened cereal such as Froot Loops (Kellogg's, Battle Creek, Michigan), chocolate chips, squares of hard cheese, or small pellets of standard chow. Particles should weigh about 1.5 grams.
Digital timer. To monitor the test length (Maximum = 15 min)
-
Stopwatches
The noise generated when the timer stopwatch is clicked is likely to distract subjects. To silence a stopwatch, cut the relevant wire inside the stopwatch with a wire cutter.
Odor familiarization
1. For two to three consecutive days before the test, put a piece of cookie (exactly the same kind as will be used in the test) in subjects' cages. Check the cages the following morning to see if the cookie has been consumed. If the cookie is not consumed, it may not be highly palatable to the mice. In that case, choose another food stimulus and repeat step 1 until a palatable food has been determined.
Food deprivation
2. 18-24 hours before the test, remove ALL chow pellets from the food hopper of the home cage. Check inside of the cage and remove any pellet fragments that may be scattered within the cage. Do not remove the water bottle.
The buried food test is more sensitive when the animals are given an overnight fast rather than moderate food deprivation (Dawson et al., 2005). Food will be replaced upon the completion of the buried food test.
Scoring the latency to find the cookie
3. The test begins by placing a subject mouse in a clean cage (46 cm L × 23.5 cm W × 20 cm H) containing 3 cm deep of clean bedding. The subject is allowed to acclimate to the cage for 5 min.
It is feasible for one investigator to test two subjects at the same time. If this is the case, make sure to place the two cages at least 0.5 m apart to ensure that mice in the two cages do not influence each other's behaviors. Prepare a timer and a stop watch for each cage. Do not remove the subject that finds the cookie first. Wait until both subjects have completed the test before removing them, to avoid disrupting the second mouse.
4. Transfer the subject to an empty clean cage. In the cage containing bedding, bury the food stimulus approximately 1 cm beneath the surface, in a random corner of the cage. Smooth out the surface.
5. Re-introduce the subject to the cage, replace the cage lid. The observer then retreats to the observation station (which should be about 2 m away from the cage). Start the timer and the stopwatch, and then stop the stopwatch when the subject mouse finds the buried food. A subject is considered to have uncovered the cookie when it starts to eat, usually holding the food with forepaws.
Record the latency to find the cookie and take notes on whether the food was consumed. If the subject fails to find the buried food after 15 minutes have elapsed, stop the test and record 900 sec as its latency score.
Another useful parameter to determine is the percentage of subjects that fail to find the cookie within the 15 min time limit. Derived statistical analysis has been described (Luo et al., 2002). If the animals are tested for multiple trials in this task, compare the latency to find the stimulus across trials as well as between groups (Dawson et al., 2005).
Basic Protocol 2
The Olfactory Habituation/Dishabituation Test
This test consists of sequential presentations of different odors. A commonly used sequence is water, two non-social odors, and two social odors. Each odor (or water) is presented in three consecutive trials for a duration of 2 min. The inter-trial interval is 1 min, which is about the amount of time needed to change the odor stimulus (Wesson et al., 2008). Habituation is defined by a progressive decrease in olfactory investigation (sniffing) towards a repeated presentation of the same odor stimulus. Dishabituation is defined by a reinstatement of sniffing when a novel odor is presented (Woodley and Baum, 2003; Wrenn et al., 2004; Wersinger et al., 2007). This test assesses whether an animal can smell and if it is able to distinguish same and different odors. The method described here involves minimal inter-trial manipulations. Since the odor stimulus (the cotton tip part of the applicator) does not touch any part of the cage or cage lid, cross-trial contamination is easily avoided. A disadvantage of the basic method is that the animals could gnaw and even pull the applicator, rendering this method unsuitable for experiments in which highly attractive odors (such as those of the opposite sex or palatable food) are used as odor stimuli. See the Alternate Protocol for a non-contact odor presentation method.
Materials
Mice: 6-weeks of age or older (sexually mature).
One stopwatch (silenced, as indicated in Basic Protocol 1) to record sniffing time.
One digital timer to register the 2 min trial duration.
-
Cotton-tipped wooden applicators (6 in. length, Solon Manufacturing Company, Solon, Maine), or similar.
Prepare a sufficient number of applicators on the day of the experiment.
Regular mouse cages, contain only clean fresh wood chip bedding.
Clean plastic cage lids, one for each cage.
Glass vials for water and solutions. Label the vials clearly and keep them tightly capped when not in use.
-
Odor stimuli
-
Non-social odors: (1) distilled water, (2) almond extract (McCormick, Hunt Valley, MD; 1:100 dilution), (3) banana extract (McCormick, Hunt Valley, MD; 1:100 dilution), or similar. Use distilled water to prepare solutions. Prepare fresh solution for each day.
Almond and banana extract are selected because they are distinct yet neutral odors. To ensure consistent odor potency, replace concentrated extract every few months. Store extracts away from direct sunlight and heat.
-
Social odors are obtained from mice of the same or opposite sex as the subject, depending on the scientific question. Two cages containing mice which have not been changed for at least three days are needed for social odors 1 and 2. Either group-housed or singly-housed animals can be used as sources of social odors; just make sure that the two cages each contain the same numbers of animals. Odor source cages should be maintained OUTSIDE the experimental testing room. Urine samples from unfamiliar mice can be used instead of cage swipes. Methods for collecting urine are described in the Alternate Protocol.
If space limit prohibits keeping social odor source cages outside the testing room, leave the cages in the vivarium. Instead, pre-swab the cages with three applicators and keep the swabs in sealed bags until needed.
-
-
A large lidded jar to dispose used applicators.
A temporary disposal container helps to prevent cross-trial contamination. Empty the jar after each animal.
Pre-test acclimation
1. Place the mouse subject in the testing cage and cover with the cage lid. Allow the animal to acclimate for 30 min. Insert a clean dry applicator through the hole on the cage lid during the pre-test acclimation period. This procedure is essential to reduce novelty-induced exploratory activity during the olfaction test. For a slant-top cage lid (left), insert the applicator through the water bottle hole. The metal piece that covers the hole will snap back and hold the applicator in place. For a flat-top cage lid (right), insert the applicator through a hole on the grid cage lid and use a small weigh boat (4.5 × 4.5 cm) to stabilize the applicator. (Figure 1).
Figure 1.

Olfactory habituation/dishabituation setup. For a slant-top cage lid (left), insert the applicator through the water bottle hole. For a flat-top cage lid (right), insert the applicator through a hole on the grid cage lid and use a small weigh boat to stabilize the applicator. In either case, always insert the wood end first through the hole. The depth of insertion is approximately 4 cm (Wrenn et al., 2004; Crawley et al., 2007).
For time efficiency, stagger acclimation and testing of successive subjects. It is highly recommended to acclimate the subjects in a dedicated room separated from the testing room. If such a set-up is not possible, make sure to keep the acclimating subjects as far as possible from the subjects being tested.
Testing olfactory habituation/dishabituation
2. To ensure accurate scoring of sniffing, position the cage on an eye-level flat surface.
3. Determine the order of odor presentation. One standard order of odor presentations is: water, water, water, almond, almond, almond, banana, banana, banana, unfamiliar social cage 1, unfamiliar social cage 1, unfamiliar social cage 1, unfamiliar social cage 2, unfamiliar social cage 2, unfamiliar social cage
4. To prepare non-social odor stimuli, dip only the cotton tip part of the applicator in the solution for 2 sec, take out the tip and recap the vial immediately. To prepare social odor stimuli, swipe the cage bottom in a zigzag fashion several times, then shake off the bedding from the cotton tip.
5. To begin a trial, insert the applicator into the cage lid as described in step 1. To avoid cross-trial contamination, it is extremely important to insert the wood end first through the hole. Cover the cage with the cage lid to which the applicator has been affixed. Retreat to the scoring station (2 m away from the cage) and start the timer.
To avoid cross-trial contamination, never insert the cotton end through the hole. Be sure to anchor the applicator at the same angle and to the same depth (about 2.5 cm) each time.
6. Use the stopwatch to record cumulative time spent sniffing the tip during the 2 min trial. Sniffing is scored when the animal is orienting towards the cotton tip with its nose 2 cm or closer to the tip.
7. After the completion of each trial, take off the cage lid and quickly change the applicator. Place the cage lid back and start the next trial. The inter-trial interval is about 1 min.
As in the buried food test, do not return a tested subject back to its home cage until all animals in that cage have been tested.
8. Analyze data for each group using repeated measures ANOVA followed by Newman-Keuls or a similar post hoc test to determine significant habituation, i.e. less time sniffing successive same smells, and dishabituation, i.e. more time sniffing a new smell.
Alternate Protocol (for BASIC PROTOCOL 2), adapted from (Wersinger et al., 2007; Jakupovic et al., 2008). Title: Testing olfactory habituation/dishabituation with non-contactable odor stimuli
The odor presentation procedure described in the Basic Protocol 2 allows the animal to have direct contact with the odor stimulus. In experiments which test the animals' ability to sense volatile components of odorants, direct contact should be prevented. Non-contact presentation is also advantageous when highly attractive odors are used. The Alternate Protocol introduces a method of non-contact odor presentation.
Materials
Mice: at least 6 weeks of age (sexually mature)
A stopwatch (silenced, as described in BASIC PROTOCOL 1)
A digital timer to register trial duration (2 min).
Clean mouse cages containing fresh wood chip bedding.
Cage lids, including the plastic top and the stainless steel grid top.
Fine wire mesh.
Small plastic weigh boats (4.5 cm × 4.5 cm).
Filter paper, cut into individual squares (2 cm × 2 cm).
Double-sided tape
Pipette and pipette tips
A large lidded jar to dispose pipette tips and pieces of filter paper
Prepare odor stimuli
Affix a piece of fine wire mesh against the grid bars of the empty stainless steel food hopper.
-
While a metal cage lid suffices to prevent the subject from gnawing or pulling the odor stimulus, nasal contact is still possible without the wire mesh. Since urine contains both volatile and non-volatile components, it is necessary to prevent direct contact if one wishes to study olfactory investigation behaviors elicited by volatile components of urine. To prepare the odor stimulus, pipette a small amount (10 μL) of solution or urine onto a piece of filter paper which is taped to a small plastic weigh boat. Collection of urine is described below.
To save time between trials, line up 15 (or the number needed for each animal) weigh boats on the table and use double-sided tape to adhere a piece of clean filter paper (2 cm × 2cm) to each weigh boat. To change the odor stimulus, simply take the next weigh boat and pipette the solution (or urine) on the filter paper and proceed to the next trial. Discard the used filter paper in the lidded jar before preparing the next stimulus.
Tape the filter paper to the weigh boat and then pipette the solution, rather than the other way around, has the advantage of reducing contamination
To avoid cross-trial contamination, it is important to make sure that the wet (odor saturated) filter paper is not touching the grid bars of the stainless steel cage lid.
Place the weigh boat on top of the wire mesh, with the filter paper facing downward. Use the stopwatch to record the time the subject spends sniffing the filter paper directly, with its nose within the perimeter of the weigh boat.
After each trial, change the odor stimulus and proceed to the next trial. The sequence of odor presentation is the same as indicated in step 3 of BASIC PROTOCOL 2.
*Data are analyzed the same way as described in BASIC PROTOCOL 2.
Urine Collection
Materials
Microcentrifuge tubes or clean glass vials.
Procedure
A urine sample is usually a mixture of urine from multiple adult mice. A standard male sample is pooled from 10 gonadally intact males. A standard female sample is pooled from 10 non-cagemates and is likely to be a mixture of urine from females at various stages of the estrus cycle (Wersinger et al., 2004; Achiraman and Archunan, 2006).
For techniques on collecting samples from gonadectomized males and/or female samples animals with stable circulating levels of sex hormones, see (Wesson et al., 2006; Martel and Baum, 2007)
Collect urine by holding the mouse by the scruff of the neck over a funnel and gently applying pressure to the abdomen. One animal typically discharges 0.2-0.3 ml of urine. Avoid fecal contamination while collecting urine.
-
Aliquot the pooled sample in 500 μl vials and store at −20°C until use.
Thaw individual aliquots as needed. Do not thaw and refreeze samples. Vortex the thawed samples thoroughly before using.
If individual urine samples are needed, collect urine evenly onto filter papers (Whatman no. 5). Seal papers in individually labeled small plastic bags and freeze immediately at −20°C until later use (Kavaliers et al., 2006).
Commentary
Background Information
The buried food test
This test was first described in the early 1970s (Alberts and Galef, 1971; Edward et al., 1972). Since then, various versions of the buried food test have been described under names such as “hidden cookie test”, “food exploration test”, or “food localization test” (Klein et al., 1996; Yamada et al., 2001; Del Punta et al., 2002; Luo et al., 2002; Dawson et al., 2005; Quiroz-Padilla et al., 2006). Cereals, chocolate chips, various kinds of cookies, and food pellets have been successfully used. The buried food test measures an animal's general ability to smell and is not to be confused with the sand-buried food test which measures an animal's ability to associate an odorant with a food reward (Wong et al., 2000; Trinh and Storm, 2003).
The olfactory habituation/dishabituation test
This test, which was first described in the early 1980s (Gregg and Thiessen, 1981), is commonly used to assess an animal's ability to detect odors and to discriminate between different odors (Alberts and Galef, 1971; Klein et al., 1996; Yamada et al., 2001; Del Punta et al., 2002; Trinh and Storm, 2003; Woodley and Baum, 2003; Wersinger et al., 2007; Stack et al., 2008). A clean cage with fresh bedding is typically used for testing, although some investigators prefer to test subjects in their home cages (Martel and Baum, 2007; Martel et al., 2007; Jakupovic et al., 2008). The pre-test acclimation phase is an important procedure because it reduces the interference of exploration of the novel environment during the test (Macknin et al., 2004). The first three presentations of water function to familiarize the subject with the testing procedure (Woodley and Baum, 2003). While olfactory habituation/dishabituation has primarily employed various non-social and social odors, it can also be used as a more sensitive test for distinguishing different concentrations of the same odor.
Critical Parameters and Troubleshooting
The buried food test
The buried food test is a simple paradigm whose main parameter is the latency to find the hidden food piece. It is critical to ensure that the subjects are calm and relatively stress-free at the time of testing. Agitated animals tend to be overly active in the testing cage and can not focus on the task, and therefore may produce false positive results. To prevent disturbances, avoid cage changing during the period from familiarization to testing. Inform the animal care staff about this requirement. On the day of testing, transfer the animals from the vivarium to the procedure room and allow them to rest in a dedicated quite place for an hour before starting the experiment. In most cases, such precautions should suffice to calm the subjects. In rare cases, the animals would still exhibit aberrant behaviors such as vigorous digging and pushing of the bedding, e.g. LP/J mice (unpublished observation). If certain aberrant behaviors are observed in a large portion of a certain group, consider testing the subjects in other olfaction tests.
To accurately record the latency score, start the stopwatch after you have placed the cage lid and retreated to the observation station. It is important to sit close enough to observe the animal clearly yet far enough to not to disturb it. An optimal distance is approximately 2 m. Stop the stopwatch when the animal picks up the food piece with its forepaws, this is the behavior most widely accepted as an indication of successful localization. In some cases, the animal can be seen eating with its head bending over the food. This behavior can also be used to indicate a success trial, even though the animal is not holding the food with its paws.
If two or more mice are to be tested from a certain cage, do not return the tested subjects back to their home cage until all animals in that cage have been tested. Instead, prepare a separate holding cage for the post-test mice. Last, cookies emit airborne odors, and should be stored in resealable bags or closed containers. Keep untested subjects outside the testing room. Only bring a small amount of the food stimulus into the testing room.
The olfactory habituation/dishabituation test
Time spent in direct olfactory investigation is the scored parameter and it is critical to score the sniffing behavior accurately. In the cotton-tipped applicator version of the test (see BASIC PROTOCOL 2), olfactory investigation is defined when the animal is orienting towards the cotton tip with its nose being 2 cm or closer to the tip. Leaning against the applicator and sniffing the wood stick part of the applicator are NOT considered as olfactory investigation directed to the odor stimulus. In the test described in the Alternate Protocol, olfactory investigation is defined when the mouse rears on its hind legs and sniffs directly below the filter paper, with its nose within the perimeter of the weigh boat. Adjust the concentration and the volume of solutions to increase or decrease the difficulty of the test.
The critical indicators of a normal ability to smell are significant habituation and dishabituation. To discover habituation, it is important to avoid “the floor effect” by selecting odors which the animals will sniff for at least a few seconds at the first encounter. To discover dishabituation, choose odor stimuli that are distinctly different. For example, orange and lemon extracts may be too similar for the animals to differentiate.
Note that sniffing time per se depends on the attractiveness of the odor in the habituation/dishabituation test. The standard non-social odors, vanilla, almond, and banana extract, only elicit modest levels of sniffing (Crawley et al., 2007; Wersinger et al., 2007). These neutral odors are preferred by investigators because they are not related to food smells that are familiar to rodents or mates. Highly attractive odors may produce gnawing or mounting of the stimulus that would confound the olfactory investigation scores.
General recommendations for running olfactory tests
Avoid interfering odor sources. The experiment should be performed in a clean room free of distinct odors such as organic smells, paints, laboratory chemicals, cleaning products, etc. The experimenter should avoid using personal products (perfume, cologne, deodorants, soap, etc.) which emit strong odors. Use odor-free laboratory gloves. Keep the trash bin outside the room and use a lidded jar as the temporal disposal container. Empty the jar after each subject.
Anticipated Results
The buried food test
Food-deprived mice with normal olfaction typically find the cookie within 1 min (Dawson et al., 2005; Wersinger et al., 2007; Jamain et al., 2008). It remains possible that a small percentage of mice with intact olfaction would fail to find the cookie within the 15 min time limit, sometimes due to novelty-induced behavioral responses such as immobility (sometimes observed in animals with high levels of anxiety) or vigorous bedding shoveling (as in LP/J mice, unpublished observation). To prevent false-positive judgments, use a complementary test, such as the olfactory habituation/dishabituation test described in this unit, to retest animals that have failed the buried food test.
The habituation/dishabituation test
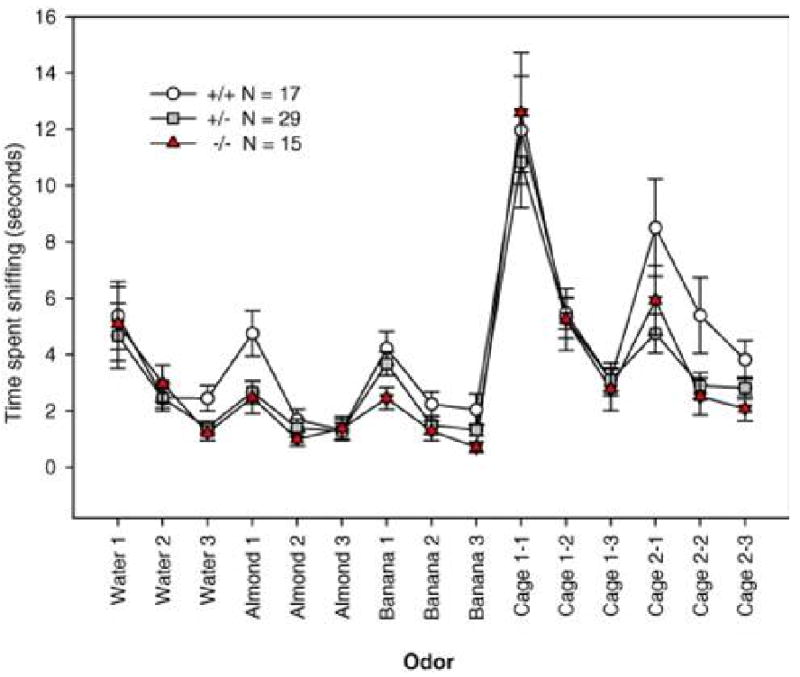
Animals with normal olfaction should show significantly reduced sniff time when an odor is re-introduced for the 2nd and 3rd time (habituation) and a reinstatement of sniffing when a novel odor is presented. Note that significant habituation-dishabituation, rather than high levels of sniffing, is the indicator of normal olfaction. As Figure 3 shows, the first presentation of a water-saturated applicator elicited moderate sniffing that declined across the second and third exposure to water (habituation). The next presentation, an applicator saturated with almond extract (1:100 dilution), then elicited significantly more sniffing (dishabituation), which declined across the second and third presentation of the almond odor. Similarly, sniffing resumed at a high level to the next new odor, an applicator saturated with in banana extract (1:100 dilution), and declined across the second and third banana presentations. Dishabituation was highly significant to a social smell, i.e. a cotton tip wiped across the bottom of cage 1 containing a mouse that had no previous contact with the subject, which declined across the second and third presentation of the social cage 1. Dishabituation to a cotton tip wiped across the bottom of cage 2 containing a different stranger mouse was seen in most mice, which declined across the second and third presentation of the social cage 2.
Figure 3.
Olfactory habituation/dishabituation results from oxytocin (OT) null mutant (triangle), heterozygote (square), and wildtype (circle) littermate mice. Generation of OT mutant mice is described in (Young et al., 1996). Figure shown here is reprinted from (Crawley et al. 2007) with permission. N = 7 male and 10 female +/+, 14 male and 15 female +/−, 6 male and 9 female −/−. All genotypes displayed significant habituation and dishabituation to non-social and social odors.
Time Considerations
With the 5 min acclimation period, a trial of the buried food test typically takes about 10-15 min for animals with normal olfaction, and 20 min if the animals fail to find the stimulus within the maximum amount of time. A trained experimenter can test two animals at the same, thereby increasing the throughput.
The basic habituation/dishabituation test consists of 30 min of acclimation and 45 min of testing, and one experimenter can only test one animal at a time. Acclimation and testing can be staggered so that each animal (except for the first one which takes 75 min) takes no more than 50 min (45 min of testing plus a few minutes to change animals) to test.
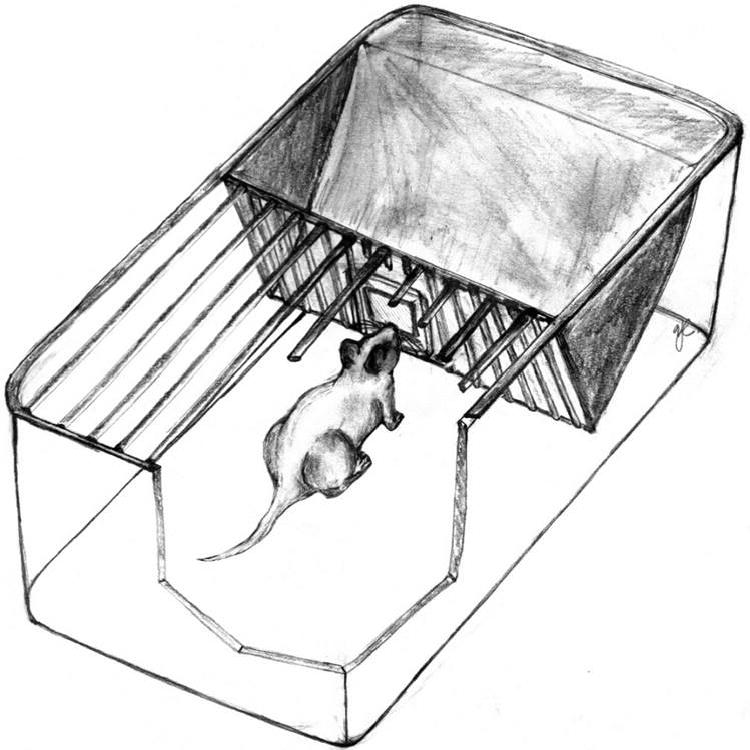
Figure 2.
Olfactory habituation/dishabituation setup as described in the Alternate Protocol. The grid bars of the stainless steel cage lid and a piece of wire mesh prevent the subject from contacting the odor stimulus. Illustration kindly contributed by Professor Michael Baum, Department of Biology, Boston University.
Literature Cited
- Achiraman S, Archunan G. 1-Iodo-2methylundecane, a putative estrus-specific urinary chemo-signal of female mouse (Mus musculus) Theriogenology. 2006;66:1913–1920. doi: 10.1016/j.theriogenology.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Alberts JR, Galef BG., Jr Acute anosmia in the rat: a behavioral test of a peripherally-induced olfactory deficit. Physiology & behavior. 1971;6:619–621. doi: 10.1016/0031-9384(71)90218-6. [DOI] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J. The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J Neurosci. 2002;22:9104–9112. doi: 10.1523/JNEUROSCI.22-20-09104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Keverne EB. Something in the air? New insights into mammalian pheromones. Curr Biol. 2004;14:R81–89. doi: 10.1016/j.cub.2003.12.052. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Zufall F. Pheromonal communication in vertebrates. Nature. 2006;444:308–315. doi: 10.1038/nature05404. [DOI] [PubMed] [Google Scholar]
- Brown RE. Mammalian social odors. Adv Stud Behav. 1979;10:107–161. [Google Scholar]
- Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, Young NB, Nadler JJ, Moy SS, Young LJ, Caldwell HK, Young WS. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Dawson PA, Steane SE, Markovich D. Impaired memory and olfactory performance in NaSi-1 sulphate transporter deficient mice. Behavioural brain research. 2005;159:15–20. doi: 10.1016/j.bbr.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Del Punta K, Leinders-Zufall T, Rodriguez I, Jukam D, Wysocki CJ, Ogawa S, Zufall F, Mombaerts P. Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature. 2002;419:70–74. doi: 10.1038/nature00955. [DOI] [PubMed] [Google Scholar]
- Demas GE, Williams JM, Nelson RJ. Amygdala but not hippocampal lesions impair olfactory memory for mate in prairie voles (Microtus ochrogaster) The American journal of physiology. 1997;273:R1683–1689. doi: 10.1152/ajpregu.1997.273.5.R1683. [DOI] [PubMed] [Google Scholar]
- Doty RL. Odor-guided behavior in mammals. Experientia. 1986;42:257–271. doi: 10.1007/BF01942506. [DOI] [PubMed] [Google Scholar]
- Edward DA, Thompson ML, Burge KG. Olfactroy bulb removal vs peripherally induced anosmia: differential effects on the aggression behavior of male mice. Behavioral Biology. 1972;7:823–828. doi: 10.1016/s0091-6773(72)80174-3. [DOI] [PubMed] [Google Scholar]
- Jakupovic J, Kang N, Baum MJ. Effect of bilateral accessory olfactory bulb lesions on volatile urinary odor discrimination and investigation as well as mating behavior in male mice. Physiology & behavior. 2008;93:467–473. doi: 10.1016/j.physbeh.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavaliers M, Choleris E, Agmo A, Braun WJ, Colwell DD, Muglia LJ, Ogawa S, Pfaff DW. Inadvertent social information and the avoidance of parasitized male mice: a role for oxytocin. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:4293–4298. doi: 10.1073/pnas.0600410103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Douhard Q, Baum MJ, Bakker J. Sexual experience does not compensate for the disruptive effects of zinc sulfate--lesioning of the main olfactory epithelium on sexual behavior in male mice. Chemical senses. 2006;31:753–762. doi: 10.1093/chemse/bjl018. [DOI] [PubMed] [Google Scholar]
- Keverne EB. Importance of olfactory and vomeronasal systems for male sexual function. Physiology & behavior. 2004;83:177–187. doi: 10.1016/j.physbeh.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Klein SL, Kriegsfeld LJ, Hairston JE, Rau V, Nelson RJ, Yarowsky PJ. Characterization of sensorimotor performance, reproductive and aggressive behaviors in segmental trisomic 16 (Ts65Dn) mice. Physiology & behavior. 1996;60:1159–1164. doi: 10.1016/0031-9384(96)00218-1. [DOI] [PubMed] [Google Scholar]
- Luo AH, Cannon EH, Wekesa KS, Lyman RF, Vandenbergh JG, Anholt RR. Impaired olfactory behavior in mice deficient in the alpha subunit of G(o) Brain research. 2002;941:62–71. doi: 10.1016/s0006-8993(02)02566-0. [DOI] [PubMed] [Google Scholar]
- Macknin JB, Higuchi M, Lee VM, Trojanowski JQ, Doty RL. Olfactory dysfunction occurs in transgenic mice overexpressing human tau protein. Brain research. 2004;1000:174–178. doi: 10.1016/j.brainres.2004.01.047. [DOI] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. Sexually dimorphic activation of the accessory, but not the main, olfactory bulb in mice by urinary volatiles. The European journal of neuroscience. 2007;26:463–475. doi: 10.1111/j.1460-9568.2007.05651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz-Padilla MF, Guillazo-Blanch G, Vale-Martinez A, Marti-Nicolovius M. Excitotoxic lesions of the parafascicular nucleus produce deficits in a socially transmitted food preference. Neurobiology of learning and memory. 2006;86:256–263. doi: 10.1016/j.nlm.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Restrepo D, Arellano J, Oliva AM, Schaefer ML, Lin W. Emerging views on the distinct but related roles of the main and accessory olfactory systems in responsiveness to chemosensory signals in mice. Hormones and behavior. 2004;46:247–256. doi: 10.1016/j.yhbeh.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Sanchez-Andrade G, James BM, Kendrick KM. Neural encoding of olfactory recognition memory. The Journal of reproduction and development. 2005;51:547–558. doi: 10.1262/jrd.17031. [DOI] [PubMed] [Google Scholar]
- Schellinck HM, Smyth C, Brown R, Wilkinson M. Odor-induced sexual maturation and expression of c-fos in the olfactory system of juvenile female mice. Brain Res Dev Brain Res. 1993;74:138–141. doi: 10.1016/0165-3806(93)90094-q. [DOI] [PubMed] [Google Scholar]
- Stack CM, Lim MA, Cuasay K, Stone MM, Seibert KM, Spivak-Pohis I, Crawley JN, Waschek JA, Hill JM. Deficits in social behavior and reversal learning are more prevalent in male offspring of VIP deficient female mice. Experimental neurology. 2008;211:67–84. doi: 10.1016/j.expneurol.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh K, Storm DR. Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nature neuroscience. 2003;6:519–525. doi: 10.1038/nn1039. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Caldwell HK, Martinez L, Gold P, Hu SB, Young WS., 3rd Vasopressin 1a receptor knockout mice have a subtle olfactory deficit but normal aggression. Genes, brain, and behavior. 2007;6:540–551. doi: 10.1111/j.1601-183X.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Kelliher KR, Zufall F, Lolait SJ, O'Carroll AM, Young WS., 3rd Social motivation is reduced in vasopressin 1b receptor null mice despite normal performance in an olfactory discrimination task. Hormones and behavior. 2004;46:638–645. doi: 10.1016/j.yhbeh.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Rissman EF. Oestrogen receptor alpha is essential for female-directed chemo-investigatory behaviour but is not required for the pheromone-induced luteinizing hormone surge in male mice. Journal of neuroendocrinology. 2000;12:103–110. doi: 10.1046/j.1365-2826.2000.00418.x. [DOI] [PubMed] [Google Scholar]
- Wesson DW, Donahou TN, Johnson MO, Wachowiak M. Sniffing Behavior of Mice during Performance in Odor-Guided Tasks. Chemical senses. 2008 doi: 10.1093/chemse/bjn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Keller M, Douhard Q, Baum MJ, Bakker J. Enhanced urinary odor discrimination in female aromatase knockout (ArKO) mice. Hormones and behavior. 2006;49:580–586. doi: 10.1016/j.yhbeh.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ST, Trinh K, Hacker B, Chan GC, Lowe G, Gaggar A, Xia Z, Gold GH, Storm DR. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. 2000;27:487–497. doi: 10.1016/s0896-6273(00)00060-x. [DOI] [PubMed] [Google Scholar]
- Woodley SK, Baum MJ. Effects of sex hormones and gender on attraction thresholds for volatile anal scent gland odors in ferrets. Hormones and behavior. 2003;44:110–118. doi: 10.1016/s0018-506x(03)00126-0. [DOI] [PubMed] [Google Scholar]
- Woodley SK, Cloe AL, Waters P, Baum MJ. Effects of vomeronasal organ removal on olfactory sex discrimination and odor preferences of female ferrets. Chemical senses. 2004;29:659–669. doi: 10.1093/chemse/bjh069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrenn CC, Kinney JW, Marriott LK, Holmes A, Harris AP, Saavedra MC, Starosta G, Innerfield CE, Jacoby AS, Shine J, Iismaa TP, Wenk GL, Crawley JN. Learning and memory performance in mice lacking the GAL-R1 subtype of galanin receptor. The European journal of neuroscience. 2004;19:1384–1396. doi: 10.1111/j.1460-9568.2004.03214.x. [DOI] [PubMed] [Google Scholar]
- Yamada K, Wada E, Wada K. Female gastrin-releasing peptide receptor (GRP-R)-deficient mice exhibit altered social preference for male conspecifics: implications for GRP/GRP-R modulation of GABAergic function. Brain research. 2001;894:281–287. doi: 10.1016/s0006-8993(01)02032-7. [DOI] [PubMed] [Google Scholar]
- Young WS, 3rd, Shepard E, Amico J, Hennighausen L, Wagner KU, LaMarca ME, McKinney C, Ginns EI. Deficiency in mouse oxytocin prevents milk ejection, but not fertility or parturition. Journal of neuroendocrinology. 1996;8:847–853. doi: 10.1046/j.1365-2826.1996.05266.x. [DOI] [PubMed] [Google Scholar]


