Abstract
In the retina, rod signal pathways process scotopic visual information. Light decrements are mediated by two distinct groups of rod pathways in the dark adapted retina that can be differentiated on the basis of their sensitivity to the glutamate agonist DL-2-amino-4-phosphonobutyric acid (APB). We have found that the APB sensitive and insensitive rod Off-pathways signal different light decrement information: the APB sensitive rod Off-pathway conveys slow and low frequency light signals, whereas the APB insensitive rod Off-pathways mediate fast and high frequency light signals (Wang, 2006). However, the mechanisms which limit the frequency following through the APB sensitive and insensitive rod Off-pathways remain unknown. In the current study, whole-cell patch-clamp recordings were made from ganglion cells in dark and light adapted mouse retina to identify the mechanisms that limit the frequency following through the APB sensitive and insensitive rod Off-pathways. The results showed that the sites from AII amacrine cells to Off cone bipolar cells are the major mechanisms that limit the frequency following through the APB sensitive rod Off-pathway. In the APB insensitive rod Off-pathways, rods themselves limited the frequency following through these pathways. Moreover, ganglion cells were able to follow higher frequencies under photopic conditions than under scotopic conditions. The Off responses followed lower frequencies than On responses under photopic conditions. This finding was observed in cells that yielded On or Off responses only as well as in On-Off cells.
Keywords: retinal ganglion cell, scotopic condition, photopic condition, APB, rod pathways, cone pathways
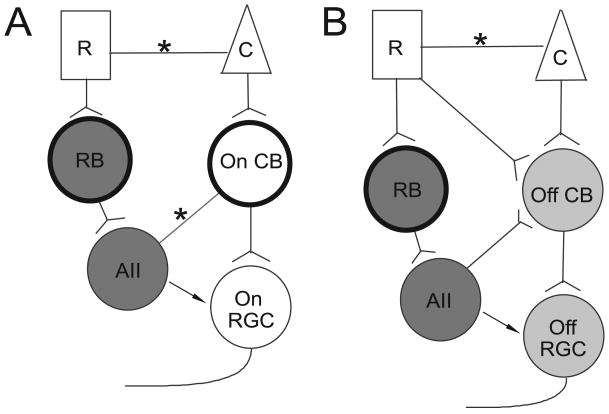
Distinct signaling pathways within the retina code for different types of visual information. The cone pathways mediate coding of visual information for photopic vision at relatively high light intensities. By contrast, the rod pathways underlie the coding for scotopic vision at relatively low light intensities (reviewed by: Dowling, 1987; Sterling and Demb, 2004). Light increments and decrements are also processed within different signaling pathways. Under photopic conditions, the light increment signals are transferred from cones to On ganglion cells via On cone bipolar cells. The light decrement signals follow the pathway from cones to Off cone bipolar cells then to Off ganglion cells (Schiller, 1992, Figure 1A&B).
Figure 1.
Schematic of rod signal pathways. Panel A, two rod pathways transmitting light increment information: rods => rod bipolar cells => AII amacrine cells => gap junctions => On cone bipolar cells => On ganglion cells; rods => gap junctions => cones => On cone bipolar cells => On ganglion cells. Panel B, three rod pathways transmitting light decrement information: rods => rod bipolar cells => AII amacrine cells => glycinergic synapses => Off cone bipolar cells => Off ganglion cells; rods => directly contacting Off cone bipolar cells => Off ganglion cells; rods => gap junctions => cones => Off cone bipolar cells => Off ganglion cells. R: rod; C: cone; RB: rod bipolar cell; On CB: On cone bipolar cell; Off CB: Off cone bipolar cell; AII: AII amacrine cell; Off RGC: Off retinal ganglion cell; On RGC: On retinal ganglion cell; *: gap junction. The dark circles represent the cells that are sensitive to APB. The dark gray, light gray and hollow cells represent the shared compartments of different signal pathways (see detail in the text). The arrows indicate the possible feedforward synapses from AII amacrine cells to ganglion cells. Note: pathways are illustrated to one ganglion cell in each panel, which does not necessarily mean these pathways converge onto the same cell.
Two DL-2-amino-4-phosphonobutyric acid (APB) sensitive rod pathways convey the light increment signal in scotopic vision (Figure 1A). In one pathway, the primary pathway, rods transmit the signal to rod bipolar cells, then to AII amacrine cells, which pass the signal to On cone bipolar cells via gap-junctions, and from there to On ganglion cells. In another pathway, the secondary pathway, rods send the signal to cones via gap-junctions, then to On cone bipolar cells, which in turn innervate On ganglion cells. APB blocks these two pathways by hyperpolarizing the rod bipolar cells and the On cone bipolar cells (Sharpe and Stockman, 1999). In contrast, distinct APB sensitive and APB insensitive rod Off-pathways process the light decrement signals in the dark adapted retina (Figure 1B). In the APB sensitive rod Off-pathway, rods transmit the signal via rod bipolar cells to AII amacrine cells then to Off cone bipolar cells (by glycinergic synapses), which in turn innervate the dendrites of Off ganglion cells (Sharpe and Stockman, 1999). This pathway is also referred to as the primary rod pathway (Völgyi et al., 2004). Two APB insensitive rod Off-pathways have been documented. In one pathway, rods pass the signal to cones by gap-junctions, then to Off cone bipolar cells (DeVries and Baylor, 1995). In another pathway, rods directly synapse on Off cone bipolar cells (Soucy et al., 1998; Hack et al., 1999; Tsukamoto et al., 2001). These two APB insensitive pathways are referred to as the secondary and the tertiary rod pathways, respectively (Völgyi et al., 2004). In addition, there are feedforward synapses from AII amacrine cells to ganglion cells as indicated by the arrows in Figure 1. It has been found that AII amacrine cells provide direct inhibitory input to Off ganglion cells (Murphy and Rieke, 2008).
To understand visual signal processing within the retina, it is essential to identify the unique functions of each signal pathway. We have found that under scotopic condition the APB sensitive and insensitive rod Off-pathways carry different light decrement information: the APB sensitive pathways convey slow and low frequency light signals, whereas the APB insensitive pathways mediate fast and high frequency light signals (Wang, 2006). However, the mechanisms which limit the frequency following through these pathways remain unknown. Moreover, since rod and cone pathways converge onto individual retinal ganglion cells, it is important to understand how each individual retinal ganglion cell changes its frequency following properties when switching from the rod pathways to the cone pathways. As of yet this has not been established. Accordingly, in this study, we identified the mechanisms that limit the frequency following through the APB sensitive and insensitive rod Off-pathways, and we investigated the frequency following properties of individual retinal ganglion cells under both scotopic and photopic conditions.
Experimental Procedures
The basic methods used in this study were similar to those used previously (Wang, 2006; Wang et al., 2007; Nemargut et al., 2009). All procedures were in compliance with National Institutes of Health guidelines and were approved by the campus animal use committees of Tulane University. Animals were dark adapted overnight prior to the experiments and all procedures, including animal surgery, dissection of retinas, and recordings from cells were made in complete darkness. Infrared goggles were used to visualize the tissue on the dissecting and recording microscopes and to maneuver in the recording room. LEDs (850 nm) were used to provide light to the dissecting microscope while the illumination from the recording microscope was passed through an ≥850 nm cut off-filter.
Retinal preparation
Retinas were obtained from three to four month old mice (C57BL/6 from Charles River Farm CA). Following a lethal dose of barbiturate (Nembutal 200 mg/kg i.p.), the eyes were removed and placed in oxygenated L15 at 37°C for 12 min. The retinas were then carefully peeled from the eyecup and stored at room temperature in Minimal essential medium eagle (MEME, sigma M-7278), continuously bubbled with 95% O2 and 5% CO2. A small piece of retina was placed ganglion cell layer up in the recording chamber and stabilized with an overlying piece of filter paper. A 2 mm hole in the filter paper provided access for the recording electrode. Cells were visualized through a 40× objective mounted on an upright epifluorescence microscope (Nikon).
During recordings, the retina was continuously perfused with MEME (1.5 ml/min) through a gravity fed line, heated with a dual channel temperature controller (Warner Instruments), and continuously bubbled with 95% O2 and 5% CO2. A calibrated thermocouple monitored the temperature in the recording chamber, and maintained it at 35°C. Recordings from each individual cell usually lasted 30-120 minutes, and retinal segments from which recordings were made typically remained viable for 8-12h. Patch electrodes were filled with a solution containing (in mM): K-gluconate, 110; KCl, 10; MgCl2, 1; CaCl2, 0.5; HEPES, 10; EGTA, 5; 1 mg/ml Nystatin; 2 mg/ml Pluronic F-68; 0.5 % Lucifer Yellow; pH 7.4; osmolarity, 290 mOsm. There were no differences in the results obtained with or without Nystatin and Pluronic, although the use of these chemicals permitted stable recordings for longer time periods (Robinson and Chalupa 1997; Wang et al., 1997). Using fluorescent microscopy, we also found that Nystatin and Pluronic facilitated the formation of the whole-cell configuration. With Nystain and Pluronic in the electrode solution, the soma is usually filled with Lucifier Yellow within five minutes after the formation of the high resistance seal, indicating the whole-cell configuration was obtained. All recordings were made with the whole-cell configuration. By the end of the experiment, the soma and the dendritic arborizations were usually completely filled. Once complete filling was achieved, the retina was removed and fixed in 4% paraformaldehyde for 6-8 hours at 4°C.
Electrophysiology
Whole-cell current-clamp recordings were made from retinal ganglion cells in dark and light adapted retinas. Patch pipettes with a tip resistance between 3 and 7 MΩ were pulled from thick-walled 1.5 mm-OD borosilicate glass on a Sutter Instruments puller (P-97). Whole-cell patch-clamp recordings were made with a MultiClamp 700B patch-clamp amplifier. The data were low-pass filtered at rates between 1 and 2 kHz and digitized at a rate of 5 kHz before storage on a computer for subsequent off-line analysis. To attain whole cell access, the vitreous and the outer limiting membrane overlying the recording area were removed by gently brushing the retinal surface with the tip of a glass pipette. Recordings were obtained by patching onto cells with clear, non-granular cytoplasm. High-resistance seals were obtained by moving the patch electrode onto the cell membrane and applying gentle suction. After formation of a high-resistance seal between the electrode and the cell membrane, transient currents caused by pipette capacitance were electronically compensated by the circuit of the MultiClamp 700B patch-clamp amplifier. Recordings from cells with a seal resistance < 1 GΩ were discarded. The series resistance was 7-16 MΩ. Recordings were terminated whenever significant increases (>20%) in series resistance occurred. After attaining a whole-cell configuration, the resting membrane potential was read off the amplifier. The value of the resting potential was monitored regularly throughout the recording, and if significant changes were observed, the recording was terminated. The sudden or gradual changes in the resting potential were considered significant if the changes were over 15% of the original values (positive or negative) and lasted longer than 10 minutes, when no electrical, light or chemical stimulations were applied. The computer software pCLAMP 9 (Axon Instruments Inc.), and Mini Analysis Program (Synaptosoft Inc.) were used to analyze the data. The results are expressed as mean±SE.
Light stimulus
Light-evoked responses were obtained by delivering square wave spots of light to the retina from a one-inch-diameter computer monitor, with a green (P43, 545 nm light) phosphor (Lucivid MR1-103; MicroBrightField, Colchester, VT), through the camera port of the microscope (Demb et al., 1999). The sizes of the spots of light were varied between 200 and 500 μm in diameters in different cells. For each cell, different sized spots were used to evoke light responses before the functional properties were tested. The size of the spot that evoked the optimal light-evoked response for this cell was selected and used to test the functional properties. The spots of light were always centered on the soma. In the dark adapted retina, stimuli were delivered once every 20 s to limit light adaptation (Xin and Bloomfield, 1999). The stimuli were programmed in Matlab (Math Works, Natick, MA) using the Psychophysics Toolbox extensions (Brainard, 1997; Pelli, 1997). The intensity of a spot of light was calibrated with a spectroradiometer/photometer (UDT instruments, S350/268R) and expressed in terms of the time-averaged rate of photoisomerizations per rod per second (Rh*/rod/s). The instrument was calibrated relative to standards of the National Institute of Standards and Technology. All stimulus intensities were calculated by using a rod cross section of 0.5 μm2 (Howes et al., 2002) and rod integration time of 0.4 s (Baylor, 1987). Under scotopic conditions, the intensities of spots of light were varied from cell to cell and ranged from 2.5 to 25 Rh*/rod/s. The intensity range fallen exclusively within the rod range (Field GD and Rieke F, 2002; Völgyi et al., 2004). The contrast of the stimuli was calculated by using the Michelson Contrast Equation: Contrast = (F-B)/(F+B) (Burkhardt and Gottesman, 1987), where F is the light intensity of the spots of light, and B is the steady background intensity. In the current study, under scotopic conditions, the steady background intensity was 0, thus, the contrast was 1.
The methods for the light adapted studies were essentially the same as our previous study (Nemargut et al., 2009). A background light of constant brightness, 1500 Rh*/rod/s, was provided full-field by the computer controlled one-inch-diameter monitor (Lucivid) for 10 minutes to allow the transition from scotopic to photopic conditions. This background light intensity completely inactivated rods (Nemargut et al., 2009). With this intensity of the background light, we found solid, consistent and reliable light-evoked responses could be recorded at least for two hours after the transition from scotopic to photopic conditions.
Light stimuli with intensities greater than that of the background light were used in the light adapted retina to evoke light responses from ganglion cells. For each cell, different intensities, ranging from 3500 to 8000 Rh*/rod/s, were used to evoke light responses. Their related contrasts varied from 0.40 to 0.68. The lowest intensity required to evoke optimal responses was used to test the frequency following properties of the ganglion cell under photopic conditions. The intensities and the contrasts are indicated in the figure legends. Within the intensity range studied, we found that the intensity differences did not affect the frequency following property for a given cell.
We have successfully established a reliable recording procedure to record from the same ganglion cell under both scotopic and photopic conditions. With this procedure, whole-cell patch-clamp recordings were made from a ganglion cell first under the scotopic conditions, then the background light was delivered to the retina to induce light adaptation, and the recording was continued from the same cell. After switching to photopic conditions, the size of the spot of light which evoked the maximal response from a given cell was determined by delivering different sizes of spots to the retina.
Drug application
DL-2-amino-4-phosphonobutyric acid (APB, Calbiochem, 100 μM) was freshly dissolved in MEME on the day of the experiment and administered through a gravity fed line. The solutions were heated with a dual channel temperature controller (Warner Instruments) and continuously bubbled with 95% O2 and 5% CO2. A six-position rotary valve (Western Analytical Products) was used to switch between bath and drug solutions.
Results
Frequency following properties of APB sensitive and insensitive rod Off-pathways
Whole-cell patch-clamp recordings were made from each ganglion cell, which was filled with Lucifer yellow. The cell class was determined based on confocal images and was classified according to the study by Doi and colleagues (1995). In our previous study (Wang, 2006), we found that APB sensitive and insensitive rod Off- pathways were not associated with a particular morphological cell type of the three major types of retinal ganglion cells in the mouse retina, identified as type I, II, and III by Doi and colleagues (Doi et al., 1995). Previous studies have validated this finding in ferret retina (Wang et al., 2001). In addition, Völgyi and colleagues have shown some Off ganglion cells with convergent rod signals from APB sensitive (primary) and APB insensitive (secondary or tertiary) rod pathways (Völgyi et al., 2004).
The major focus of the current study was to determine the mechanisms that limit the frequency following properties for each signal pathway. We recorded from Off, On, and On-Off ganglion cells under scotopic and photopic conditions to dissect the sites (components) at which the frequency following property is limited in a given signal pathway. The examples of confocal images from recorded ganglion cells are shown in Figure 2. The top panels show the top views of the dendritic branching patterns and the lower panels show the side views of the dendritic stratification patterns of On, Off, and On-Off ganglion cells.
Figure 2.
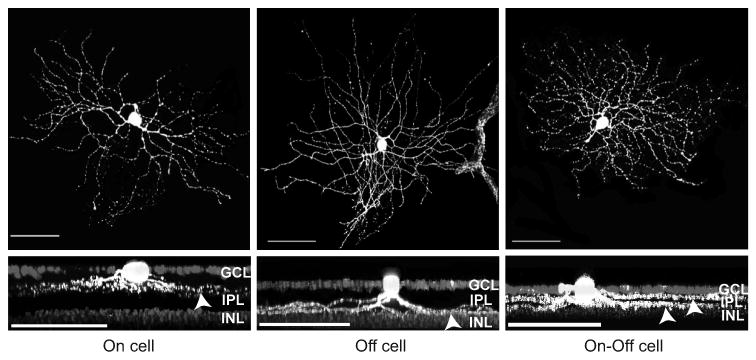
Confocal reconstructions of three ganglion cells from which recordings were made. The cells were filled with Lucifer Yellow during the course of recording. An On cell is shown in the left panels, an Off cell is shown in the middle panels (a blood vessel with auto fluorescence is included in the right of the meddle up panel), and an On-Off cell is shown in the right panels. Top panels: top views of the dendritic arborization of these cells. Lower panels: the 90 degree rotated confocal stacked images to show the dendritic stratifications, indicated by the arrowheads, of these cells in the IPL. The nuclei of the ganglion cell layer and inner nuclear layer were stained with DAPI. Scale bars = 50 μm. GCL=ganglion cell layer, INL=inner nuclear layer.
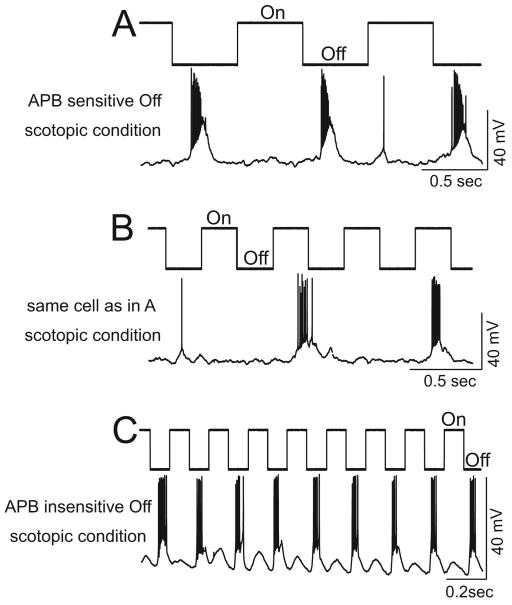
To test the frequency following properties of retinal ganglion cells, various frequencies of square wave flashing spots of light at a selected intensity were used to stimulate retinas. The light stimuli were always delivered in order from the lowest to the highest frequency. The frequency following property is defined as the highest frequency of light stimuli that each cell can follow with a one to one stimulus to response ratio (see examples in Figure 3). Note: the frequency following property differs from the temporal resolution which is usually quantified by the response amplitudes as a function of temporal frequencies. The frequency following property is not related to the amplitude of each light-evoked response; rather, it determines if a ganglion cell can generate a response corresponding to each light onset or light offset of square wave flashing spots of light, with a given intensity, at different frequencies. We have previously found that the APB sensitive rod Off-pathway manifested a much lower frequency following property than the APB insensitive rod Off-pathways (Wang et al., 2006). Examples of recordings of frequency following properties of ganglion cells from the APB sensitive and insensitive rod Off-pathways are shown in Figure 3. APB was used to differentiate the ganglion cell in the APB sensitive rod Off-pathway from those in the APB insensitive rod Off-pathways. Figures 3A&B illustrate recordings of an Off ganglion cell from the APB sensitive rod Off-pathway. At a low stimulus frequency (1Hz), the cell responded to every light offset (Figure 3A). However, when the stimulus frequency increased to 2 Hz, this cell failed to follow each light offset (Figure 3B). In contrast, an Off cell in the APB insensitive rod Off-pathway was found to be capable of following a substantially higher stimulus frequency of a flashing light, up to 5 Hz (Figure 3C).
Figure 3.
Off responses of the APB insensitive rod Off-pathways were found to be capable of following substantially higher stimulus frequencies of a flashing light under scotopic conditions. The light onset and offset are indicated above each recording trace. A&B: recordings from a retinal ganglion cell in the APB sensitive rod Off-pathway. The stimulus light intensity was 18 Rh*/rod/s and the contrast was 1. The Off responses of this cell could follow the low stimulus frequency (A, 1 Hz), but failed to follow the 2 Hz stimulus frequency (B). C: an example of an Off cells in the APB insensitive rod Off-pathway. Note that the Off responses followed a relatively high stimulus frequency of 5 Hz very well. The stimulus light intensity was 20 Rh*/rod/s and the contrast was 1.
Mechanism that limits the frequency following through the APB sensitive rod Off-pathway
Our hypothesis was that the sites from the AII amacrine cell to the Off cone bipolar cell may provide the mechanism that limits the high frequency light stimuli from following through the APB sensitive rod Off-pathway. Two sets of experiments were performed to test this hypothesis. The first set of experiments was to test if Off cone bipolar cells/Off ganglion cells in the APB sensitive rod Off-pathways are the sites that limit the high light stimuli frequency from flowing through the pathway. The second set of experiments was to test if rod/rod bipolar cells/AII amacrine cells are the sites that limit the frequency following property of the APB sensitive rod Off-pathway.
In the first set of experiments, we tested the frequency following properties of the same Off ganglion cells under scotopic and photopic conditions. As may be seen in Figure 1B, the APB sensitive rod Off-pathway in the dark adapted retina consists of the following sequence: rods => rod bipolar cells => AII amacrine cells => glycinergic synapses => Off cone bipolar cells => Off ganglion cells. By contrast, the cone Off-pathway in the light adapted retina consists of cones => Off cone bipolar cells => Off ganglion cells. Because these two signal pathways share the same Off cone bipolar cells and Off ganglion cells (light gray cells in Figure 1B), by switching the recordings from scotopic to photopic conditions we were able to localize the sites limiting the frequency following properties to either the Off cone bipolar/ganglion cells or to the rod/rod bipolar/AII amacrine cells.
Current-clamp recordings were made from ganglion cells in the APB sensitive rod Off-pathway under scotopic conditions to test their frequency following properties. APB was used to confirm that the cells were in the APB sensitive rod Off-pathway under scotopic conditions. After confirmation, APB was washed out. The recordings were then switched to photopic conditions by projecting a background light onto the retina (see methods section). The frequency following properties of the same cells, now receiving inputs from Cone => Off cone bipolar cell => Off ganglion cell pathway, were established under photopic conditions.
Our working hypothesis was that if a ganglion cell follows higher frequencies under photopic conditions than under scotopic conditions, the result would indicate that the Off cone bipolar cells and the Off ganglion cells are intrinsically capable of following higher frequencies. Therefore, the Off cone bipolar cells and the Off ganglion cells are not the sites that limit the frequency following property in the APB sensitive rod Off-pathway. Our data showed that the Off responses from ganglion cells under photopic conditions followed higher frequencies than they did when in the APB sensitive rod Off-pathways (Figure 4A&B). The average highest frequency of light stimuli that the cells from the APB sensitive rod Off-pathway were able to follow was 1.11±0.08 Hz (n=33). This is consistent with our previous report (Wang, 2006). However, after switching to photopic conditions the average highest frequency of light stimuli that those cells were able to follow increased to 6.15±0.39 Hz (n=13, tested).
Figure 4.
Current-clamp recordings from an Off (A&B) and an On (C) ganglion cells. A and B: recordings from an Off ganglion cell under scotopic and photopic conditions, respectively. Under scotopic conditions the Off ganglion cell was in the APB sensitive rod Off-pathway, determined by using APB. The light onset and offset are indicated above the recording trace. The stimulus light intensity was 20 Rh*/rod/s and the contrast was 1. The highest frequency of the flashing spot stimuli that this cell followed was 1 Hz (A). After switching to photopic condition, this cell is capable of following flashing spots of light stimuli of up to 5Hz (B). The stimulus light intensity was 5000 Rh*/rod/s, the background light intensity was 1500 Rh*/rod/s, and the contrast was 0.54. C: A current-clamp recording from an On ganglion cell under scotopic conditions. This cell followed light stimulus frequency of up to 5 Hz. The stimulus light intensity was 22 Rh*/rod/s and the contrast was 1.
In the second set of experiments, we compared the frequency following properties of the APB sensitive rod Off-pathways with those of the rod On-pathways. In the dark adapted retina, the rod On-pathway consists of rods => rod bipolar cells => AII amacrine cells => gap junction => On cone bipolar cells => On ganglion cells. This pathway shares the same rods/rod bipolar cells/AII amacrine cells (dark gray cells in Figure 1) with the APB sensitive rod Off-pathway. We applied a similar hypothesis as described above. If the rod On-pathway can follow higher frequencies than that of the APB sensitive rod Off-pathway, the result will indicate that the rod/rod bipolar cell/AII amacrine cells are intrinsically capable of following high frequencies of light stimuli. Our results showed that indeed the rod On-pathway can follow higher frequencies than the APB sensitive rod Off-pathway under scotopic conditions (Figure 4A&C). The average highest frequency that the cells from the rod On-pathways were able to follow was 4.93±0.30 Hz (n=14). This value is significantly higher than that of the cells in the APB sensitive rod Off-pathway (p<0.05, two tailed t-test).
Mechanism that limits the frequency following through the APB insensitive rod Off-pathway
Similar experiments, as described above, were also conducted to study the mechanism that limits the frequency following properties of APB insensitive rod Off-pathways. As diagrammed in Figure 1B, there are two APB insensitive rod Off-pathways. Because the two APB insensitive rod Off-pathways share the same Off cone bipolar cells and Off ganglion cells (light gray cells in Figure 1B) with the cone Off-pathway, by switching from scotopic to photopic conditions, we tested if the rods or the Off cone bipolar cells/Off ganglion cells serve as the limiting mechanism in the APB insensitive rod Off-pathways. We found that the Off ganglion cells, which were originally in the APB insensitive rod Off-pathways under scotopic conditions, followed higher frequencies after the switch to photopic conditions.
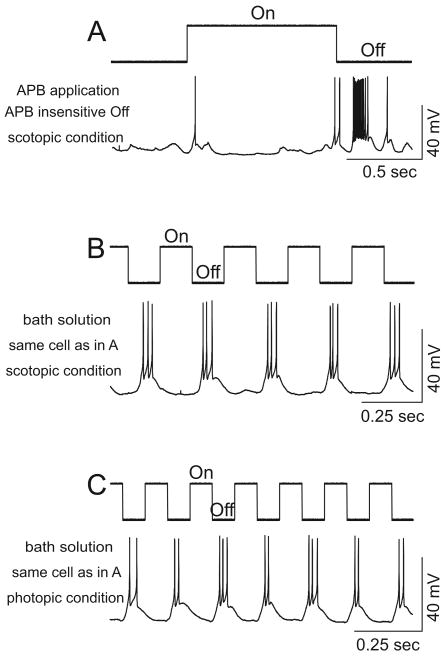
Recordings of an Off cell from the APB insensitive rod Off-pathway were shown in Figure 5. Bath application of APB did not abolish the Off response (panel A). The highest frequency that this cell was able to follow under scotopic conditions was 4 Hz (panel B). After switching to photopic conditions, this cell followed frequencies as high as 6 Hz (panel C). In total, we obtained 30 Off cells from the APB insensitive rod Off-pathways. The frequency following properties of these cells under scotopic and photopic conditions are shown in Figure 6. The panel A shows the distribution of recorded cells as a function of the highest frequency of light stimuli that they were able to follow. After switching to photopic conditions, the frequency following properties of these cells were higher than under scopotic conditions. The average highest frequency that the Off cells from the APB insensitive rod Off-pathways were able to follow was 4.20±0.23 Hz (n=30). After switching to photopic conditions it significantly increased to 5.71±0.43 Hz (n=24 tested, Figure 6 panel B, p<0.05, two tailed t-test). These results showed that the Off cone bipolar/ganglion cells in the APB insensitive rod Off-pathways were intrinsically capable of following higher frequencies. Thus, they are not the sites that limit the frequency following through the APB insensitive rod Off-pathways. Therefore, rods were not able to follow high frequencies in the APB insensitive rod Off-pathways, thus they serve as the mechanism that limits the frequency following through these pathways.
Figure 5.
Current-clamp recordings from an Off ganglion cell under scotopic and photopic conditions. The light onset and offset are indicated on the top of each panel. A: Under scotopic conditions, bath application of APB did not abolish the Off response, indicating this Off response was mediated by the APB insensitive rod Off-pathways. B: Under scotopic condition, this cell followed a flashing light at a frequency up to 4 Hz. The stimulus light intensity was 19 Rh*/rod/s and the contrast was 1 in A&B. C: After switching to photopic condition, this cell was able to follow flashing light frequencies up to 6 Hz. The recordings in B&C were obtained without the presence of APB. The stimulus light intensity was 6000 Rh*/rod/s, the background light intensity was 1500 Rh*/rod/s, and the contrast was 0.6 in C.
Figure 6.
A: the distribution of APB insensitive cells as a function of the highest stimulus frequency that those cells were capable of following under scotopic and photopic conditions. After switching to photopic conditions these cells were able to follow higher frequencies of flashing light than they were under scotopic conditions. As may be seen in the figure, the highest frequencies that these cells were able to follow under scotopic condition were around 4 Hz, whereas they were around 6 Hz after switching to photopic condition. B: There was a significant difference between the average highest frequencies that the APB insensitive Off cells can follow under scotopic and photopic conditions (* p<0.05, frequency following under photopic condition compared with frequency following under scotopic condition, two-tailed t-test).
Frequency following properties of On responses of retinal ganglion cells under scotopic and photopic conditions
We previously focused on the frequency following properties of Off ganglion cells under scotopic conditions (Wang, 2006), but the frequency following properties of On ganglion cells have not been established. Here, we investigated the frequency following properties of individual On ganglion cells under scotopic and photopic conditions.
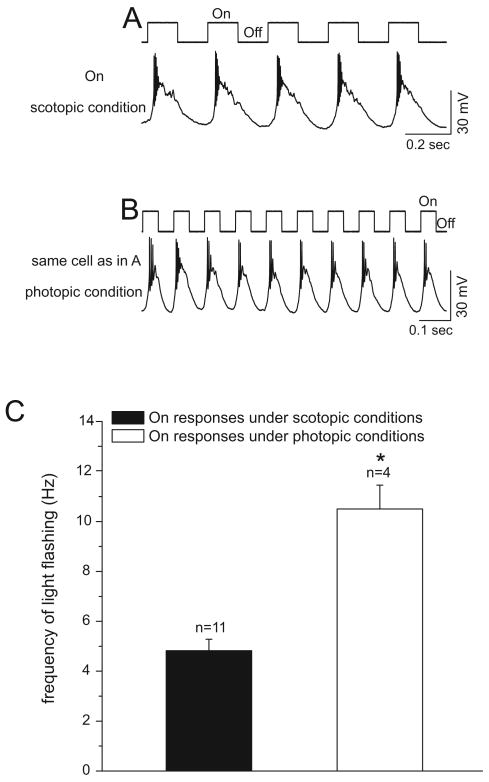
Successful recordings were made from a total of 11 retinal ganglion cells that only yielded On responses. Recordings from an On cell under scotopic and photopic conditions are shown in Figure 7. The highest frequency that this cell was able to follow under scotopic conditions was 4 Hz (panel A). After switching to photopic conditions, this cell followed the highest frequencies of up to 12 Hz (panel B). Each tested On cell followed higher frequencies under photopic conditions than under scotopic conditions (panel C). The average highest frequency of light stimuli that the On cells were able to follow under scotopic conditions was 4.82±0.46 Hz (n=11). By contrast, it was 10.50±0.96 Hz (n=4, tested) under photopic conditions. This difference in the frequency following properties of these cells under scotopic and photopic conditions was significant (panel C, p<0.05, two tailed t-test).
Figure 7.
Current-clamp recordings from an On ganglion cell under scotopic and photopic conditions. The light onset and offset are indicated on the top of each panel. A: Under scotopic conditions, this cell followed a flashing light of up to 4 Hz. The stimulus light intensity was 23 Rh*/rod/s and the contrast was 1. B: After switching to photopic conditions, this cell was able to follow a flashing light stimulus of 12 Hz. The stimulus light intensity was 6500 Rh*/rod/s, the background light intensity was 1500 Rh*/rod/s, and the contrast was 0.63. C: There is a significant difference between the average highest frequencies that On cells can follow under scotopic and photopic conditions (* p<0.05, frequency following under photopic condition compared with that under scotopic condition, two-tailed t-test).
We compared the frequency following properties of On responses (from On cells and On-Off cells) and Off responses (from Off cells and On-Off cells) under photopic conditions. We found that the On responses followed higher frequencies than the Off responses under photopic conditions. The average frequency following properties of On and Off responses under photopic conditions differed significantly (7.45±0.85 Hz, n=11 and 5.86±0.31 Hz, n=37, respectively, p<0.05, two tailed t-test). However, under scotopic conditions, the frequency following properties of On responses were similar to the Off responses from the APB insensitive rod Off-pathways (4.93±0.30 Hz, n=14 and 4.20±0.23 Hz, n=30, respectively). These findings were observed in On-Off cells as well as in the cells that yield only On or Off responses.
Frequency following properties of On-Off retinal ganglion cells under scotopic and photopic conditions
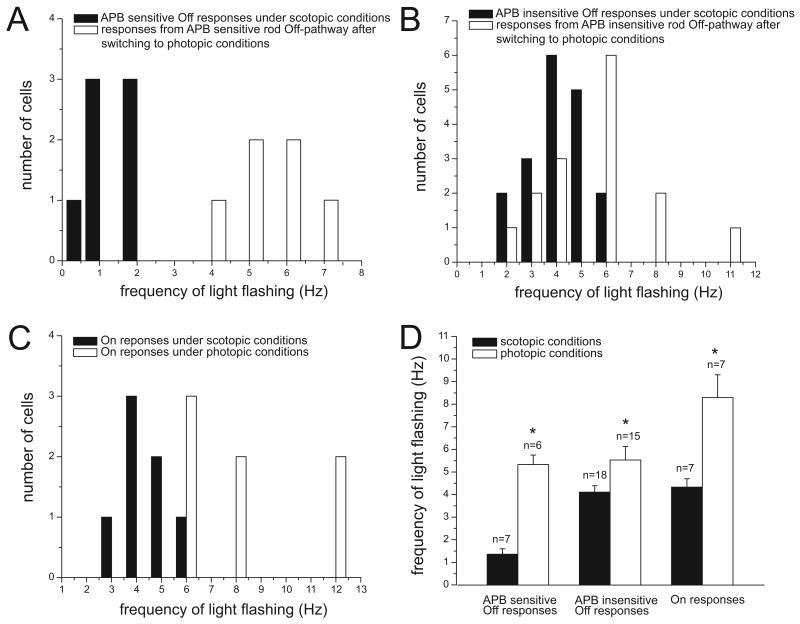
The frequency following properties of 25 On-Off cells were tested under scotopic and photopic conditions. The Off responses from these cells under scotopic conditions were differentiated into the APB sensitive and insensitive rod Off-pathways by using APB. Under scotopic conditions, the APB sensitive and insensitive rod Off-pathways followed significantly different maximal frequencies of light stimuli at 1.36± 0.24 Hz (n=7) and 4.11±0.28 Hz (n=18), respectively (p<0.05, two tailed t-test). These results are in line with those of our previous findings (Wang, 2006). However, after photopic conditions, the frequency following properties of these two groups were not significantly different at 5.33±0.42 Hz (n=6, tested) and 5.53±0.60 Hz (n=15, tested), respectively (p>0.05, two tailed t-test).
The frequency following properties of the On responses from On-Off cells under scotopic and photopic conditions were similar to the On responses from On cells. The average highest frequencies followed by the On response from On-Off cells were significantly different under scotopic and photopic conditions at 4.43±0.37 Hz (n=7) and 8.29±1.01 Hz (n=7, tested), respectively (Figure 8D). The distributions of the frequency following properties of On-Off cells under scotopic and photopic conditions are shown in Figure 8A-C. We also found that among the On-Off cells under photopic conditions, the On responses followed significantly higher frequencies than the Off responses from the same cells. The average frequency following properties of On and Off responses from On-Off cells were 8.29±1.01 Hz (n=7) and 5.57±0.37 Hz (n=7), respectively (p<0.05, two tailed t-test).
Figure 8.
The frequency following properties of On-Off ganglion cells under scotopic and photopic conditions. The distribution of APB sensitive Off responses (A), APB insensitive Off responses (B) and On responses (C) are shown as a function of the highest stimulus frequency that they were capable of following under scotopic and photopic conditions. As it may be seen in the figure, in each case frequency following capability is higher after switching to photopic conditions. D: There are significant differences between the average highest frequencies that those cells can follow under scotopic and photopic conditions (* p<0.05, frequency following under photopic condition compared with that under scotopic condition, two-tailed t-test).
Discussion
Mechanism that limits the frequency following through the APB sensitive rod Off-pathways
In the APB sensitive rod Off-pathway under scotopic conditions, rods transmit the signals via rod bipolar cells to AII amacrine cells then to Off cone bipolar cells (by glycinergic synapses), which in turn innervate the dendrites of Off ganglion cells (Sharpe and Stockman, 1999). Völgyi and colleagues have shown that this pathway has a high sensitivity to light offset (Völgyi et al., 2004). We found that this pathway conveys slow and low frequency signals (Wang, 2006). These findings indicate that this pathway may represent the rod pathway that transfers slow and low threshold signals in human psychophysical studies (Blakemore and Rushton, 1965; Conner, 1982).
However, the mechanism that limits the frequency following through this pathway remains unknown. Our hypothesis is that the sites from the AII amacrine cells to the Off cone bipolar cells may serve as the limiting mechanism. By switching recordings of each individual ganglion cell from scotopic to photopic conditions, we were able to dissect the frequency following properties of different components within this pathway. We found that within this pathway the components of rod/rod bipolar cells/AII amacrine cells and Off cone bipolar cells/Off ganglion cells were capable of following high frequencies of light stimuli (Figure 4B&C). However, the APB sensitive Off ganglion cells were not capable of following a high frequency light stimulus under scotopic conditions. These results indicate that the glycinergic synapse between AII amacrine cells and Off cone bipolar cells may limit the frequency following through this pathway.
In the APB sensitive rod Off-pathway, rods stop releasing glutamate when the light turns on, rod bipolar cells are depolarized, then AII amacrine cells are activated, AII amacrine cells release glycine to inhibit Off cone bipolar cells, and thus the synaptic inputs from Off cone bipolar cells to Off ganglion cells are reduced during illumination (light on). When the light turns off, however, rods begin to release glutamate, glutamate binds to mGluR6 receptors on rod bipolar cells, rod bipolar cells are hyperpolarized, AII amacrine cells are inhibited, and stop releasing glycine to Off cone bipolar cells; thus, the inhibition from glycine to Off cone bipolar cells is removed, allowing Off cone bipolar cells to release glutamate to Off ganglion cells and Off ganglion cells to generate Off responses. The removal of glycine inhibition from Off cone bipolar cells is the trigger for Off responses in the APB sensitive rod Off-pathway (reviewed by Sharpe and Stockman, 1999).
There are two possible explanations for the glycinergic synapses limiting the frequency following properties of the APB sensitive rod Off-pathway. First, as discussed above, the inhibition from the AII amacrine cells to the Off cone bipolar cells during light illumination is critical for the Off signals passing through the APB sensitive rod Off-pathways. Without the pre-inhibition before Off signal arrive, the Off cone bipolar cells could not generate a burst release onto ganglion cells when the Off signals arrive, therefore, disallowing Off responses from the Off ganglion cells. Under high frequency stimuli, each On cycle is short, therefore the AII amacrine cells may not have enough time to release enough glycine onto the Off cone bipolar cells and to generate the effective pre-inhibition on Off cone bipolar cells before Off signals arrive, thereby limiting the high frequency stimuli following through the APB sensitive rod Off-pathways.
Alternatively, under high frequency stimuli each Off cycle is also short. The short Off cycle may limit the removal of glycine from the Off cone bipolar cells. Since the removal of glycine inhibition from Off cone bipolar cells is the trigger for Off responses in the APB sensitive rod Off-pathway (reviewed by Sharpe and Stockman, 1999), the insufficient removal of glycine inhibition may limit the high frequency stimuli following through the APB sensitive rod Off-pathways. Further experiments are needed to validate the two above possibilities.
The postsynaptic mGluR6 receptors at rod bipolar cells have been shown to have slower kinetic properties than AMPA/KA receptors at Off cone bipolar cells (Ashmore and Copenhagen, 1980). Since the rod On-pathways share the rod bipolar cells with the APB sensitive rod Off-pathways (Figure 1), we tested the frequency following properties of the APB sensitive rod On-pathways to determine whether the slower kinetic properties of mGluR6 at rod bipolar cells limit the frequency following properties of the APB sensitive rod Off-pathways. If the mGluR6 receptors at rod bipolar cells limit the frequency following properties of the APB sensitive rod On-pathways, one would expect the frequency following property of the APB sensitive rod On-pathways to be similar to those of the APB sensitive rod Off-pathways. However, our results showed that the frequency following properties of the APB-sensitive rod On-pathways are significantly higher than the APB sensitive rod Off-pathways (Figure 4C). These results indicate that the rod bipolar cells are not the components which have the slowest response properties in the APB sensitive rod Off-pathways. Therefore, the slower kinetic properties of the mGluR6 receptors at rod bipolar cells do not play a critical role in limiting the stimulus frequency following through the APB sensitive rod Off-pathways.
Light adaptation does not only change the retinal circuitry from rod to cone pathways, it also changes the response properties of retinal neurons. Thus, it is possible that the Off cone bipolar cells may follow only low frequencies under scotopic conditions, and the properties of the Off cone bipolar cells may change during light adaptation. Therefore, the Off cone bipolar cell may serve as the site that limits high frequency following through the APB sensitive rod Off-pathway under scotopic conditions. However, this possibility is not favored by the previous findings. Völgyi and colleagues have shown that some Off ganglion cells receive convergent rod signals from primary (APB sensitive) and secondary or tertiary (APB insensitive) rod pathways (Völgyi et al., 2004). As diagramed in the Figure 1B of the current study, the convergence occurs at the Off cone bipolar cells. We previously found that the APB insensitive rod Off-pathways followed higher stimulus frequencies than the APB sensitive rod Off-pathways (Wang, 2006). These previous findings suggest that the Off cone bipolar cells may be capable of following high stimulus frequencies under scotopic conditions.
Rods limit the frequency following through the APB insensitive rod Off-pathways
As may be seen in Figure 1B, there are two APB insensitive rod Off pathways: rod => gap junction => cone => Off cone bipolar cell => ganglion cell (secondary rod Off-pathway); and rod => directly contacting Off cone bipolar cell => ganglion cell (tertiary rod Off-pathway). It has been found that the tertiary pathway is rare in the mouse retina (Protti et al., 2005). In the current study, we did not separate these two pathways. After switching the recordings from scotopic to photopic conditions, the ganglion cells which were part of the APB insensitive rod Off-pathway were able to follow significantly higher frequencies of light stimuli. The results indicate that the components of Off cone bipolar cells and ganglion cells in the APB insensitive rod Off-pathways are intrinsically capable of following high frequencies. Thus, in the APB insensitive rod Off-pathways (Figure 1B) only rods cannot follow high frequencies; therefore, rods serve as the mechanism that limits the frequency following through the APB insensitive rod Off-pathways.
Frequency following properties of On and Off responses under scotopic and photopic conditions
It has been documented that cones have faster rates of response than rods (Attwell, 1986; Miller et al., 1994; Yau 1994; Rabl et al., 2005; Johnson et al., 2007). How the difference between the cones and rods contributes to the frequency following properties of the entire cone signaling pathway (from cones to ganglion cells) and the entire rod signaling pathway (from rods to ganglion cells) remains to be established. In the current study we sought to determine if the cone and the rod signaling pathways convey different frequency following properties. We found On responses from On and On-Off ganglion cells were capable of following higher frequencies under photopic conditions than under scotopic conditions. Similar results were also found with Off responses. These results reveal that cone pathways are able to convey a much higher frequency of visual signals than rod signaling pathways.
In a rod or cone signaling pathway, if a component has the lowest frequency following property, this component serves as the limiting mechanism that controls the high stimulus frequencies flowing through the signaling pathway. To determine if the rods or cones serve as the limiting mechanisms in rod and cone signaling pathways, we compared the secondary rod On-pathway (rod => via gap junction => cone => cone On bipolar cell => On ganglion cell) and the cone On-pathway (cone => On cone bipolar cell => On ganglion cell). These two pathways share the On cone bipolar cells and ganglion cells (hollow circle cells in Figure 1A). The fact that the cone On-pathway followed higher frequencies than the rod On-pathway indicates that cones and rods are the limiting mechanisms in cone and rod signaling pathways, respectively. This indication is supported by the fact that Off cells can follow higher frequencies under photopic conditions than under scotopic conditions (see discussion above).
In the present study, we found that the frequency following properties of On responses were significantly higher than Off responses under photopic conditions. This was observed in cells that yielded just On or Off responses as well as in On-Off cells. The underlying mechanisms for this difference are unknown. Based on the fact that the difference was evident in individual On-Off cells, cones and ganglion cells are not the sites that underlie this difference. The difference between the frequency following properties of On and Off responses may be due to the functional differences between On cone bipolar cells and Off cone bipolar cells. In fact, it has been shown that On cone bipolar cells and Off cone bipolar cells have different properties in response to light stimulation and glycinerigic input (Ivanova et al., 2006; Rieke 2001; Euler and Masland, 2000; Zhou and Dacheux, 2005). These different properties between On cone and Off cone bipolar cells may contribute to the differences of frequency following properties between On and Off responses of ganglion cells under photopic conditions.
Acknowledgments
We thank Dr. Catherine G. Cusick for reading and commenting on this manuscript, Joseph P. Nemargut and Wei Huang for technical support. This research was supported by grant EY13301 from the National Eye Institute, Tulane Research Enhancement Fund, and Tulane New Faculty Startup Fund.
Abbreviations
- APB
DL-2-amino-4-phosphonobutyric acid
- MEME
Minimal essential medium eagle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashmore JF, Copenhagen DR. Different postsynaptic events in two types of retinal bipolar cell. Nature. 1980;288:84–86. doi: 10.1038/288084a0. [DOI] [PubMed] [Google Scholar]
- Attwell D. The Sharpey-Schafer lecture. Ion channels and signal processing in the outer retina. Q J Exp Physiol. 1986;71:497–536. [PubMed] [Google Scholar]
- Baylor DA. Photoreceptor signals and vision. Proctor lecture. Investigative ophthalmology & visual science. 1987;28:34–49. [PubMed] [Google Scholar]
- Blakemore CB, Rushton WA. Dark adaptation and increment threshold in a rod monochromat. J Physiol. 1965;181:612–628. doi: 10.1113/jphysiol.1965.sp007786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard GC, Rollag MD, Hanifin JP. Photic regulation of melatonin in humans: ocular and neural signal transduction. J Biol Rhythms. 1997;12:537–546. doi: 10.1177/074873049701200608. [DOI] [PubMed] [Google Scholar]
- Burkhardt DA, Gottesman J. Light adaptation and responses to contrast flashes in cones of the walleye retina. Vision Res. 1987;27:1409–1420. doi: 10.1016/0042-6989(87)90151-9. [DOI] [PubMed] [Google Scholar]
- Conner JD. The temporal properties of rod vision. J Physiol. 1982;332:139–155. doi: 10.1113/jphysiol.1982.sp014406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Haarsma L, Freed MA, Sterling P. Functional circuitry of the retinal ganglion cell's nonlinear receptive field. J Neurosci. 1999;19:9756–9767. doi: 10.1523/JNEUROSCI.19-22-09756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH, Baylor DA. An alternative pathway for signal flow from rod photoreceptors to ganglion cells in mammalian retina. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:10658–10662. doi: 10.1073/pnas.92.23.10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M, Uji Y, Yamamura H. Morphological classification of retinal ganglion cells in mice. The Journal of comparative neurology. 1995;356:368–386. doi: 10.1002/cne.903560305. [DOI] [PubMed] [Google Scholar]
- Dowling JE. The retina : an approachable part of the brain. Cambridge, Mass: Belknap Press of Harvard University Press; 1987. [Google Scholar]
- Euler T, Masland RH. Light-evoked responses of bipolar cells in a mammalian retina. J Neurophysiol. 2000;83:1817–1829. doi: 10.1152/jn.2000.83.4.1817. [DOI] [PubMed] [Google Scholar]
- Field GD, Rieke F. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron. 2002;35:773–785. doi: 10.1016/s0896-6273(02)00700-6. [DOI] [PubMed] [Google Scholar]
- Hack I, Peichl L, Brandstatter JH. An alternative pathway for rod signals in the rodent retina: rod photoreceptors, cone bipolar cells, and the localization of glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:14130–14135. doi: 10.1073/pnas.96.24.14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes KA, Pennesi ME, Sokal I, Church-Kopish J, Schmidt B, Margolis D, Frederick JM, Rieke F, Palczewski K, Wu SM, Detwiler PB, Baehr W. GCAP1 rescues rod photoreceptor response in GCAP1/GCAP2 knockout mice. The EMBO journal. 2002;21:1545–1554. doi: 10.1093/emboj/21.7.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Müller U, Wässle H. Characterization of the glycinergic input to bipolar cells of the mouse retina. Eur J Neurosci. 2006;23:350–364. doi: 10.1111/j.1460-9568.2005.04557.x. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Jr, Perkins GA, Giddabasappa A, Chaney S, Xiao W, White AD, Brown JM, Waggoner J, Ellisman MH, Fox DA. Spatiotemporal regulation of ATP and Ca2+ dynamics in vertebrate rod and cone ribbon synapses. Mol Vis. 2007;13:887–919. [PMC free article] [PubMed] [Google Scholar]
- Miller JL, Picones A, Korenbrot JI. Differences in transduction between rod and cone photoreceptors: an exploration of the role of calcium homeostasis. Curr Opin Neurobiol. 1994;4:488–495. doi: 10.1016/0959-4388(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Rieke F. Signals and noise in an inhibitory interneuron diverge to control activity in nearby retinal ganglion cells. Nat Neurosci. 2008;11:318–326. doi: 10.1038/nn2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemargut J, Zhu J, Savoie B, Wang GY. Differential effects of charybdotoxin on the activity of retinal ganglion cells in the dark- and light-adapted mouse retina. Vision Res. 2009;49:388–397. doi: 10.1016/j.visres.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- Protti DA, Flores-Herr N, Li W, Massey SC, Wässle H. Light signaling in scotopic conditions in the rabbit, mouse and rat retina: a physiological and anatomical study. J Neurophysiol. 2005;93:3479–3488. doi: 10.1152/jn.00839.2004. [DOI] [PubMed] [Google Scholar]
- Rabl K, Cadetti L, Thoreson WB. Kinetics of exocytosis is faster in cones than in rods. J Neurosci. 2005;25:4633–4640. doi: 10.1523/JNEUROSCI.4298-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F. Temporal contrast adaptation in salamander bipolar cells. J Neurosci. 2001;21:9445–9454. doi: 10.1523/JNEUROSCI.21-23-09445.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DW, Chalupa LM. The intrinsic temporal properties of alpha and beta retinal ganglion cells are equivalent. Curr Biol. 1997;7:366–374. doi: 10.1016/s0960-9822(06)00184-9. [DOI] [PubMed] [Google Scholar]
- Schiller PH. The ON and OFF channels of the visual system. Trends Neurosci. 1992;15:86–92. doi: 10.1016/0166-2236(92)90017-3. [DOI] [PubMed] [Google Scholar]
- Sharpe LT, Stockman A. Rod pathways: the importance of seeing nothing. Trends Neurosci. 1999;22:497–504. doi: 10.1016/s0166-2236(99)01458-7. [DOI] [PubMed] [Google Scholar]
- Soucy E, Wang Y, Nirenberg S, Nathans J, Meister M. A novel signaling pathway from rod photoreceptors to ganglion cells in mammalian retina. Neuron. 1998;21:481–493. doi: 10.1016/s0896-6273(00)80560-7. [DOI] [PubMed] [Google Scholar]
- Sterling P, Demb JB. Retina. In: Shepherd GM, editor. The Synaptic Organization of the Brain. New York: Oxford University Press; 2004. pp. 217–269. [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for night vision in mouse retina. J Neurosci. 2001;21:8616–8623. doi: 10.1523/JNEUROSCI.21-21-08616.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völgyi B, Deans MR, Paul DL, Bloomfield SA. Convergence and segregation of the multiple rod pathways in mammalian retina. J Neurosci. 2004;24:11182–11192. doi: 10.1523/JNEUROSCI.3096-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GY, Ratto G, Bisti S, Chalupa LM. Functional development of intrinsic properties in ganglion cells of the mammalian retina. J Neurophysiol. 1997;78:2895–2903. doi: 10.1152/jn.1997.78.6.2895. [DOI] [PubMed] [Google Scholar]
- Wang GY, Liets LC, Chalupa LM. Unique functional properties of on and off pathways in the developing mammalian retina. J Neurosci. 2001;21:4310–4317. doi: 10.1523/JNEUROSCI.21-12-04310.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GY. Unique functional properties of the APB sensitive and insensitive rod pathways signaling light decrements in mouse retinal ganglion cells. Vis Neurosci. 2006;23:127–135. doi: 10.1017/S0952523806231110. [DOI] [PubMed] [Google Scholar]
- Wang GY, van der List DA, Nemargut JP, Coombs JL, Chalupa LM. The sensitivity of light-evoked responses of retinal ganglion cells is decreased in nitric oxide synthase gene knockout mice. J Vis. 2007;7(7):1–13. doi: 10.1167/7.14.7. [DOI] [PubMed] [Google Scholar]
- Xin D, Bloomfield SA. Comparison of the responses of AII amacrine cells in the dark- and light-adapted rabbit retina. Vis Neurosci. 1999;16:653–665. doi: 10.1017/s0952523899164058. [DOI] [PubMed] [Google Scholar]
- Yau KW. Phototransduction mechanism in retinal rods and cones. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1994;35:9–32. [PubMed] [Google Scholar]
- Zhou C, Dacheux RF. Glycine- and GABA-activated inhibitory currents on axon terminals of rabbit cone bipolar cells. Vis Neurosci. 2005;22:759–767. doi: 10.1017/S095252380522607X. [DOI] [PubMed] [Google Scholar]