Abstract
The ability to select and integrate relevant information in the presence of competing irrelevant information can be enhanced by advance information to direct attention and guide response selection. Attentional preparation can reduce perceptual and response conflict, yet little is known about the neural source of conflict resolution, whether it is resolved by modulating neural responses for perceptual selection to emphasize task-relevant information or for action selection to inhibit pre-potent responses to interfering information. We manipulated perceptual information that either matched or did not match the relevant color feature of an upcoming Stroop stimulus and recorded hemodynamic brain responses to these events. Longer reaction times to incongruent than congruent color-word Stroop stimuli indicated conflict; however, conflict was even greater when a color cue correctly predicted the Stroop target’s color (match) than when it did not (nonmatch). A predominantly anterior network was activated for Stroop-match and a predominantly posterior network was activated for Stroop-nonmatch. Thus, when a stimulus feature did not match the expected feature, a perceptually-driven posterior attention system was engaged, whereas when interfering, automatically-processed semantic information required inhibition of pre-potent responses, an action-driven anterior control system was engaged. These findings show a double dissociation of anterior and posterior cortical systems engaging in different types of control for perceptually-driven and action-driven conflict resolution.
Keywords: Attention, Conflict, Control, fMRI, Perceptual Cueing
INTRODUCTION
Selective perception and goal-directed action depend on an interaction of executive, motor, and sensory control processes (Fuster, 2007). A fundamental aspect of goal-directed actions involves the ability to select relevant and inhibit irrelevant information. Selection is especially challenging in conflict situations when information becomes overlearned as occurs when stimuli have semantic value and responses become “automatic.” In this case, overlearned information can interfere with appropriate response selection (Kahneman and Chajczyk, 1983; Jacoby et al., 2003; Langenecker et al., 2004). Advance information from valid cueing can improve and accelerate effective selection of relevant stimulus attributes and inhibition of irrelevant information during cognitive operations (Damasio, 1996; Meiran, 1996; Posner et al., 1980; Sudevan and Taylor, 1987). Functional imaging studies have shown that the prefrontal cortex (PFC) mediates many aspects of conflict processing, but it is not fully understood how perceptual cueing influences conflict resolution or how the brain processes conflict with perceptual cueing (Stern et al., 2007).
In monkeys, recording from single neurons demonstrated a necessary role of the PFC in a match-to-sample task (Wallis et al., 2001). In humans, neuroimaging studies have identified a predominant role of the PFC in tasks that require overriding pre-potent responses, such as in Stroop conflict tasks (McLeod, 1991; Stroop, 1935), when the semantic property of a word (e.g., the word RED written in blue ink) involuntarily or automatically interferes with perceptual stimulus feature processing required to name its ink color (Bush et al., 2003; Lungu et al., 2007).
Here, we used functional magnet resonance imaging (fMRI) to examine whether processing perceptual information to resolve an impending conflict is unique to the PFC or extends to posterior attentional and sensory systems. Accordingly, we developed a Stroop Match-to Sample task that required matching the color of a cue stimulus to the color of a Stroop target stimulus to assess perceptual-driven and action-driven conflict processing and to determine whether these processes engage separate or shared neural correlates of conflict processing. Previously, we demonstrated that reaction times were faster and more accurate for cue-target color matches than nonmatches, whereas Stroop conflict was actually greater for match than nonmatch trials (Schulte et al., 2008; Schulte et al., 2006). This finding is consistent with a study by Chen (2003), who observed greater Stroop interference with valid than invalid cues in a Stroop task that employed spatial cues and lateralized stimulus presentation. Chen (2003) argued that extending the attentional focus with invalid spatial cues limits processing resources otherwise available to process the Stroop word’s meaning, whereas narrowing the attentional focus with valid cues leaves resources for distractor processing and increases interference. The attentional focus hypothesis, however, cannot explain the differential degree of inhibition across conditions observed for color cues. Nevertheless, the validity of prior perceptual information may influence resources available to process distracting information. Processing load may be higher with nonmatching cues than matching color cues, owing to suppression of invalid color information, updating and selecting of incoming relevant information (Lavie, 1995).
Imaging studies have shown greater activity in anterior cingulate cortex (ACC), presupplementary motor areas (SMA), dorsolateral prefrontal cortex (DLPFC), and inferior parietal lobe (IPL) when monitoring and resolving conflict (Botvinick et al., 1999; Casey et al., 2000). It has been argued that anterior and posterior brain regions are differentially sensitive to stimulus and response conflict (Davelaar, 2008; Liu et al., 2004; Milham et al., 2001; van Veen et al., 2001). For stimulus-stimulus conflict between the relevant color-attribute and the irrelevant word-attribute (Stroop task), posterior regions (e.g., IPL) involved in biasing the processing toward the task-relevant feature were activated, whereas for stimulus-response conflict between irrelevant spatial stimulus information and response to task relevant nonspatial information (Simon-task), anterior regions (e.g., ACC, SMA) sensitive to detection of response conflict, response selection, and planning were activated (Liu et al., 2004). Conflict-related activity in these areas, however, can be reduced when cognitive conflict occurs consecutively (Kerns et al., 2004) and when cues enable preparation to resolve conflict (Egner and Hirsch, 2005; Blasi et al., 2007; Luks et al., 2007). For example, the ACC was activated during resolving response conflict but not during stimulus conflict (van Veen et al., 2001) whereas DLPFC was also activated with stimulus conflict (Liu et al., 2004; Milham et al., 2001). Although neuroimaging findings suggest that anterior brain regions are activated in response conflict processing, recent behavioral studies indicate that conflict processing is driven more by the amount of stimulus conflict than by the amount of response conflict (Notebaert and Verguts, 2006; Verbruggen et al., 2006). Thus, prior conflict and attentional preparation can reduce the experience of conflict, yet little is known about how prior valid and invalid perceptual information prepares attentional systems for color-word conflict processing.
We hypothesized that different cognitive mechanisms and brain systems control conflict resolution for perception and action selection. Specifically, when the validity of advance perceptual information modulates the visual system’s response to relevant features, automatic processing of interfering information with valid cues requires inhibition of inaccurate responses for goal-directed actions, we predicted engagement of an action-driven anterior control system. By contrast, when a stimulus feature does not match the expected feature and increases processing load, we predicted engagement of a perceptually-driven posterior attention system.
MATERIALS AND METHODS
Subjects
Adult volunteers (12 women, 12 men; mean age = 23.5 ± 2.9 years, range 19-30 years) underwent fMRI while performing the Stroop Match-to-Sample task. All subjects were neurologically healthy, highly educated (15.6 ± 1.1 years, range 14-18 years), right-handed, and were free of history of illicit substance or alcohol abuse or dependence according to DSM-IV criteria. Subjects gave written informed consent to participate in this study, which was approved by the Institutional Review Boards at Stanford University School of Medicine and SRI International.
Stimuli and experimental design
Stroop Match-to-Sample Task. Stimuli were created and presented with PsyScope software. Subjects matched the color of a cue stimulus displayed for 450 ms in the center of the screen to the color of a Stroop target stimulus that appeared for 1100 ms after an interstimulus interval of 300 ms and was followed by a blank screen for about 1450 ms (Figure 1). Thus, subjects had 2550 ms to respond. The total trial duration was 3.3 sec. The color cue either matched or did not match the color of the Stroop target, which was either congruent (word blue written in blue ink) or incongruent (word blue written in red ink). Cue and target colors were red, green or blue. The congruent color-word condition is the non-Stroop control condition, and Stroop effects were examined by comparing congruent and incongruent conditions (Pardo et al., 1990; Koch and Brown, 1994; Melcher and Gruber, 2006). In incongruent-nonmatch trials the word always matched the cue color (e.g., red cue, word RED written in green ink). Subjects pressed a YES-key for cue-target color matches and a NO-key for nonmatches, yielding accuracy and reaction time measures (Figure 1, top). To mix YES- and NO responses four blocks were presented, two containing incongruent match and non-match trials (incongruent, INC) and the other two containing congruent match and nonmatch trials (congruent, CON) in addition to four same-response blocks (congruent-match, congruent-nonmatch, incongruent-match, incongruent-nonmatch) (Figure 1, bottom). Trials presented in same- and mixed-response blocks were the same; only the order of trials differed. Two runs were presented with 18 blocks each (1 block = 9 TRs or 6 trials; TR = 2.2 sec) including two rest condition blocks at the end of each run. In the rest condition, subjects passively viewed Stroop Match-to-Sample trials.
Figure 1.
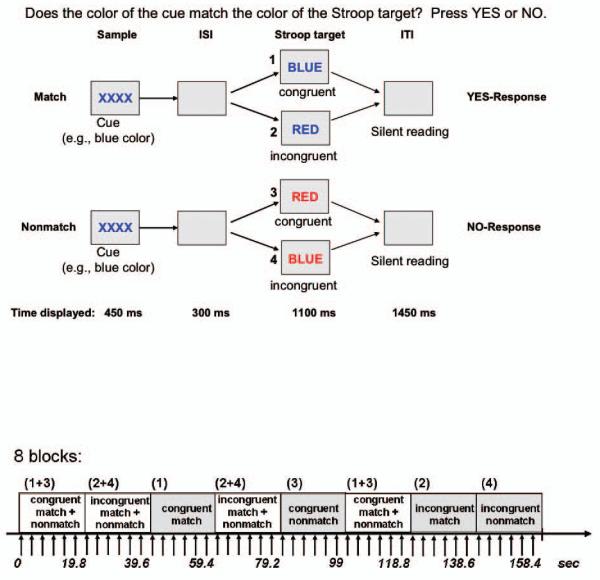
Top: Stroop Match-to-Sample design, illustrating 4 conditions: incongruent-match, congruent-match, incongruent-nonmatch, and congruent-nonmatch. A color cue (XXXX) presented for 450ms was followed by an incongruent or congruent Stroop target stimulus that appeared for 1100ms after an inter-stimulus interval (ISI) of 300ms. The inter-trial interval (ITI) was 1450 ms. Subjects matched the color of the cue to the ink color of the Stroop stimulus. The example shown uses two colors although over trials three colors were used (red, green, and blue).
Bottom: fMRI block design illustrated for 8 blocks. Each block consisted of 6 trials (9 TRs). Each block lasted for 19.8 sec. Stroop stimuli in each block were either congruent (e.g., word BLUE written in blue ink) or incongruent (e.g., word BLUE written in red ink). In half of the blocks cue-target color either matched or did not match, in the other half of the blocks match and nonmatch trials were mixed. In total 36 blocks were presented in pseudo-random order ensuring that each condition was equally often represented.
The start of the scan was triggered automatically from PsyScope software. Test instructions were reviewed with the subject by the examiner in a short practice session before entering the scanner and also via the scanner intercom system before the onset of each run. Subjects had a short break after ~ 6 minutes, i.e., between run 1 and run 2, but remained in the scanner. In the Stroop Match-to-Sample task, we chose subvocalization, as done by others (Adleman et al., 2002; Blumberg et al., 2003; Mead et al., 2002; Peterson et al., 1999; 2002), because overt speech can cause significant fMRI signal artifacts (Barch et al., 1999). By combining a subvocal response with a matching task, we were able to measure task performance and task compliance during scanning.
MRI data acquisition
Imaging was performed with a 3.0-T whole body MRI scanner (General Electric Medical Systems, Signa, Waukesha, WI, USA) using the Array Spatial Sensitivity Encoding Technique (ASSET) 3T head coil. Structural MRI protocols consisted of a spin-echo localizer scan and a T2-weighted fast spin-echo anatomical scan (axial acquisition; TE = 17 ms; TR = 5000 ms; FOV = 24 cm; 256 × 192 matrix; NEX = 1.0; slice thickness = 5 mm; 36 slices) used for spatially registering the fMRI data. Whole-brain fMRI data were acquired with a T2*-weighted gradient echo planar pulse sequence (axial, mode = 2D, Scan timing: TE = 30 ms, TR = 2200 ms, flip angle = 90°, matrix = 64 × 64, slice thickness = 5 mm, 36 slices). Image preprocessing and statistical analyses were performed using the SPM2 software package (Wellcome Department of Cognitive Neurology, University College London, UK).
The functional images were subjected to motion correction, and the T2-weighted FSE structural images were coregistered to the motion-corrected functional mean images for each subject. The images were then normalized to MNI (Montreal Neurological Institute, Quebec, Canada) space, and the volumes were smoothed with a Gaussian kernel of 8 mm (FWHM).
Data analysis
Individual statistics were computed using a general linear model approach (Friston et al., 1995) as implemented in SPM2. Statistical preprocessing consisted of high pass filtering at 39.6s, low pass filtering through convolution with the SPM2 canonical hemodynamic response function, and global scaling. A random effect analysis was conducted for group averaging and population interference, where one image per contrast was computed for each subject, and these images were subjected to t-tests, which produced a statistical image for the following contrasts for each subject: Stroop-match (INC > CON, for match (M) trials), Stroop-nonmatch (INC > CON, for nonmatch (NM) trials). The contrasts (INC > CON) for match and nonmatch trials for each individual were entered in one-sample t-tests. Finally, ANOVAs between Stroop-nonmatch and Stroop-match were performed to compare the activity of brain regions preferentially involved in either subprocess. Analyses were carried out with an uncorrected P value threshold of 0.001, and k = 10 voxels as extent threshold. We additionally tested whether our findings were robust when using a threshold that corrects for multiple comparisons. Accordingly, we used a statistical threshold with a joint-expected probability of p = .01 for height and p = .05 for extent corrected for the whole brain (Poline et al. 1997). For display purposes, group activations were superimposed onto a single subject’s T2-weighted SPM2- template image. Brain areas were determined by using the MNI coordinate function in MRICro, Version 1.40, from Chris Rorden (http://www.mricro.com). For validation, SPM-MNI coordinates in tables were transformed into the coordinate system of the Talairach and Tournoux (1988) stereotaxic atlas using the transformation from Matthew Brett (http://www.mrc.cbu.cam.ac.uk/Imaging/mnispace.html). Activations in the cerebellum were characterized using the atlas of Schmahmann et al. (2000).
To investigate individual differences concerning the brain-behavior relationship in the presence of attentional cueing, we performed region-of-interest (ROI) analyses. ROIs were selected from the contrasts Stroop-match vs. Stroop-nonmatch and incongruent-match vs. incongruent-nonmatch, where we hypothesized the existence of associations with Stroop-match and Stroop-nonmatch task performance. For this exploratory analysis, we used a statistical threshold of a height threshold of p < .05 family-wise error (FWE) corrected. Correlations were considered significant when corrected for multiple comparisons PFWE-corrected = .05, and considered at trend level when PFWE-corrected > .05 and ≤ .1. For display purposes of correlations, we extracted the mean parameter estimates from these clusters using the MarsBaR region of interest (ROI) analysis toolbox (marsbar.sourceforge.net/) implemented in SPM2.
RESULTS
Behavioral results
Incidence of errors was less than 2% (3.2 ± 3.3), and misses and reaction time outliers less than 0.5% (misses: 0.6 ± 1.5; outliers: 0.9 ± 1; outlier = RTs ± 3 SD from mean for each condition), indicating high accuracy levels while performing the Stroop Match-to-Sample task in the scanner. A repeated measures ANOVA with Stroop (incongruent, congruent), Match (match, nonmatch), and response block (mix, same) as within-subject variables revealed a significant Stroop effect with RTs to incongruent trials longer than to congruent trials (F(1,23) = 55.8, p < .0001), a cue-target match effect with RTs faster to match trials than to nonmatch trials (F(1,23) = 19.11, p < .0001), and a significant Stroop-by-match interaction (F(1,23) = 5.53, p = .028). Response times to congruent-match (CON-M) trials were shorter than RTs to incongruent-match (INC-M) and congruent-nonmatch (CON-NM) trials, which were shorter than RTs to incongruent-nonmatch (INC-NM) trials.
The Stroop effect was on group average 69.7 ms for match and 29.9 ms for nonmatch trials. RTs did not significantly differ between mixed- and same-response blocks (F(1,23) = 1.59, p = .22), and there were no significant interactions between response block and Stroop (F(1,23) = 2.01, p = .17), between response block and match (F(1,23) = 0.26, p = .62), or among the three factors (F(1,23) = 1.11, p = .31). Thus, neither reaction time nor Stroop conflict or match effects differed significantly between response blocks (Figure 2). Follow-up t-tests comparing same and mixed response blocks for each condition showed no significant RT difference (congruent-match t(23) = 0.21, p = .83; congruent-nonmatch t(23) = 0.11, p = .92; incongruent-match t(23) = 1.89, p = .07; incongruent-nonmatch t(23) = 0.49, p = .63).
Figure 2.
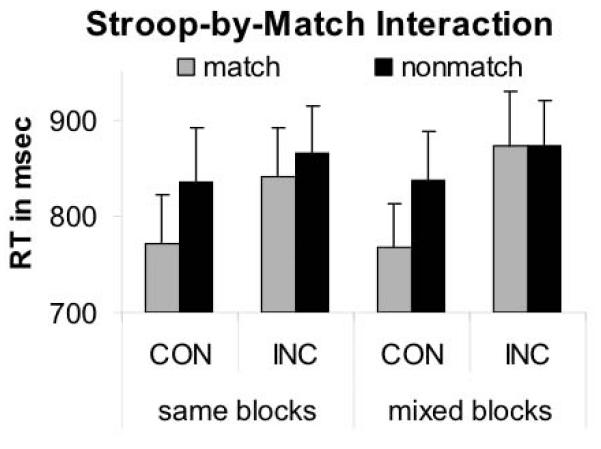
Stroop Match-to-Sample performance. Illustration of the Stroop-by-Match interaction effect: Stroop effects (incongruent — congruent) were greater for match than nonmatch trials.
Neural correlates of Stroop Match-to-Sample effects
To localize brain areas that were more active during incongruent than congruent Stroop target processing, we generated Stroop contrast images (INC > CON) for match and nonmatch trials for each subject. Contrast images were then entered into one sample t-tests for second-level group analyses for Stroop-match and Stroop-nonmatch.
Stroop-match contrast
Stroop processing (INC > CON) with valid pretrial color cueing (match) was associated with an increased BOLD response in the frontal and parietal brain regions including right superior, middle and inferior frontal gyri, bilateral inferior parietal cortex, right superior parietal lobe and right middle temporal gyrus. The opposite contrast (CON > INC) yielded several significant activations, including bilateral visual and parahippocampal areas, bilateral ventral middle (i.e., junction of posterior and anterior) cingulate cortex, bilateral supplementary motor areas and left superior temporal gyrus (Table 1).
Table 1.
Activation table for Stroop contrasts for cue-target match and nonmatch trials (p < .001 uncorrected, extent threshold k = 10 voxels); BA = Brodmann area; kE = number of voxels in a cluster
| Brain region | BA | kE | MNI- coordinates |
T | Z≡ | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Stroop-Match: INC > CON | |||||||
| R. superior frontal gyrus | 6 | 85 | 18 | 10 | 74 | 5.02 | 4.09 |
| L. inferior parietal lobe — supramarginal gyrus |
40 | 149 | −56 | −46 | 42 | 4.72 | 3.91 |
| R. middle temporal gyrus, fusiform | 37 | 30 | 58 | −60 | 2 | 4.10 | 3.84 |
| R. DLPFC — middle frontal gyrus | 46 | 147 | 38 | 24 | 34 | 4.58* | 3.82 |
| R. inferior frontal lobe — pars opercularis | 44 | 19 | 58 | 14 | 32 | 4.52* | 3.78 |
| R. superior parietal lobe — angular gyrus | 7 | 145 | 32 | −60 | 50 | 4.37* | 3.69 |
| 7 | 26 | 58 | −58 | 26 | 4.24* | 3.61 | |
| R. inferior parietal lobe | 40 | 15 | 56 | −50 | 44 | 3.74* | 3.27 |
| CON > INC | |||||||
| R. | 30 | 482 | −10 | −38 | 2 | 5.34* | 4.26 |
| L. parahippocampal / lingual gyrus | 18 | 6 | −62 | 2 | 5.28* | 4.23 | |
| L. | 30 | 12 | −40 | 0 | 4.07* | 3.49 | |
| L. ventral anterior cingulate cortex | 24 | 624 | −6 | −4 | 44 | 5.32* | 4.25 |
| L. supplementary motor area | 6 | 0 | −8 | 62 | 4.68* | 3.88 | |
| R. ventral posterior cingulate cortex | 23 | 6 | −12 | 48 | 4.58 | 3.82 | |
| R. supplementary motor area | 6 | 57 | 8 | −12 | 76 | 4.68* | 3.88 |
| L. rolandic operculum / sup. temporal lobe | 48,22 | 189 | −62 | −2 | 8 | 5.05 | 4.10 |
| L. cuneus | 19 | 80 | −6 | −80 | 38 | 4.56 | 3.81 |
| L. precentral gyrus | 4/6 | 110 | −36 | −26 | 66 | 4.15 | 3.55 |
|
Stroop-Nonmatch: INC > CON |
|||||||
| L. middle temporal gyrus | 37 | 48 | −44 | −66 | 6 | 4.11 | 3.52 |
| R. precentral gyrus | 6 | 17 | 54 | −4 | 40 | 3.88 | 3.37 |
| CON > INC | |||||||
| R. dorsal anterior cingulate cortex | 32 | 27 | 2 | 38 | 30 | 3.84 | 3.34 |
regions significant at p < .05 corrected for the whole brain
Stroop-nonmatch contrast
Stroop processing (INC > CON) with invalid pretrial color cueing (nonmatch) was associated with an increased BOLD response in left middle temporal (BA 37) and right precentral gyri (BA 6). The opposite contrast (CON > INC) showed a significant activation in the right dorsal anterior cingulate cortex (Table 1).
Comparison between Stroop-match and Stroop-nonmatch
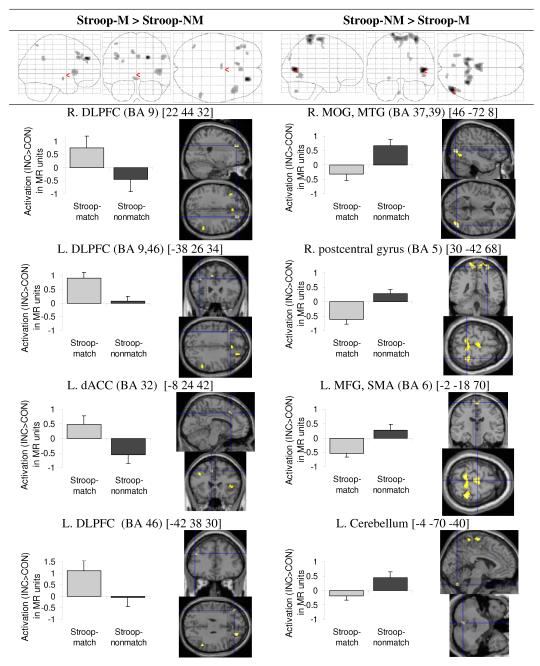
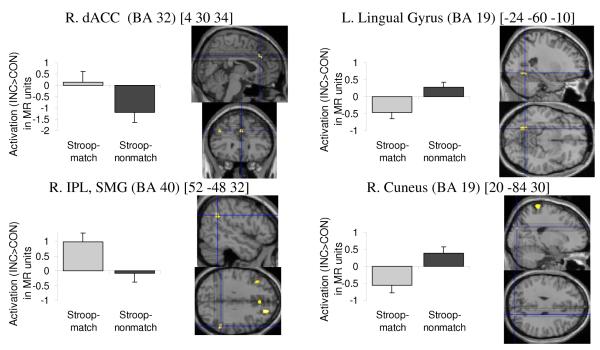
Increased BOLD response specific to Stroop-match (vs. Stroop-nonmatch) occurred mainly in anterior brain areas, including bilateral dorsolateral prefrontal cortex (DLPFC) (BA 9, 46), dorsal anterior cingulate cortex (ACC) (BA 32), ventrolateral prefrontal cortex (VLPFC) (BA 45), and parietal areas (left BA 7, 40; right BA 40) (Figure 3). Increased BOLD response specific to Stroop-nonmatch (vs. Stroop-match) occurred mainly in posterior brain areas, including bilateral visual association (BA 19), right middle and inferior temporal gyrus (BA 37, 39), somatosensory (BA 2, 3) and somatosensory association (BA 5) areas, and also left motor (BA 4) and supplementary motor (SMA, BA 6) areas, and uvula of the cerebellum (Table 2, Figure 3).
Figure 3.
Illustration of two dissociated neural networks: fronto-parietal network showing higher activation for Stroop-match than Stroop-nonmatch trials (left) and visuo-motor network showing higher activation for Stroop-nonmatch than Stroop-match trials (right). Using MarsBaR ROI data extraction tool, regional activations were extracted for Stroop (INC > CON) contrasts for match and nonmatch trials, and illustrated to the left of regional activations.
Table 2.
Comparison of Stroop-match (M) and Stroop-nonmatch (NM): Activity of brain regions preferentially invoked in either subprocess; repeated measures ANOVA (p < .001 uncorrected, extent threshold k = 10 voxels); BA = Brodmann area; kE = number of voxels in a cluster
| Brain region | BA | kE | MNI-coordinates | T | Z≡ | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| (A) Stroop-M > Stroop-NM | |||||||
| R. DLPFC — superior frontal gyrus | 9 | 58 | 22 | 44 | 32 | 5.76 | 4.49 |
| 9,46, | 51 | -38 | 26 | 34 | 4.46~* | 3.75 | |
| L. DLPFC — middle frontal gyrus | 45 | -42 | 34 | 38 | 3.84~* | 3.34 | |
| 46 | 11 | -28 | 44 | 22 | 3.86~* | 3.36 | |
| R. VLPFC — inferior frontal gyrus | 45 | 162 | 46 | 16 | 6 | 4.32 | 3.66 |
| L. dorsal anterior cingulate cortex | 32 | 25 | -8 | 24 | 42 | 4.14 | 3.54 |
| L. DLPFC — middle frontal gyrus | 46 | 16 | -42 | 38 | 30 | 4.04 | 3.48 |
| L. superior, inferior parietal lobe | 7,40 | 12 | -40 | -62 | 60 | 4.01 | 3.45 |
| R. inferior parietal lobe — supramarginal gyrus |
40 | 50 | 52 | -48 | 32 | 3.99 | 3.44 |
| R. dorsal anterior cingulate cortex | 32 | 29 | 4 | 30 | 34 | 3.98 | 3.44 |
| (B) Stroop-NM > Stroop-M | |||||||
| R. middle and inferior temporal gyri | 39 | 181 | 46 | -72 | 8 | 5.77~* | 4.49 |
| 37 | 46 | -60 | -4 | 3.97~* | 3.43 | ||
| R. cuneus, superior occipital gyrus — extrastriate area V3 |
19 | 19 | 20 | -84 | 30 | 3.80~* | 3.31 |
| R. | 5 | 30 | -42 | 68 | 5.37* | 4.28 | |
| L. postcentral gyrus, precuneus | 3 | 543 | -16 | -40 | 78 | 5.20* | 4.19 |
| L. | 4,5 | -6 | -42 | 70 | 4.97* | 4.05 | |
| L. medial frontal gyrus, supplementary motor area |
6 | 168 | -2 | -18 | 70 | 4.89 | 4.01 |
| -4 | -12 | 52 | 3.60 | 3.17 | |||
| L. inferior cerebellum, lobule IX (uvula) | 26 | -4 | -70 | -40 | 4.03 | 3.47 | |
| L. lingual gyrus — extrastriate area V3 | 19 | 34 | -24 | -60 | -10 | 3.88* | 3.37 |
regions significant at p < .05 corrected for the whole brain
trend at p ≤ .1 corrected for the whole brain
Processing incongruent information was associated with increased BOLD signal for match compared to nonmatch in mainly anterior brain areas, including insula (bilateral BA 48), dorsal (right BA 32) and ventral anterior cingulate cortex (left BA 24), dorsolateral prefrontal cortex (right BA 9, 46), supramarginal gyrus (bilateral BA 40) and bilateral thalamus. The opposite contrast for processing incongruent information with nonmatch compared to match color cues was associated with activation of posterior visual and motor processing areas including middle temporal and occipital gryus (right BA 37, 19), motor cortex (right BA 4), somatosensory (left BA 3) and somatosensory association (left BA 5) areas, and the cerebellum (Table 3). The results were significant even after correcting for multiple comparisons (Tables 1-3).
Table 3.
Comparison of incongruent-match (M) and incongruent-nonmatch (NM): Activity of brain regions preferentially invoked in either subprocess; repeated measures ANOVA (p < .001 uncorrected, extent threshold k = 10 voxels); BA = Brodmann area; kE = number of voxels in a cluster
| Brain region | BA | kE | MNI-coordinates | T | Z≡ | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| (A) Incongruent M > Incongruent NM | |||||||
| R. insula | 48 | 683 | 44 | 14 | 0 | 6.70* | 4.94 |
| R. d- | 6 | 28 | 36 | 5.67* | 4.44 | ||
| anterior cingulate cortex | 32,24 | 351 | |||||
| L. v- | 0 | 18 | 28 | 4.56* | 3.81 | ||
| R. dorsolateral prefrontal cortex | 9 | 128 | 24 | 42 | 34 | 5.61 | 4.41 |
| 34 | 32 | 34 | 3.71 | 3.25 | |||
| L. insula | 48,47 | 84 | -46 | 12 | 0 | 5.37 | 4.28 |
| L. supramarginal gyrus | 40 | 71 | -58 | -48 | 24 | 4.92 | 4.03 |
| L. | -6 | -12 | 6 | 4.53 | 3.79 | ||
| medial dorsal and anterior thalamus | 88 | ||||||
| R. | 6 | -8 | 6 | 3.90 | 3.38 | ||
| R. DLPFC | 8,9 | 35 | 42 | 8 | 46 | 4.20 | 3.58 |
| R. cingulate cortex | 23,24 | 42 | 6 | -20 | 42 | 4.13 | 3.54 |
| R. DLPFC | 9 | 20 | 34 | 42 | 46 | 4.07 | 3.50 |
| R. supramarginal gyrus | 40 | 55 | 54 | -46 | 34 | 3.92 | 3.40 |
| (B) Incongruent NM > Incongruent M | |||||||
| R. middle temporal and occipital gyri | 37,19 | 263 | 48 | -74 | 10 | 6.94 | 4.49 |
| 46 | -58 | -6 | 4.52 | 3.79 | |||
| R. | 6 | -38 | 70 | 4.95* | 4.04 | ||
| L. para-and postcentral gyri, precuneus | 3,4,5 | 111 | -16 | -40 | 78 | 4.20 | 3.58 |
| L. | -6 | -42 | 70 | 3.87 | 3.36 | ||
| L. postcentral gyrus, somatosensory cortex |
3 | 280 | -40 | -30 | 50 | 4.70* | 3.89 |
| -48 | -28 | 58 | 3.95 | 3.42 | |||
| R. inferior cerebellum, lobule VIII/IX (pyramis/uvula) |
96 | 10 | -68 | -48 | 4.66* | 3.87 | |
| 2 | -62 | -48 | 4.01* | 3.46 | |||
| R. superior cerebellum, lobule IV/V (declive/culmen) |
39 | 20 | -58 | -20 | 4.12* | 3.53 | |
| L. inferior cerebellum, lobule VIII/IX (pyramis/uvula) |
44 | -8 | -66 | -40 | 3.88 | 3.37 | |
| L. postcentral gyrus | 4 | 17 | -56 | -2 | 42 | 3.79 | 3.30 |
regions significant at p < .05 corrected for the whole brain
Correlation analyses
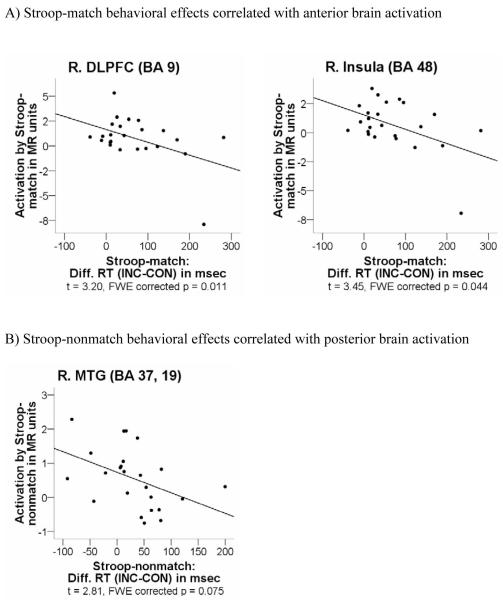
Correlation analyses tested the relationships between BOLD signal and performance differences involving Stroop conflict for cue-target color matches and nonmatches. Stroop-match behavioral effects indicating greater conflict were correlated with lower activation in the right DLPFC (BA 9) (t = 3.20; PFWE corrected = .011), and right insula (BA 48) (t = 3.45; PFWE corrected = .044), but not with activation in posterior brain regions (PFWE corrected > .1). One subject appeared to be an outlier (Figure 4); analyses without this subject showed a trend toward a negative correlation between Stroop-match effects and right DLPFC activation (t =2.17, PFWE corrected = .079). By contrast, Stroop-nonmatch behavioral effects indicating greater conflict showed trend correlates with lower activation in the right middle temporal gyrus (MTG) (BA 37, 19) (t = 2.81; PFWE corrected = .075), but not with activation in anterior brain regions (PFWE corrected > .1) (Figure 4).
Figure 4.
Significant correlations between behavioral Stroop-match and Stroop-nonmatch effects and regional brain activations; 24 right-handed young healthy subjects (12 male, 12 female).
DISCUSSION
We used a novel Stroop Match-to-Sample task to examine how perceptual cueing influences behavior and modulates neural activity during conflict processing. Both behavioral and fMRI results indicate that color cueing influenced conflict processing: Greater Stroop effects in match relative to nonmatch trials were associated with increased activation in anterior brain regions, whereas smaller Stroop effects in nonmatch relative to match trials were associated with increased activation in posterior brain regions.
Effects of perceptual color cueing on Stroop performance
Perceptual cueing produced greater behavioral Stroop effects, manifest as longer reaction times for incongruent than congruent stimuli, when the color cue correctly predicted the Stroop target’s color (match) than when it did not (nonmatch). Reaction times were longest for incongruent-nonmatch trials, intermediate for congruent-nonmatch and incongruent-match trials, and shortest for congruent-match trials. This replicates our earlier behavioral Stroop Match-to-Sample study where conditions were randomly intermixed (Schulte et al., 2005) and is consistent with other studies demonstrating a cueing benefit for congruent trials using cues that provided information about which type of target is coming next (Aarts et al., 2008; Crump et al., 2006; Gratton et al., 1992; Logan and Zbrodoff, 1982). Prior information from cues can facilitate the processing of an upcoming conflict (Luks et al., 2007; Stern et al., 2007); similarly, prior conflict reduces subsequent conflict in following trials (Egner and Hirsch, 2005; Gratton et al., 1992). It has been argued that such “conflict adaptation” occurs because cognitive control is enhanced after detecting an incongruent trial or when a cue prepares for conflict and, as a consequence, decreases subsequent experience of conflict (Botvinick et al., 2001; Egner, 2007; Kerns et al., 2004; Larson et al, 2009; Ullsperger et al, 2005). However, in our paradigm cues carried color information only and provided no specific information about whether the upcoming target would be congruent or incongruent. Yet, cueing a specific feature, such as color, can benefit processing stimuli with that feature by creating a memory representation (Awh and Jonides, 2001; Funes et al., 2007). Hence, the resulting processing enhancement can then amplify processing of congruent information, thereby increasing Stroop effects for match trials.
With incongruent and nonmatch information, cognitive control adjustments include action-driven control for monitoring and resolving response conflict from incongruent Stroop targets in addition to perceptually-driven control for disengaging attention from the incorrectly cued color (nonmatch) (Carter et al. 2000; Pardo et al. 1990; MacDonald et al. 2000). Specifically, incongruent and nonmatch trials constitute a challenging condition for subjects, because such trials require both inhibiting a pre-potent response to the Stroop word’s meaning and making a nonmatch decision, where the cue color does not match the targets ink color. Thus, resolving nonmatch and incongruency involves separate component processes of action- and perceptual-driven cognitive control, which provides one explanation why responses in incongruent-nonmatch trials were longer than responses to incongruent-match and congruent-nonmatch trials (Schulte et al., 2005; Schulte et al., 2008). The paradox of greater Stroop effects for match than nonmatch trials may be explained by the operation of different cognitive control demands for processing incongruency only (Stroop-match) from those used for processing both incongruency and nonmatch (Stroop-nonmatch).
Neural correlates of Stroop conflict with matching and nonmatching perceptual cues
Consistent with our proposal that different cognitive control mechanisms are involved in resolving Stroop conflict for match and nonmatch conditions, we found different brain systems associated with Stroop-match and Stroop-nonmatch processing.
Fronto-parietal Stroop-match activity
With matching cues, incongruent Stroop targets activated a lateral fronto-parietal network including DLPFC and bilateral parietal lobes, whereas congruent Stroop targets activated a medial frontal-occipital network including middle cingulate cortex, supplementary motor, extrastriate and parahippocampal areas. Fronto-parietal network activation has been previously reported for processing of incongruency with parietal areas implicated in processing stimulus-conflict and frontal areas in processing response conflict (Davelaar, 2008). However, our findings of fronto-parietal network activation for incongruent-match trials together with enhanced Stroop-match behavioral effects indicate that matching perceptual cues do not assist in resolving conflict from incongruent Stroop targets even though they benefited responses for congruent Stroop targets. Hence, activation in parahippocampal, medial cingulate and supplementary motor areas for congruent-match conditions may reflect working memory processes required to maintain the color cue information for effective response selection (Cavina-Pratesi et al., 2006; Desimone, 1996; Gonzalez-Hernandez et al., 2002; Ungerleider et al., 1998), whereas activation of occipito-temporal areas may be indicative of color and language processing when matching stimulus properties of cue and congruent targets (Barrett et al., 2001; Donohue et al., 2008; Simon and Baker, 1995).
Posterior visuo-motor Stroop-nonmatch activity
With non-matching cues, fewer areas (occipito-temporal and precentral gyri) with smaller cluster size were activated when processing incongruent than congruent Stroop targets. This pattern of only modest activations in nonmatch conditions is consistent with smaller Stroop-nonmatch behavioral effects.
Dissociation of frontal executive control and posterior visuo-motor networks in conflict processing
A direct comparison of regional BOLD responses engaged in Stroop-match and Stroop-nonmatch elicited a double dissociation with a predominantly anterior network activated during Stroop-match (DLPFC, VLPFC, dACC, parietal cortex) and a predominantly posterior network activated for Stroop-nonmatch (extrastriate cortex, somatosensory association cortex, motor and supplementary motor areas, cerebellum). Evidence that these differential anterior-posterior activation patterns are Stroop conflict-related is provided by the fact that incongruent-match trials activated a fronto-parietal control network (DLPFC, ACC, SMG), whereas incongruent-nonmatch trials activated a cortico-cerebellar motor and visual processing network.
Given greater behavioral Stroop conflict for match than nonmatch trials, our results complement previous studies showing greater activity in prefrontal and parietal brain areas for high relative to low conflict processing (Botvinick et al., 1999; Casey et al., 2000). Involvement of the anterior cingulate cortex (ACC) in Stroop-match processing is consistent with the contention that ACC activity reflects conflict detection at the decision stage, particularly in trials eliciting high-conflict (Pochon et al., 2008). Carter and van Veen (2007) postulated that the specific role of the ACC in cognitive control is to detect conflict between competing stimulus attributes and to engage the DLPFC to resolve such conflict. DLPFC activation has been further implicated in working memory functions (Curtis and D’Esposito, 2003; Edin et al., 2009) and may have been recruited during Stroop-match processing to hold information online while conflicts were being resolved. Our finding that smaller behavioral Stroop-match effects correlated with greater activation in the DLPFC is consistent with this prediction.
With nonmatch trials, processing Stroop conflict invoked a posterior network involving occipital-temporal gyri, postcentral gyri, cerebellar and extrastriate cortex areas. These results are similar to those of Zhang et al. (2008) and Curtis and D’Esposito (2003), who found occipito-temporal cortex activation for mismatch conditions. Yet, in our paradigm, nonmatching cue colors matched the Stroop word’s content. Thus, the finding that Stroop conflict was reduced with perceptual cueing of the interfering feature suggests that cognitive control mechanisms can operate on specific stimulus features at early perceptual stages of conflict processing (Scerif et al., 2006). Alternatively, smaller Stroop-nonmatch behavioral conflict may reflect greater perceptual processing demands when nonmatch trials limit resources available to process incongruent information (Lavie, 1995; Lavie, 2006).
Effect of response repetition on Stroop conflict
An inherent limitation of our study is use of a block design, precluding the opportunity to distinguish cue from target-related activity or to measure trial-by-trial variations in conflict and control to isolate their neural correlates. To minimize this limitation, we manipulated the order of responses in the mixed response trials and the order of mixed and same response blocks within a run. Post-scan debriefing revealed that none of the subjects recognized the blocked stimulation pattern of the experiment. When a stimulus sequence obeys an underlying regularity, conflict adaptation from implicit learning can occur even though participants may not be aware of it (Nissen and Bullemer, 1987). Then, one would expect more conflict in blocks with an irregular stimulus-response (SR) sequence because irregular, less automatic SR mappings rules lead to more conflict than repetitive stimulus-response mappings (Mayr et al., 2003; Hommel, 2004; Verguts and Notebaert, 2008). Yet, neither reaction time nor Stroop conflict differed between blocks with irregular (mixed-response blocks) and regular (same-response blocks) stimulus-response mappings, providing evidence that implicit sequence learning did not play a role for conflict processing in our block paradigm. Furthermore, during incongruency processing (same-response blocks) we found activation of prefrontal, anterior cingulate, and parietal cortices typically associated with conflict monitoring and conflict resolution, as have others (Botvinick et al., 2004; Kerns et al., 2004); this activation pattern was modulated by perceptual cueing, consistent with behaviorally greater Stroop-match than Stroop-nonmatch effects. Thus, it appears that pretrial cueing of task-relevant perceptual information can modulate conflict by invoking control mechanism of attentional preparation and working memory to maintain the color cue information for high-level perceptual and motor response selection in the Stroop Match-to-Sample task (Simon and Baker, 1995; Donohue et al., 2008).
Conclusion
Our findings demonstrate that different brain systems underlie action-driven and perception-driven conflict resolution. An action-driven anterior control system was engaged when interfering semantic information required inhibition of pre-potent responses, whereas a perceptually driven posterior attention system was engaged when perceptual processing was required to resolve conflict when cue and target colors did not match. Thus, this study shows a double dissociation of anterior and posterior networks engaging in different sets of cognitive control for Stroop conflict resolution depending on prior perceptual information. This distinction has ramifications for clinical research in populations, such as patients with substance abuse disorder who show deficits in resolving conflict or exerting executive control over impulsive responses to automatically processed stimuli (Garavan and Hester, 2007; Oscar-Berman and Marinković, 2007; Schulte et al., 2008; Uslaner and Robinson, 2006).
ACKNOWLEDGEMENTS
This work was supported by NIAAA grants: AA10723, AA05965, AA018022
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aarts E, Roelofs A, van Turennout M. Anticipatory activity in anterior cingulate cortex can be independent of conflict and error likelihood. J Neurosci. 2008;28:4671–4678. doi: 10.1523/JNEUROSCI.4400-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends Cogn Sci. 2001;5:119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Perlstein W, Baird J, Cohen JD, Schooler N. Increased stroop facilitation effects in schizophrenia are not due to increased automatic spreading activation. Schizophr Res. 1999;23:51–64. doi: 10.1016/s0920-9964(99)00025-0. [DOI] [PubMed] [Google Scholar]
- Barrett NA, Large MM, Smith GL, Michie PT, Karayanidis F, Kavanagh DJ, Fawdry R, Henderson D, O’Sullivan BT. Human cortical processing of colour and pattern. Hum Brain Mapp. 2001;13:213–225. doi: 10.1002/hbm.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi G, Goldberg TE, Elvevåg B, Rasetti R, Bertolino A, Cohen J, Alce G, Zoltick B, Weinberger DR, Mattay VS. Differentiating allocation of resources and conflict detection within attentional control processing. Eur J Neurosci. 2007;25:594–602. doi: 10.1111/j.1460-9568.2007.05283.x. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Leung HC, Skudlarski P, Lacadie CM, Fredericks CA, Harris BC, Charney DS, Gore JC, Krystal JH, Peterson BS. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA. The Multi-Source Interference Task: validation study with fMRI in individual subjects. Mol Psychiatry. 2003;8:60–70. doi: 10.1038/sj.mp.4001217. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;15:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Welsh TF, Badgaiyan RD, Eccard CH, Jennings JR, Crone EA. Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proc Natl Acad Sci U S A. 2000;97:8728–8733. doi: 10.1073/pnas.97.15.8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavina-Pratesi C, Valyear KF, Culham JC, Kohler S, Obhi SS, Marzi CA, Goodale MA. Dissociating arbitrary stimulus-response mapping from movement planning during preparatory period: evidence from event-related functional magnetic resonance imaging. J Neurosci. 2006;26:2704–2713. doi: 10.1523/JNEUROSCI.3176-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. Attentional focus, processing load, and Stroop interference. Percept Psychophys. 2003;65:888–900. doi: 10.3758/bf03194822. [DOI] [PubMed] [Google Scholar]
- Crump MJ, Gong Z, Milliken B. The context-specific proportion congruent Stroop effect: location as a contextual cue. Psychon Bull Rev. 2006;13:316–321. doi: 10.3758/bf03193850. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Success and failure suppressing reflexive behavior. J Cogn Neurosci. 2003;15:409–418. doi: 10.1162/089892903321593126. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Davelaar EJ. A computational study of conflict-monitoring at two levels of processing: reaction time distributional analyses and hemodynamic responses. Brain Res. 2008;2:109–119. doi: 10.1016/j.brainres.2007.06.068. [DOI] [PubMed] [Google Scholar]
- Desimone R. Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci U S A. 1996;93:13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue SE, Wendelken C, Bunge SA. Neural correlates of preparation for action selection as a function of specific task demands. J Cogn Neurosci. 2008;20:694–706. doi: 10.1162/jocn.2008.20042. [DOI] [PubMed] [Google Scholar]
- Edin F, Klingberg T, Johansson P, McNab F, Tegnér J, Compte A. Mechanism for top-down control of working memory capacity. Proc Natl Acad Sci U S A. 2009 Apr 1; doi: 10.1073/pnas.0901894106. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T. Congruency sequence effects and cognitive control. Cogn Affect Behav Neurosci. 2007;7:380–390. doi: 10.3758/cabn.7.4.380. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci. 2005;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R. Analysis of fMRI time-series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Funes MJ, Lupiáñez J, Milliken B. Separate mechanisms recruited by exogenous and endogenous spatial cues: evidence from a spatial Stroop paradigm. J Exp Psychol Hum Percept Perform. 2007;33:348–362. doi: 10.1037/0096-1523.33.2.348. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Jackson and the frontal executive hierarchy. Int J Psychophysiol. 2007;64:106–107. doi: 10.1016/j.ijpsycho.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol Rev. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Hernandez JA, Pita-Alcorta C, Cedeno I, Bosch-Bayard J, Galan-Garcia L, Scherbaum WA, Figueredo-Rodriguez P. Wisconsin Card Sorting Test synchronizes the prefrontal, temporal and posterior association cortex in different frequency ranges and extensions. Hum Brain Mapp. 2002;17:37–47. doi: 10.1002/hbm.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. Optimizing the use of information: strategic control of activation of responses. J Exp Psychol Gen. 1992;121:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Hommel B. Coloring an action: intending to produce color events eliminates the Stroop effect. Psychol Res. 2004;68:74–90. doi: 10.1007/s00426-003-0146-5. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Lindsay DS, Hessels S. Item-specific control of automatic processes: stroop process dissociations. Psychon Bull Rev. 2003;10:638–644. doi: 10.3758/bf03196526. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Chajczyk D. Test of the automaticity of reading. Dilution of Stroop effects by color irrelevant stimuli. J Exp Psychol. 1983;9:497–509. doi: 10.1037//0096-1523.9.4.497. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Koch C, Brown JM. Examining the time course of prime effects on Stroop processing. Percept Mot Skills. 1994;79:675–687. doi: 10.2466/pms.1994.79.1.675. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Nielson KA, Rao SM. fMRI of healthy older adults during Stroop interference. Neuroimage. 2004;21:192–200. doi: 10.1016/j.neuroimage.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Kaufman DA, Perlstein WM. Neural time course of conflict adaptation effects on the Stroop task. Neuropsychologia. 2009;47:663–670. doi: 10.1016/j.neuropsychologia.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Lavie N. Perceptual load as a necessary condition for selective attention. J Exp Psychol Hum Percept Perform. 1995;21:451–468. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- Lavie N. The role of perceptual load in visual awareness. Brain Res. 2006;1080:91–100. doi: 10.1016/j.brainres.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Logan GD, Zbrodoff NJ. Constraints on strategy construction in a speeded discrimination task. J Exp Psychol Hum Percept Perform. 1982;8:502–520. doi: 10.1037//0096-1523.8.4.502. [DOI] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event-related fMRI. Neuroimage. 2004;22:1097–1106. doi: 10.1016/j.neuroimage.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Luks TL, Simpson GV, Dale CL, Hough MG. Preparatory allocation of attention and adjustments in conflict processing. Neuroimage. 2007;35:949–958. doi: 10.1016/j.neuroimage.2006.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungu OV, Binenstock MM, Pline MA, Yeaton JR, Carey JR. Neural changes in control implementation of a continuous task. J Neurosci. 2007;27:3010–3016. doi: 10.1523/JNEUROSCI.5051-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mayr U, Awh E, Laurey P. Conflict adaptation effects in the absence of executive control. Nat Neurosci. 2003;6:450–452. doi: 10.1038/nn1051. [DOI] [PubMed] [Google Scholar]
- McLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Mead LA, Mayer AR, Bobholz JA, Woodley SJ, Cunningham JM, Hammeke TA, Rao SM. Neural basis of the Stroop interference task: response competition or selective attention? J Int Neuropsychol Soc. 2002;8:735–742. doi: 10.1017/s1355617702860015. [DOI] [PubMed] [Google Scholar]
- Meiran N. Reconfiguration of processing mode prior to task performance. J Exp Psychol Learn Mem Cogn. 1996;22:1423–1442. [Google Scholar]
- Melcher T, Gruber O. Oddball and incongruity effects during Stroop task performance: a comparative fMRI study on selective attention. Brain Res. 2006;1121:136–149. doi: 10.1016/j.brainres.2006.08.120. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Res Cogn Brain Res. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer PT. Attentional requirements for learning: Evidence from performance measures. Cognitive Psychology. 1987;19:1–32. [Google Scholar]
- Notebaert W, Verguts T. Stimulus conflict predicts conflict adaptation in a numerical flanker task. Psychon Bull Rev. 2006;13:1078–1084. doi: 10.3758/bf03213929. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinković K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci U S A. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Kane MJ, Alexander GM, Lacadie C, Skudlarski P, Leung HC, May J, Gore JC. An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Brain Res Cogn Brain Res. 2002;13:427–440. doi: 10.1016/s0926-6410(02)00054-x. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC. An fMRI study of Stroop word-color interference: evidence for cingulated subregions subserving multiple distributed attentional systems. Biol Psychiatry. 1999;15:1237–1258. doi: 10.1016/s0006-3223(99)00056-6. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Riis J, Sanfey AG, Nystrom LE, Cohen JD. Functional imaging of decision conflict. J Neurosci. 2008;28:3468–3473. doi: 10.1523/JNEUROSCI.4195-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Evans AC, Friston KJ. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage. 1997;5:83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. J Exp Psychol. 1980;109:160–174. [PubMed] [Google Scholar]
- Scerif G, Worden MS, Davidson M, Seiger L, Casey BJ. Context modulates early stimulus processing when resolving stimulus-response conflict. J Cogn Neurosci. 2006;18:781–792. doi: 10.1162/jocn.2006.18.5.781. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, Toga AW, Petrides M, Evans AC. MRI Atlas of the Human Cerebellum. Academic Press; San Diego: 2000. [DOI] [PubMed] [Google Scholar]
- Schulte T, Mueller-Oehring EM, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Differential effect of HIV infection and alcoholism on conflict processing, attentional allocation, and perceptual load: evidence from a Stroop Match-to-Sample task. Biol Psychiatry. 2005;57:67–75. doi: 10.1016/j.biopsych.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Pfefferbaum A, Sullivan EV. Callosal compromise differentially affects conflict processing and attentional allocation in alcoholism, HIV infection, and their comorbidity. Brain Imaging Behav. 2008;2:27–38. doi: 10.1007/s11682-007-9014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Salo R, Pfefferbaum A, Sullivan EV. Callosal involvement in a lateralized stroop task in alcoholic and healthy subjects. Neuropsychology. 2006;20:727–736. doi: 10.1037/0894-4105.20.6.727. [DOI] [PubMed] [Google Scholar]
- Simon JR, Baker KL. Effect of irrelevant information on the time to enter and retrieve relevant information in a Stroop-type task. J Exp Psychol Hum Percept Perform. 1995;21:1028–1043. [Google Scholar]
- Stern ER, Wager TD, Egner T, Hirsch J, Mangels JA. Preparatory neural activity predicts performance on a conflict task. Brain Res. 2007;1176:92–102. doi: 10.1016/j.brainres.2007.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;12:643–662. [Google Scholar]
- Sudevan P, Taylor DA. The cuing and priming of cognitive operations. J Exp Psychol Hum Percept Perform. 1987;13:89–103. doi: 10.1037//0096-1523.13.1.89. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. A Co-Planar Stereotaxic Atlas of a Human Brain. Thieme-Verlag; Stuttgart: 1988. [Google Scholar]
- Ullsperger M, Bylsma LM, Botvinick MM. The conflict adaptation effect: it’s not just priming. Cogn Affect Behav Neurosci. 2005;5:467–472. doi: 10.3758/cabn.5.4.467. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Courtney SM, Haxby JV. A neural system for human visual working memory. Proc Natl Acad Sci U S A. 1998;95:883–890. doi: 10.1073/pnas.95.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner JM, Robinson TE. Subthalamic nucleus lesions increase impulsive action and decrease impulsive choice - mediation by enhanced incentive motivation? Eur J Neurosci. 2006;24:2345–254. doi: 10.1111/j.1460-9568.2006.05117.x. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage. 2001;4:1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Notebaert W, Liefooghe B, Vandierendonck A. Stimulus- and response conflict-induced cognitive control in the flanker task. Psychon Bull Rev. 2006;13:328–333. doi: 10.3758/bf03193852. [DOI] [PubMed] [Google Scholar]
- Verguts T, Notebaert W. Hebbian learning of cognitive control: dealing with specific and nonspecific adaptation. Psychol Rev. 2008;115:518–525. doi: 10.1037/0033-295X.115.2.518. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Anderson KC, Miller EK. Single neurons in prefrontal cortex encode abstract rules. Nature. 2001;411:953–956. doi: 10.1038/35082081. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ma L, Li S, Wang Y, Weng X, Wang L. A mismatch process in brief delayed matching-to-sample task: an fMRI study. Exp Brain Res. 2008;186:335–341. doi: 10.1007/s00221-008-1285-0. [DOI] [PubMed] [Google Scholar]