Abstract
Molecular sensing by gastrointestinal (GI) cells plays a critical role in the control of multiple fundamental functions including digestion, regulation of caloric intake, pancreatic insulin secretion and metabolism, as well as protection from ingested harmful drugs and toxins. These processes are likely to be mediated by the initiation of humoral and/or neural pathways through the activation of endocrine cells. However, the initial recognition events and mechanism(s) involved are still largely unknown. This article reviews the current evidence that the chemosensory machinery discovered in specialized neuroepithelial taste receptor cells of the lingual epithelium is operational in enteroendocrine open GI cells that sense the chemical composition of the luminal contents of the gut.
Keywords: T2R family, transducin, brush cells, gastrointestinal peptides, enteroendocrine cells
The gastrointestinal (GI) tract is a sensory organ that responds to a variety of signals originating in the lumen, including nutrient and non-nutrient chemicals, mechanical factors, microorganisms, drugs and toxins. Detection of intraluminal contents by GI cells initiates a cascade of events involving hormonal and neural pathways and culminating in functional responses that ultimately regulate nutrient digestion and absorption, food intake, pancreatic insulin secretion and metabolism. Despite its physiological importance, the molecular recognition events sensing the chemical composition of the luminal contents of the GI tract have yet to be elucidated.
Here, we discuss recent findings demonstrating fundamental similarities between the molecular pathways that mediate oral taste signaling and the chemosensory mechanisms that operate in endocrine cells of the GI tract and in enteroendocrine cell lines. These discoveries might have important clinical implications for developing therapeutic strategies for the treatment of pathological conditions, including feeding disorders and metabolic diseases triggered by luminal chemosensing.
Expression of transcripts encoding members of taste receptor family of G protein coupled receptors (GPCRs) in the GI tract
A family of bitter taste receptors (referred as T2Rs), which are expressed in taste receptor cells organized within taste buds in the lingual epithelium, has been identified in humans and rodents [1-3]. These taste receptors belong to the G protein-coupled receptor (GPCR) superfamily, which are characterized by seven transmembrane α-helices. Similarly, the GPCRs of the T1R family, namely T1R1, T1R2 and T1R3, have been identified as the receptors that perceive sweet substances and L-amino acids [4,5]. We determined a total of 36 true T2R genes in the mouse and rat genome with small variations in pseudogene numbers and locations, and identified 25 T2Rs in the human genome [6].
An important step in the elucidation of the chemosensory mechanisms that operate in the GI tract has been the detection of multiple T2R transcripts in the mucosa of the GI tract of mouse and rat **[7]. Amplified products were cloned and sequenced, confirming that they are identical to known taste receptor sequences. Examples include transcripts encoding for mT2R108 and mT2R138, whose putative ligands are denatonium benzoate (DB) and phenylthiocarbamide (PTC), respectively [6]. In contrast, RT-PCR using RNA isolated from a variety of other tissues and organs, including liver, heart, kidney, and brain as well as from undifferentiated intestinal epithelial cell lines (IEC-6 and IEC-18) did not detect any of these transcripts **[7].
More recently, we have identified the expression of multiple hT2R transcripts in the human colon, including hT2R3, hT2R4, hT2R5, hT2R10, hT2R13, HT2R38, hT2R39, hT2R40, hT2R42, hT2R43, hT2R44, hT2R45, hT2R46, hT2R47, hT2R49, hT2R50, and hT2R60 *[8]. Some of the hT2Rs have known ligands, examples being hT2R38, the PTC receptor [9], hT2R47, a DB receptor [10], and hT2R43 and hT2R44, which function as saccharin and acesulfame K receptors and which are activated by aristolochic acid [11].
Expression of T1R2 and T1R3, the heterodimer receptor broadly tuned to sweet substances [4], has been demonstrated in the mouse and rat intestine [12-14]. The transcript for hT1R3, the common subunit of the sweet (T1R2/T1R3) and umami (T1R1/T1R3) receptors, was also detected in human intestine [8,15]. These studies clearly demonstrated the expression of bitter and sweet taste GPCRs in the mouse, rat and human GI tract.
Expression of the α subunits of gustducin (Gαgust) and transducin (Gαt-2) in GI cells from mouse, rat and humans
Genetic and biochemical evidence indicate that the G protein gustducin mediates T1R and T2R signaling in the taste buds of the lingual epithelium [16] though other G proteins (e.g. transducin and Gi) are also likely be involved in taste receptor signaling [10]. Outside the lingual epithelium, the α subunit of gustducin (Gαgust) has been localized to gastric [7,17], intestinal [7,18] and pancreatic [19] cells, in agreement with the notion that a taste-sensing mechanism also operates in the GI tract [6,7]. The mRNA encoding Gαgust was detected in mucosal scrapings of the whole GI tract of mice and rats [7,15] and confirmed by in situ hybridization [14].
Gαgust has been described in a distinct population of GI epithelial cells in rodents, called brush or caveolated cells *[17]. These cells are characterized by apical and basolateral expression of villin and lack of intracellular secretory vesicles. We confirmed that solitary Gαgust-positive cells contain villin immunoreactivity in the mucosa of the gastric corpus of rodents, but also identified Gαgust-positive cells that do not contain basolateral villin, especially in the antrum and in the intestine [20,21]. Further studies also identified Gαgust-positive cells with properties of brush cells but also showed expression of Gαgust in other cell types that did not express brush cell markers other than villin [15,18]. Collectively, these results indicate that there are distinct sub-populations of Gαgust-positive cells interspersed in the mouse GI epithelium [20,21].
The α subunit of transducin 2 (Gαt-2), which is implicated in taste signal transduction [22], was also detected in the GI mucosa [6,7]. The distribution and morphology of Gαt-2-positive cells in the rodent gastrointestinal mucosa appeared different from those of Gαgust-positive cells [7], implying that Gαgust and Gαt-2 might be expressed by different epithelial cell types in the GI tract of mice and rats [6,7].
Expression of Gαgust by endocrine cells of the GI tract
Gαgust immunoreactivity has been also detected in individual human cells distributed throughout the GI tract [8,14,23]. Interestingly, we found that most Gαgust-positive cells co-expressed chromogranin A but not basolateral villin immunoreactivity *[8], indicating that Gαgust functions in endocrine rather than brush cells of the human GI tract. A similar conclusion was reached in two recent independent studies [14,23]. Gαgust expression was detected in enteroendocrine cells but not in other epithelial cells of the intestine.
The endocrine cells of the GI tract represent less than 1% of the intestinal epithelium, but as a whole, they constitute the largest endocrine organ of the body, producing and releasing multiple hormones [24]. In the context of molecular sensing, the open enteroendocrine cells respond to luminal signals in the apical side by releasing GI peptides at the basolateral side. For instance, the enteroendocrine L cells, prominently localized in the distal region of the small intestine and the colon, are characterized by production of peptide YY (PYY) and glucagon-like peptide-1 (GLP-1), an incretin that induces glucose-dependent insulin secretion from pancreatic β cells [25,26]. PYY and GLP-1 also regulate food intake [27,28].
Recent studies demonstrated that Gαgust-positive cells contain immunoreactive GLP-1 and PYY *[8]. We also detected co-localization of Gαgust with CCK in I cells (unpublished results), another GI peptide that regulates GI motility and food intake [28]. Studies from other laboratories confirmed co-localization of Gαgust with GLP-1 in mouse *[18] and human duodenal L cells **[14] and showed co-localization of Gαgust with GIP (glucose-dependent insulinotropic peptide), another incretin that induces glucose-dependent insulin secretion from pancreatic β cells **[23].
Although the differentiation pathways leading to specific enteroendocrine cells remain incompletely understood [29], it appears that there are, at least, two major branches for enteroendocrine differentiation [29,30]. One leads to cells that express PYY and/or GLP-1 (L cells) or CCK (I cells) while the other corresponds to cells that express serotonin and substance P [29,30]. Gαgust is expressed by enteroendocrine cells that contain PYY and GLP-1 in humans [8], but it has also been reported in serotonin-containing cells in the mouse jejunum *[18]. Gαgust-dependent signaling may be operational in both lineages of enteroendocrine cells.
Expression of taste-specific markers in cultures of enteroendocrine cell lines
Cultured cell lines have been used extensively to investigate the organization, signaling pathways and function of intestinal cells. Using RT-PCR and sequencing analysis, we demonstrated that enteroendocrine STC-1 cells express multiple T2Rs, Gαgust, phospholypase Cβ2 (PLCβ2) and transient receptor potential channel M5 (TRPM5) [6,7,31]. All these signal transduction molecules have been implicated in taste perception in the lingual epithelium. Recent studies revealed that another mouse enteroendocrine line, GLUTag, also expresses taste-specific signaling elements, including T1R2, T1R3 and Gαgust [14]. Thus, murine STC-1 and GLUTag cells provide good model systems to elucidate signaling pathways and secretory responses elicited by taste stimuli in GI cells.
The rat pancreatic acinar tumor cell line AR42J has neuroendocrine properties, including the presence of microvesicles containing neurotransmitters. Transcripts encoding T2Rs (including rT2R38), Gαgust and Gαt-2, were also found in these cells [6]. Thus, rat AR42J cells provide another model to elucidate signaling pathways and secretory responses elicited by bitter stimuli in a GI cell system.
Endocrine human intestinal cell lines, including HuTu-80 (secretin-producing) and NCI-H716 (GLP-1 producing), also coexpress Gαgust and receptors implicated in intracellular taste signal transduction [8]. HuTu-80 cells express hT1R3 and multiple hT2Rs, including hT2R38. NCI-H716 also express Gαgust and hT2R38 [8] as well as T1R1, T1R2, T1R3 and TRPM5 [23].
The fact that enteroendocrine STC-1, GLUTag, AR42J, HuTu-80 and NCI-H716 cells express multiple components of taste signaling is consistent with the hypothesis that endocrine cells of the gut play a critical role in sensing the chemical composition of luminal contents via elements of the chemosensory machinery used by taste receptor cells of the lingual epithelium.
Signal transduction pathways activated by bitter stimuli in GI cells
The precise coupling of T1Rs, T2Rs, gustducin, PLCβ2 and TRPM5 in taste receptor cells remains incompletely understood. The βγ subunits of gustducin, transducin and Gi are thought to stimulate PLCβ2-mediated synthesis of Inositol (1,4,5)phosphate P3 (IP3) leading to the release of Ca2+ from intracellular stores, whereas Gαgust appears to reduce the intracellular level of cAMP through activation of phosphodiesterases. An increase in the intracellular Ca2+ concentration ([Ca2+]i) could trigger the activation of TRPM5, which in turn promotes membrane depolarization in taste receptor cells. In addition, bitter tastants can also modulate the gating of voltage-sensitive ion channels that mediate depolarization and Ca2+ influx into the cell (see Fig. 1).
Figure 1.
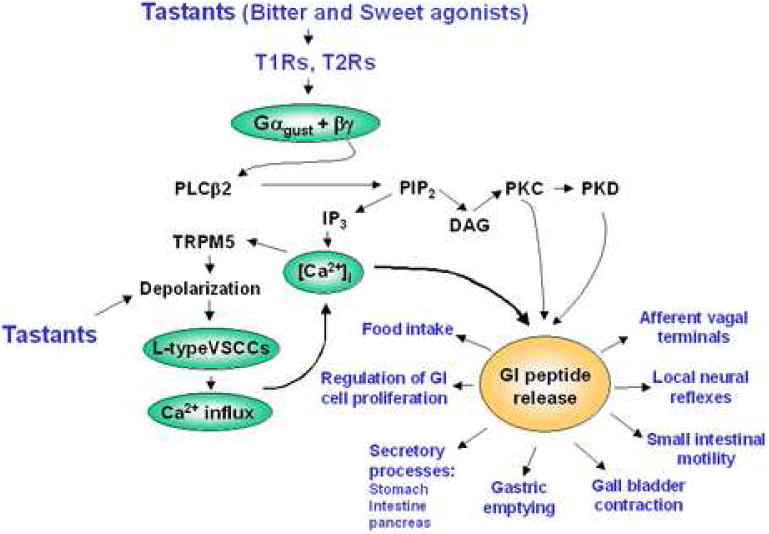
A scheme showing that tastant-induced signaling via T2Rs and T1Rs in the apical portion of an open enteroendocrine cell engages gustducin, PLCβ2, TRPM5 and L-type VSCCs leading to an elevation of the intracellular concentration of Ca2+. This second messenger in combination with diacylglycerol (DAG)-mediated activation of protein kinase C (PKC) and protein kinase D (PKD) triggers the release of GI peptides into the basolateral portion of the cell. The gut hormones, acting in an endocrine and/or paracrine manner, exert multiple physiological effects. Detailed description and abbreviations can be found in the text.
Although little is known about the signal transduction cascades initiated by bitter or sweet stimuli in GI endocrine cells, initial results suggest that taste-specific signaling is operational in these cells. For example, STC-1 cells showed a marked increase in [Ca2+]i in response to bitter stimuli, including DB, PTC and cycloheximide (CYX) **[7]. Subsequent studies confirmed that bitter stimuli induce Ca2+ signaling in STC-1 cells [31,32] and other enteroendocrine cells [6,8].
The increase in [Ca2+]i induced by DB or PTC in STC-1 cells is mediated primarily by an increase in Ca2+ influx from the extracellular medium *[31]. L-type voltage-sensitive Ca2+ channels (VSCCs) mediate influx of extracellular Ca2+ into neuronal and neuroendocrine cells in response to membrane depolarization [33]. Accordingly, the L-type VSCCs blockers nitrendipine and diltiazem prevented the increase in [Ca2+]i elicited by PTC and DB in STC-1 cells *[31], indicating that bitter tastants increase [Ca2+]i through Ca2+ influx mediated by the opening of L-type VSCCs in enteroendocrine STC-1 cells, as indicated in Fig. 1.
AR42J cells, like STC-1 cells, show a marked increase in [Ca2+]i in response to the addition of PTC, DB or CYX [6]. PTC also induced a rapid increase in Ca2+ signaling in cultures of either HuTu-80 or NCI-H761 cells [8]. Interestingly, different bitter agonists promoted Ca2+ signaling through different pathways in AR42J cells [6] and STC-1 cells (unpublished results), implying that T2R agonists activate multiple signal transduction pathways in GI cells.
DB, PTC, and CYX, did not induce Ca2+ signaling in multiple cell lines that do not express T2Rs and G proteins implicated in taste reception [6-8,31]. These results reinforce the notion that the effects of bitter and sweet compounds on second messenger production in enteroendocrine cell lines are mediated by taste-specific receptors and signal transducers that are expressed in these cells.
Bitter and sweet agonists induce release of GI peptides in vitro and in vivo
We hypothesized that the robust increase in [Ca2+]i induced by bitter and/or sweet tastants in enteroendocrine cells triggers the release of GI peptides, including CCK, PYY and GLP-1. These peptides activate local neural reflexes and/or vagal afferent pathways and modulate the activity of adjacent or distant target cells, including pancreatic β cells (Fig. 1).
In line with this hypothesis, DB was shown to be a potent stimulant of CCK release from enteroendocrine STC-1 cells *[31]. Treatment with either EGTA to prevent Ca2+ influx or nitrendipine prevented the secretory response [31]. We conclude that DB-elicited CCK release is mediated by an increase in [Ca2+]i produced by the opening of L-type VSCCs. Stimulation of NCI-H716 or GLUTag cells with sweet receptor (T1R2/T1R3) agonists, including glucose, sucrose or sucralose, induced an increase in [Ca2+]i and promoted the release of GLP-1 and GIP from these cells **[14,23].
Recent studies have made important contributions to test the operation of these pathways in vivo. Release of GLP-1 leading to an increase in the concentration of this peptide in the bloodstream in response to carbohydrate gavage was not observed in Gαgust-deficient mice and concomitantly, these mice exhibited an impaired release of insulin and more pronounced hyperglycemia **[23]. In addition, sweet receptor activation also stimulated the expression of the Na+/glucose cotransporter SGLT1 by enterocytes, a response abrogated in mice deficient in either Gαgust or T1R3 **[14]. In a different study using perfused rat jejunum in vivo, sweet receptor agonists, including artificial sweeteners, induced a rapid increase in the expression of the glucose transporter GLUT2 instead of SGLT1 [13]. These studies provide evidence that expression of taste-specific signaling molecules in the GI tract mediate physiological responses leading to carbohydrate absorption by enterocytes and subsequent regulation of pancreatic insulin release.
Conclusions and Implications
Recent studies have provided striking support for the hypothesis that the molecular pathways that mediate oral taste signaling operate in specific cells of the GI tract. It is now established that taste-specific molecular transducers, including T1Rs, T2Rs, Gαgust, Gαt-2, PLCβ2 and TRPM5, are expressed by a variety of cell types interspersed in the epithelium of the mammalian GI tract [7,8,12,15,17,18,20,21], including endocrine cells. These transducers are also expressed by mouse, rat and human enteroendocrine cell lines that serve as model systems to define signal transduction pathways that mediate chemosensory responses in GI cells [6-8,12,20,31,32].
Recent results demonstrating co-localization of Gαgust with GLP-1 and PYY in enteroendocrine L cells from both human *[8] and rodent mucosa [8,18] are of interest and potential importance. Several lines of evidence indicate that sweet and bitter agonists trigger secretory responses in enteroendocrine cell in culture leading to the output of CCK, GLP-1 and GIP into the medium and sweet agonists administered to mice by gavage elicit a rapid increase in the plasma levels of GLP-1, a response absent in animals lacking Gαgust or T1R3 **[23]. Interestingly, the peptides released from L cells (GLP-1 and PYY) and I cells (CCK) reduce food intake [28,34-37] and mediate an aversive food response in mice [38,39]. GLP-1 and PYY are implicated in fundamental mechanisms of regulation in response to food intake and may participate in the pathogenesis of the most common metabolic disorders, namely obesity and Type 2 diabetes [28].
In view of the fact that GPCRs are among the most common targets for currently used therapeutic drugs, the identification of chemosensory GPCRs in GI cells could pave the way for developing novel therapeutic compounds that modify their function in the gut. These results raise the attractive possibility of exploiting the stimulation of the endogenous release of these GI peptides by non-permeable tastants as a novel approach for therapeutic intervention in obesity and Type 2 diabetes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJP. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 2.Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJP, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 3.Matsunami H, Montmayeur J-P, Buck LB. A family of candidate taste receptors in human and mouse. Nature (London) 2000;404:601–604. doi: 10.1038/35007072. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 6.Wu SV, Chen MC, Rozengurt E. Genomic organization, expression, and function of bitter taste receptors (T2R) in mouse and rat. Physiol Genomics. 2005;22:139–149. doi: 10.1152/physiolgenomics.00030.2005. [DOI] [PubMed] [Google Scholar]
- 7**.Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci U S A. 2002;V99:2392–2397. doi: 10.1073/pnas.042617699.. Expression of multiple T2R transcripts in the mucosa of the gastrointestinal tract of mouse and rat and in enteroendocrine cells in culture was demonstrated. This study also showed that bitter stimuli, including denatonium, induce a rapid increase in calcium signaling in enteroendocrine cells
- 8*.Rozengurt N, Wu SV, Chen MC, Huang C, Sternini C, Rozengurt E. Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G792–802. doi: 10.1152/ajpgi.00074.2006.. The α-subunit of gustducin is localized to enteroendocrine L cells that express peptide YY and glucagon-like peptide-1 (GLP-1) in the human colonic mucosa. Expression of multiple T2R transcripts in the human gastrointestinal tract and human enteroendocrine cells was also demonstrated.
- 9.Bufe B, Breslin PA, Kuhn C, Reed DR, Tharp CD, Slack JP, Kim UK, Drayna D, Meyerhof W. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol. 2005;15:322–327. doi: 10.1016/j.cub.2005.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sainz E, Cavenagh MM, Gutierrez J, Battey JF, Northup JK, Sullivan SL. Functional characterization of human bitter taste receptors. Biochem J. 2007;403:537–543. doi: 10.1042/BJ20061744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn C, Bufe B, Winnig M, Hofmann T, Frank O, Behrens M, Lewtschenko T, Slack JP, Ward CD, Meyerhof W. Bitter Taste Receptors for Saccharin and Acesulfame K. J Neurosci. 2004;24:10260–10265. doi: 10.1523/JNEUROSCI.1225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans. 2005;33:302–305. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- 13.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582:379–392. doi: 10.1113/jphysiol.2007.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14**.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0706678104. in press.. The sweet taste receptor subunit T1R3 and α-gustducin, expressed in enteroendocrine cells, underlie intestinal sugar sensing and regulation of SGLT1 mRNA and protein. Dietary sugar and artificial sweeteners increased SGLT1 expression, and glucose absorptive capacity in wild-type mice, but not in knockout mice lacking T1R3 or α-gustducin.
- 15.Bezencon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2007;32:41–49. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz-Avila L, Wong GT, Damak S, Margolskee RF. Dominant loss of responsiveness to sweet and bitter compounds caused by a single mutation in alpha -gustducin. Proc Natl Acad Sci U S A. 2001;98:8868–8873. doi: 10.1073/pnas.151235798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Hoefer D, Pueschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci U S A. 1996;93:6631–6634. doi: 10.1073/pnas.93.13.6631.. The α- subunit of gustducin was localized to a population of brush or caveolated cells in rodent gastrointestinal cells.
- 18*.Sutherland K, Young RL, Cooper NJ, Horowitz M, Blackshaw LA. Phenotypic characterization of taste cells of the mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1420–1428. doi: 10.1152/ajpgi.00504.2006.. The expression of a-gustducin is documented in a variety of gut cells in the mouse. The study consolidates the notion that taste signaling operates in different cell types, at least in the rodent gut.
- 19.Hoefer D, Drenckhahn D. Identification of the taste cell G-protein, alpha-gustducin, in brush cells of the rat pancreatic duct system. Histochem Cell Biol. 1998;110:303–309. doi: 10.1007/s004180050292. [DOI] [PubMed] [Google Scholar]
- 20.Rozengurt E. Taste Receptors in the Gastrointestinal Tract. I. Bitter taste receptors and {alpha}-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol. 2006;291:G171–177. doi: 10.1152/ajpgi.00073.2006. [DOI] [PubMed] [Google Scholar]
- 21.Sternini C. Taste receptors in the gastrointestinal tract. IV. Functional implications of bitter taste receptors in gastrointestinal chemosensing. Am J Physiol Gastrointest Liver Physiol. 2007;292:G457–461. doi: 10.1152/ajpgi.00411.2006. [DOI] [PubMed] [Google Scholar]
- 22.Margolskee RF. Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem. 2002;277:1–4. doi: 10.1074/jbc.R100054200. [DOI] [PubMed] [Google Scholar]
- 23**.Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0706890104. in press.. Ingestion of glucose by α-gustducin null mice revealed deficiencies in secretion of GLP-1 and the regulation of plasma insulin and glucose. The results indicate that carbohydrate sensing in the small intestine is mediated by sweet receptors and signaling pathways similar to those functioning in oral glucose sensing.
- 24.Rehfeld JF. The New Biology of Gastrointestinal Hormones. Physiol Rev. 1998;78:1087–1108. doi: 10.1152/physrev.1998.78.4.1087. [DOI] [PubMed] [Google Scholar]
- 25.Gautier JF, Fetita S, Sobngwi E, Salaun-Martin C. Biological actions of the incretins GIP and GLP-1 and therapeutic perspectives in patients with type 2 diabetes. Diabetes Metab. 2005;31:233–242. doi: 10.1016/s1262-3636(07)70190-8. [DOI] [PubMed] [Google Scholar]
- 26.Hansotia T, Drucker DJ. GIP and GLP-1 as incretin hormones: lessons from single and double incretin receptor knockout mice. Regulatory Peptides. 2005;128:125–134. doi: 10.1016/j.regpep.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 27.Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24–32. doi: 10.1172/JCI30076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schonhoff SE, Giel-Moloney M, Leiter AB. Minireview: Development and differentiation of gut endocrine cells. Endocrinology. 2004;145:2639–2644. doi: 10.1210/en.2004-0051. [DOI] [PubMed] [Google Scholar]
- 30.Roth KA, Kim S, Gordon JI. Immunocytochemical studies suggest two pathways for enteroendocrine cell differentiation in the colon. Am J Physiol. 1992;263:G174–180. doi: 10.1152/ajpgi.1992.263.2.G174. [DOI] [PubMed] [Google Scholar]
- 31*.Chen MC, Wu SV, Reeve JR, Jr, Rozengurt E. Bitter stimuli induce Ca2+ signaling and CCK release in enteroendocrine STC-1 cells: role of L-type voltage-sensitive Ca2+ channels. Am J Physiol Cell Physiol. 2006;291:C726–739. doi: 10.1152/ajpcell.00003.2006.. The bitter substance denatonium was shown to be a potent stimulant of CCK release from enteroendocrine STC-1 cells via L-type voltage-sensitive calcium channels.
- 32.Masuho I, Tateyama M, Saitoh O. Characterization of bitter taste responses of intestinal STC-1 cells. Chem Senses. 2005;30:281–290. doi: 10.1093/chemse/bji022. [DOI] [PubMed] [Google Scholar]
- 33.Catterall WA, Striessnig J, Snutch TP, Perez-Reyes E. International Union of Pharmacology. XL. Compendium of Voltage-Gated Ion Channels: Calcium Channels. Pharmacol Rev. 2003;55:579–581. doi: 10.1124/pr.55.4.8. [DOI] [PubMed] [Google Scholar]
- 34.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 35.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 36.Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral Exendin-4 and Peptide YY3-36 Synergistically Reduce Food Intake through Different Mechanisms in Mice. Endocrinology. 2005;146:3748–3756. doi: 10.1210/en.2005-0473. [DOI] [PubMed] [Google Scholar]
- 37.Neary NM, Small CJ, Druce MR, Park AJ, Ellis SM, Semjonous NM, Dakin CL, Filipsson K, Wang F, Kent AS, Frost GS, Gathei MA, Bloom SR. Peptide YY3-36 and Glucagon-Like Peptide-17-36 Inhibit Food Intake Additively. Endocrinology. 2005;146:5120–5127. doi: 10.1210/en.2005-0237. [DOI] [PubMed] [Google Scholar]
- 38.Schirra J, Goke B. The physiological role of GLP-1 in human: incretin, ileal brake or more? Regulatory Peptides. 2005;128:109–115. doi: 10.1016/j.regpep.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 39.Halatchev IG, Cone RD. Peripheral administration of PYY3-36 produces conditioned taste aversion in mice. Cell Metabolism. 2005;1:159–168. doi: 10.1016/j.cmet.2005.02.003. [DOI] [PubMed] [Google Scholar]


