Abstract
We generated and characterized Salmonella enterica serovar Typhimurium mutants that were deleted for the genes encoding Braun lipoprotein (lpp) alone or in conjunction with the msbB gene, which codes for an enzyme required for the acylation of the lipid A moiety of lipopolysaccharide. Two copies of the lpp gene, designated as lppA and lppB, exist on the chromosome of S. Typhimurium. These mutants were highly attenuated in a mouse infection model and induced minimal histopathological changes in mouse organs compared to those seen in infection with wild-type (WT) S. Typhimurium. The lppB/msbB and the lppAB/msbB mutants were maximally attenuated, and hence further examined in this study for their ability to induce humoral and cellular immune responses. Importantly, infection of out-bred Swiss-Webster mice with the mutant S. Typhimurium generated superior T helper cell type 2 (Th2) responses compared to WT S. Typhimurium, as determined by measuring IgG subclasses and cytokines. WT S. Typhimurium induced higher levels of IgG2a in sera of infected mice, while the lppB/msbB and lppAB/msbB mutants mounted higher levels of IgG1 as determined by an enzyme-linked immunosorbent assay. Mice immunized with lppB/msbB and lppAB/msbB mutants rapidly cleared WT S. Typhimurium upon subsequent rechallenge, and naïve mice passively immunized with sera from animals infected with S. Typhimurium mutants were protected against subsequent challenge with WT S. Typhimurium. Splenic T cells produced higher levels of interferon-gamma following ex vivo exposure to WT S. Typhimurium, while splenic T cells infected with the above-mentioned two mutants evoked higher levels of interleukin-6. Further, mice infected with lppB/msbB and lppAB/msbB mutants showed much higher levels of splenic T cell activation as measured by CD44+ expression on CD4+ T cells by flow cytometry and by incorporation of 3H-thymidine compared to mice that were infected with WT S. Typhimurium. We expect the lppB/msbB and lppAB/msbB mutants to be excellent live-attenuated vaccine candidates, because they induced minimal inflammatory responses and evoked stronger and specific antibody and cellular immune responses.
Keywords: Salmonella Typhimurium, vaccine, immune responses, mouse model
1. Introduction
Salmonella enterica, which belongs to the Enterobacteriaceae family, contains 2300 serogroups based on the structure of O-antigen of lipopolysaccharide (LPS). Although most of these serogroups are of animal origin, S. Typhi is strictly a human pathogen [1]. Diseases associated with Salmonella infections include self-limiting gastroenteritis and septicemia, and humans could be asymptomatic carriers of this pathogen for several years with the organism residing in hepatocytes and the gall bladder [2]. Although infections with salmonellae are of concern in both developing and developed countries, typhoid fever is highly prevalent in developing countries with an annual global incidence of approximately 16 million cases and 600,000 deaths [3]. Among different serogroups of Salmonella, S. enterica serovar Typhimurium is most commonly associated with human infections after consuming contaminated food and water. The organism specifically invades M cells and is then taken up by macrophages before being released into the blood stream to infect other organs [4,5].
Braun (murein) lipoprotein (Lpp) represents one of the most abundant components present in the outer membrane of bacteria belonging to the family Enterobacteriaceae [6,7]. Maturation of Lpp requires modification of the lipid moiety, which is catalyzed by enzymes, specifically glycerol transferase, O-acyl transferase, signal peptidase II, and N-acyl transferase [8]. Two functional copies of the lpp gene (designated as lppA and lppB) exist on the chromosome of S. Typhimurium 14028 located in tandem and separated by 82 bp [9]. Deletion of both copies of the lpp gene results in a S. Typhimurium mutant that is minimally invasive to epithelial cells, non-motile, and severely impaired in its ability to induce cytotoxicity in murine macrophages (RAW 264.7 cells) and T84 human colonic epithelial cells, possibly due to the reduced production of proinflammatory cytokines and chemokines (e.g., tumor necrosis factor-alpha [TNF-α] and interleukin [IL]-8) [9]. The lpp (lppAB) double knockout (DKO) mutant was avirulent in mice following oral and intraperitoneal (i.p.) challenges. Mice immunized with the lppAB mutant were protected from death when rechallenged with a lethal dose of wild-type (WT) S. Typhimurium [9].
All Gram-negative bacteria possess LPS with lipid A representing the biological active domain and containing fatty acids believed to contribute to the low-permeability barrier of the outer membrane of Gram-negative bacteria [10]. As is the case with Lpp, lipid modification of LPS by the addition of fatty acids is catalyzed by enzymes encoded by the genes msbB (multicopy suppressor of htrB), htrB (high temperature requirement), and pagP (PhoP-activated gene) that attach myristic, lauric, and palmitic acids, respectively, to lipid A [11,12].
Deletion of the msbB gene reduces toxicity associated with LPS by preventing the addition of a terminal myristyl group to the lipid A domain [13]. As a result of a reduced production of proinflammatory cytokines and nitric oxide synthase, the msbB mutant of S. Typhimurium evokes less mortality and tissue damage in mice compared to that seen with WT S. Typhimurium [14,15]. Therefore, msbB single knockout (SKO) mutants show a reduced septic shock response and hence increase the safety of these S. Typhimurium mutants for potential use as live-attenuated vaccines in humans [15].
WT S. Typhimurium releases LPS during both in vitro and in vivo growth [16]. LPS release is significantly enhanced during lysis of S. Typhimurium following exposure to antibiotics or human serum and this enhanced LPS release causes septic shock [17]. Likewise, Lpp is also a critical bacterial component in the induction and pathogenesis of septic shock. It induces the production of TNF-α and IL-6 in mouse and human macrophages ex vivo [5] and leads to lethal shock as a result of the production of these cytokines in both LPS-responsive and non-responsive mice [18–20]. More importantly, Lpp synergizes with LPS to induce production of proinflammatory cytokines in mice, because Lpp binds to the toll-like receptor (TLR)-2, whereas LPS binds to TLR-4 and CD14 to activate host cells [20–22].
Therefore, we predicted that lpp mutants of S. Typhimurium with or without the deletion of the msbB gene would be excellent live-attenuated vaccine candidates. We reported that such lpp mutants (e.g., lppA and lppB SKO, lppAB, lppA/msbB and lppB/msbB DKO, and lppAB/msbB triple knockout [TKO]) are highly attenuated in ex vivo and in vivo models of S. Typhimurium infections [23]. In the present study, we investigated the immunological responses of lpp/msbB mutants and demonstrated that these S. Typhimurium mutants induced significantly higher IL-6 and minimal interferon-gamma (IFN-γ) from mouse splenic T cells and evoked superior T cell activation compared to WT S. Typhimurium. Further, lppB/msbB and lppAB/msbB mutant-infected mice showed significantly higher levels of IgG1, and mice immunized with the S. Typhimurium mutants rapidly cleared a subsequent infection with WT S. Typhimurium. Finally, passive immunization of naïve mice with sera from animals infected with S. Typhimurium mutants protected recipient mice against infection with WT S. Typhimurium.
2. Results
2.1. Mortality in Swiss-Webster mice after infection
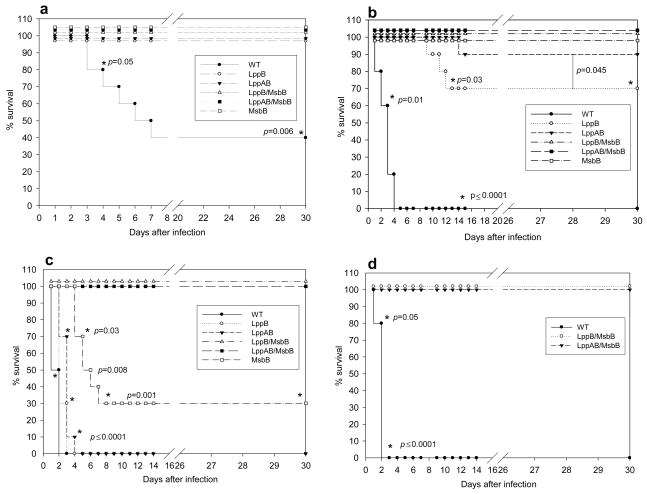
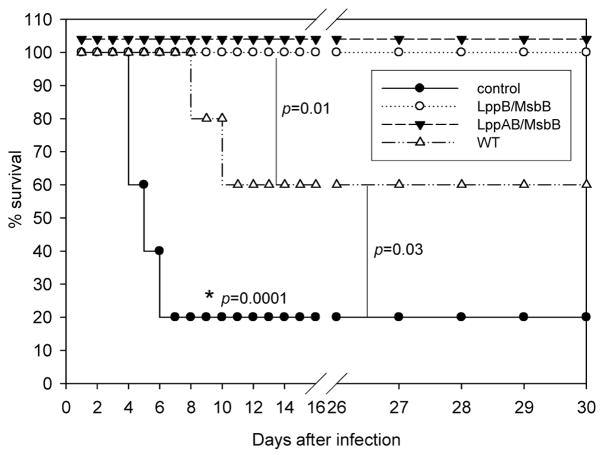
Both Lpp and LPS of S. Typhimurium contribute to septic shock and mortality in a mouse model of salmonellosis [9,19,24]. Similar to in-bred C57BL/6 mice used in the salmonellosis model, out-bred Swiss-Webster mice are also sensitive to infection by S. Typhimurium [22]. We first compared mortality in mice infected with WT S. Typhimurium or the various mutant S. Typhimurium strains. Each experimental group contained ten mice, and animals were observed over a period of 30 days following infection via the intraperitoneal (i.p.) route. At a dose of 1 × 103 cfu, 60% of mice infected with WT S. Typhimurium died within 4 to 8 days. In contrast, animals infected with the same dose of msbB, lppB, lppAB, lppB/msbB, or lppAB/msbB mutants did not die (Fig. 1a). At a higher dose of 1 × 104 cfu, mice infected with the msbB, lppB/msbB, or lppAB/msbB mutants showed 100% survival rates (Fig. 1b). In contrast, all of the mice infected with 1 × 104 cfu of WT S. Typhimurium died within 4–5 days. The survival rate of mice was 90% and 70%, respectively, when animals were infected with 1 × 104 cfu of lppAB or lppB mutant strains (Fig. 1b). Since these latter mutants did not provide 100% survival to animals, these strains were not considered sufficiently attenuated to serve as vaccine candidates.
Figure 1.
Survival of Swiss-Webster mice following infection with WT S. Typhimurium or various mutant strains of S. Typhimurium. a) Mice were infected via i.p. injection with 1 × 103 cfu, and observed for 30 days; b) mice were injected with 1 × 104 cfu.; c) mice were injected with 1 × 106 cfu; d) mice were injected with 1 × 107 cfu of WT or lppB/msbB or lppAB/msbB mutant S. Typhimurium; The actual p-values (Fisher’s exact test) are provided. The p-values shown for the WT or the mutant(s) S. Typhimurium were in comparison to those mutants that showed 100% protection. Asterisks denote statistically significant differences.
An even higher infectious dose (1 × 106 cfu) led to death in WT S. Typhimurium-infected mice within three days (Fig. 1c). At this dose, all of the mice infected with the lppB or lppAB mutant of S. Typhimurium also died, but death was somewhat delayed compared to WT S. Typhimurium-infected mice (three to four vs. two to three days). In contrast, 30% of the mice infected with 1 × 106 cfu of the msbB mutant survived, and importantly, 100% survival was observed in mice that were infected with either the lppB/msbB or the lppAB/msbB mutant (Fig. 1c). The lppB/msbB and lppAB/msbB mutants of S. Typhimurium were highly avirulent; 100% of the mice infected with even 1 × 107 cfu survived (Fig. 1d). At this dose, WT S. Typhimurium-infected mice died within three days.
2.2. Protection provided by mutants against subsequent challenge with WT S. Typhimurium
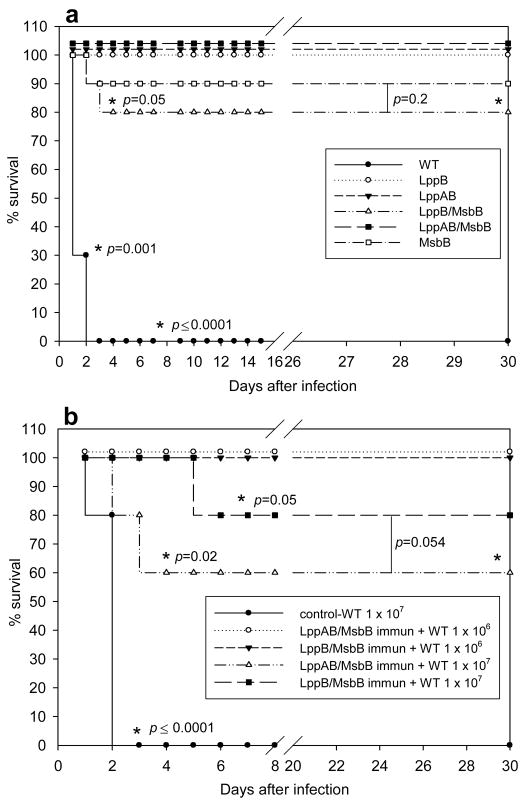
To determine whether initial infection with mutant S. Typhimurium induced protective immunity, we rechallenged mice that survived infection by 1 × 104 cfu of any of the mutant S. Typhimurium strains (Fig. 1b) with WT S. Typhimurium. For the rechallenge, 1 × 105 cfu of WT S. Typhimurium were injected 30 days after the first infection with mutant S. Typhimurium. Age-matched mice initially injected with phosphate-buffered saline (PBS) served as a control group. All of the control mice injected with 1 × 105 cfu of WT S. Typhimurium died, whereas mice that survived infection with a mutant S. Typhimurium strain were protected (80 to 100% survival following rechallenge with WT S. Typhimurium) (Fig. 2a).
Figure 2.
Protection of Swiss-Webster mice following infection with mutant strains of S. Typhimurium and challenge with WT S. Typhimurium. a) mice were injected with 1 × 104 cfu of various mutant strains of S. Typhimurium and, after 30 days, rechallenged with 1 × 105 cfu of WT S. Typhimurium; b) mice were injected with 1 × 106 cfu of lppB/msbB or lppAB/msbB mutant S. Typhimurium and, after 30 days, rechallenged with 1 × 106 or 1 × 107 cfu of WT S. Typhimurium. The control groups of mice were given a primary challenge dose of 1 × 107 cfu of WT S. Typhimurium. The actual p-values (Fisher’s exact test) are provided. The p-values shown for the WT or the mutant(s) S. Typhimurium were in comparison to those mutants that showed 100% protection. Asterisks denote statistically significant differences.
We also determined the protective effect of a higher dose of lppB/msbB and lppAB/msbB mutants of S. Typhimurium (1 × 106 cfu) in a mouse model. Thirty days after the initial infection, mice were rechallenged with WT S. Typhimurium. All of the mice initially infected with 1 × 106 cfu of lppB/msbB or lppAB/msbB mutant S. Typhimurium survived a rechallenge with 1 × 106 cfu of WT S. Typhimurium, while 60% (for lppAB/msbB) or 80% (for lppB/msbB) protection was achieved in mice rechallenged with 1 × 107 cfu of WT S. Typhimurium (Fig. 2b). We chose to initially infect mice with the mutants at a higher dose (i.e., 1 × 106 cfu) as all of the mice infected with 1 × 104 cfu of the lppB/msbB or lppAB/msbB mutant of S. Typhimurium died when rechallenged with 1 × 106 cfu of WT S. Typhimurium (data not shown). For the studies presented in Fig. 2b, we specifically used mutants lppAB/msbB and lppB/msbB as they were highly attenuated and did not kill mice even at a dose as high as 1 × 107 cfu (Fig. 1d).
2.3. Splenic bacterial load after infection
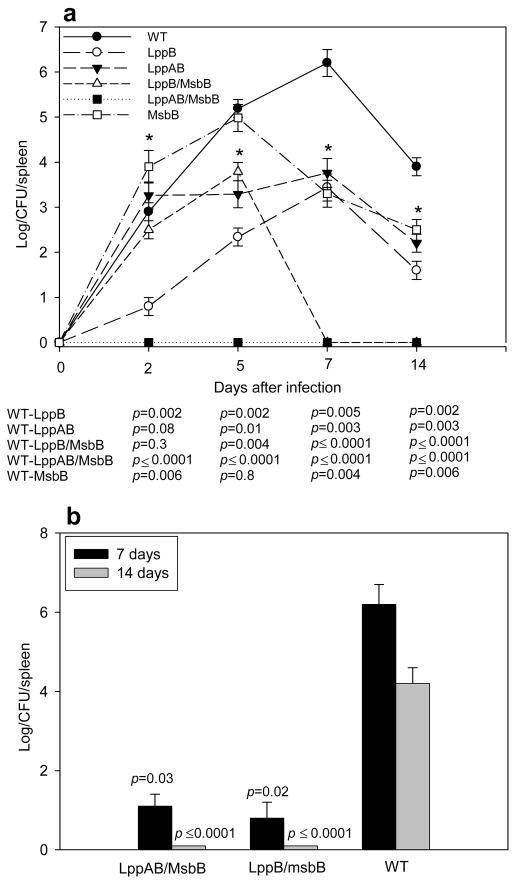
In early time points, we expected to find a reduced bacterial load in the spleens of mice infected with lpp mutants of S. Typhimurium, because our previous studies showed that deletion of the lpp genes affected invasiveness of S. Typhimurium into host cells [9]. To determine whether WT and mutant strains of S. Typhimurium differed in their ability to grow in infected hosts, we measured bacterial loads in the spleens of mice infected with 1 × 103 cfu of the various S. Typhimurium strains. At various times after infection, spleens were harvested from three mice per group. The spleens were homogenized, and bacteria were enumerated in serial log dilutions. The number of WT S. Typhimurium increased dramatically, reaching a maximal level of more than 1 × 106 cfu seven days after infection (Fig. 3a). The spleens of mice infected with mutant S. Typhimurium varied in their maximal bacterial loads, but the decline in splenic bacterial numbers was more rapid in mice infected with mutant S. Typhimurium than in mice infected with WT S. Typhimurium. Importantly, lppAB/msbB mutant S. Typhimurium did not survive in host cells, and no bacteria were detectable two days after infection. The second S. Typhimurium mutant strain that was highly protective in rechallenge experiments, the lppB/msbB mutant (Fig. 2b), reached maximal splenic numbers by day five and was completely eliminated from infected spleens by day seven (Fig. 3a).
Figure 3.
Bacterial growth in the spleens of infected Swiss-Webster mice. a) Mice were infected via i.p. injection with 1 × 103 cfu of WT or various mutant strains of S. Typhimurium (symbols are defined in the figure). At the indicated times, cfu in the spleens were determined. Data are expressed as mean ± S.D. and p-values < 0.05 are considered statistically significant (*). b) Mice were injected with 1 × 103 cfu of lppB/msbB or lppAB/msbB mutant S. Typhimurium, and rechallenged with 1 × 103 cfu of WT S. Typhimurium after 30 days. Seven or 14 days after the rechallenge, cfu in the spleens were determined. The p-values were determined for the comparison between immunized groups vs. a control group given a primary challenge with 1 × 103 cfu of WT S. Typhimurium. The p-values were < 0.05 for all immunized groups compared to a control group given a primary challenge with 1 × 103 cfu of WT S. Typhimurium for 7 and 14 days. The actual p-values (Student’s t-test) are provided.
To determine whether immunization with mutant S. Typhimurium prevented or retarded growth of WT S. Typhimurium upon rechallenge, we infected mice initially immunized with 1 × 103 cfu of either the lppB/msbB or the lppAB/msbB mutant, with 1 × 103 cfu of WT S. Typhimurium, 30 days after the first infection. Unimmunized mice (injected with PBS alone) were used as a control and were challenged with WT S. Typhimurium at the same dose. Seven days after rechallenge, we encountered very low bacterial numbers in the spleens of immunized mice, and we could not detect any bacterial growth 14 days after rechallenge (Fig. 3b). In contrast, we observed normal bacterial growth in the spleens of unimmunized control mice over a period of 14 days (Figs. 3a and b). To further evaluate the protective effect of immunization with 1 × 103 cfu of either the lppB/msbB or the lppAB/msbB mutant, we rechallenged immunized mice with 1 × 106 cfu of WT S. Typhimurium. As we observed at the lower dose of rechallenge, no surviving WT S. Typhimurium bacteria could be detected after 14 days in the spleens (data not shown). Our data indicated that WT S. Typhimurium was either rapidly cleared or killed in immunized mice.
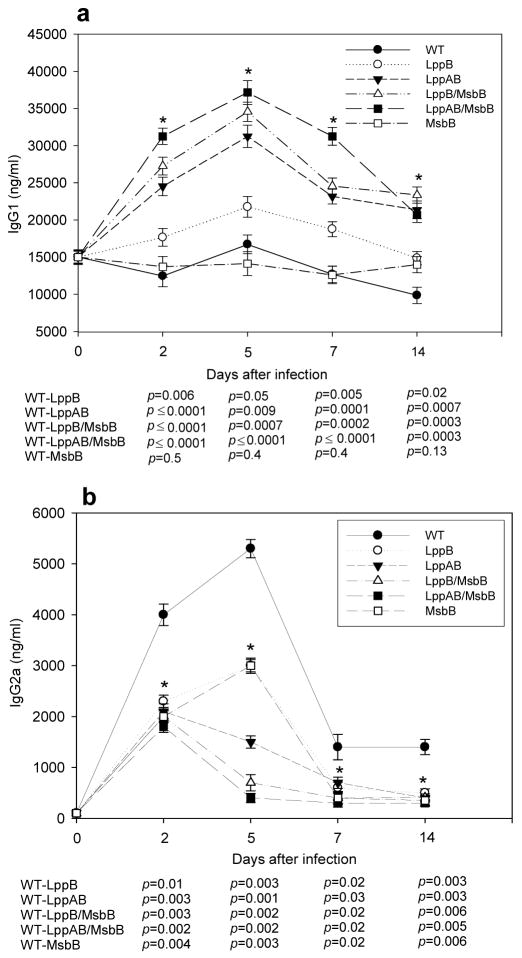
2.4. Serum IgG levels in mice infected with WT or mutant strains of S. Typhimurium
In order to be an effective vaccine strain, the attenuated S. Typhimurium mutants would have to induce protective humoral immune responses. To determine whether infected mice developed humoral immunity, we injected mice with 1 × 103 cfu of WT or mutant S. Typhimurium. We expected that activation of the immune system would be detectable by increases in total serum IgG. Therefore, we measured total IgG1 and IgG2a in the sera of mice before infection and 2, 5, 7, and 14 days post-infection. Total serum IgG1 antibody titers increased significantly in mice that were infected with lppAB/msbB, lppB/msbB, and lppAB mutant S. Typhimurium (Fig. 4a). Peak levels were reached five days after infection. In contrast, serum IgG1 levels did not increase in mice infected with WT, msbB mutant, or lppB mutant S. Typhimurium. The findings were very different for IgG2a (Fig. 4b). Serum levels of total IgG2a increased in all infected mice, but the strongest and most rapid increase was observed in mice infected with WT S. Typhimurium. The lppB and msbB mutant strains also demonstrated an ability to increase total serum IgG2a with a similar kinetics as observed for WT S. Typhimurium (peak level five days post-infection). Importantly, the mutant S. Typhimurium strains (lppAB/msbB, lppB/msbB, and lppAB) that elicited the most robust IgG1 response (Fig. 4a) were least potent in inducing a stable IgG2a response (Fig. 4b). These three bacterial strains showed a moderate increase in IgG2a serum levels two days after infection, but five days after infection, serum IgG2a titers were almost back to normal levels.
Figure 4.
Swiss-Webster mice were infected with 1 × 103 cfu of WT or various mutant strains of S. Typhimurium. a) Serum IgG1 and b) serum IgG2a concentrations were determined by ELISA at the indicated times after infection. Data are expressed as mean ± S.D. The p-values of < 0.05 for all mutant strains except msbB (only IgG1) compared to WT at all time points were considered statistically significant (*). The actual p-values (Student’s t-test) are provided.
2.5. Protective effect of sera from mice infected with mutant S. Typhimurium strains
It was important to determine whether the increase in total serum IgG1 was associated with protection from WT S. Typhimurium. Therefore, we infected/immunized Swiss-Webster mice with 1 × 103 cfu of either WT or lppB/msbB or lppAB/msbB mutant S. Typhimurium. Ten days after the infection when the IgG1 titers were still significantly higher in mutant-infected mice compared to the WT control (Fig. 4a), immune sera were collected. A 120-μl serum aliquot was injected intravenously into uninfected mice. After 24 h, the passively immunized mice were infected with 1 × 104 cfu of WT S. Typhimurium, a dose that killed 100% of unprotected mice within 4–5 days (Fig. 1b). The serum from mice infected with either lppB/msbB or lppAB/msbB mutant S. Typhimurium provided complete protection; 100% of passively immunized mice survived infection with a lethal dose of WT S. Typhimurium (Fig. 5). In contrast, 80% of the mice that either did not receive serum or were injected with 120 μl of pre-immune serum died within 7 days following infection with WT S. Typhimurium (indicated as a control group). Sera from animals that were infected with WT S. Typhimurium provided 60% protection in naïve mice against subsequent challenge with WT S. Typhimurium.
Figure 5.
Sera from Swiss-Webster mice immunized once with 1 × 103 cfu of lppB/msbB or lppAB/msbB mutant S. Typhimurium completely protected animals from rechallenge with 1 × 104 cfu of WT S. Typhimurium. Mice were injected i.v. with 120 μl of sera from mice infected with either WT or lppB/msbB or lppAB/msbB mutant strains of S. Typhimurium. Control groups received PBS or pre-immune sera. After 24 h, mice were challenged with 1 × 104 cfu WT S. Typhimurium and observed for 30 days. The actual p-values (Fisher’s exact test) between different groups are provided. Asterisks denote statistically significant differences.
2.6. T cell responses to a primary exposure to WT or mutant strains of S. Typhimurium
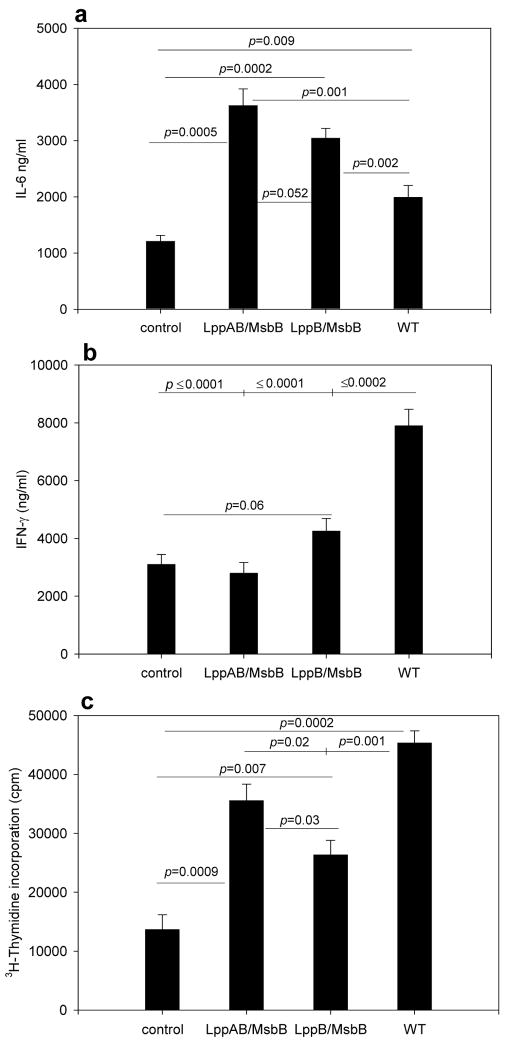
The SKO (either lpp or msbB) mutants still induce TNF-α and IL-8 in mouse macrophages and human epithelial cells, respectively, at levels comparable to those induced by WT S. Typhimurium [23,25]. Further, the lppB SKO mutant induces inflammatory cytokine responses and tissue damage as indicated by histopathology studies, albeit at a much reduced level compared to the WT S. Typhimurium-infected mice [23]. Therefore, it was important to determine the ability of the two prospective vaccine strains of S. Typhimurium in inducing inflammatory T cell responses during a primary infection. To this end, we cultured splenocytes from uninfected mice with WT, lppB/msbB, or lppAB/msbB mutant of S. Typhimurium at a multiplicity of infection (MOI) of 1 for 16 h. Following this incubation period, T cells were enriched by passage through nylon-wool columns and cultured for 72 h with irradiated splenocytes from uninfected mice. Culture supernatants were collected and IL-4, IL-6, and IFN-γ levels were measured by ELISA (Figs. 6a and b). Culture supernatants from T cells infected with either the lppB/msbB or the lppAB/msbB mutant contained significantly higher levels of IL-6 than did supernatants from T cells that were infected with WT S. Typhimurium. In contrast, levels of IFN-γ in conditioned media from T cells infected with WT S. Typhimurium were significantly higher than those in culture supernatants from T cells infected with either of the two mutant strains (Fig. 6b). In fact, the two mutant S. Typhimurium strains did not significantly induce IFN-γ production in infected cultures. The IL-4 levels were in the range of 430 ± 65 and 380 ± 40 pg/ml in lppAB/msbB and lppB/msbB mutant-infected T cells (p < 0.0001) compared to that of WT S. Typhimurium-infected T cells (150 ± 30 pg/ml). The latter were not statistically different from IL-4 produced from control T cells (120 ± 14 pg/ml).
Figure 6.
Cytokine production by T cells following in vitro culture of splenocytes from Swiss-Webster mice with WT or mutant S. Typhimurium at an MOI of 1 for 16 h. T cells were then isolated and incubated with irradiated splenocytes from uninfected mice for 72 h. a) IL-6 and b) IFN-γ concentrations in culture supernatants. T cell proliferation following in vitro culture of splenocytes from Swiss-Webster mice with WT or mutant S. Typhimurium at an MOI of 1 for 16 h. c) T cells were then isolated and incubated with irradiated splenocytes from uninfected mice for 72 h. 3H-thymidine was added during the last 10 h of incubation. Controls were cultured in the absence of S. Typhimurium. The p-values (student’s t-test) are given for statistically significant differences.
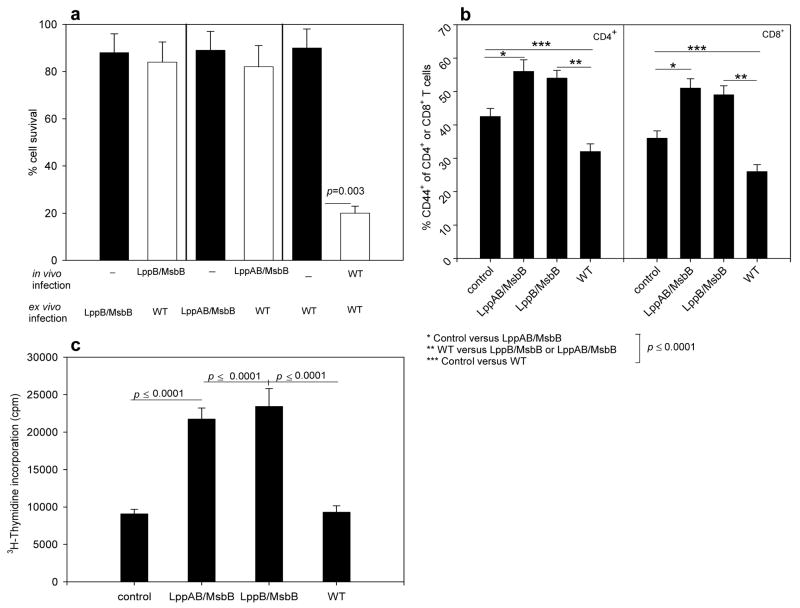
To determine whether the differences in splenocyte survival and T cell proliferative responses was dependent on the expression of genes encoding Lpp and LPS of S. Typhimurium, we exposed splenocytes from unimmunized mice to WT or mutant strains of S. Typhimurium for 16 h (MOI of 1) and then determined splenocyte survival. Splenocyte survival was consistently >90%, independent of the S. Typhimurium strain used in the ex vivo infection (Fig. 7a, black bars). We then enriched T cells from the S. Typhimurium-exposed cultures and incubated them with irradiated splenocytes. Instead of harvesting the supernatants, we added [3H]-thymidine to the cultures for the last 10 h of incubation. Subsequently, we measured incorporated radioactivity as a measurement of proliferation. Importantly, WT S. Typhimurium induced the strongest T cell proliferation in this primary ex vivo infection (Fig. 6c).
Figure 7.
T cell activation in infected Swiss-Webster mice. a) Survival of splenocytes. Splenocytes were harvested five days post-infection with 1 × 103 cfu of lppB/msbB, lppAB/msbB, or WT S. Typhimurium (white bars). Splenocytes were then incubated with WT S. Typhimurium at an MOI of 1 for 16 h, and cell survival was determined. Controls were from uninfected mice and were incubated in vitro with lppB/msbB, lppAB/msbB, or WT S. Typhimurium (black bars). b) Expression of the activation and memory T cell marker CD44 in T cells from Swiss-Webster mice infected with WT or mutant S. Typhimurium. Cells were analyzed by flow cytometry five days post-infection with 1 × 103 cfu. Controls were from uninfected mice. c) Swiss-Webster mice were infected with 1 × 103 cfu of WT or mutant S. Typhimurium or left uninfected (control). After five days, splenocytes were isolated and incubated with WT S. Typhimurium at an MOI of 1 for 16 h. T cells were then isolated and incubated with irradiated splenocytes from uninfected mice for 72 h. T cell proliferation was measured by incorporation of [3H]-thymidine during the last 10 h of incubation. The p-values (Student’s t-test) are given for statistically significant differences. Asterisks denote statistically significant differences.
Because the timeframe of the ex vivo infection did not allow for the development of specific T cell responses, the observed T cell proliferation and cytokine secretion were presumably due to the mitogenic stimulation of splenocytes by Lpp and LPS. Interestingly, not only were T cell proliferative responses lower in cultures exposed to lppB/msbB or the lppAB/msbB mutant of S. Typhimurium than in those exposed to WT S. Typhimurium, but cytokine secretion also differed: WT S. Typhimurium-exposed cultures secreted significantly higher levels of inflammatory IFN-γ than did cultures exposed to either of the mutant strains, suggesting that the mutant strains would induce less tissue damage and thus could possibly serve as vaccine candidates.
2.7. T cell activation in mice infected with WT or mutant strains of S. Typhimurium
To determine whether T cells were activated in Swiss-Webster mice immunized with the immunogenic mutant strains of S. Typhimurium (i.e., the lppB/msbB and the lppAB/msbB mutant), we isolated splenocytes from mice that were infected with 1 × 103 cfu of either WT, lppB/msbB mutant, or lppAB/msbB mutant S. Typhimurium. Spleens were collected at 3, 5, 7, or 14 days post-infection and the splenocytes stained with antibodies against CD4 or CD8 in combination with anti-CD44 antibodies. These markers served to distinguish naïve from activated or memory T cells: naïve CD4+ T cells express little CD44 while activated and memory CD4+ T cells express high levels of CD44 [26]. Five days post-infection, the percentage of CD44+ cells was significantly increased in both CD4+ and CD8+ T cells from mice infected with lppB/msbB or lppAB/msbB mutant S. Typhimurium, in comparison to either uninfected mice or mice infected with WT S. Typhimurium (Fig. 7b).
To further investigate whether the lppB/msbB and lppAB/msbB mutant S. Typhimurium induced specific T cell immunity, we infected Swiss-Webster mice with 1 × 103 cfu of WT, lppB/msbB mutant, or lppAB/msbB mutant S. Typhimurium. Spleens were collected five days post-infection and splenocytes isolated and cultured with WT S. Typhimurium at an MOI of 1 for 16 h. Splenocyte survival was determined and T cells were enriched and incubated for another 72 h with irradiated splenocytes. During the last 10 h of incubation, proliferating T cells were labeled with [3H]-thymidine. Importantly, we detected statistically significant lower cell survival in splenocytes from mice infected with WT S. Typhimurium than in splenocytes from mice infected with either the lppB/msbB or lppAB/msbB mutant strains (Fig 7a, white bars). Along the same lines, we measured significantly increased proliferative responses in T cell cultures from mice that were infected with either the lppB/msbB or lppAB/msbB mutant S. Typhimurium (Fig. 7c). In contrast, proliferation of T cells from mice infected with WT S. Typhimurium did not differ from the spontaneous proliferation observed in control cultures that contained T cells from uninfected mice and were cultured without bacteria throughout the experiment (Fig. 7c). Earlier studies indicated that mice infected with a live avirulent S. Typhimurium SL3235 strain caused more than 90% reduction in the proliferative responses of splenocytes from immunized mice to a panel of B- and T-cell mitogens [27,28]
3. Discussion
We recently reported that the deletion of lpp genes from either the WT S. Typhimurium 14028 or from the msbB-negative background strain resulted in mutants that were highly attenuated in ex vivo and in vivo models of salmonellosis [23]. In our present study, among several mutants that we generated (e.g., lppA, lppB, and msbB SKO, lppAB, lppA/msbB and lppB/msbB DKO, and the lppAB/msbB TKO), we believe that the lppB/msbB and lppAB/msbB mutants are the best candidates for a new live-attenuated vaccine. An important link between innate and adaptive immune responses is established by the activation of TLRs [29]. Lpp activates TLR-2 [19,24], and in the lppB/msbB mutant, one intact copy of the lpp gene (lppA) remains. LPS acts via both TLR-4 and the CD14 receptor [29]. Although the biological potency of lipid A is significantly reduced in the lppB/msbB mutant, this mutant still expresses LPS. Therefore, we predicted that the lppB/msbB mutant strain of S. Typhimurium would induce minimal proinflammatory cytokine responses making it an attractive vaccine candidate with improved safety for human use. A similar argument could be made for the lppAB/msbB mutant strain of S. Typhimurium. However, our previous studies using ex vivo and in vivo models of Salmonella infections showed that the lppAB/msbB mutant could not survive in host cells [23] and, hence, might not trigger sufficiently strong cell-mediated immune responses. Here, we show that despite reduced viability in the host, both the lppB/msbB DKO and the lppAB/msbB TKO strain of S. Typhimurium induced excellent humoral and cellular protective immunity.
Our present study indicated that infection by i.p. injection with either the lppB/msbB or the lppAB/msbB mutant strains of S. Typhimurium did not cause death in mice at doses as high as 1 × 107 cfu (Fig. 1d) whereas lppB and lppAB mutants killed all mice within 4 days when initially infected at a dose of 1 × 106 cfu (Fig. 1c). We also showed that mice that were first immunized with either of these two mutant strains (lppB/msbB and lppAB/msbB) at a dose of 1 × 106 cfu before being challenged with WT S. Typhimurium at the same dose were protected (Fig. 2b). Further, significant protection was achieved in mice immunized with these mutant strains of S. Typhimurium if the mice were subsequently challenged with 1 × 107 cfu of WT S. Typhimurium 14028 (Fig. 2b). These data clearly indicated that although our lpp/msbB mutants were highly attenuated in their virulence, they still induced protective immunity against subsequent challenges with WT S. Typhimurium. We obtained confirming evidence that T cells were activated and may have differentiated to memory T cells from flow cytometric analyses: the percentage of CD4+ and CD8+ T cells expressing the CD44 cell surface markers was significantly higher in mice infected with mutant S. Typhimurium strains than in mice that were infected with WT S. Typhimurium (Fig. 7b).
Further, we noted increased proliferation of the T cells from mice infected with the mutants (lppB/msbB and lppAB/msbB) compared to WT S. Typhimurium-infected animals as measured by thymidine update (Fig. 7C). Mittrucker et al. [30] demonstrated that mice infected with WT S. Typhimurium SL1344 had higher percentage of activated T cells in the spleen, however, they did not observe any proliferation of the T cells. In our studies, we noted activation and proliferation of T cells only with the mutant-infected mice. Taken together, our data suggested that immunization of mice with the lppAB/msbB or the lppB/msbB mutant S. Typhimurium induced the activation and proliferation of T cells reactive against WT S. Typhimurium.
We chose to infect mice in these studies via the i.p. route as salmonellae lead to systemic infection in humans and animals. However, in order to demonstrate efficacy of our vaccine candidates, we plan to perform future studies in which animals will be infected by the oral route and shedding of the bacteria in stool specimens will also be examined. The results presented in this study illustrated first evidence of immune responses generated by lpp/msbB mutants in a mouse model and provided proof-of-principle that such mutants could lead to the development of excellent live attenuated vaccine candidates.
Because S. Typhimurium is a facultative intracellular pathogen, cellular immune responses are important components of the protective immunity against this organism. This protective cellular immunity is mediated via CD4+ T cells and causes activation of macrophages and delayed-type hypersensitivity responses [31]. Our data suggested that the tested lppB/msbB and lppAB/msbB mutants activated antigen-presenting cells to mount cell-mediated and humoral immune responses before they were cleared from the animals (Figs. 3, 4, and 7). Protective immune responses against S. Typhimurium in mice have been attributed to a correct balance between antibody and cellular immunity with an emphasis on the development of Th1 [32] and Th2 T cell subsets [33]. In order to evaluate the immune response elicited after immunization with the mutant strains of S. Typhimurium, we examined sera of immunized mice for the production of IgG2a and IgG1 antibodies as indicative of Th1 and Th2 subsets, respectively. Our results showed lower levels of IgG2a antibodies in mice infected with the lppAB/msbB or the lppB/msbB mutant strains than in mice that were infected with the WT S. Typhimurium (Fig. 4b).
Although SKO mutants such as msbB and lppB showed higher levels of IgG2a (Fig. 4b) in the serum than the lppAB/msbB or lppB/msbB mutants, they were not considered for further studies because of their higher virulence in mice (Figs. 1b and c) as compared to lppB/msbB and lppAB/msbB mutants. Conversely to IgG2a, levels of IgG1, an indicator of Th2 responses, were higher in mice infected with the lppAB/msbB or the lppB/msbB mutant strains than in mice infected with WT S. Typhimurium with a peak at 5 days post-infection and a slow decline thereafter (Fig. 4a). Serum levels of IgG1 were still significantly higher after 14 days, indicating the generation of a strong neutralizing antibody for an appropriate anti-bacterial response. Similarly supporting a predominant influence of Th2 cells on antibody production in mice infected with the lppAB/msbB or the lppB/msbB mutant strains was our observation that these mice had higher serum levels of IgG2b and IgA than did mice infected with WT S. Typhimurium (data not shown).
Further, we examined cytokines such as IFN-γ, IL-4, and IL-6 in T cells. IFN-γ, a major cytokine produced by CD4+ Th1 and NK cells, has been demonstrated to play an important role in the protection against Salmonella infection in avian hosts and is an appropriate indicator of an adequate inflammatory response [24,34,35]. During infections with intracellular pathogens, IFN-γ derived from activated Th1 cells is critical for host resistance by mediating the activation of effector cells and promoting inflammatory responses. Nevertheless, overproduction of the potent antimicrobial and inflammatory Th1 cytokines can lead to tissue damage [36–39]. One important regulator of LPS-induced pathology is IFN-γ [37,40]. The multi-functional cytokine IL-6 exerts effects on many different target cell types, and the main biological activities include the induction of terminal differentiation in B cells and the activation of T cells [41]. Important for our attempts to limit inflammatory Th1 responses is the inhibitory effect of IL-6 on the differentiation of CD4+ T cells into effector Th1 cells. IL-6 induces the expression of suppressor of cytokine signaling (SOCS)1 which inhibits IFN-γ signaling [42]. In addition, IL-6 directs the differentiation of CD4+ T cells into IL-4–producing effector Th2 cells [43].
In our experiments, the IFN-γ response was higher in splenic T cells challenged with WT S. Typhimurium compared to responses following exposure to the mutant strains lppB/msbB or lppAB/msbB (Fig. 6). Conversely, IL-6 levels were significantly higher in splenic T cells exposed to the lppB/msbB or lppAB/msbB mutant strains. Likewise, we noted increased production of IL-4 from T cells exposed to the above two mutants. Important for our study is to recognize that Th1 cells produce preferentially IFN-γ and tumor necrosis factor (TNF)- β, whereas Th2 cells preferentially produce IL-4 and IL-5 [44]. A consequence of this selective cytokine production by Th cells is the differential regulation of immunoglobulin isotype switching where Th1 and Th2 cells specifically induce antigen-specific B cells to secrete IgG2a and IgG1, respectively [45]. We noted that both IL-4 and IL-5 levels were difficult to measure, presumably due to their consumption by activated T cells and their instability. In general, the levels of inflammatory cytokines, as measured by Bio-Plex, were very low in the supernatant of T cells infected with lppB/msbB and lppAB/msbB mutants compared to WT S. Typhimurium exposed T cells (data not shown).
Several vaccines against Salmonella, including whole-cell killed and live vaccines, have been developed with variable results [19]. Killed Salmonella vaccines induce strong antibody responses but are poor triggers of cell-mediated immunity [19,46,47]. In addition, killed Salmonella vaccines require parenteral immunization, whereas live vaccines can be administered orally. Further, T-cell or antibody responses alone confer only a moderate level of protection against virulent Salmonella strains [48–51]. Mutants of S. Typhimurium deficient in the biosynthesis of aromatic metabolites (e.g. aroA, aroC and aroD) [52–56] or purines (e.g. purA and purE) [57,58], in the production of adenylate cyclase (cya) or the cyclic AMP receptor protein (crp) [59], or with mutations affecting the global regulatory system (phoP/phoQ) [60] or the gene encoding Cdt (colonization of deep tissues) [61] have been investigated in mice as potential live vaccine candidates. Strains of S. Typhimurium with mutations in the Salmonella pathogenicity island-2 (SPI-2) are also attenuated in mice [62–65]. Further, the phoP/phoQ and the ΔaroC ΔssaV S. Typhimurium mutant strains have been reported to be safe and elicit specific antibodies and IgA antibody-secreting cell responses to the heterologous antigens as well as to the carriers LPS [56,66,67] in human volunteers. The ssaV gene codes for a protein involved in protein secretion via the type 3 secretion system (T3SS) on the SPI-2. However, these mutants were heavily colonized in the tested volunteers, as indicated by prolonged shedding, and induced a significant acute-phase response, indicative of inflammation, at the highest dose levels. Most importantly, these mutants still have intact LPS and Lpp which induce strong proinflammatory cytokine responses. Therefore, further live-attenuated oral vaccine strains generated by mutating genes that contribute to shedding and the generation of proinflammatory cytokines are needed for safer vaccine development.
Importantly, we found that the lppB/msbB and lppAB/msbB mutant S. Typhimurium strains did not persist in vivo, and were rapidly cleared from the infected mice. In fact, the lppAB/msbB mutant strain could not be cultured from spleens collected two days post-infection, and the lppB/msbB mutant strain persisted less than seven days in the spleen of infected mice (Fig. 3a). The time-frame of this bacterial clearance, however, is sufficient for the induction of protective immunity as has been recently demonstrated by Ravindran et al. using an in vivo system of Salmonella infection with subsequent visualization of bacterial antigen and T cell activation [68]. We believe that the new mutant strains of S. Typhimurium (lppB/msbB and lppAB/msbB) that have been tested in this investigation provided three important features that are crucial for a good vaccine candidate: 1) significantly enhanced specific antibody and cellular responses against WT S. Typhimurium in a mouse model, 2) ability to clear rapidly from infected mice, and 3) inability to generate significant pro-inflammatory cytokine responses. In future studies, we will compare immunological responses of our lpp/msbB mutants with other live attenuated S. Typhimurium mutants (e.g., PhoP/Q, AroA, HtrA), which could be crucial for evaluating their potential use as vaccine candidates in humans and animals.
Taken together, we have developed and characterized new mutants of S. Typhimurium that could be excellent live vaccine candidates. Because lpp genes are found in S. Typhi as well, such mutants could also be developed in the S. Typhi background strain for their possible use against typhoid.
4. Materials and Methods
4.1. Bacterial culture
S. Typhimurium strains used in this study are listed in Table 1. The organisms were grown in Luria-Bertani (LB) broth and on LB agar plates in the presence of the appropriate antibiotics. For the growth of msbB mutants, we used a special MsbB medium as previously described [23]. The MsbB medium/liter consisted of the following: Tryptone 10 g, Yeast Extract 5 g, 1 M MgSO4 (1 ml), and 1 M CaCl2 (1 ml). The bacteria were cultivated at 37°C overnight with shaking at 200 rpm. Bacteria were harvested by centrifugation (6,000 rpm for 5 min), washed with PBS, and resuspended in a minimal amount of PBS. Bacteria were enumerated by determining colony-forming units (cfu) in triplicate, and expressed as cfu/ml.
TABLE 1.
Strains used in this study
| Strain | Relevant characteristic(s) | Reference |
|---|---|---|
| S. enterica serovar Typhimurium strains | ||
| 14028 | S. enterica serovar Typhimurium | 9 |
| Mutant lppB | Isogenic mutant in which only one copy of the lpp gene (lppB) was deleted; Nalr Knr | 9 |
| Mutant msbB | 14028 in which the msbB gene was truncated; Tcr | 23 |
| Mutant lppAB | Isogenic mutant in which both copies of the lpp gene (lppAB) were deleted; Nalr Knr | 9 |
| Mutant lppB/msbB | Isogenic mutant in which the lppB gene was deleted from the msbB minus background strain of 14028; Nalr Tcr Knr | 25 |
| Mutant lppAB/msbB | Isogenic mutant in which the lppAB genes were deleted from the msbB minus background strain of 14028; Nalr Tcr Knr | 23 |
Nalr = Nalidixic acid resistance; Tcr = Tetracycline resistance; Knr = Kanamycin resistance
4.2. Animals
We used 6- to 8-week-old Swiss-Webster mice that were purchased from Taconic Farms, Germantown, NY. These mice are susceptible to infection with S. Typhimurium [69], and we chose these out-bred mice over in-bred mice to examine true heterogeneity in immune responses to vaccine candidates. We believe that this model better represents the situations to be encountered in human populations. Mice were kept under specific pathogen-free conditions in filter-topped cages with sterile bedding, and were fed sterile food and water.
4.3. Infection of mice
Mice were infected via the intraperitoneal (i.p.) route with the following bacterial strains: WT S. Typhimurium 14028 and its msbB, lppB, lppAB, lppB/msbB, and lppAB/msbB mutants as prepared and characterized previously [9,23,25]. We chose lppB and lppB/msbB mutants over lppA and lppA/msbB mutants because our earlier studies indicated that the lppB mutant, retaining expression of lppA, is more potent than the lppA mutant in inducing inflammatory cytokines necessary to activate an adaptive immune response [23]. Mice were infected with bacterial doses ranging from 1 × 103–1 × 108 cfu, and observed for 30 days. In some cases, mice initially infected with mutant S. Typhimurium were rechallenged with WT S. Typhimurium (1 × 104–1 × 107 cfu) 30 days after the initial infection.
4.4. Enumeration of bacteria
At indicated time intervals after infection/immunization of mice, the spleens were removed, homogenized in PBS, and serial dilutions of the homogenate were plated on LB and Salmonella-Shigella agar plates [23,25] for determining bacterial counts. The plates were incubated at 37°C for 24 to 48 h and colonies were counted.
4.5. Serum immunoglobulins
Mice were infected with WT or mutant S. Typhimurium. Total serum IgG1 and IgG2a were measured after 2, 5, 7, or 14 days by enzyme-linked immunosorbent assay (ELISA). Highbinding ELISA plates (flat bottom, Evergreen Scientific, Los Angeles, CA) were coated with 1 μg/ml of capture antibody (anti-IgG1 or anti-IgG2a; Bethyl Inc., Montgomery, TX) diluted in 0.05 M carbonate/bicarbonate buffer (pH 9.6) for 1 h at room temperature. Subsequently, the wells of the microtiter plates were washed three times with washing buffer (50 mM Tris, 0.14 M NaCl, and 0.05% Tween 20). Sera from mice were diluted 1:50 in sample buffer (50 mM Tris, 0.14 M NaCl, 1% bovine serum albumin [BSA], and 0.05% Tween 20). Mouse Reference Serum (Bethyl Inc.) was diluted two-fold starting at a concentration of 250 ng/ml and used for plotting a standard curve. Subsequently, the plates were incubated at room temperature for 1 h and washed three times with washing buffer. The plates were then blocked with blocking buffer (50 mM Tris, 0.14 M NaCl, and 1% BSA). After washing, a 1:150,000 dilution of horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Bethyl Inc.) was added to the wells of the microtiter plate. Finally, 3, 3′, 5, 5′ tetramethylbenzidine (TMB) substrate was added to visualize antigen-antibody reactions. The reaction was stopped with 2 M H2SO4, and the optical density (OD) was measured at 450 nm using an ELISA reader. Serum from uninfected mice was used as a control. The concentration of IgG subclass antibodies was expressed in ng/ml.
4.6. Collection of blood
Blood was obtained by retro-orbital bleeding. Samples were kept at 4°C for 4–6 h, and then centrifuged at 4,000 rpm for 5 min to separate the serum. Sera were stored at −20°C.
4.5. Flow cytometric analysis
Spleen cells were obtained from mice infected with WT or mutant S. Typhimurium. Splenocytes (0.5–1.0 × 106) were suspended in PBS and 1% fetal calf serum (FCS), and incubated with mouse CD16/CD32 monoclonal antibodies (0.25 μg/100 μl) (BD Bioscience, Franklin Lakes, NJ) for 15 min at room temperature. These antibodies were added to block antibody binding to mouse Fc-γ receptor-bearing cells. Then, the cells were washed twice with PBS plus 1% FCS. To quantitate activated T cells, the cell suspension was incubated with either anti-CD4 or anti-CD8 antibody conjugated to fluorescein isothiocyanate [FITC] (0.25 μg/100 μl) and anti-CD44 antibody conjugated to phycoerythrin (PE) (0.1 μg/100 μl) for 60 min at 4°C. Subsequent to incubation for 60 min at 4°C, the cells were washed twice with PBS, and then analyzed using a FACScan flow cytometer and CellQuest (Becton Dickinson, Mountain View, CA) software.
4.7. Cytokine assays
Cytokine production from T cells was measured as previously described [9]. Briefly, splenocytes were isolated from naïve mice and plated at a density of 1 × 107/ml in 24-well tissue culture plates. These cells were then incubated with WT S. Typhimurium or the lppB/msbB or lppAB/msbB mutants at a multiplicity of infection (MOI) of 1 for 16 h. Subsequently, splenocytes were washed extensively with PBS, and T cells were enriched by passing splenocytes through nylon-wool columns (108 splenocytes were incubated in the column at 37°C in the presence of 5% CO2 for 1 h before eluting the cells with 9 ml of RPMI medium) [70]. The T cells were plated at a density of 2 × 105 with irradiated splenocytes from uninfected mice (2 × 104) as feeder cells in 96-well microtiter plates in 200 μl of RPMI medium for 72 h at 37°C in the presence of 5% CO2. The culture supernatants were then examined by ELISA for the presence of IFN-γ, IL-6, and IL-4.
For the ELISA, the wells of microtiter plates were coated with 100 μl of purified anti-mouse monoclonal capture antibody (eBioscience, San Diego, CA) against either IFN-γ, IL-4, or IL-6 at a concentration of 1 μg/ml in coating buffer (PBS plus 0.1% BSA). The plates were incubated overnight at 4°C. Subsequently, the plates were washed three times with washing buffer (PBS plus 0.05% Tween 20), followed by blocking with 200 μl of blocking buffer (PBS plus 1% BSA) per well. The plates were incubated at room temperature for 60 min, washed three times with washing buffer, and then 100 μl of standard or sample was added to each well. Recombinant mouse IL-4, IL-6, and IFN-γ were diluted two-fold starting at a concentration of 0.1–0.5 μg/ml and used as a standard. Subsequently, the plates were incubated at room temperature for 1 h, washed three times with washing buffer, and incubated with 100 μl of detection antibody (1 μg/ml of biotinylated anti-mouse IL-6/IL-4 or anti-IFN-γ antibody; eBioscience) prepared in PBS plus 0.1% BSA. The plates were incubated at room temperature for 1 h, washed six times with washing buffer, followed by the addition of streptavidin-conjugated HRP (from eBioscience) at a 1:500 dilution in blocking buffer. The plates were incubated at room temperature for 30 min, washed six times with washing buffer, and then 100 μl of TMB substrate was added. The color was developed at room temperature for 20–30 min followed by the addition of 50 μl of 2 M H2SO4 to stop the reaction. The OD was measured at 450 nm. The supernatants from T cells were also examined using the multiplex assay with Bio-Plex (Bio-Rad, Hercules, CA), which detects 23 cytokines/chemokines.
4.8. T cell proliferation assay
Mice were infected/immunized with mutant or WT S. Typhimurium at a dose of 1 × 103 cfu by the i.p. route. After 2, 5, 7, and 14 days, splenocytes from three infected/immunized mice were isolated, pooled, and incubated with WT S. Typhimurium at an MOI of 1 for 16 h. After the incubation period, the number of live splenocytes was determined by the trypan blue exclusion technique and counting with a hemocytometer. Live T cells were enriched using nylon-wool columns, and incubated at a cell density of 2 × 105/well with irradiated splenocytes (2 × 104) from uninfected mice in 96-well U-bottom microtiter plates for 72 h. Cells were pulsed with [3H]-thymidine for the last 10 h, and incorporation of radioactivity was measured by liquid scintillation counting in triplicate. Data were expressed as arithmetic means ± standard deviation (S.D.).
In another set of experiments, splenocytes were isolated from naïve mice and incubated with WT or lppB/msbB or lppAB/msbB mutant S. Typhimurium for 16 h at an MOI of 1. Subsequently, live cells were counted by the Trypan Blue exclusion technique, and T cells were enriched using nylon-wool columns and incubated with irradiated splenocytes for proliferation measurements as described above.
4.9. Statistics
At least three independent experiments were performed, and the data were analyzed using Student’s t test. p-values of ≤0.05 were considered significant. Animal mortality was analyzed using Fisher’s exact test.
Acknowledgments
We acknowledge grant support from the NIH/NIAID (AI064389) and John Sealy Endowment Fund for Biomedical Research, UTMB, to AKC for accomplishing this research. SLA is funded by the NIH T32 pre-doctoral training grant in Emerging and Tropical Infectious Diseases. Help provided by Ms. Mardelle Susman in editing the manuscript is highly appreciated. We thank Mark Griffin for his help in providing the facilities of the flow cytometry core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaufmann SH, Raupach B, Finlay BB. Introduction: microbiology and immunology: lessons learned from Salmonella. Microbes Infect. 2001;3:1177–81. doi: 10.1016/s1286-4579(01)01498-8. [DOI] [PubMed] [Google Scholar]
- 2.Kabra SK, Madhulika, Talati A, Soni N, Patel S, Modi RR. Multidrug-resistant typhoid fever. Trop Doct. 2000;30:195–7. doi: 10.1177/004947550003000404. [DOI] [PubMed] [Google Scholar]
- 3.Pang T, Levine MM, Ivanoff B, Wain J, Finlay BB. Typhoid fever--important issues still remain. Trends Microbiol. 1998;6:131–3. doi: 10.1016/s0966-842x(98)01236-0. [DOI] [PubMed] [Google Scholar]
- 4.Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J Exp Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glauser MP, Zanetti G, Baumgartner JD, Cohen J. Septic shock: pathogenesis. Lancet. 1991;338:732–6. doi: 10.1016/0140-6736(91)91452-z. [DOI] [PubMed] [Google Scholar]
- 6.Braun V, Hantke K. Biochemistry of bacterial cell envelopes. Annu Rev Biochem. 1974;43:89–121. doi: 10.1146/annurev.bi.43.070174.000513. [DOI] [PubMed] [Google Scholar]
- 7.Juncker AS, Willenbrock H, Von Heijne G, Brunak S, Nielsen H, Krogh A. Prediction of lipoprotein signal peptides in Gram-negative bacteria. Protein Sci. 2003;12:1652–62. doi: 10.1110/ps.0303703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi S, Wu HC. Lipoproteins in bacteria. J Bioenerg Biomembr. 1990;22:451–71. doi: 10.1007/BF00763177. [DOI] [PubMed] [Google Scholar]
- 9.Sha J, Fadl AA, Klimpel GR, Niesel DW, Popov VL, Chopra AK. The two murein lipoproteins of Salmonella enterica serovar Typhimurium contribute to the virulence of the organism. Infect Immun. 2004;72:3987–4003. doi: 10.1128/IAI.72.7.3987-4003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neidhardt F, Ingraham J, Low K, Magasanik B, Schaechter M, Umbarger H. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington D.C.: American Society for Microbiology; 1987. [Google Scholar]
- 11.Guo L, Lim KB, Gunn JS, Bainbridge B, Darveau RP, Hackett M, Miller SI. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science. 1997;276:250–3. doi: 10.1126/science.276.5310.250. [DOI] [PubMed] [Google Scholar]
- 12.Clementz T, Zhou Z, Raetz CR. Function of the Escherichia coli msbB gene, a multicopy suppressor of htrB knockouts, in the acylation of lipid A. Acylation by MsbB follows laurate incorporation by HtrB. J Biol Chem. 1997;272:10353–60. doi: 10.1074/jbc.272.16.10353. [DOI] [PubMed] [Google Scholar]
- 13.Post DM, Ketterer MR, Phillips NJ, Gibson BW, Apicella MA. The msbB mutant of Neisseria meningitidis strain NMB has a defect in lipooligosaccharide assembly and transport to the outer membrane. Infect Immun. 2003;71:647–55. doi: 10.1128/IAI.71.2.647-655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan SA, Everest P, Servos S, Foxwell N, Zahringer U, Brade H, Rietschel ET, Dougan G, Charles IG, Maskell DJ. A lethal role for lipid A in Salmonella infections. Mol Microbiol. 1998;29:571–9. doi: 10.1046/j.1365-2958.1998.00952.x. [DOI] [PubMed] [Google Scholar]
- 15.Low KB, Ittensohn M, Le T, Platt J, Sodi S, Amoss M, Ash O, Carmichael E, Chakraborty A, Fischer J, Lin SL, Luo X, Miller SI, Zheng L, King I, Pawelek JM, Bermudes D. Lipid A mutant Salmonella with suppressed virulence and TNFα induction retain tumor-targeting in vivo. Nat Biotechnol. 1999;17:37–41. doi: 10.1038/5205. [DOI] [PubMed] [Google Scholar]
- 16.Evans ME, Pollack M. Effect of antibiotic class and concentration on the release of lipopolysaccharide from Escherichia coli. J Infect Dis. 1993;167:1336–43. doi: 10.1093/infdis/167.6.1336. [DOI] [PubMed] [Google Scholar]
- 17.Dofferhoff AS, Nijland JH, de Vries-Hospers HG, Mulder PO, Weits J, Bom VJ. Effects of different types and combinations of antimicrobial agents on endotoxin release from gram-negative bacteria: an in-vitro and in-vivo study. Scand J Infect Dis. 1991;23:745–54. doi: 10.3109/00365549109024303. [DOI] [PubMed] [Google Scholar]
- 18.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 19.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–9. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Peterson JW, Niesel DW, Klimpel GR. Bacterial lipoprotein and lipopolysaccharide act synergistically to induce lethal shock and proinflammatory cytokine production. J Immunol. 1997;159:4868–78. [PubMed] [Google Scholar]
- 21.Means TK, Golenbock DT, Fenton MJ. The biology of Toll-like receptors. Cytokine Growth Factor Rev. 2000;11:219–32. doi: 10.1016/s1359-6101(00)00006-x. [DOI] [PubMed] [Google Scholar]
- 22.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–92. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 23.Fadl AA, Sha J, Klimpel GR, Olano JP, Niesel DW, Chopra AK. Murein lipoprotein is a critical outer membrane component involved in Salmonella enterica serovar Typhimurium systemic infection. Infect Immun. 2005;73:1081–96. doi: 10.1128/IAI.73.2.1081-1096.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, Brennan PJ, Bloom BR, Godowski PJ, Modlin RL. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–6. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 25.Fadl AA, Sha J, Klimpel GR, Olano JP, Galindo CL, Chopra AK. Attenuation of Salmonella enterica Serovar Typhimurium by altering biological functions of murein lipoprotein and lipopolysaccharide. Infect Immun. 2005;73:8433–6. doi: 10.1128/IAI.73.12.8433-8436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sprent J. Immunological memory. Current Opinion in Immunology. 1997;9:371. doi: 10.1016/s0952-7915(97)80084-2. [DOI] [PubMed] [Google Scholar]
- 27.Eisenstein TK, Killar LM, Stocker BA, Sultzer BM. Cellular immunity induced by avirulent Salmonella in LPS-defective C3H/HeJ mice. J Immunol. 1984;133:958–61. [PubMed] [Google Scholar]
- 28.Lee JC, Gibson CW, Eisenstein TK. Macrophage-mediated mitogenic suppression induced in mice of the C3H lineage by a vaccine strain of Salmonella typhimurium. Cell Immunol. 1985;91:75–91. doi: 10.1016/0008-8749(85)90033-4. [DOI] [PubMed] [Google Scholar]
- 29.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 30.Mittrucker H-W, Kohler A, Kaufmann SHE. Characterization of the murine T-lymphocyte response to Salmonella enterica serovar Typhimurium infection. Infect. Immun. 2002;70:199–203. doi: 10.1128/IAI.70.1.199-203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufmann SH. Immunity to intracellular bacteria. In: Paul WE, editor. Fundamental Immunology. Philadelphia: Lippincott-Raven; 2003. pp. 1229–61. [Google Scholar]
- 32.Mizuno Y, Takada H, Nomura A, Jin CH, Hattori H, Ihara K, Aoki T, Eguchi K, Hara T. Th1 and Th1-inducing cytokines in Salmonella infection. Clin Exp Immunol. 2003;131:111–7. doi: 10.1046/j.1365-2249.2003.02060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ugrinovic S, Menager N, Goh N, Mastroeni P. Characterization and development of T-Cell immune responses in B-cell-deficient (Igh-6(−/−)) mice with Salmonella enterica serovar Typhimurium infection. Infect Immun. 2003;71:6808–19. doi: 10.1128/IAI.71.12.6808-6819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lalmanach AC, Lantier F. Host cytokine response and resistance to Salmonella infection. Microbes Infect. 1999;1:719–26. doi: 10.1016/s1286-4579(99)80073-2. [DOI] [PubMed] [Google Scholar]
- 35.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–95. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 36.Beutler B, Cerami A. The biology of cachectin/TNF -- a primary mediator of the host response. Annual Review of Immunology. 1989;7:625–55. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 37.Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–71. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 38.Billiau A, Dijkmans R. Interferon-γ: mechanism of action and therapeutic potential. Biochem Pharmacol. 1990;40:1433–9. doi: 10.1016/0006-2952(90)90437-p. [DOI] [PubMed] [Google Scholar]
- 39.Heinzel FP. The role of IFN-gamma in the pathology of experimental endotoxemia. J Immunol. 1990;145:2920–4. [PubMed] [Google Scholar]
- 40.Kohler J, Heumann D, Garotta G, LeRoy D, Bailat S, Barras C, Baumgartner JD, Glauser MP. IFN-γ involvement in the severity of gram-negative infections in mice. J Immunol. 1993;151:916–21. [PubMed] [Google Scholar]
- 41.Le JM, Vilcek J. Interleukin 6: a multifunctional cytokine regulating immune reactions and the acute phase protein response. Lab Invest. 1989;61:588–602. [PubMed] [Google Scholar]
- 42.Diehl S, Anguita J, Hoffmeyer A, Zapton T, Ihle JN, Fikrig E, Rincon M. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity. 2000;13:805–15. doi: 10.1016/s1074-7613(00)00078-9. [DOI] [PubMed] [Google Scholar]
- 43.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–9. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cherwinski HM, Schumacher JH, Brown KD, Mosmann TR. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987;166:1229–44. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, Vitetta ES. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–8. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 46.Collins FM. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974;38:371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galdiero M, De Martino L, Marcatili A, Nuzzo I, Vitiello M, Cipollaro de l’Ero G. Th1 and Th2 cell involvement in immune response to Salmonella typhimurium porins. Immunology. 1998;94:5–13. doi: 10.1046/j.1365-2567.1998.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guilloteau L, Buzoni-Gatel D, Bernard F, Lantier I, Lantier F. Salmonella abortusovis infection in susceptible BALB/cby mice: importance of Lyt-2+ and L3T4+ T cells in acquired immunity and granuloma formation. Microb Pathog. 1993;14:45–55. doi: 10.1006/mpat.1993.1005. [DOI] [PubMed] [Google Scholar]
- 49.Mastroeni P, Villarreal-Ramos B, Hormaeche CE. Adoptive transfer of immunity to oral challenge with virulent salmonellae in innately susceptible BALB/c mice requires both immune serum and T cells. Infect Immun. 1993;61:3981–4. doi: 10.1128/iai.61.9.3981-3984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paul C, Shalala K, Warren R, Smith R. Adoptive transfer of murine host protection to salmonellosis with T-cell growth factor-dependent, Salmonella-specific T-cell lines. Infect Immun. 1985;48:40–3. doi: 10.1128/iai.48.1.40-43.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whittle BL, Verma NK. The immune response to a B-cell epitope delivered by Salmonella is enhanced by prior immunological experience. Vaccine. 1997;15:1737–40. doi: 10.1016/s0264-410x(97)00119-9. [DOI] [PubMed] [Google Scholar]
- 52.Coynault C, Norel F. Comparison of the abilities of Salmonella typhimurium rpoS, aroA and rpoS aroA strains to elicit humoral immune responses in BALB/c mice and to cause lethal infection in athymic BALB/c mice. Microb Pathog. 1999;26:299–305. doi: 10.1006/mpat.1999.0273. [DOI] [PubMed] [Google Scholar]
- 53.Brennan FR, Oliver JJ, Baird GD. Differences in the immune responses of mice and sheep to an aromatic-dependent mutant of Salmonella typhimurium. J Med Microbiol. 1994;41:20–8. doi: 10.1099/00222615-41-1-20. [DOI] [PubMed] [Google Scholar]
- 54.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–9. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 55.Mastroeni P, Chabalgoity JA, Dunstan SJ, Maskell DJ, Dougan G. Salmonella: immune responses and vaccines. Vet J. 2001;161:132–64. doi: 10.1053/tvjl.2000.0502. [DOI] [PubMed] [Google Scholar]
- 56.Roberts M, Chatfield SN, Dougan G. Salmonella as carriers of heterologous antigens. In: O’Hagan DT, editor. Novel delivery systems for oral vaccines. Boca Raton, FL: CRC Press, Inc; 1994. pp. 27–58. [Google Scholar]
- 57.Sigwart DF, Stocker BA, Clements JD. Effect of a purA mutation on efficacy of Salmonella live-vaccine vectors. Infect Immun. 1989;57:1858–61. doi: 10.1128/iai.57.6.1858-1861.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Callaghan D, Maskell D, Liew FY, Easmon CS, Dougan G. Characterization of aromatic-and purine-dependent Salmonella typhimurium: attention, persistence, and ability to induce protective immunity in BALB/c mice. Infect Immun. 1988;56:419–23. doi: 10.1128/iai.56.2.419-423.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Curtiss R, 3rd, Kelly SM. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987;55:3035–43. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galan JE, Curtiss R., 3rd Virulence and vaccine potential of phoP mutants of Salmonella typhimurium. Microb Pathog. 1989;6:433–43. doi: 10.1016/0882-4010(89)90085-5. [DOI] [PubMed] [Google Scholar]
- 61.Zhang X, Kelly SM, Bollen WS, Curtiss R., 3rd Characterization and immunogenicity of Salmonella typhimurium SL1344 and UK-1 Δcrp and Δcdt deletion mutants. Infect Immun. 1997;65:5381–7. doi: 10.1128/iai.65.12.5381-5387.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khan SA, Stratford R, Wu T, McKelvie N, Bellaby T, Hindle Z, Sinha KA, Eltze S, Mastroeni P, Pickard D, Dougan G, Chatfield SN, Brennan FR. Salmonella typhi and S typhimurium derivatives harbouring deletions in aromatic biosynthesis and Salmonella Pathogenicity Island-2 (SPI-2) genes as vaccines and vectors. Vaccine. 2003;21:538–48. doi: 10.1016/s0264-410x(02)00410-3. [DOI] [PubMed] [Google Scholar]
- 63.Hensel M, Shea JE, Waterman SR, Mundy R, Nikolaus T, Banks G, Vazquez-Torres A, Gleeson C, Fang FC, Holden DW. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol Microbiol. 1998;30:163–74. doi: 10.1046/j.1365-2958.1998.01047.x. [DOI] [PubMed] [Google Scholar]
- 64.Shea JE, Beuzon CR, Gleeson C, Mundy R, Holden DW. Influence of the Salmonella typhimurium pathogenicity island 2 type III secretion system on bacterial growth in the mouse. Infect Immun. 1999;67:213–9. doi: 10.1128/iai.67.1.213-219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shea JE, Hensel M, Gleeson C, Holden DW. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1996;93:2593–7. doi: 10.1073/pnas.93.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Angelakopoulos H, Hohmann EL. Pilot study of phoP/phoQ-deleted Salmonella enterica serovar Typhimurium expressing Helicobacter pylori urease in adult volunteers. Infect Immun. 2000;68:2135–41. doi: 10.1128/iai.68.4.2135-2141.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hindle Z, Chatfield SN, Phillimore J, Bentley M, Johnson J, Cosgrove CA, Ghaem-Maghami M, Sexton A, Khan M, Brennan FR, Everest P, Wu T, Pickard D, Holden DW, Dougan G, Griffin GE, House D, Santangelo JD, Khan SA, Shea JE, Feldman RG, Lewis DJ. Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type III secretion system (ssaV) mutations by immunization of healthy volunteers. Infect Immun. 2002;70:3457–67. doi: 10.1128/IAI.70.7.3457-3467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ravindran R, Rusch L, Itano A, Jenkins MK, McSorley SJ. CCR6-dependent recruitment of blood phagocytes is necessary for rapid CD4 T cell responses to local bacterial infection. Proc Natl Acad Sci U S A. 2007;104:12075–80. doi: 10.1073/pnas.0701363104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hanna EE, Hale M. Deregulation of mouse antibody-forming cells in vivo in cell culture by streptococcal pyrogenic exotoxin. Infect Immun. 1975;11:265–72. doi: 10.1128/iai.11.2.265-272.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Julius MH, Simpson E, Herzenberg LA. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973;3:645–9. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]