SUMMARY
The lateral geniculate nucleus and pulvinar are examples of two different types of relay: the former is a first order relay, transmitting information from a subcortical source (retina), while the latter is mostly a higher order relay, transmitting information from layer 5 of one cortical area to another cortical area. First and higher order thalamic relays can also be recognized for much of the rest of thalamus, and most of thalamus seems to be comprised of higher order relays. Higher order relays seem especially important to general corticocortical communication, and this challenges and extends the conventional view that such communication is based on direct corticocortical connections.
The thalamus has traditionally been thought of as a necessary link in the flow of information from the periphery to the cortex. However, once one accounts for the thalamic relays of peripheral sensory information (visual, auditory, and somatosensory) plus the various other forms of relayed information (e.g., from cerebellum and the mamillary body), the majority of thalamus remains unaccounted for. For instance, the pulvinar is generally thought to be a visual thalamic nucleus, because it innervates extrastriate visual areas, but what is its role? That is, if the lateral geniculate nucleus relays all retinal information to cortex, what more is there for the pulvinar to relay? The argument made here is that pulvinar, like most of the thalamus, is not chiefly involved in relaying peripheral information to cortex, but rather plays a heretofore unappreciated role as a key link in relaying information between cortical areas. To understand this, it is necessary to identify the information routes through thalamus and what sort of information the relevant thalamic afferents actually relay.
The answers to the questions raised above are far from clear and complete, but the purpose here is to frame the questions more clearly and suggest the form some of the answers may take. A starting point is a consideration of thalamic circuitry and the various types of afferents to relay cells. It is important here to distinguish the afferents that bring the information to be relayed, called the drivers, from the other inputs, known as the modulators [1;2]. We shall start with the lateral geniculate nucleus as a convenient model, because many of the main cell and circuit properties seen here are found throughout thalamus. One clear difference between various thalamic nuclei will be emphasized when we compare the lateral geniculate nucleus with the pulvinar.
Drivers and modulators
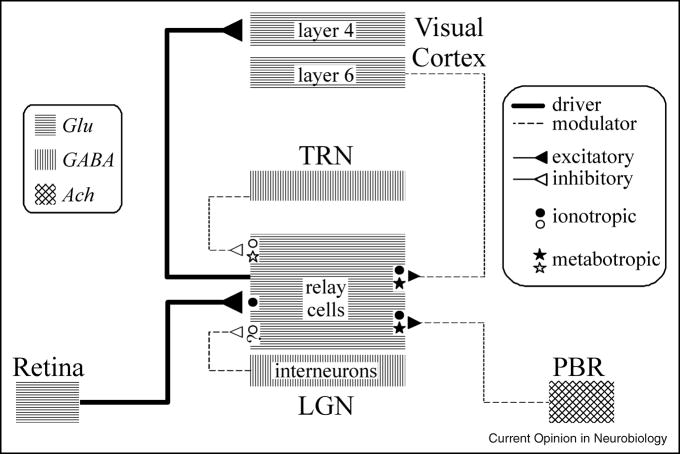
Figure 1 schematically shows the major inputs to geniculate relay cells. In addition to the glutamatergic retinal input, there are local GABAergic inputs (from interneurons and cells of the thalamic reticular nucleus), feedback glutamatergic inputs from layer 6 of cortex, and inputs from various brainstem sites, these mostly being cholinergic inputs from the parabrachial region. For further details of these and other inputs, see [1]).
Figure 1.
Schematic diagram of circuitry for the lateral geniculate nucleus. The inputs to relay cells are shown along with the relevant neurotransmitters and postsynaptic receptors (ionotropic and metabotropic) Abbreviations: ACh, acetylcholine; GABA, γ-aminobutyric acid; Glu, glutamate; LGN, lateral geniculate nucleus; PBR, parabrachial region; TRN, thalamic reticular nucleus.
These afferents all end on relay cell dendrites with conventional chemical synapses, meaning that their postsynaptic effects are dependent on postsynaptic receptors. These receptors come in two basic flavors: ionotropic and metabotropic. Ionotropic receptors include AMPA receptors for glutamate, GABAA receptors, and nicotinic receptors for acetylcholine; the respective metabotropic receptors, are various metabotropic glutamate receptors, GABAB receptors, and various muscarinic receptors. Ionotropic and metabotropic receptors differ along many parameters (for details, see [3;4]), but one important to the present account is duration of effect: postsynaptic potentials from ionotropic receptors tend to be very brief, over within 10 or a few 10s of msec, whereas those from metabotropic receptors last 100s of msec to several sec.
Figure 1 shows that retinal input activates only ionotropic receptors, whereas all nonretinal inputs activate metabotropic receptors as well. This is good for information transfer of the retinal input, because the fast excitatory postsynaptic potentials can be matched one to one to retinal spikes, thereby maximizing information transfer for higher firing rates in the input. The advantage for nonretinal inputs may be the lengthy postsynaptic effects associated with metabotropic receptor activation. For example, this can have relatively long term effects on excitability of relay cells. Probably more germane is the fact that these relay cells, like cells throughout the central nervous system, have many voltage- and time-gated ion channels [5], meaning that transmembrane ionic currents can flow when membrane potentials change sufficiently in amplitude and time. For instance, T-type Ca2+ channels determine the firing mode of relay cells—burst or tonic—and these have a time and voltage dependency well controlled by the combination of metabotropic receptors activated by nonretinal afferents to relay cells. That is, these T channels inactivate if held depolarized more positive than about −60 mV for ≥ 100 msec or so, but they de-inactivate if held more negative than about −70 mV for ≥100 msec or so, and once de-inactivated, they can be activated by a suitable depolarization, or EPSP. When these T channels become active, the relay cell responds in burst mode, and when they are inactive, the cell responds in tonic mode. These different response modes strongly affect the nature of information relayed [6;7]. The point here is that the nonretinal inputs, by virtue of their activation of metabotropic receptors, can effectively control the activation of voltage- and time-gated ion channels.
Postsynaptic receptors are only one feature that distinguish retinal from nonretinal input. Table 1 provides a more complete list of differences. From the pattern of differences, we have classified inputs to relay cells as driver or modulator. For the lateral geniculate nucleus, the driver input is the retinal input, and this represents the main information to be relayed. All the nonretinal inputs are modulators, and these serve to modulate retinogeniculate transmission. Other thalamic nuclei for which there is sufficient information have a similar classification of inputs to relay cells. For instance, the ventral posterior nucleus and the ventral portion of the medial geniculate nucleus have driver inputs from the medial lemniscus and inferior colliculus, respectively, and modulator inputs from most of the same sources as in Figure 1.
TABLE 1.
Drivers and Modulators in LGN plus layer 5 Drivers
| Criteria | Retinal (Driver) | Layer 5 to HO (Driver) | Modulator: layer 6 | Modulator: PBR | Modulator: TRN & Int |
|---|---|---|---|---|---|
| Criterion 1 | Determines relay cell receptive field | * Determines relay cell receptive field | Does not determine relay cell receptive field | Does not determine relay cell receptive field | Does not determine relay cell receptive field |
| Criterion 2 | Activates only ionotropic receptors | Activates only ionotropic receptors | Activates metabotropic receptors | Activates metabotropic receptors | TRN: Activates metabotropic receptors; Int: † |
| Criterion 3 | Large EPSPs | Large EPSPs | Small EPSPs | † | TRN: small IPSPs; Int: † |
| Criterion 4 | Large terminals on proximal dendrites | Large terminals on proximal dendrites | Small terminals on distal dendrites | Small terminals on proximal dendrites | Small terminals; TRN: distal; Int: proximal |
| Criterion 5 | Each terminal forms multiple contacts | Each terminal forms multiple contacts | Each terminal forms single contact | Each terminal forms single contact | Each terminal forms single contact |
| Criterion 6 | Little convergence onto target | * Little convergence onto target | Much convergence onto target | † | † |
| Criterion 7 | Very few synapses onto relay cells (~5%) | Very few synapses onto relay cells (~5%) | Many synapses onto relay cells (~30%) | Many synapses onto relay cells (~30%) | Many synapses onto relay cells (~30%) |
| Criterion 8 | Often thick axons | Often thick axons | Thin axons | Thin axons | Thin axons |
| Criterion 9 | Glutamatergic | Glutamatergic | Glutamatergic | Cholinergic | GABAergic |
| Criterion 10 | Synapses show paired-pulse depression (high p) | * Synapses show paired-pulse depression (low p) | Synapses show paired-pulse facilitation | † | † |
| Criterion 11 | Well localized, dense terminal arbors | Well localized, dense terminal arbors | Well localized, dense terminal arbors | Sparse terminal arbors | Well localized, dense terminal arbors |
| Criterion 12 | Branches innervate subtelencephalic targets | Branches innervate subtelencephalic targets | Subcortically known to innervate thalamus only | † | Subcortically known to innervate thalamus only |
| Criterion 13 | Innervates dorsal thalamus but not TRN | Innervates dorsal thalamus but not TRN | Innervates dorsal thalamus and TRN | Innervates dorsal thalamus and TRN | TRN: both; Int: dorsal thalamus only |
Very limited data to date
No relevant data available
The important point to make here is that not all inputs to relay cells are equal, and they should not be treated as some sort of anatomical democracy. More to the point, if one can identify the driver input to a thalamic nucleus, one can at least gain insight into the source and type of information relayed by that nucleus. Identifying the driver inputs to certain thalamic nuclei, like most of the pulvinar, has also led to a division of thalamic relays into first order and higher order.
First and higher order thalamic relays
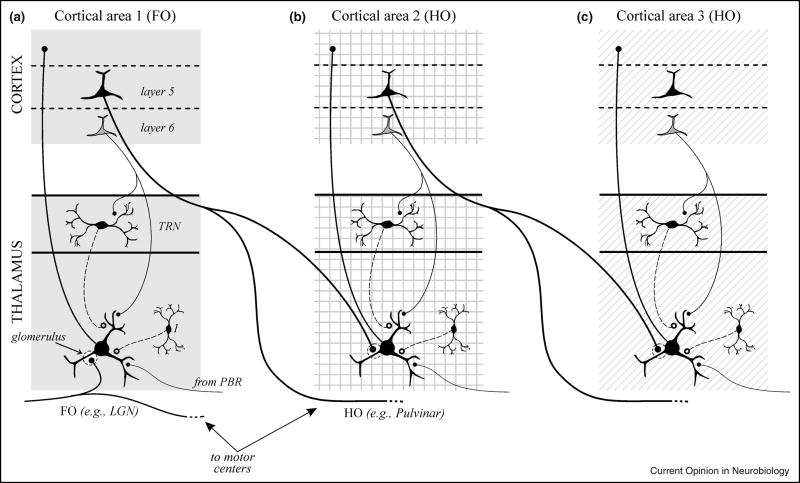
A consideration of driver input sources to different thalamic nuclei has led to their division into first and higher order types (Figure 2). All thalamic nuclei receive similar modulator inputs to those shown in Figure 1. However, the driver input to first order relays derives from a subcortical source, like the retina for the first order lateral geniculate nucleus, and this represents the first relay of a particular kind of information to cortex. As Figure 2 shows, the driver input to higher order relays derives from layer 5 of cortex itself [8;9], but unlike the layer 6 input, this layer 5 input is not a feedback projection [10] and instead is presumed to be feedforward. By this reasoning, these higher order relays can be viewed as an essential link in a cortico-thalamo-cortical circuit for information processing.
Figure 2.
Schematic diagrams showing organizational features of first and higher order thalamic nuclei. A first order nucleus (A) represents the first relay of a particular type of subcortical information to a first order or primary cortical area. A higher order nucleus (B, C) relays information from layer 5 of one cortical area to another cortical area. This relay can be from a primary area to a higher one (B) or between two higher order cortical areas (C). The important difference between them is the driver input, which is subcortical (A) for a first order thalamic nucleus and from layer 5 of cortex (B, C) for a higher order nucleus. Note that all thalamic nuclei receive an input from layer 6 of cortex, which is mostly feedback, but higher order nuclei in addition receive a layer 5 input from cortex, which is feedforward. Note in A–C that the driver inputs, both subcortical and from layer 5, are typically from branching axons, the significance of which is elaborated in the text. Abbreviations: FO, first order; HO, higher order; LGN, lateral geniculate nucleus; TRN, thalamic reticular nucleus. Redrawn from [23].
An important proviso is that, while first order relays like the lateral geniculate nucleus appear to be purely first order, many higher order nuclei may have mixed first and higher order circuits. For instance, while most of the pulvinar receives driver input from layer 5, part of it receives an input from the midbrain that appears to have the anatomical characteristics of a driver [11]. We need much more data to sort out the details of possible first and higher order circuits in thalamic nuclei, and for this reason, we use the terminology below of higher order relay rather than higher order nucleus.
In any case, evidence to support this hypothesis that higher order relays are an important link in a cortico-thalamo-cortical information route—and it is a hypothesis that remains to be fully tested—is summarized in Table 1. The main support for this is that the synaptic properties of the layer 5 thalamocortical synapses and all thalamocortical synapses, including those from higher order relays, have the signature of driver synapses. It is further interesting that, while layer 4 cells receive thalamic input with driver characteristics, this same population receives input from layer 6 cells that have the same modulator characteristics as do thalamocortical synapses from layer 6 (C.C. Lee and S.M. Sherman, unpublished). Synaptic numbers match up here as well: only about 5% of the synapses to geniculate relay cells are from retina [12], and they, being the driver input, determine the basic receptive field properties of relay cells; likewise, only about 6% of the synapses onto layer 4 cells in visual cortex derive from geniculocortical afferents [13], which impart the basic receptive field properties, such as orientation selectivity, to their layer 4 targets [14;15]. This last point has the further implication that the driver/modulator classification that works so well for inputs to relay cells may be extended outside thalamus, and specifically to cortical circuitry.
The main conclusion here is that higher order thalamic relays represent part of a cortico-thalamo-cortical route of corticocortical communication. If so, what of the large direct corticocortical projections? These have been used as the basis for determining a hierarchical relationship for the various visual cortical areas in the monkey [16]. However, if the driver/modulator classification has any validity for cortical circuits, then the functional significance of these direct corticocortical pathways must be reconsidered, because they are based exclusively, or almost so, on anatomical identification. The assumption that all are information bearing may be wrong. Thus it may be important to identify which among them have driver characteristics and then reconsider the cortical hierarchy based on the identity of these driver pathways. To put this point in the strongest relief, consider the possibility that all direct corticocortical pathways are modulatory, which would mean that information routes between cortical areas depend on higher order thalamic relays. This would imply that all information reaching a cortical area, whether originating in the periphery (e.g., retina) or another cortical area, must pass through the thalamus. In other words, just as retinal information is relayed by thalamus, so is corticocortical information. Another, perhaps more likely, suggestion is that a subset of direct corticocortical pathways are drivers, and these transmit information between cortical areas in parallel with cortico-thalamo-cortical pathways involving higher order thalamic nuclei.
Nature of information carried by driver afferents to thalamus
The notion that direct corticocortical and cortico-thalamo-cortical pathways convey information in parallel implies that there are important functional differences between these routes. One difference may be related to the fact that, with few exceptions, direct corticocortical projections are strictly cortical, meaning that the axons involved have no branches to subcortical targets, whereas, as indicated in Figure 2, many and perhaps all driver afferents to thalamus have branches innervating numerous extrathalamic, subcortical sites. For instance, many or all retinogeniculate axons branch to also innervate the midbrain [17], and most or all layer 5 axons that innervate thalamus also innervate sites in the midbrain, pons, and even spinal cord [18;19]. Thus one important difference between these routes of information transfer in cortex is that the route involving higher order thalamic relays involves information that is shared with additional subcortical sites.
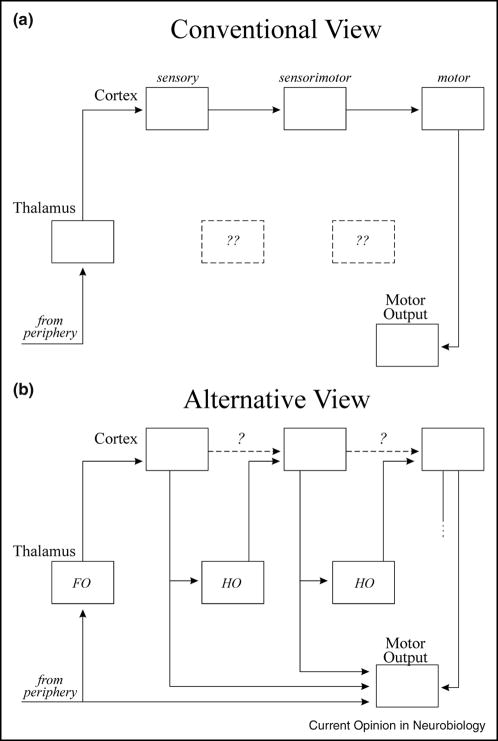
It is also interesting that the extrathalamic targets of drivers to thalamus seem to be involved in motor control. That is, the midbrain targets of retinogeniculate axons are implicated in eye movements, pupillary control, etc., and the targets of layer 5 corticothalamic axons are also involved in eye movements as well as various head and body movements. Guillery [20;21] has suggested from such observations that the information relayed through thalamus to cortex may be a efference copy of motor commands to keep higher cortical areas informed of such commands sent from lower areas. This idea and its departure from conventional thinking of the organization of cortical processing is underscored by Figure 3. Thus, Figure 3A shows the conventional scheme whereby input comes into cortex from thalamus and is relayed through various cortical areas via direct connections, starting with sensory areas through to sensorimotor areas and ending in motor areas, and having a distinct input (at primary sensory cortex) and output (from motor cortex). This scheme has no use for most of thalamus that we have identified as higher order. In contrast, Figure 3B shows a scheme whereby right from the beginning a first order thalamic relay passes on information about a presumably crude motor command. Further cortical processing upgrades these commands, and these upgrades are passed onto higher cortical areas via higher order thalamic relays. The scheme in Figure 3B has no one input to or output from cortex, but instead, these are reflected by all areas. In this regard, it is important to note that all cortical areas for which sufficient information is available has a layer 5 output to subcortical motor structures. For example, this is true for primary visual cortex, and electrical stimulation here produces eye movements [22]. In this regard, it seems inappropriate to refer to any cortical area as primarily or essentially sensory.
Figure 3.
Comparison of conventional view (A) with the alternative view proposed here (B). The role of the direct corticocortical connections in B (dashed lines) is questioned (see text for details). Abbreviations: FO, first order; HO, higher order. Reproduced from [23].
This difference in the nature of direct corticocortical versus cortico-thalamo-cortical pathways, namely that the latter reflects information regarding motor command, offers a possible rationale for parallel processing of information between cortical areas. That is, the direct pathways may carry the basic information needed to analyze the relevant information, such as information about the visual scene analyzed by the various visual cortical areas, whereas the cortico-thalamo-cortical pathways are used to update target cortical areas about motor commands. For instance, for the visual system, it is important as visual information is analyzed to distinguish between movements in the visual environment and those induced by self movement of the head or eyes. Nonetheless, the fact remains that we still know less about the nature of direct corticocortical connections, meaning that the (slim) possibility exists that all are modulators, and perhaps the vast bulk of information is carried between cortical areas via higher order thalamic relays.
Finally, this idea that information relayed through thalamus to cortex is a copy of motor commands also suggests that our perceptual experiences are built on these commands. Details of this additional hypothesis are beyond the scope of this account but can be found in Guillery [20;21]. Whatever the interpretation, the anatomical fact that many or all inputs relayed by thalamus are branches of axons that also target motor structures requires some further consideration.
Acknowledgments
Supported by NIH Grant EY03038
The authors laboratory has been supported by grants from the USPHS: EY03038 and DC008794.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- 1.Sherman SM, Guillery RW. Exploring the Thalamus and its Role in Cortical Function. 2. Cambridge, MA: MIT Press; 2006. [Google Scholar]
- 2.Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: Distinguishing “drivers” from “modulators”. Proc Natl Acad Sci USA. 1998;95:7121–7126. doi: 10.1073/pnas.95.12.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annual Review of Pharmacology & Toxicology. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 4.Nicoll RA, Malenka RC, Kauer JA. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990;70:513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- 5.Huguenard JR, McCormick DA. Electrophysiology of the Neuron. New York: Oxford University Press; 1994. [Google Scholar]
- 6.Swadlow HA, Gusev AG, Bezdudnaya T. Activation of a cortical column by a thalamocortical impulse. J Neurosci. 2002;22:7766–7773. doi: 10.1523/JNEUROSCI.22-17-07766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherman SM. Dual response modes in lateral geniculate neurons: mechanisms and functions. Visual Neurosci. 1996;13:205–213. doi: 10.1017/s0952523800007446. [DOI] [PubMed] [Google Scholar]
- 8.Reichova I, Sherman SM. Somatosensory corticothalamic projections: Distinguishing drivers from modulators. J Neurophysiol. 2004;92:2185–2197. doi: 10.1152/jn.00322.2004. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Guido W, Bickford ME. Two distinct types of corticothalamic EPSPs and their contribution to short-term synaptic plasticity. J Neurophysiol. 2003;90:3429–3440. doi: 10.1152/jn.00456.2003. [DOI] [PubMed] [Google Scholar]
- 10.Van Horn SC, Sherman SM. Differences in projection patterns between large and small corticothalamic terminals. J Comp Neurol. 2004;475:406–415. doi: 10.1002/cne.20187. [DOI] [PubMed] [Google Scholar]
- 11.Kelly LR, Li J, Carden WB, Bickford ME. Ultrastructure and synaptic targets of tectothalamic terminals in the cat lateral posterior nucleus. J Comp Neurol. 2003;464:472–486. doi: 10.1002/cne.10800. [DOI] [PubMed] [Google Scholar]
- 12.Van Horn SC, Erişir A, Sherman SM. The relative distribution of synapses in the A-laminae of the lateral geniculate nucleus of the cat. J Comp Neurol. 2000;416:509–520. [PubMed] [Google Scholar]
- 13.Ahmed B, Anderson JC, Martin KAC, Nelson JC. Map of the synapses onto layer 4 basket cells of the primary visual cortex of the cat. J Comp Neurol. 1997;380:230–242. [PubMed] [Google Scholar]
- 14.Ferster D. Assembly of receptive fields in primary visual cortex. In: Chalupa LM, Werner JS, editors. In The Visual Neurosciences. Cambridge MA: MIT Press; 2004. pp. 695–703. [Google Scholar]
- 15.Alonso JM, Usrey WM, Reid RC. Rules of connectivity between geniculate cells and simple cells in cat primary visual cortex. J Neurosci. 2001;21:4002–4015. doi: 10.1523/JNEUROSCI.21-11-04002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Essen DC, Anderson CH, Felleman DJ. Information processing in the primate visual system: an integrated systems perspective. Science. 1992;255:419–423. doi: 10.1126/science.1734518. [DOI] [PubMed] [Google Scholar]
- 17.Tamamaki N, Uhlrich DJ, Sherman SM. Morphology of physiologically identified retinal X and Y axons in the cat’s thalamus and midbrain as revealed by intra-axonal injection of biocytin. J Comp Neurol. 1994;354:583–607. doi: 10.1002/cne.903540408. [DOI] [PubMed] [Google Scholar]
- 18.Bourassa J, Pinault D, Deschênes M. Corticothalamic projections from the cortical barrel field to the somatosensory thalamus in rats: A single-fibre study using biocytin as an anterograde tracer. Eur J Neurosci. 1995;7:19–30. doi: 10.1111/j.1460-9568.1995.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 19.Bourassa J, Deschênes M. Corticothalamic projections from the primary visual cortex in rats: A single fiber study using biocytin as an anterograde tracer. Neurosci. 1995;66:253–263. doi: 10.1016/0306-4522(95)00009-8. [DOI] [PubMed] [Google Scholar]
- 20.Guillery RW. Anatomical pathways that link action to perception. Prog Brain Res. 2005;149:235–256. doi: 10.1016/S0079-6123(05)49017-2. [DOI] [PubMed] [Google Scholar]
- 21.Guillery RW. Branching thalamic afferents link action and perception. J Neurophysiol. 2003;90:539–548. doi: 10.1152/jn.00337.2003. [DOI] [PubMed] [Google Scholar]
- 22.Tehovnik EJ, Slocum WM, Schiller PH. Saccadic eye movements evoked by microstimulation of striate cortex. Eur J Neurosci. 2003;17:870–878. doi: 10.1046/j.1460-9568.2003.02489.x. [DOI] [PubMed] [Google Scholar]
- 23.Sherman SM. Thalamic relays and cortical functioning. Prog Brain Res. 2005;149:107–126. doi: 10.1016/S0079-6123(05)49009-3. [DOI] [PubMed] [Google Scholar]