Abstract
Background
Most clinical studies have used autologous bone marrow (BM) stem cells for myocardial regeneration in elderly patients. We hypothesize that aging impairs the survival and differentiation potential of BM stem cells thus limiting their therapeutic efficacy.
Methods and Results
BM derived MSCs from young (YngMSCs; 8–12 weeks) and old (oldMSCs; 24–26 weeks) rats were purified and assessed for their responsiveness to anoxia and reparability of infarcted heart. Higher expression of angiogenic growth factors was observed by YngMSCs under anoxia as compared to oldMSCs, cultured either alone or in co-culture (Co-oldMSCs) with YngMSCs. Likewise, YngMSCs were more tolerant to apoptotic stimuli and showed higher ability to form tubular structures during in vitro Matrigel assay as compared to oldMSCs and Co-oldMSCs with a possible role of p21 and p27 as contributory survival factors. For in vivo studies, acute myocardial infarction model was developed in Fisher-344 rats (n= 38). The animals were grouped to receive 70 µl basal DMEM without cells (group-1) or containing 2×106 YngMSCs (PKH67 labeled; group-2), or oldMSCs (PKH26 labeled; group-3) and mixture of YngMSCs + oldMSCs (1×106 cells each; group-4). Histological studies revealed that by day 7, YngMSCs showed elongated morphology with orientation similar to the host muscle architecture. Electron microscopy and confocal imaging after fluorescent immunostaining showed superior angiomyogenic potential of YngMSCs. Echocardiography showed significantly preserved heart function indices in the animals transplanted with YngMSCs.
Conclusions
Aging impairs the responsiveness of oldMSCs to anoxia and their differentiation potential. YngMSCs fail to alter the survival of oldMSCs under in vitro as well as in vivo conditions. It is therefore concluded that transplantation of stem cells from young donors would be a better option for heart cell therapy in future clinical studies.
Keywords: Aging, angiogenesis, myocardium, myogenesis, myocardial infarction, Stem cells, Transplantation
Introduction
Bone marrow derived mesenchymal stem cells (MSCs) differentiate into specialized tissues including cardiomyocytes, endothelial cells and smooth muscle cells [1–3]. Indeed, their demonstrated multi-lineage differentiation potential, lower immunoreactivity and availability from autologous source make these cells superior candidates for heart cell therapy [4,5]. In models of myocardial infarction, transplantation of MSCs improve cardiac function through myocyte regeneration, scar area reduction, and sympathetic nerve regeneration [6–8]. At least a part of this improved cardiac function has also been attributed to the angiogenic potential of MSCs ensued by paracrine effects via release of a broad spectrum of growth factors and cytokines [9–11]. The released bioactive molecules provide a microenvironment that is supportive for cell survival, inhibit scarring and apoptosis, and stimulate host progenitors to divide and differentiate into functional units [12,13]. Nevertheless, most of these studies used MSCs from young animals [14].
Like other cells of an organism, MSCs are also subject to aging effects associated with decline in function and an imbalance between cell loss and cell renewal [15]. Aging of MSCs is characterized by reduction in the number of small colony forming cells, loss of multi-potentiality, attainment of comparatively large and mature appearance, and propensity of slowly replicating cells [16]. Changes in the telomere length have also been observed in the aging MSCs [17]. MSCs from young (YngMSCs) and old (oldMSCs) donors may also differ in their response to stress stimuli [18]. Stimulation of YngMSCs by transient exposure to hypoxia modulate their angiogenic potential via overexpression of angiogenic growth factors [19]. The aim of our present study was to investigate the effect of MSCs aging for their response to normoxia and anoxia in terms of their survival, and ability to secrete angiogenic growth factors. Besides in vitro studies, the study also involved simultaneous administration of YngMSCs and oldMSCs in the same heart in order to assess their in vivo behavior and reparability of the injured myocardium. A novel aspect of our study is simultaneous engraftment of YngMSCs and oldMSCs in the same heart to see if the presence of YngMSCs can somehow influence the reparative potential of oldMSCs. The study results gave insight into the basic mechanism of the role of senescent MSCs either alone or after possible physiological transformation, if any, by their younger counterparts in the myocardial repair process.
Materials and Methods
Purification of bone marrow MSCs
MSCs were isolated and purified from bone marrow obtained from young (2–3 months) and old (24–26 months) male Fischer-344 rats as described earlier [20]. The adherent, spindle-shaped MSCs were expanded and cultured for no more than 4–5 passages before transplantation.
In vitro characterization of MSCs for cytokine and growth factor expression
YngMSCs and oldMSCs were suspended and seeded separately (in triplicate) at a density of 1×105 cells/ml in Dulbecco’s modified Eagle medium (DMEM) supplemented with 15% fetal bovine serum (FBS) in 6-well cell culture plates. For co-culture experiments, 1× 105 oldMSCs per well were seeded in 6 wells with 1×105 YngMSCs in the transwells. After co-culture for 24 h, the YngMSCs containing transwells were removed and the oldMSCs remaining in the wells were used as the co-cultured old MSCs (Co-oldMSCs). YngMSCs, oldMSCs and Co-oldMSCs were incubated under normoxia or anoxia (Forma Scientific Corp. USA) for 6 h at 37°C in 0.2% FBS. The cells and their supernatant from each of the group were harvested either immediately or after overnight cultivation (re-oxygenation) for assessment of cytokine and growth factor expression by real-time polymerase chain reaction (real-time PCR), enzyme linked immunosorbent assay (ELISA) and Western blotting.
Real-time PCR
Isolation of total RNA from the different groups of MSCs, and their subsequent first-strand cDNA synthesis, was performed using an RNeasy mini kit (Qiagen) and an Omniscript Reverse Transcription kit (Qiagen), respectively, per the instructions of manufacturer. Real-time PCR was performed using iQ SYBR-Green supermix (Bio-Rad) in a Bio-Rad iQ5 optical module. The final concentration of MgCl2 used in the PCR mix was 3.5 mM. The cycling conditions were set at 3 min at 95°C for initial denaturation, 40 cycles of denaturation at 95°C for 30 sec, annealing at 59.5°C for 40 sec and extension at 72°C for 50 sec. The data was acquired during the extension step. Melting curves were obtained at the end of the reaction by gradually raising the temperature by 1°C/min from 59.5°C to 95°C over a time period of 35 min. The primer sequences used have been shown in Table-1.
Table-1.
Sequence of the primers used in the study.
| Gene Symbol | Primer sequence | Gene ID | Product size |
|---|---|---|---|
| HIF-1α | 5`tcaagtcagcaacgtggaag 3` | NM_024359 | 198 |
| 5`tatcgaggctgtgtcgactg 3` | |||
| HO-1 | 5`cacgcatatacccgctacct 3` | NM_012580 | 227 |
| 5`aaggcggtcttagcctcttc 3` | |||
| VEGF-A | 5`caatgatgaagccctggagt 3` | NM_031836 | 211 |
| 5`tttcttgcgctttcgttttt 3` | |||
| Ang-1 | 5`cagcacaaaggacgctgata 3` | NM_053546 | 232 |
| 5`atagcgccttcagaagtcca 3` | |||
| FGF-2 | 5`ccagttggtatgtggcactg 3’ | NM_019305 | 225 |
| 5`cagggaagggtttgacaaga 3’ |
Western blot studies
Cells were lysed with RIPA buffer (150 mM sodium chloride, 0.5 % deoxycholic acid, 1 % Nonidet P40, 0.1 % SDS, 50 mM Tris hydrochloride pH 7.5 and 2 mM freshly prepared PMSF) and centrifuged at 14000 rpm for 30 min at 4°C to remove cell debris. Protein concentration in the supernatant was measured using the Bio-Rad DC Protein Assay kit using the Shimadzu UV-spectrophotometer (model: BIOSPEC-1601). Equal amounts of protein samples (40–100 µg) were fractionated on 4–12% pre-cast polyacrylamide gel (Invitrogen Corp., USA) and electroblotted on to Immun-Blot PVDF membrane (Bio-Rad) as described earlier [21]. Finally, the membrane was incubated in sufficient SuperSignal West Femto Maximum Sensitivity Substrate (Pierce, USA) and exposed to Kodak Scientific Imaging Film for detection of the signals. The antibodies used included; anti hypoxia inducible factor (HIF-1α) (Abcam Inc., USA, 1:500), anti hemeoxygenase-1 (HO-1) (Abcam Inc., USA, 1:500) and detected by using Pierce ImmunoPure Peroxidase Conjugated goat anti-rabbit IgG (H+L) (1:2000).
ELISA
VEGF concentration was measured in the 24h conditioned medium obtained from YngMSCs, oldMSCs and Co-oldMSCs, each grown under normoxia, 6h anoxia or anoxia-re-oxygenation (6h anoxia followed by 24h re-oxygenation) using a rat VEGF ELISA kit per the instructions of manufacturer (R&D Systems, USA).
Cell viability assay and LDH release
YngMSCs, oldMSCs and Co-oldMSCs were plated and grown in 10% DMEM containing 10% FBS for 24 h before the experiment in 35 mm dishes (1×105 cells per dish). The serum containing medium was replaced with glucose and serum free DMEM and subjected to anoxia (5% CO2/95% N2) for 8h in anoxia chamber (Forma Scientific Corp. USA). The cells were later suspended using trypsin and mixed with 0.4% trypan blue solution (Sigma Chemical, USA). The samples of cell supernatant were removed for measurement of LDH leakage using LDH assay kit (Diagnostic Chemicals Ltd. USA). The experiments were performed in triplicate during 3 independent experiments. Percentage viability in each group of cells was evaluated by dye exclusion method using Trypan blue (Sigma, USA).
In vitro apoptosis assay
Terminal dUTP nick-end labeling (TUNEL) assay was performed in different groups of MSCs to evaluate apoptosis induced by glucose and serum starvation under anoxia. After 24h incubation, the cells were fixed in 1% paraformaldehyde. TUNEL was performed using In-Situ Cell Death Detection kit (Roche Inc., USA) per manufacturer’s instructions for the detection of apoptotic nuclei. The cells were then mounted in the medium containing 4′,6-diamidino-2-phenylindole (DAPI). Randomly selected microscopic fields (n=8) were evaluated to calculate the ratio of TUNEL-positive cells to the total number of cells in three independent experiments.
In vitro tube formation assay
The assay was performed on Matrigel coated 96-well plates (Becton Dickinson) to investigate whether conditioned medium from YngMSCs, oldMSCs and Co-oldMSCs induced tube formation. Human umbilical vein endothelial cells (HUVEC) were seeded (1×104 cells per well) on 96-well Matrigel plates with 200 µl fresh DMEM supplemented with 10% FBS as a control or conditioned DMEM collected from YngMSCs, oldMSCs and Co-oldMSCs grown under normoxia, or immediately after 6h anoxia or after 24 h re-oxygenation following 6h anoxia. The formation of tubular structures in the respective wells was examined at 6h and 16h after incubation of cells at 37°C in 5% CO2 with a phase-contrast microscope (BX41-Olympus, Japan).
In vivo studies
Labeling of donor cells
For identification and tracking the fate of the engrafted cells, YngMSCs and oldMSCs were labeled with PKH67 (green fluorescence) and PKH26 (red fluorescence) cell tracker dyes respectively using their respective Fluorescent Cell Linker Kits as per instructions of the manufacturer (Sigma, USA).
Rat model of myocardial infarction and cell engraftment
The present study conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (H Publication No. 85-23, revised 1985) and protocol approved by the Institutional Animal Care and Use Committee, University of Cincinnati.
A model of acute myocardial infarction was established in young female Fischer-344 rats (n=38) each weighing 180–200g as described previously [20]. Immediately after left anterior descending coronary artery ligation, the animals were grouped to receive intra-myocardial injections of 70 µl of basal DMEM without cells (group-1; n=8) or containing 2×106 YngMSCs (group-2; n=8), or oldMSCs (group-3; n=8) and Yng+oldMSCs (1×106 each of young and old MSCs; group-4; n=14). Each animal received multiple injections (n=4 on average) in the centre and border zone of the infarct. The chest was closed and the animals were allowed to recover. The animals were maintained on Buprinex after surgery for 24 h to alleviate pain.
Physiologic assessment of heart function
Transthoracic echocardiography was performed to study change in the heart function at 4 weeks after respective treatment using Compact Linear Array probe CL10-5 on HDI-5000 SONOS CT (HP) as described earlier [21]. Anterior and posterior end-diastolic and end-systolic wall thickness and left ventricle (LV) internal dimensions; LV end-systolic (LVESD) and end-diastolic (LVEDD) diameters were measured from at least three consecutive cardiac cycles. Indices of LV systolic functions including LV fractional shortening (LVFS) and LV ejection fraction (LVEF) were calculated using LVFS= (LVEDD-LVESD)/LVEDd × 100 and LVEF= [(LVEDD3-LVESD3)/ LVEDD3] ×100 relations respectively and the results were expressed as percentage.
Histochemical and Immunohistochemical studies
For measurement of infarction size and area of fibrosis, the heart was arrested in diastole by intravenous injection of cadmium chloride and fixed in 10% buffered formalin. The heart was then excised, cut transversely, and embedded in paraffin. Histological sections of 6 µm thickness were cut and used for hematoxylin-eosin and Mason’s trichome staining for visualization of muscle architecture and area of fibrosis. Infarct size was defined as sum of the epicardial and endocardial infarct circumference divided by sum of the total LV epicardial and endocardial circumferences using computer-based planimetry with Image-J analysis software (version 1.6065; NIH).
Blood vessel density was assessed as previously described [21]. Briefly, cryosections (6 µm thick) were immunostained using vonWillebrand Factor-VIII (vWFactor-VIII) specific primary antibody (1:50; Dako, Denmark) and detected with fluorescently labeled secondary antibody (Molecular Probes). The number of blood vessels positive for vWFactor-VIII were counted in both infarct and peri-infarct regions. At least 50 microscopic fields each in infarct and peri-infarct regions were randomly selected and counted in each treatment group. Blood vessel density was expressed as the number of vessels per microscopic surface area (0.74 mm2) at 200× magnification.
Ultra-structure studies
After measurement of contractile function, tissue strips was immersed in 2% glutaraldehyde and dissected into small cubes <1 mm. The tissue was fixed overnight, post-fixed for 1 h in 1% osmium tetroxide at room temperature, en-bloc stained with uranyl acetate and dehydrated through a graded alcohol series followed by acetone and embedded in Spurr medium. Semi-thick sections were stained with toluidine blue and examined by light microscopy. Thin sections (600 A in thicknesses) were cut from selected blocks, stained with uranyl acetate and counterstained with lead citrate, and examined in Hitachi H-600 transmission electron microscope.
Statistical analysis
All data were described as mean ±SEM. To analyze the data statistically, we performed Student’s t-test and one-way ANOVA with post hoc analysis and a value of P<0.05 was considered statistically significant.
Results
In vitro studies
Compared to the more elongated and spindle-shape morphology of YngMSCs, oldMSCs showed more spread-out and flattened appearance and larger size. oldMSCs were slower in adherence with the plastic surface in vitro culture conditions. Even when they were attached, they maintained their poorly expanded, round appearance for until 4–5 days after isolation and were slower to expand. The yield of oldMSCs from bone marrow was lower. We observed substantial proliferative loss with aging which was evident from their higher doubling time as compared with their younger counterparts and were less clonogenic in nature. Co-culture of oldMSCs with their younger counterparts did not change their culture characteristics.
LDH analysis and TUNEL Assay
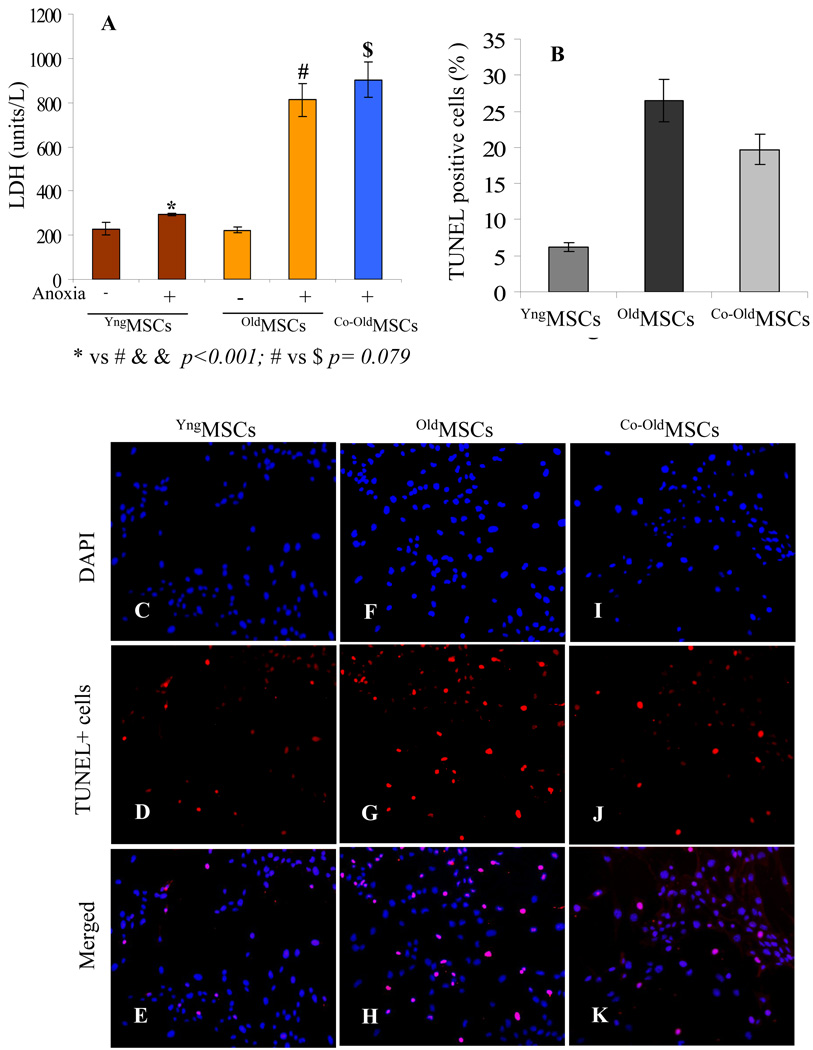
After 8h incubation under glucose and serum-free anoxia conditions, YngMSCs, oldMSCs and Co-oldMSCs revealed morphological changes evident from cytoplasmic shrinkage. However, these morphological changes were markedly less pronounced in YngMSCs. LDH analysis revealed significantly low levels of LDH release from YngMSCs as compared with oldMSCs and Co-oldMSCs (Figure 1A). TUNEL staining showed that glucose and serum free culture conditions under anoxia markedly induced cell apoptosis in oldMSCs and Co-oldMSCs as compared to the YngMSCs (Figure 1B–K). The percentage of TUNEL+ cells was significantly lower in YngMSCs as compared with oldMSCs and Co-oldMSCs (YngMSCs vs all other groups p<0.05). On the contrary, although the percentage of TUNEL+ cells was lower in Co-oldMSCs as compared with oldMSCs, the difference was only insignificant (Co-oldMSCs vs oldMSCs p>0.068).
Figure 1.
(A) LDH release from YngMSCs, oldMSCs and Co-oldMSCs as an indicator of cellular injury. YngMSCs were highly resistant to 8h anoxia as was indicated by the significantly low level LDH release as compared with the oldMSCs. oldMSCs in the presence of their younger counterparts (Co-oldMSCs) failed to show improved resistance to anoxia. YngMSCs and oldMSCs without exposure to anoxia were used as controls. (B–K) TUNEL assay on YngMSCs, oldMSCs and Co-oldMSCs after exposure to 8h anoxia showed that the number of TUNEL positive cells was significantly less in YngMSCs (C–E) as compared to oldMSCs (F–H) and Co-oldMSCs (I–K).
Angiogenic growth factor expression
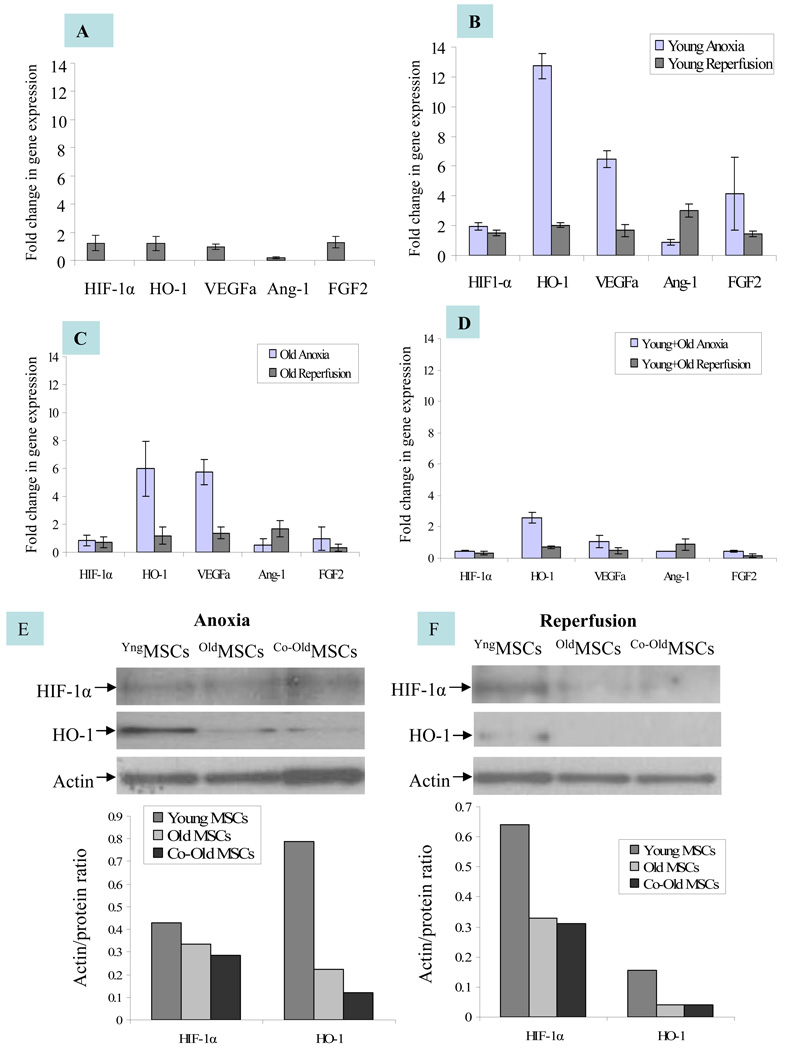
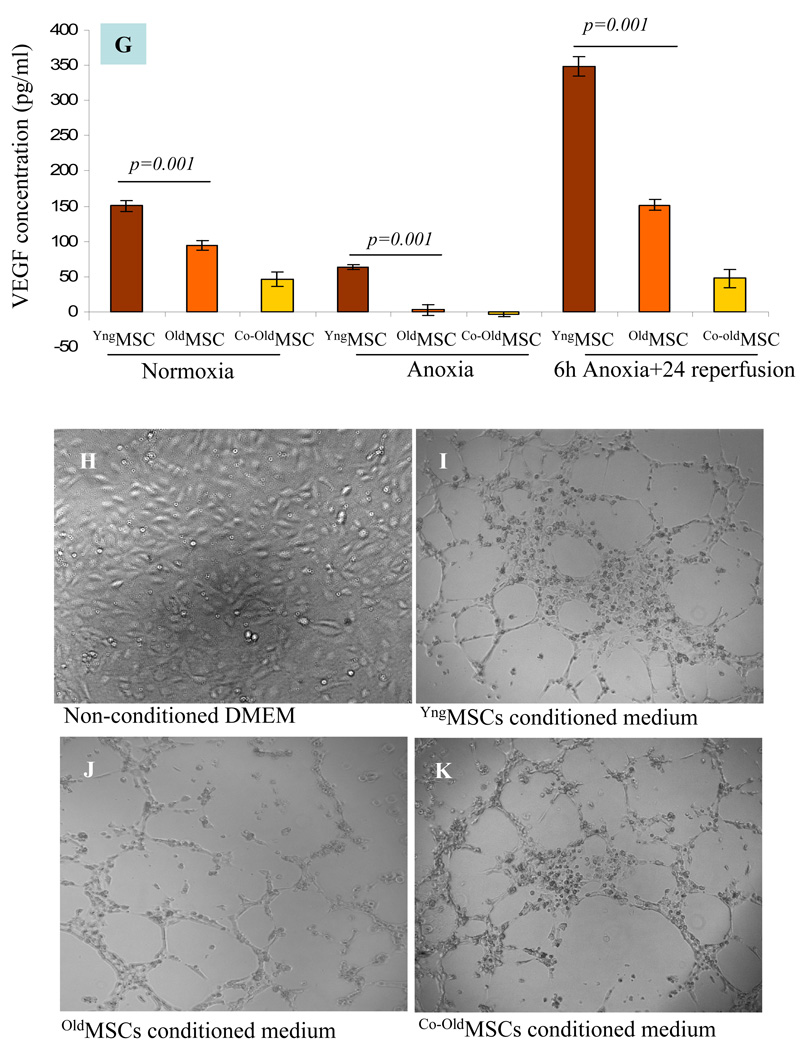
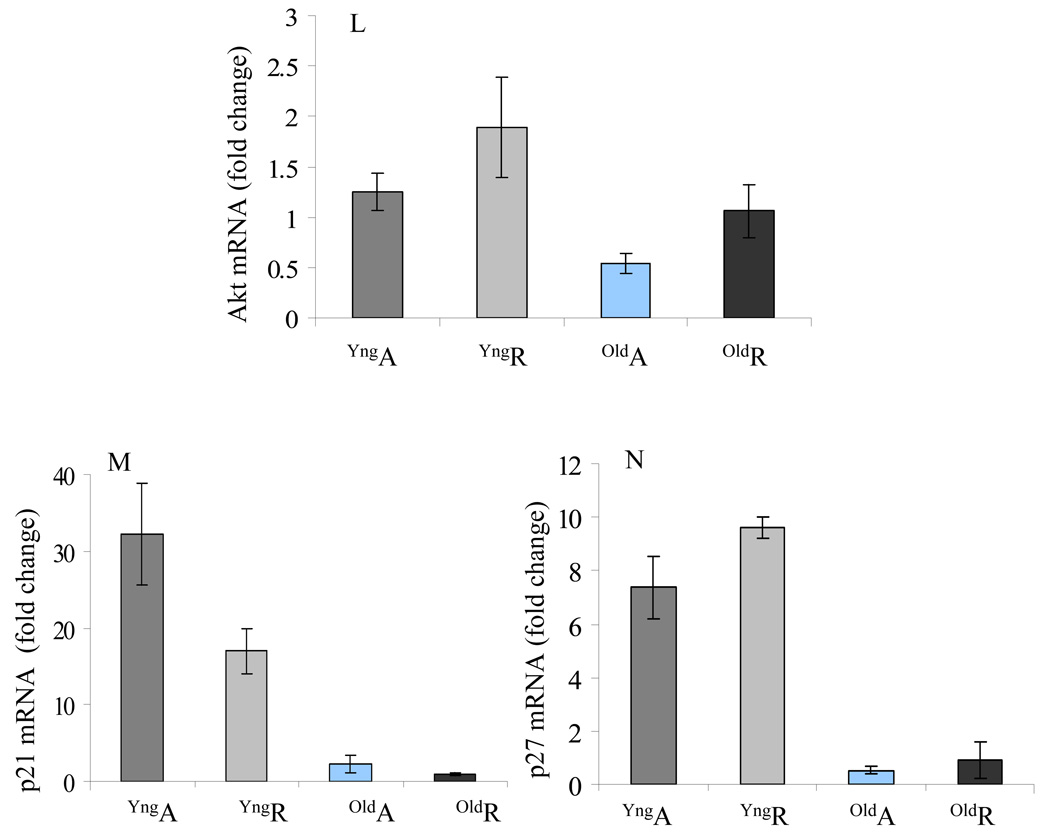
The expression of angiogenic growth factors was observed from different groups of MSCs under different set of culture conditions. Real time-PCR results showed that the expression of the angiogenic growth factors under normoxia was insignificantly higher in oldMSCs as compared with YngMSCs (Figure 2A). When subjected to anoxia, mRNA expression of HIF-1α (2 fold), HO-1 (>13 fold), VEGF (Ϧ fold), and FGF-2 (>4 fold) was significantly increased in the YngMSCs as compared with its normoxia levels (Figure 2B). On the contrary, oldMSCs showed decreased expression of HIF-1α but HO-1 and VEGF levels increased by ~6 fold each, as compared to their normoxia levels whereas the expression of Ang-1, and FGF-2 was abolished (Figure 2C). YngMSCs continued to over express angiogenic growth factors during re-oxygenation phase 24h after exposure to anoxia (including Ang-1 expression which did not show change during anoxia), the re-oxygenation phase expression of all the growth factors under study was significantly reduced or remained unchanged in case of oldMSCs. In short, YngMSCs emerged as better responders to anoxia and continued with their response during re-oxygenation phase until 24h of observation in comparison to oldMSCs. The presence of YngMSCs in co-culture with oldMSCs failed to induce improvement in their response to anoxia. Instead, the expression of growth factors from oldMSCs in the presence of YngMSCs was significantly suppressed (Figure 2D). Consistent with these results, protein level expression of the growth factors by Western blot followed the trend similar to mRNA expression under different set of culture conditions (Figure 2E–F). VEGF secretion was significantly decreased under anoxia as compared to normoxia and significantly increased during re-oxygenation phase in all the cell groups. However, VEGF protein expression was highest in the YngMSCs during the re-oxygenation phase subsequent to anoxia (Figure 2G). Tube formation experiments showed that after 6h incubation on Matrigel, extensive tubular network was observed in HUVEC treated with conditioned medium from YngMSC, as compared with oldMSCs and Co-oldMSCs which showed poor morphological change (Figure H–K). Real time PCR results showed increased Akt mRNA in YngMSC and oldMSCs when subjected to anoxia which continued to increase during re-oxygenation phase (Figure 2L). Similarly, p21 and p27 downstream of Akt were significantly increased in YngMSC during both anoxia and re-oxygenation phases as compared with oldMSCs (Figure 2M–N).
Figure 2.
(A–D) Real time-PCR on YngMSCs, oldMSCs and Co-oldMSCs for various angiogenic growth factors expression. The cells were cultured under normoxia, 6h anoxia and 6h anoxia+ 24h re-oxygenation. The results were normalized to angiogenic growth factor expression in YngMSCs under normoxia assigned an arbitrary value of 1. (A) The normoxia level expression of various angiogenic growth factors in oldMSCs were normalized with that of YngMSCs. HIF-1α, HO-1 and FGF-2 were insignificantly higher in oldMSCs as compared with YngMSCs. On the contrary, gene expression of HIF-1α, HO-1, VEGF and FGF-2 were significantly increased in response to anoxia in YngMSCs (B) as compared with oldMSCs (C). Co-culture of oldMSCs with YngMSCs did not change their response (D). Figures E & F show representative Western immunoblots of HIF-1α and HO-1 immediately after 6h anoxia and 24h re-oxygenation after 6h anoxia respectively. (G) ELISA for VEGF protein in cell lysate samples from YngMSCs, oldMSCs and Co-oldMSCs grown under normoxia, immediately after anoxia and 24h re-oxygenation after 6h anoxia. VEGF protein expression decreased significantly under anoxia and increased during re-oxygenation phase in all the cell groups. However, VEGF protein expression was highest in the YngMSCs. (H–K) Representative photographs of in vitro tube formation assay on HUVEC grown on Matrigel using conditioned medium from the cells used for VEGF ELISA. After 6h incubation, HUVEC showed migration to form typical tube-like structures under the influence of conditioned medium from YngMSC as compared with oldMSCs whereas the non-conditioned medium did not produce any morphological change. (L–N) Real-time PCR results showing fold change in mRNA expression of Akt, p21 and p27 in different groups of cells (A= anoxia; R= reoxygenation).
In vitro tube formation studies
In vitro formation of tubular structures from HUVECs on Matrigel revealed that morphological changes were most obvious at 6h after incubation with conditioned medium obtained from YngMSCs grown under normoxia, 6h anoxia (followed by immediate harvest of cells) and, 6h anoxia followed by 24h re-oxygenation as compared with the conditioned medium obtained from oldMSCs and Co-oldMSCs grown under the same set of conditions (Figure 2H–K). Similarly, highest activity was observed in the wells in which conditioned medium from YngMSCs, oldMSCs and Co-oldMSCs (6h anoxia followed by 24h re-oxygenation) was added. These results clearly showed that in vitro release of pro-angiogenic growth factors was highest in YngMSCs during their early response to anoxia as well as during the re-oxygenation phase until 24h of observation.
In vivo studies
All animals survived full length of studies and there were no deaths related with cell transplantation. The animals (n=8 per group) were harvested at 4 weeks after respective treatment. Additionally, in group-4, three animals each were harvested on day 3 and day 7 after cell transplantation to study the fate of combined transplanted YngMSCs as well as oldMSCs. Both YngMSCs as well as oldMSCs showed survival in the infarcted rat heart until 4 weeks of observation.
Direct comparison of the myogenic potential of YngMSCs and oldMSCs
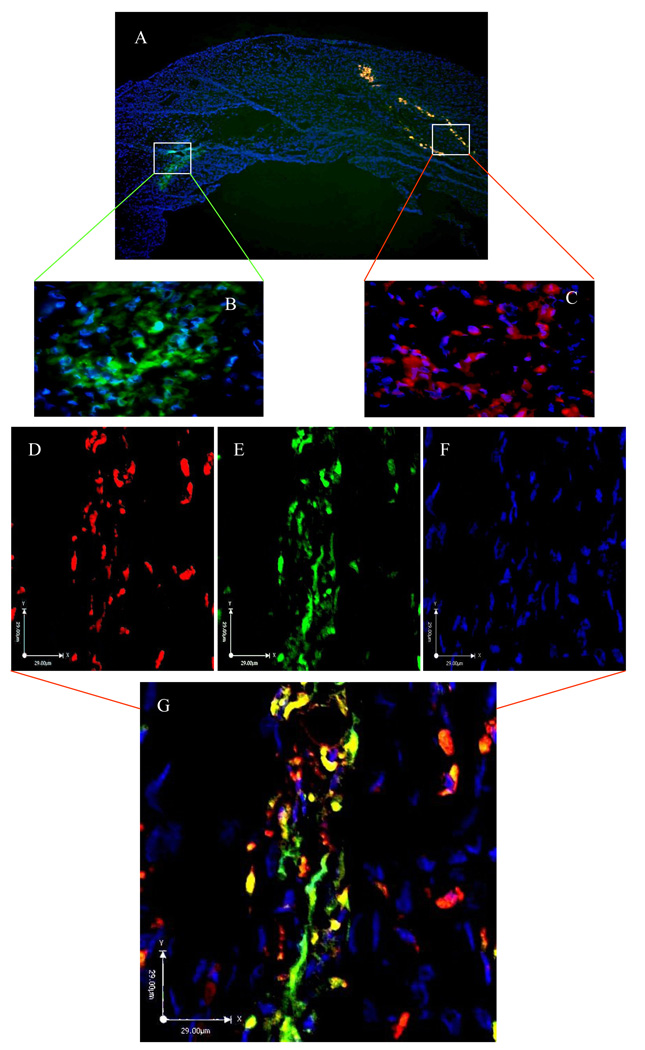
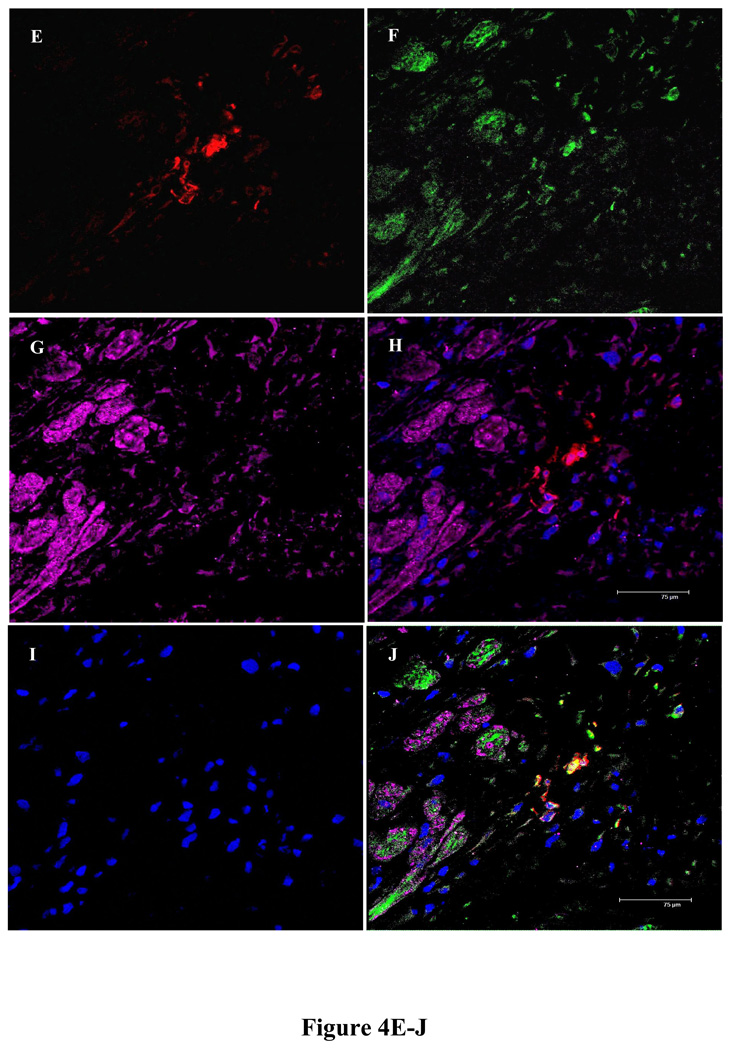
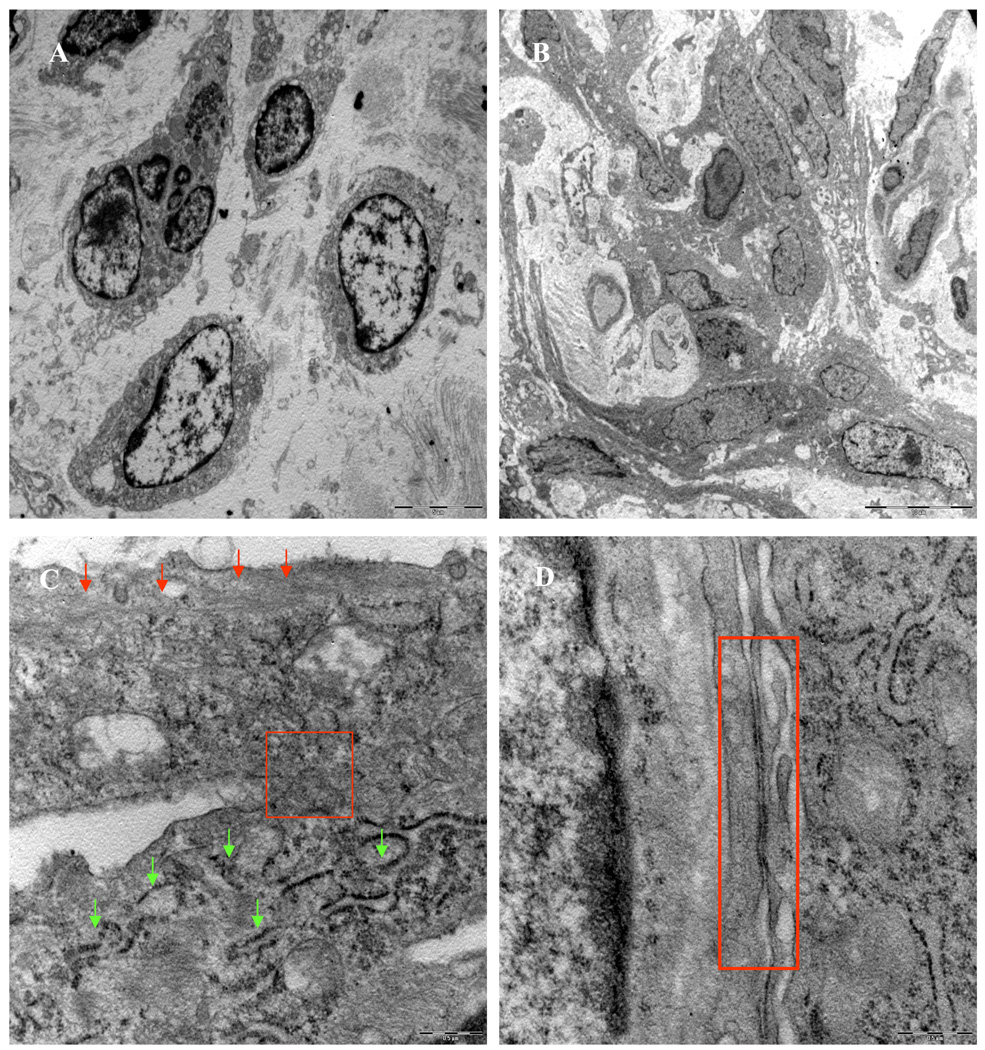
Histological studies of rat heart tissues showed that 3 days after engraftment, YngMSCs (PKH67 green fluorescence) and oldMSCs (PKH27 red fluorescence) were clumped together at the injection sites (Figure 3A–C) and by day 7, they had already spread out and traversed across from the site of injection (Figure 3D–G). Whereas oldMSCs mostly appeared undifferentiated circular entities during confocal imaging, YngMSCs gave elongated appearance, adopting the architectural organization of the surrounding cardiac tissue. At 4 weeks time-point, actin specific fluorescent immunostaining of the heart tissue in different treatment animal groups was performed to study the myogenic differentiation of the transplanted cells. Confocal imaging revealed that oldMSCs (red fluorescence) exhibited meager actin expression (green fluorescence) in the cell transplanted region (Figure 4A–D). On the contrary, YngMSCs (green fluorescence) showed remarkable myogenic potential and developed into muscle fibers in the infarcted myocardium. Figure 4E–J shows rat heart tissue from group-4 which was transplanted with YngMSCs (green) developing into mature muscle fibers immunostaining positively for actin expression (magenta) whereas the oldMSCs could be seen lying undifferentiated in the vicinity (red). These findings were further confirmed by ultra-structural studies. Significant metabolic activity was evident in the YngMSCs transplanted heart as compared with oldMSCs (Figure 5 A–B). Higher magnification images showed myogenic differentiation of the cells which was evident from the development of neo-filaments. An interesting feature of ultra-structural studies was the presence of tight junctions between the newly forming muscle fibers and abundant endoplasmic reticulum with ribosomes (green arrows) (Figure 5 C–D).
Figure 3.
(A) Fluorescent photomicrographs of rat heart tissue sections harvested on day 3 after simultaneous engraftment of YngMSCs (green) and oldMSCs (red). Nuclei were visualized by DAPI staining (blue). Figures B and C are magnifications from oldMSCs and YngMSCs engrafted regions shown in white boxes in (A) respectively. (D–G) Confocal images taken on day 7 revealed that oldMSCs mostly remained un-changed as rounded entities (red; D) as compared with the YngMSCs (green; E) which showed elongated morphology and adopted host myocardial tissue architecture. The nuclei were visualized by DAPI staining (blue; F). Figure G represents the merged image of D–F.
Figure 4.
Fluorescent immunostaining of rat heart tissue for α-actinin expression (green; A) at 4 weeks after engraftment of PKH26 labeled oldMSCs (red; B) in group-3. Nuclei were visualized by DAPI staining (blue; C). The red fluorescence labeled oldMSCs mostly survived as the poorly differentiated rounded structures with poor expression of actinin (merged image; D). On the other hand, immunostaining of rat heart tissue sections from group-4 for α-actinin expression at 4 weeks after transplantation of PKH26 labeled oldMSCs (red; E) and PKH67 labeled YngMSCs (F; green) showed that α-actinin expression (magenta; G) poorly co-localized with oldMSCs (red; H) thus indicating their scanty myogenic differentiation. DAPI staining (blue; I) was used for visualization of nuclei. As compared to the oldMSCs, YngMSCs (green; J) showed marked co-localization with α-actinin expression (magenta; J) thus indicating their extensive myogenic differentiation.
Figure 5.
Ultra-structure studies of rat heart at 4 weeks after transplantation of oldMSCs (A) and YngMSCs (B–D). Whereas oldMSCs survived in the infarcted myocardium without differentiation and their nuclear chromatin appeared extensively marginated, YngMSCs were seen elongated and spread out, replacing the fibrous infarct. (C) Two differentiating YngMSCs at 4 weeks after transplantation. New filaments can be seen in the differentiating cells (red arrows). Considerable metabolic activity was observed as indicated by ribosomes in the endoplasmic reticulum (green arrows) and formation of tight junction was observed between the two adjacent cells (red box in C) which has been magnified in (D).
Infarct size and Angiogenesis
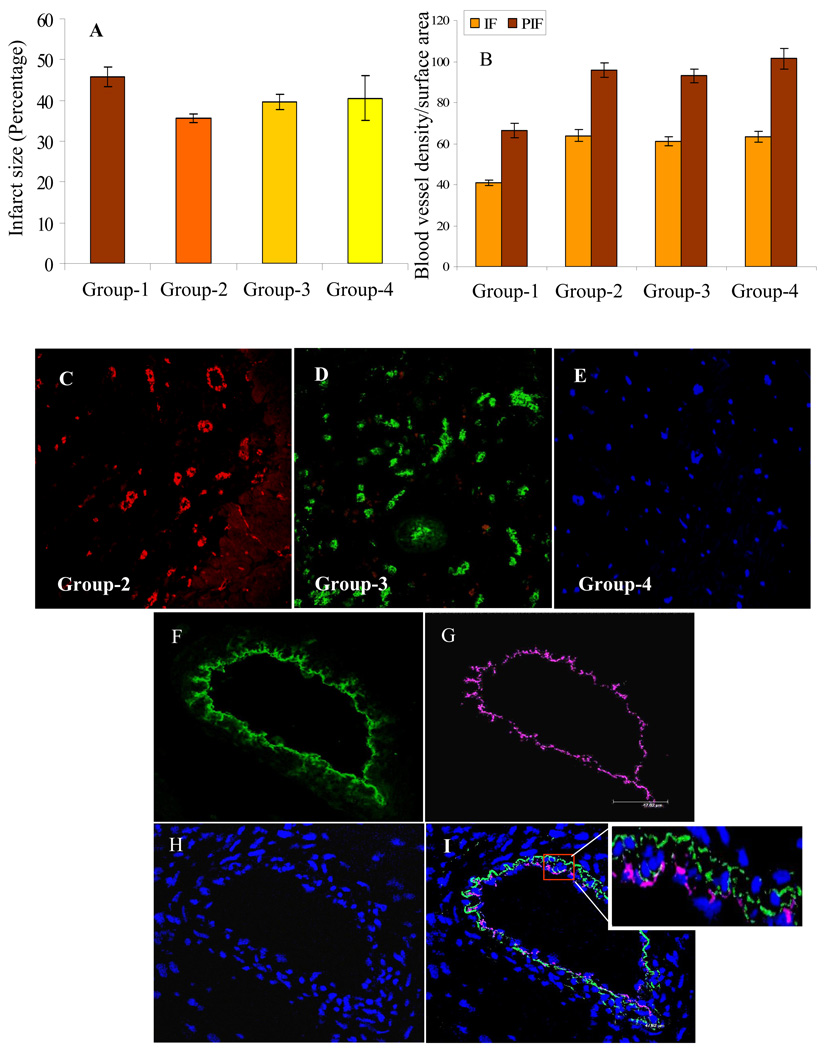
Infarct size was attenuated in all the experimental groups as compared with control group-1. However, only group-2 showed significant attenuation of infarct size in comparison with control group-1 (Figure 6A). Direct intramyocardial injection of MSCs into cardiac tissue promoted vascular activity in the infarcted myocardium. Quantification of blood vessel density per unit area (0.74 mm2) between various groups at 4 weeks after their respective treatment was performed. Significantly higher blood vessel density was observed in both infarct and peri-infarct regions in all the cell transplanted experimental groups (groups 2–4) in comparison with the control group-1 (41± 1.3; 66.4± 3.6). However, blood vessel density was insignificantly different between group-2 (63.8± 2.73; 95.8± 3.6) group-3 (61± 2.2; 93.1± 3.3) and group-4 (63.4± 2.6; 101.4± 5.1) in both infarct area and peri-infarct areas respectively (Figure 6B–E). Nevertheless, blood vessel density was higher in group-2 and group-4 as compared with group-3 possibly due to the reason that YngMSCs which were better responder to anoxia, were transplanted in both these groups. We observed occasional integration of YngMSCs into the lumen of blood vessels as was evident from co-localization of green fluorescence (PK67 label of the transplanted YngMSCs) and red fluorescence of vWFactor-VIII immunostaining in group-2 (shown as magenta color in Figure 6F–I).
Figure 6.
(A) Infarct size was significantly reduced in group-2 as compared with group-1. However, infarct size between group-1 vs groups-3 and 4 was almost similar. (B–E) Fluorescent immunostaining of rat heart tissue for vWFactor-VIII showed that blood vessel density was significantly higher in the infarct and peri-infarct regions in group-2, 3, and 4 as compared with group-1. However, no significant difference was observed amongst groups 2, 3 and 4 themselves. (F–I) Confocal images of rat heart tissue showed that the transplanted PKH67 labeled YngMSCs (green; F) were integrated into the blood vessels as determined by immunostaining for vWFactor-VIII (magenta; G). DAPI staining showed the nuclei (H; blue). YngMSCs integration into blood vessels was evident from co-localization of green and magenta fluorescence (I; merged image with white box showing magnified part of the blood vessel) (magnification= oil immersion).
Heart function
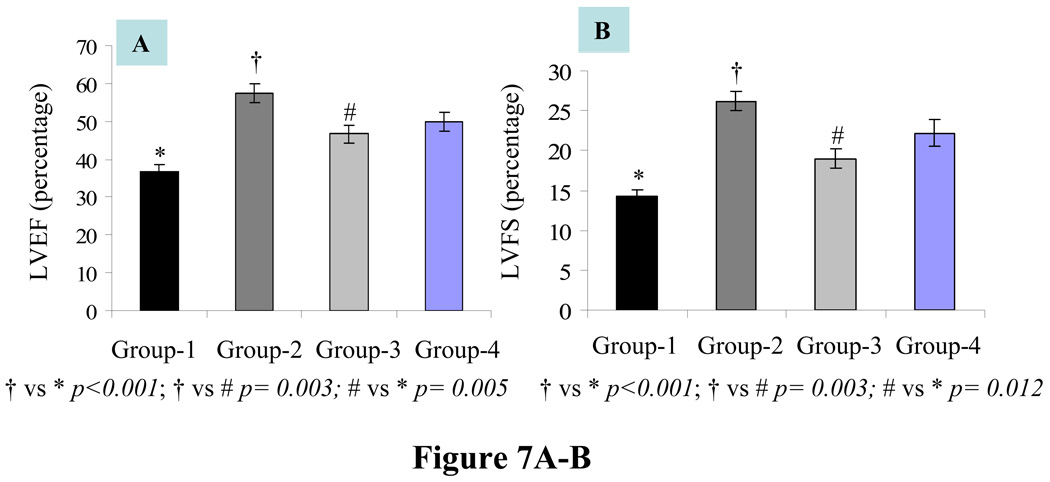
Assessment of LV heart function by echocardiography revealed significant improvement in systolic function indices. LVEF and LVFS respectively were highest in group-2 (57.34±2.5 p< 0.001 and 26.19±1.2 p< 0.001) as compared with group-1 (36.7±1.8 and 14.2±0.8) (Figure 7A–B). On the other hand, group-3 (46.7±2.3 p=0.005 and 19±1.2 p= 0.012) and group-4 (49.95±2.8 p<.001 and 22.1±1.68 p< 0.001) also showed improvement as compared with group-1. LVEF and LVFS between group-2 and group-3 also showed significant difference (p=0.003). These data indicated that YngMSCs exerted greater cardioprotective effects as compared with oldMSCs transplanted either alone or together with YngMSCs. As shown in Table-2, the LV remodeling indices of LVEDd (mm) and LVEDs (mm) in DMEM group-1 were increased to 7.2±0.1 and 6.2±0.1 respectively. As compared with group-1, there was a limited increment of LVEDd (p=0.022) and LVEDs (p<0.001) in group-2, group-3 (p=0.09 and p=0.009) and group-4 (p=0.1 and p=0.002) respectively. Similarly, LV anterior wall thickness (mm) at end-diastole (AWTd) and end-systole (AWTs) were best maintained in animal group-2 (p<0.001) and (p<0.001) respectively followed by animal group-4 (p= 0.003 and p<0.001) and group-3 (p= 0.014 and p= 0.012) respectively. DMEM injection after acute myocardial infarction resulted in significant reduction of AWTd (0.76±0.06) and AWTs (0.81±0.06) in animal group-1 as compared with the cell treatment groups. LV posterior wall thickness (mm) at end-diastole (PWTd) and end-systole (PWTs) were best maintained in animal group-2 (p=0.003 and p=0.004) respectively followed by animal group-4 (p= 0.002 and p=0.05) and group-3 (p= 0.004 and p= 0.125) respectively.
Figure 7.
Heart function studies showed that LV contractile function parameters including (A) LVEF and (B) LVFS showed significant improvement in all the cell injected groups-2, 3 and 4 as compared with group-1. However, highest level of improvement was observed in group-2. Similarly, group-2 showed significant improvement as compared with group-3. Table-2 shows improved chamber dimensions and diastolic and systolic LV wall thickness in group-2.
Table-2.
Echocardiography data for rat heart function at 4weeks post treatment.
| Parameter | Group-1 | Group-2 | Group-3 | Group-4 |
|---|---|---|---|---|
| LVEF (%) | 36.72±1.83 | 57.34±2.49 | 46.66±2.28 | 49.95±2.47 |
| LVFS(%) | 14.2±0.83 | 26.19±1.22 | 19.01±1.16 | 22.17±1.68 |
| LVEDd (mm) | 7.24±0.13 | 6.39±0.23 | 6.63±0.29 | 6.64±0.3 |
| LVEDs (mm) | 6.23±0.12 | 4.72±0.2 | 5.36±0.23 | 5.19±0.29 |
| AWTd (mm) | 0.76±0.06 | 1.12±0.06 | 0.99±0.06 | 1.05±0.05 |
| PWTd (mm) | 1.36±0.15 | 1.81±0.09 | 1.78±0.07 | 1.81±0.05 |
| AWTs (mm) | 0.81±0.06 | 1.36±0.08 | 1.1±0.09 | 1.24±0.06 |
| PWTs (mm) | 1.73±0.15 | 2.54±0.15 | 2.12±0.17 | 2.23±0.22 |
AWTd= Anterior wall thickness diastolic; AWTs= Anterior wall thickness systolic; LVEF= Left ventricle ejection fraction; LVFS= Left ventricle fractional shortening LVEDd= Left ventricle end diastolic dimension; LVEDs= Left ventricle end systolic dimension; PWTd= posterior wall thickness diastolic; PWTs= posterior wall thickness systolic
Discussion
The main findings of our study were that; 1- YngMSCs and oldMSCs were divergent in their response to stress stimulus in terms of survival and angiogenic growth factor expression; 2- oldMSCs co-cultured with YngMSCs did not show significant change in their functionality; 3- Age of the donor significantly influenced the reparability of the stem cells in the infarcted heart after engraftment.
Secretion of a broad spectrum of growth factors and cytokines provides a plausible mechanism for improved cardiac function after MSCs transplantation in the infarcted heart [5, 22]. Conceivably, cytokines secreted from the engrafted MSCs mediated pleiotropic effects in the heart, including enhanced angiogenesis and prevention of cell apoptosis [23]. The capability of MSCs to produce different growth factors is significantly influenced by various pathological conditions and age [24]. Moreover, like any other cell type, their response to stress stimuli is also altered over the passage of time. Exposure of YngMSCs to lower temperature enhanced heat shock proteins (HSP) expression [18]. Similar observations were made while exploring hepatic hypoxia responsive gene regulation which showed that aging activated HIF-1α, accompanied by remarkable up-regulation of HIF-1α dependent genes including HO-1, VEGF, erythropoietin, and inducible nitric oxide synthase [25]. MSCs which normally show low level expression of interleukin (IL)-6, IL-11, leukemia inhibitory factor and granulocyte-macrophage colony stimulating factor, elicited higher expression of these cytokines when challenged with ILβ1 [26,27]. Our results provide a direct comparison of YngMSCs and oldMSCs in their ability to secrete angiogenic growth factors under normoxia and anoxia. We observed that the expression of angiogenic growth factors in both YngMSCs and oldMSCs was insignificantly different under normoxia. However, YngMSCs were superior responders to anoxia both during the acute phase after exposure to anoxia and upon re-oxygenation for 24 h. Despite the observed difference in response to lack of oxygen in terms of growth factor expression, YngMSCs and oldMSCs did not differ significantly in induction of angiogenesis in the heart. These observations are in agreement with the published results which showed that capillary density was maintained irrespective of the down-regulation of several angiogenesis-related factors in aging animals [28].
Aging also makes the cells more sensitive to apoptotic stimuli. In a comparative study between YngMSCs and oldMSCs (from 6 and 56 weeks old rats), low temperature stress was cytoprotective for YngMSCs which responded by up-regulated expression of anti-apoptotic HSP-27, HSP-70, and HSP-90 and down-regulation of pro-apoptotic HSP-60. Contrarily, oldMSCs cultured at low temperature generally produced no beneficial changes in these parameters, rather they showed detrimental effects [18]. A recent report on gene expression profile showed increased activity of p53 and related genes in aging cells which supported a role for p53-mediated transcriptional program in their increased sensitivity to oxidant stress [29]. Our results showed that serine/threonine kinase PKB (Akt) was increased following anoxia–reoxygenation as part of the survival mechanism which was more prominent in YngMSCs as compared with the oldMSCs. Akt is a major player in the cell survival signaling and all three of its isoforms contribute to it’s activity to promote cell proliferation, differentiation and survival. Moreover, Akt executes its canonical anti-apoptotic actions by phosphorylation of multiple downstream target molecules including p21 and p27. Upon phosphorylation by Akt in the cytoplasm, cytoplasmic stability of p21 and p27 is enhanced which acquire an anti-apoptotic gain of function. On the other hand, increased expression of p21 and p27 in the nucleus inhibits Cdk2 thus leading to the arrest of cell cycle progression which is essential for maintenance of cell viability under oxygen stress [30]. Incidentally, we observed significantly higher levels of both p21 and p27 in YngMSCs, both of which have anti-apoptotic activity. Further molecular studies are warranted to elucidate their involvement in anti-apoptotic effect on YngMSCs and oldMSCs under anoxia.
The higher susceptibility of oldMSCs to anoxia may also be attributed to their poor responsiveness to oxygen stress in terms of growth factor expression. HO-1 is an inducible form of heme oxygenase in response to various stimuli, and has a major role cytoprotection against oxygen stress. We observed that HO-1 increased during oxygen stress and during re-oxygenation phase in YngMSCs while HO-1 up-regulation in oldMSCs was significantly low, especially in the re-oxygenation phase, thus indicating that HO-1 production under oxygen stress was greatly affected by aging. These results implied that the defense mechanisms against oxygen stress were severely attenuated in oldMSCs which made them susceptible to apoptosis.
Different corrective measures have been adopted to counter the effects of aging in a variety of cells. Ex-vivo IGF-1 treatment of endothelial progenitor cells from elderly individuals improved their age-related dysfunction and attenuated cellular senescence [31]. The reversal of age-related dysfunction was mediated in IGF-1/phosphoinositide-3-kinase/Akt dependent manner. We hypothesized that the paracrine factors released from YngMSCs in co-culture will positively influence the oldMSCs to alleviate their age-related dysfunction. However, that was not the case. We did not observe any difference between oldMSCs and Co-oldMSCs with respect to their resistance to oxygen stress as well as their ability to express angiogenic growth factors. Similar observations were made in vivo when YngMSCs and oldMSCs were co-transplanted in the infartced rat heart. The possible explanation for this failure of oldMSCs to respond positively to the presence of YngMSCs is due to the age associated diminished responsiveness of these cells to growth and differentiation factors.
Despite extensive research in the use of MSCs for myocardial repair, there is no definite statement on the differentiation potential of these cells taken from young and aging animals [32]. Most of the previous work in this regard has been focused on their osteogenic potential which is markedly reduced in oldMSCs. Aging causes a decrease in the commitment of MSCs to osteoblast lineage and increases their commitment to adipocyte lineage [33]. This is reflected by changes in the expression of phenotype-specific gene markers. A study on human CD105+ MSCs from young and old patients however, showed that aging incurred no significant switch in their myogenic differentiation capability. The cells treated with 5-azacytidine expressed myosin heavy chain (β-isoform) and connexin-43 [34]. Nevertheless, these observations lacked convincing evidence for their adoption of cardiac phenotype because the differentiated cells failed to show spontaneous contractility and electrophysiological activity. Moreover, it is difficult to extrapolate these results to predict the outcome of in vivo cell transplantation. We observed extensive myogenic differentiation of YngMSCs in the infarcted heart whereas the oldMSCs failed to show induction of differentiation at 4 weeks after transplantation. Our results suggest that care should be exercised following autologous bone marrow transplantation in clinical settings. In most of the clinical studies, autologous cells were obtained from the elderly patients and transplanted in their aging infarcted hearts. We therefore argue that the approach was inherently flawed due to two possible reasons. Firstly, the advancing age is associated with functional deficit of the stem cells. The compromised proliferative kinetics together with depleted phenotypic plasticity and reduced regenerative potential of the autologous engrafted bone marrow stem cells is a hallmark of aging process. The number of bone marrow-derived MSCs declines dramatically with aging while the existing cells drastically lose their effectiveness in the repair process. Secondly, the age-induced dysfunctional conditions is directly related to the biology of aging heart, and that too under ischemic stress, provide a not so conducive microenvironment to the transplanted stem cells. The combined effect of these effects has led to less than the expected functional outcome during the past clinical trials. Hence, the use of stem cells from young donors will be more effective for myocardial repair. It is therefore concluded that stem cells from young donors is an attractive option for heart cell therapy in future clinical trials.
Acknowledgements
This work was supported by National Institutes of Health grants # R37-HL074272; HL-23597; HL70062 and HL-080686 (to M.A).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 2.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 3.Kawada H, Fujita J, Kinjo K, Matsuzaki Y, Tsuma M, Miyatake H, Muguruma Y, Tsuboi K, Itabashi Y, Ikeda Y, Ogawa S, Okano H, Hotta T, Ando K, Fukuda K. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–3587. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 6.Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M, Tokudome T, Mori H, Miyatake K, Kitamura S. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 7.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 8.Pak HN, Qayyum M, Kim DT, Hamabe A, Miyauchi Y, Lill MC, Frantzen M, Takizawa K, Chen LS, Fishbein MC, Sharifi BG, Chen PS, Makkar R. Mesenchymal stem cell injection induces cardiac nerve sprouting and increased tenascin expression in a Swine model of myocardial infarction. J Cardiovasc Electrophysiol. 2003;14:841–848. doi: 10.1046/j.1540-8167.2003.03124.x. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, Matsuzaki M, Hamano K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol. 2006;291:H886–H893. doi: 10.1152/ajpheart.00142.2006. [DOI] [PubMed] [Google Scholar]
- 10.Tang YL, Zhao Q, Qin X, Shen L, Cheng L, Ge J, Phillips MI. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg. 2005;80:229–236. doi: 10.1016/j.athoracsur.2005.02.072. discussion 236–7. [DOI] [PubMed] [Google Scholar]
- 11.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 12.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 13.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 14.Edelberg JM, Tang L, Hattori K, Lyden D, Rafii S. Young adult bone marrow-derived endothelial precursor cells restore aging-impaired cardiac angiogenic function. Circ Res. 2002;90:E89–E93. doi: 10.1161/01.res.0000020861.20064.7e. [DOI] [PubMed] [Google Scholar]
- 15.Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fehrer C, Laschober G, Lepperdinger G. Aging of murine mesenchymal stem cells. Ann N Y Acad Sci. 2006;1067:235–242. doi: 10.1196/annals.1354.030. [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann S, Voss M, Kaiser S, Kapp U, Waller CF, Martens UM. Lack of telomerase activity in human mesenchymal stem cells. Leukemia. 2003;17:1146–1149. doi: 10.1038/sj.leu.2402962. [DOI] [PubMed] [Google Scholar]
- 18.Stolzing A, Sethe S, Scutt AM. Stressed stem cells: Temperature response in aged mesenchymal stem cells. Stem Cells Dev. 2006;15:478–487. doi: 10.1089/scd.2006.15.478. [DOI] [PubMed] [Google Scholar]
- 19.Li TS, Hamano K, Suzuki K, Ito H, Zempo N, Matsuzaki M. Improved angiogenic potency by implantation of ex vivo hypoxia prestimulated bone marrow cells in rats. Am J Physiol Heart Circ Physiol. 2002;283:H468–H473. doi: 10.1152/ajpheart.00261.2002. [DOI] [PubMed] [Google Scholar]
- 20.Jiang S, Haider H, Idris NM, Salim A, Ashraf M. Supportive interaction between cell survival signaling and angiocompetent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Circ Res. 2006;99:776–784. doi: 10.1161/01.RES.0000244687.97719.4f. [DOI] [PubMed] [Google Scholar]
- 21.Niagara MI, Haider H, Jiang S, Ashraf M. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res. 2007;100:545–555. doi: 10.1161/01.RES.0000258460.41160.ef. [DOI] [PubMed] [Google Scholar]
- 22.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 23.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RV, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 24.Li TS, Furutani A, Takahashi M, Ohshima M, Qin SL, Kobayashi T, Ito H, Hamano K. Impaired potency of bone marrow mononuclear cells for inducing therapeutic angiogenesis in obese diabetic rats. Am J Physiol Heart Circ Physiol. 2006;290:H1362–H1369. doi: 10.1152/ajpheart.00766.2005. [DOI] [PubMed] [Google Scholar]
- 25.Kang MJ, Kim HJ, Kim HK, Lee JY, Kim DH, Jung KJ, Kim KW, Baik HS, Yoo MA, Yu BP, Chung HY. The effect of age and calorie restriction on HIF-1-responsive genes in aged liver. Biogerontology. 2005;6:27–37. doi: 10.1007/s10522-004-7381-z. [DOI] [PubMed] [Google Scholar]
- 26.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166:585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 27.Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 28.Wagatsuma A. Effect of aging on expression of angiogenesis-related factors in mouse skeletal muscle. Exp Gerontol. 2006;41:49–54. doi: 10.1016/j.exger.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Edwards MG, Anderson RM, Yuan M, Kendziorski CM, Weindruch R, Prolla TA. Gene expression profiling of aging reveals activation of a p53-mediated transcriptional program. BMC Genomics. 2007;8:80. doi: 10.1186/1471-2164-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blagosklonny MV. Are p27 and p21 cytoplasmic oncoproteins? Cell Cycle. 2002;1:391–393. doi: 10.4161/cc.1.6.262. [DOI] [PubMed] [Google Scholar]
- 31.Thum T, Hoeber S, Froese S, Klink I, Stichtenoth DO, Galuppo P, Jakob M, Tsikas D, Anker SD, Poole-Wilson PA, Borlak J, Ertl G, Bauersachs J. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ Res. 2007;100:434–443. doi: 10.1161/01.RES.0000257912.78915.af. [DOI] [PubMed] [Google Scholar]
- 32.Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5:91–116. doi: 10.1016/j.arr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 34.Roura S, Farre J, Soler-Botija C, Llach A, Hove-Madsen L, Cairo JJ, Godia F, Cinca J, Bayes-Genis A. Effect of aging on the pluripotential capacity of human CD105+ mesenchymal stem cells. Eur J Heart Fail. 2006;8:555–563. doi: 10.1016/j.ejheart.2005.11.006. [DOI] [PubMed] [Google Scholar]