Abstract
Neurally intact (NI) rats and chronic spinal cord injured (SCI) rats were studied to determine how activation of mechanosensory or cholinergic receptors in the bladder urothelium evokes ATP release from afferent terminals in the bladder as well as in the spinal cord. Spinal cord transection was performed at the T9-T10 level 2–3 weeks prior to the experiment and a microdialysis fiber was inserted in the L6-S1 lumbosacral spinal cord. Mechanically evoked (i.e. 10cm/w bladder pressure) ATP release into the bladder lumen was approximately 6.5 fold higher in SCI compared to NI rats (p<0.05). Intravesical carbachol (CCh) induced a significantly greater release of ATP in the bladder from SCI as compared to NI rats (3424.32 ± 1255.57 vs. 613.74 ± 470.44 pmol/ml, respectively, p<0.05). However, ATP release in NI or SCI rats to intravesical CCh was not affected by the muscarinic antagonist atropine (Atr). Spinal release of ATP to bladder stimulation with 10cm/w pressure was 5-fold higher in SCI compared to NI rats (p<0.05). CCh also induced a significantly greater release of spinal ATP in SCI rats compared to controls (4.3 ± 0.9 vs. 0.90 ± 0.15 pmol, p < 0.05). Surprisingly, the percent inhibitory effect of Atr on CCh-induced ATP release was significantly less in SCI as compared to NI rats (49% vs. 89%, respectively). SCI induces a dramatic increase in intravesical pressure and cholinergic receptor evoked bladder and spinal ATP release. Muscarinic receptors do not mediate intravesical CCh induced ATP release into the bladder lumen in NI or SCI rats. In NI rats sensory muscarinic receptors are the predominant mechanism by which CCh induces ATP release from primary afferents within the lumbosacral spinal cord. Following SCI, however, nicotinic or purinergic receptor mechanisms become active, as evidenced by the fact that Atr was only partially effective in inhibiting CCh-induced spinal ATP release.
Keywords: ATP, purinergic, cholinergic, spinal cord injury, microdialysis, urinary bladder, urothelium
1. Introduction
Normal micturition in the rat is triggered by sensory input conveyed by myelinated Aδ nerve fibers and requires intact spinobulbospinal pathways that integrate bladder and urethral reflexes at the level of the pontine micturition center (Kruse et al., 1990). Mounting evidence suggests that purinergic signaling may play an important role in regulating micturition reflexes. The significance of purinergic receptors in bladder mechanosensory transduction is underscored by the marked decrease in reflex bladder activity found in P2X3- and P2X2- deficient mice (Cockayne et al., 2005; Vlaskovska et al., 2001). Homomeric P2X3 and heteromeric P2X2/3 channels, prominently associated with sensory mechanisms (Burnstock, 2000; Ruggieri, 2006), are expressed on DRG cell bodies as well as on central and peripheral terminals of primary bladder afferents (Cockayne et al., 2005). In fact, ATP activation of presynaptic P2X3 and P2X2/3 receptors was even shown to facilitate release of another sensory transmitter, glutamate, from spinal afferent nerve terminals (Nakatsuka et al., 2001).
Sensory purinergic receptors have even been expressed on epithelial tissues such as bladder urothelium (Cockayne et al., 2005). In addition, studies have demonstrated that urothelial cells not only respond to chemical stimuli, but can synthesize and release the neurotransmitters like ATP when stimulated by stretch (Sperlagh et al., 1996; Ferguson et al., 1997; Sun et al., 2001; Birder et al., 2002). Released ATP could activate suburothelially located P2X3 and P2X2/3 receptors to increase sensory nerve transmission, possibly in a non-synaptic manner, a phenomenon that has been previously described regarding the interaction between efferent nerve terminals (Vizi, 1979). Thus, ATP might modulate bladder sensation via actions on purinergic receptors at peripheral (i.e. urothelial or bladder afferent nerve terminals) and central (i.e. spinal afferent nerve terminals) locations.
Spinal cord injury (SCI) induces significant plasticity within neural pathways innervating the lower urinary tract that leads to functional changes such as increased bladder activity (e.g. detrusor hyperreflexia) and bladder outlet obstruction (e.g. detrusor sphincter dyssynergia). Underlying these changes in efferent outflow are significant increases in bladder afferent activity (de Groat et al., 1990). ATP levels and P2X receptor mediated responses are also increased in pathologic states associated with heightened bladder sensory mechanisms. For example, urothelial ATP release is significantly enhanced in chronic SCI rats and in rats subjected to chemically induced chronic bladder inflammation (Khera et al., 2004; Smith et al., 2005). Both of these conditions are associated with bladder hyperreflexia. Increased ATP release has also been described in other models including augmented urothelial ATP release in cats with a naturally occurring cystitis (Birder et al., 2003) as well as in urothelial cell cultures of humans suffering from interstitial cystitis (Sun et al., 2001). The significance of elevated ATP levels in conditions of enhanced bladder sensory nerve activation was further illustrated by immunohistochemical studies in human bladders demonstrating a higher expression of suburothelial P2X3 receptors under pathologic conditions (i.e. SCI) (Brady et al., 2004).
This study had two primary aims: (1) to examine whether intravesical pressure or chemical stimulation evokes ATP release within the bladder lumen as well as in the dorsal lumbosacral spinal cord and, (2) to examine whether the quantity of ATP release and the mechanism by which these stimuli evoke spinal and bladder ATP release are different in neurally intact versus chronic spinal cord injured rats.
2. Materials and methods
2.1. Animals
The studies were carried out on female Sprague-Dawley rats (210 to 250 g), obtained from Harlan Sprague-Dawley, Inc. The animals were housed three per cage in a temperature- (22°C) and humidity-controlled environment, with a light/dark cycle of 12 h/12 h. Food and water were available ad libitum. The animals were allowed to adapt to the environment for at least 3 days before surgery. Twelve rats were divided into 2 groups: a neurally intact (NI) group and a spinal cord injured (SCI) group. All the experimental procedures were approved by the Institutional Animal Care and Use Committee (IACAUC) at Baylor College of Medicine, Houston, TX and were conducted in accordance with National Institutes of Health guidelines for the care and use of laboratory animals.
2.2. Surgery
2.2.1. Spinal cord injury
Two to three weeks prior to the microdialysis experiment, animals in the SCI group were anesthetized with isoflurane (1.5%). After a midline dorsal incision, laminectomies were performed to expose spinal segments T9-T10. The dura and the spinal cord were then completely transected using a microscope to ensure that the transection was complete. The overlying muscle and skin were closed and the rats were given 10 ml of Ringers Lactate solution subcutaneously. All animals received amoxicillin (150mg/kg) intramuscularly once a day for 14 days. Rat bladders were manually expressed 2 to 3 times per day until spinal reflex micturition developed (i.e. 10–14 days).
2.2.2. Microdialysis probe preparation and insertion
Forty-eight hours before experimentation, microdialysis fibers (Filtral AN 69 microdialysis fiber, 200 μm o.d., Hospal Industrie, Gambro) were prepared by coating hollow porous dialysis fibers with a thin layer of epoxy, except for a central 2-mm dialysis zone. One day prior to the experiments, animals of both groups were anesthetized with isoflurane and an incision was made to expose spinal segments L1-L2. The microdialysis fiber was directed using a stainless steel guidewire through drilled holes on each side of the L1 vertebral body into the L6-S1 dorsal spinal cord. Each end of the microdialysis fiber was connected to polyethylene tubing (PE-20, VWR, Suwanee, GA) and exteriorized in the dorsal cervical region through a subcutaneous tunnel. The microdialysis probe was primed by infusing Krebs solution at a rate of 5 μL/min with a syringe pump (CMA 102, Stockholm, Sweden) for one hour after which the inlet and outlet tubing were mechanically sealed.
2.3. ATP sampling
On the day of the experiment, animals were anesthetized with urethane (1.2g/kg) and the probe was perfused with oxygenated Krebs solution (in mM) 113 NaCl, KCl 4.7, CaCl2 2.5, MgSO4 1.2, NaHCO3 25, KH2PO4 1.2, and d-glucose 11.5 at a rate of 5μL/min for 1 h to allow for equilibration. The bladder was catheterized transurethrally using polyethylene tubing (PE-90) that was connected via a 3-way stopcock to both a pressure transducer and a large-surface fluid reservoir. The catheter was secured by tying a suture around the urethra to create isobaric conditions. The initial bladder pressure was set at 10cm/w and held constant throughout the experiments.
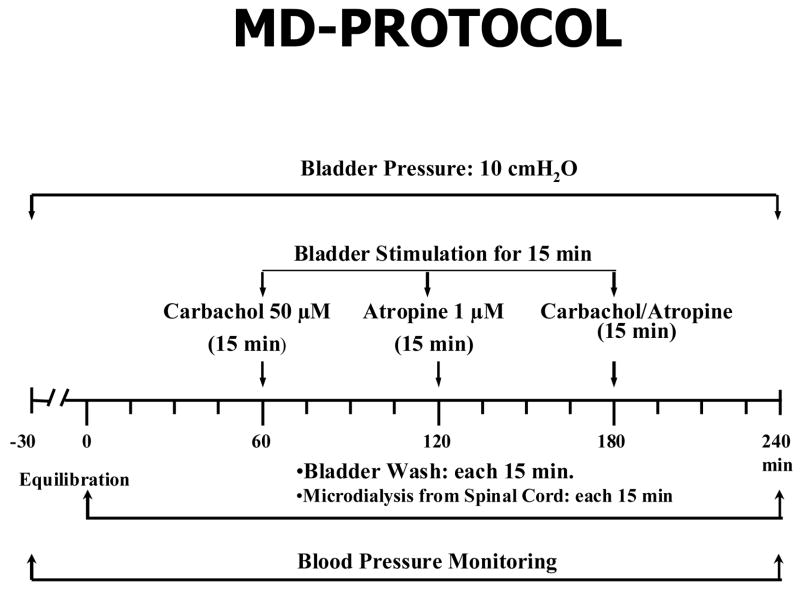
Subsequently, the bladder was stimulated for 15-min with each of the following drugs: carbachol 50 μM (CCh), atropine 1 μM (ATR), and CCh + ATR (50 μM + 1 μM, respectively). Before and during stimulation with each drug, 4 consecutive 15-min samples were collected from the bladder and spinal cord (Figure 1). A 50 μL aliquot from each 15 min effluent was placed into a luminometer (Turner, Sunnyvale, CA) and the ATP content was measured using the luciferin-luciferase assay. The luminescence of the collected sample was compared to that of standard ATP and the evoked release by each stimulus was calculated. Baseline ATP release from 10cm/w pressure was calculated by averaging ATP values from the 1st two samples at the start of each experiment. Drug evoked release of ATP was computed by subtracting the mean of ATP release of two samples before and after recovery from stimulation. The sum of these figures gave the area of the increased efflux curve, which represented the amount of ATP released.
Figure 1.
Schematic representation showing the protocol used for the measurement of ATP released into the bladder lumen and spinal cord.
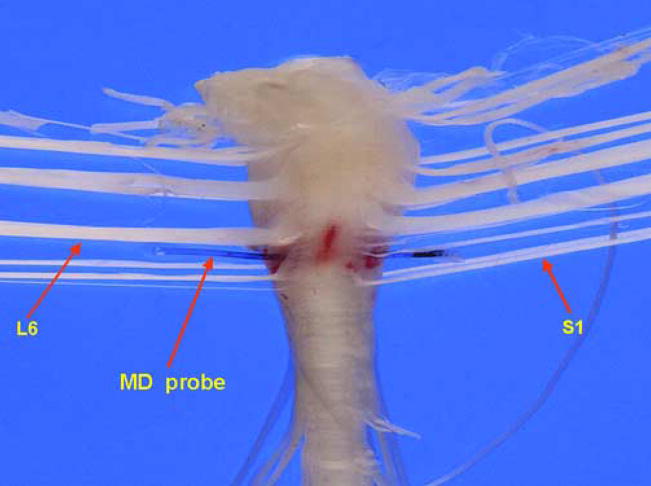
At the end of the experiment, the position of the probe in the spinal cord of each animal was macroscopically confirmed (Figure 2). In a subset of investigations, the probe was placed cranial to the L6-S1 segments (i.e. L4-5) to determine whether spinal cord ATP could be evoked by bladder stimulation. No measurable spinal cord ATP release was observed for either pressure or chemical bladder stimulation under these conditions. Probe recovery was measured by placing the microdialysis fiber in a solution of 5μM ATP and perfusing it with Krebs solution at a rate of 5μL/min. ATP recovery through the microdialysis probe was consistently 0.3% of its outside concentration.
Figure 2.
Macroscopic view of the rat spinal cord showing the microdialysis probe placed between the L6-S1 spinal cord segments.
2.4. Drugs
Carbachol, ampicillin, atropine, urethane, and all constituents of the Krebs solution were purchased from Sigma–Aldrich, Inc Co. (St. Louis, MO). Isoflurane was obtained from IsoFlo (Abbott Lab, North Chicago, IL).
2.5. Statistical Analysis
The data are expressed as mean ± S.E.M. Results obtained from different groups were compared using the Student’s t-test or the non-parametric Mann-Whitney test. Results obtained within groups were compared using the paired t-test. A level of p< 0.05 was considered statistically significant.
3. Results
3.1. ATP release into the bladder lumen
3.1.1. Pressure stimulation
The bladder pressure of NI and SCI rats was elevated to 10cm/w under isobaric conditions. As shown in figure 3, the mechanically evoked ATP release into the bladder lumen was approximately 6.5 fold higher in SCI compared to NI rats (914.53 ± 243.22 vs. 140.16 ± 33.38 pmol/ml, respectively, p < 0.05).
Figure 3.
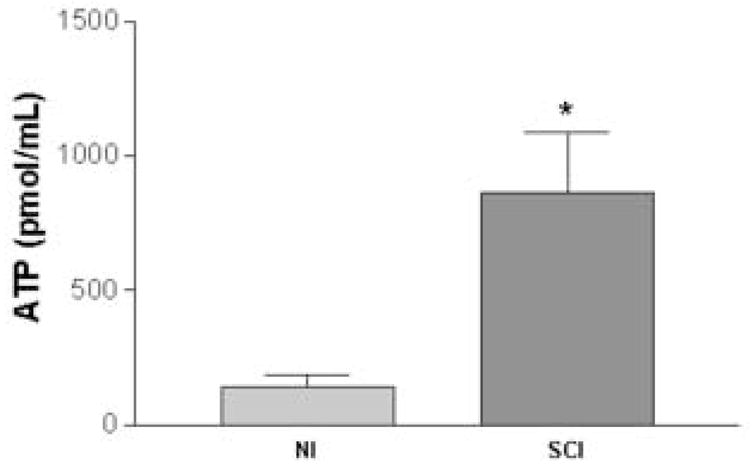
Release of ATP into the bladder lumen in neurally intact (NI) (n=5) and spinal cord injured (SCI) (n=6) rats after raising the intravesical pressure to 10cm/w. Bladder ATP concentration was significantly higher in SCI compared to NI rats (p<0.05, unpaired t-test).
3.1.2. Cholinergic receptor activation
Under isobaric (i.e. 10cm/w) bladder pressure, the mixed nicotinic-muscarinic agonist carbachol (CCh, 50μM) was instilled intravesically for a period of 15 minutes. Activation of afferent cholinergic receptors induced a significantly greater release of bladder lumen ATP from SCI as compared to NI rats (3424.32 ± 1255.57 vs. 613.74 ± 470.44 pmol/ml, respectively, p <0.05) (Figure 4). However, ATP release in bladders of NI or SCI rats evoked by chemical stimulation with CCh was not affected by the muscarinic antagonist atropine (Atr, 1μM).
Figure 4.
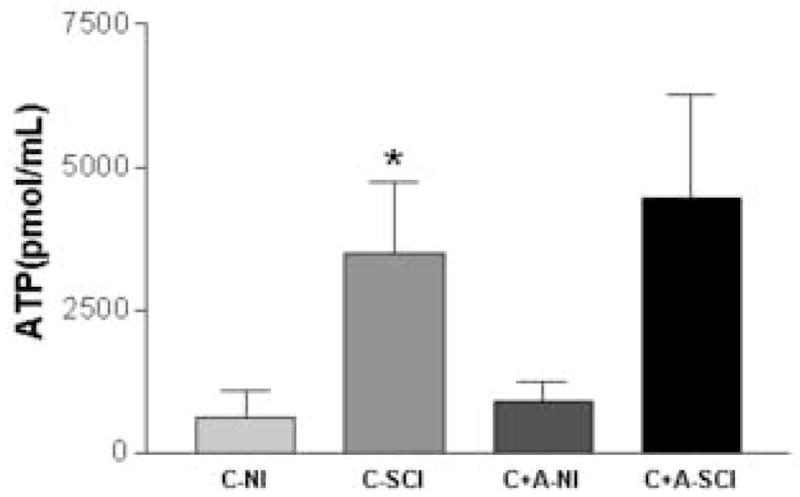
Bladder lumen ATP release to chemical stimulation with carbachol. NI=neurally intact rat; SCI=spinal cord injured rat; C=carbachol; A=atropine. Carbachol stimulation evoked a greater increase in bladder ATP concentration in SCI (n=6) compared to NI (n=5) rats (p<0.05, unpaired t-test). Atropine, however, did not alter bladder ATP concentration to carbachol stimulation in either group tested.
3.2. Spinal ATP release
3.2.1. Pressure stimulation
Bladder pressure in NI or SCI rats was maintained at 10cm/w while the microdialysis probe was perfused with oxygenated Krebs solution at a rate of 5μL/min. The results shown in the Fig. 5 indicate that the pressure evoked (i.e. 10cm/w) release of ATP from the dorsal L6-S1 segments was significantly higher in SCI compared to NI rats (1.2 ± 0.24 vs. 0.23 ± 0.008 pmol, respectively, p < 0.01).
Figure 5.
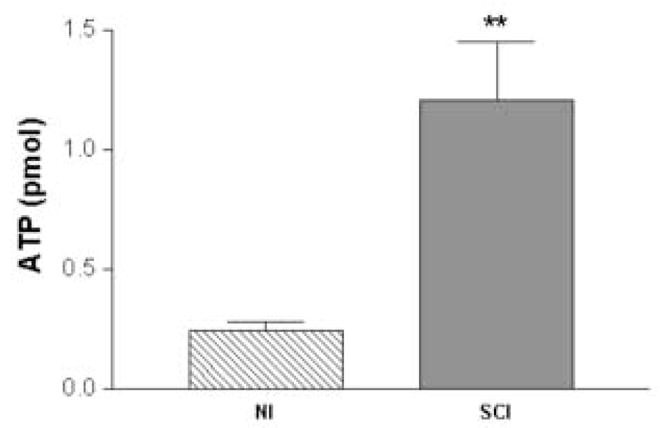
ATP release measured by a microdialysis probe implanted in the L6-S1 spinal cord of rats during 10cm/w pressure in neurally intact (NI) and spinal cord injured (SCI) rats. Note the greater than 5-fold increase in ATP levels with pressure stimulation in SCI rats (n=6) compared to NI rats (n=5) (p<0.01, unpaired t-test).
3.2.2. Cholinergic receptor activation
When the cholinergic agonist CCh (50μM) was intravesically administered under isobaric conditions, spinal ATP release was markedly enhanced in SCI rats versus NI rats (3.59 ± 0.75 vs. 0.77 ± 0.14 pmol, respectively) (p< 0.01) (Figure 6). In contrast to its effects on ATP release in the bladder lumen, Atr significantly inhibited CCh evoked spinal ATP release in both NI and SCI rats (p<0.05). However, the percent inhibitory effect by atropine was much less pronounced in SCI compared to NI rats (49% vs. 89%, respectively).
Figure 6.
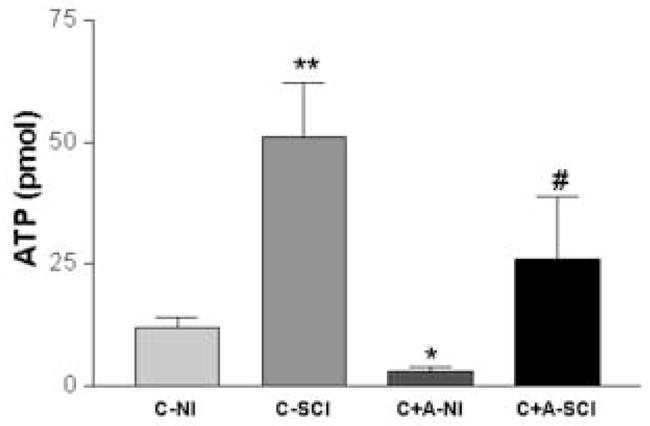
Bar graph depicting L6-S1 spinal cord ATP release collected with a microdialysis probe during intravesical chemical stimulation with carbachol or carbachol + atropine in NI (n=5) and SCI (n=6) rats. NI=neurally intact rat; SCI=spinal cord injured rat; C=carbachol; A=atropine. Carbachol evoked a significantly higher spinal ATP release in SCI versus NI rats (p<0.01, unpaired t-test). In addition, atropine significantly inhibited carbachol evoked spinal ATP release in both groups (p<0.05, paired t-test). * denotes significant change compared to C-NI group. # denotes significant change compared to C-SCI group.
4. Discussion
The purpose of this study was to examine the effects of intravesical pressure and chemical activation of bladder and spinal ATP release in neurally intact (NI) and spinal cord transected (SCI) rats. The main findings of these experiments are that: (1) bladder and spinal ATP release can be evoked by intravesical mechanical (i.e. pressure) or chemical (i.e. carbachol) stimulation, (2) the bladder stimulation evoked ATP release in the bladder lumen and spinal cord is significantly enhanced following SCI and, (3) spinal cord ATP release to intravesical cholinergic receptor stimulation with carbachol is inhibited by intravesical administration of the muscarinic antagonist atropine in both NI and SCI rats, but by a markedly higher percentage in NI rats.
Since discovering that ATP release can be generated by increasing the hydrostatic pressure on the urothelial side of the bladder or in the lumen of the ureter, it has become increasingly evident that urothelium plays a significant role as a pressure sensing system, utilizing the neurotransmitter ATP in response to mechanical stimulation (Khera et al., 2004; Smith et al., 2005; Ferguson et al., 1997; Birder et al., 2002; Knight et al., 2002). Previous research within our lab examined the effects of in vitro mechanoreceptor stimulation and subsequent ATP release in SCI and normal bladders to determine how disruption of supraspinal control of micturition modulates the evoked release of ATP from bladder urothelium (Khera et al., 2004). While epithelial release of ATP in normal rats was minimal; ATP release was significantly higher in chronic SCI rat bladders (Khera et al., 2004). The current studies in normal and SCI rats prove that mechanical stimulation induced bladder ATP release is also enhanced in SCI rats under in vivo conditions. For practical reasons, we applied a constant bladder pressure of 10cm/w so that drugs could be adequately delivered to the bladder mucosal surface, and also so that reliable volumes could be recovered from the bladder at the end of each stimulation. Because ATP release in the bladder or spinal cord was not measured under truly basal conditions (i.e. 0 cm/w bladder pressure), the exact contribution of ATP evoked by pressure stimulation could not be determined. In addition to intravesical pressure stimulation, we also utilized intravesical chemical stimulation with the cholinergic agonist carbachol to identify whether bladder lumen and spinal cord ATP could be evoked by a different stimulation paradigm. Carbachol is a mixed nicotinic-muscarinic agonist that, when given intravesically, induces bladder overactivity (Kim et al., 2005). Since carbachol is not subject to metabolism by the enzyme acetylcholinesterase, its actions could represent the effects of endogenous acetylcholine, especially at high tissue concentrations. The fact that carbachol induced bladder wash ATP release in both NI and SCI rats suggests that a physiologic link exists between afferent cholinergic receptor activation and release of ATP within the bladder lumen and, moreover, that this connection is strengthened following SCI.
Intravesical atropine, however, did not prevent carbachol induced bladder ATP release in either rat group. These results imply that intravesical carbachol induces release of ATP within the bladder lumen by activating nicotinic receptors on urothelium or superficial afferent nerve terminals. Beckel and colleagues postulated that urothelial nicotinic receptor activation could have excitatory or inhibitory effects on reflex bladder activity depending upon whether excitatory α3* (i.e. α3β4, α3β3β4, α3α5β4, α3α5β3β4) heteromeric receptors or inhibitory homomeric α7 nicotinic receptors were activated (Beckel et al., 2006). Thus, bladder lumenal ATP release to intravesical carbachol and, perhaps, intravesical pressure stimulation may be enhanced following SCI because of greater α3* nicotinic receptor activation within bladder urothelium.
Spinal ATP release was reliably measured with microdialysis to either mechanical (i.e. 10cm/w pressure) or chemical (i.e. carbachol) bladder stimulation in both NI and SCI rats. Microdialysis has been extensively described as a method to sample transmitter release and metabolism both in the brain and spinal cord. We have previously demonstrated the technical ability to localize micturition nuclei and perform microdialysis experiments in both acute SCI and irritated bladder models (Smith et al., 2002; Rocha et al., 2003). In the current experiments, we targeted probe placement to the dorsal horn of the L6-S1 spinal cord segments, a location where bladder afferent nerve fibers in the rat are known to project (Vizzard, 2000). Because of the small size of the microdialysis probe relatively small areas can be targeted, and released substances can be collected from the immediate vicinity of the probe.
Probe recovery rate is dependent upon various factors including the length and type, the charge and the pore size of the membrane, as well as the perfusion rate and the diffusion coefficient of the compounds in question. However, the microdialysis probe sees only a fraction of the released transmitters in the extracellular space, a value that varies for different transmitters but is fairly constant between experiments for a given transmitter. For example, while McAdoo and colleagues demonstrated a 10% recovery rate for amino acids in the spinal cord, the recovery rate for ATP release in our experiments was comparably smaller (i.e. 0.3%) (McAdoo et al., 1999). In addition, because the microdialysis probe only collects the transmitters in its immediate vicinity, a high spatial resolution was observed. This finding was supported by our results demonstrating no change in ATP release to bladder stimulation when the microdialysis probe was located outside the L6-S1 spinal cord segments.
Reliable measurement of a given transmitter depends not only on local tissue concentrations and probe recovery rates but also on the sensitivity of the analytical method used. For example, previous attempts to measure adenosine release from the spinal cord failed because the applied HPLC method was not sensitive enough to measure low concentrations of purines (Patterson et al., 2001). Fortunately, the luminometric method used in this study was at least 100 times more sensitive than HPLC, and allowed us to obtain meaningful results despite the relatively low recovery rate (0.3%) through the microdialysis probe. When calculating actual spinal cord ATP release on the basis of probe recovery, we determined that ATP levels would be in the high pmol and low nmol range for NI and SCI rats, respectively. Given the constrained volume of the rat spinal cord, these calculated values are indicative of high extracellular tissue concentrations of ATP and could signify amplification of central purinergic responses to bladder stimulation, especially after SCI.
The higher ATP release from the bladder urothelium of the SCI rats to bladder pressure or chemical stimulation might explain the significantly greater spinal ATP release in the dorsal lumbosacral spinal cord segments (L6–S1) of the SCI rat. In other words, ATP released from the bladder urothelium could activate P2X3 or P2X2/3 receptors in epithelial and subepithelial layers to increase afferent nerve activity and subsequent ATP release from central primary afferent nerve terminals. However, intravesical atropine inhibited spinal cord ATP release to carbachol stimulation by 89% and 49%, respectively, in NI and SCI rats. On the one hand, these results suggest that a significant component of carbachol induced spinal ATP release in both groups result from muscarinic receptor activation of bladder afferent pathways. On the other hand, spinal ATP release persisted to a greater degree (i.e. expressed as percentage) in SCI compared to NI rats when subjected to carbachol stimulation in the presence of atropine.
Two possibilities could explain this apparent plasticity in response to intravesical cholinergic receptor stimulation in SCI rats: 1) enhanced activation of excitatory nicotinic receptors (i.e. α3*) within bladder urothelium and/or superficial afferent nerve terminals or; 2) a prominent role for ATP released within the bladder lumen in activating bladder reflex mechanisms following spinal cord injury. Prior investigations in chronic SCI rats demonstrated that intravesical treatment with the potent neurotoxin botulinum toxin A (BoNT-A) not only markedly reduced reflex bladder activity, but also significantly decreased mechanically evoked in vitro ATP release from bladder urothelium (Khera et al. 2004; Khera et al., 2005). Our current finding that intravesical atropine only partially inhibits spinal ATP release in SCI rats as well as the fact that atropine has minimal effect on carbachol induced ATP release into the bladder lumen suggests that intravesical carbachol may be indirectly activating peripheral purinergic afferent pathways that drive the development of bladder overactivity.
What is the significance of elevated spinal ATP release in SCI rats? While most attention has focused on the role of purinergic receptor mechanisms in peripheral tissues (i.e. bladder), recent evidence shows that spinal purinergic receptor activation: 1) transmits peripheral nociceptive signals and plays a functional role in detrusor overactivity induced by acute bladder inflammation, 2) promotes development of central sensitization in conditions of chronic inflammation and, 3) aggravates excitotoxicity-based neuronal degeneration following acute spinal cord injury (Masuda et al., 2006; Schwab et al., 2005; Wang et al., 2004; Sperlagh et al., 2006). Our findings support a functional role for enhanced spinal purinergic receptor activation following chronic SCI. After spinal cord injury, both ascending and descending supraspinal connections eventually regress. The loss of spinal synaptic connectivity induced by chronic SCI is thought to promote sprouting of afferent and interneuronal pathways within the lumbosacral spinal cord that ultimately leads to the development of aberrant spinal reflexes and bladder overactivity (de Groat et al., 1990). It is plausible that an amplification of evoked spinal ATP release was observed in SCI rats because of an increase in the number of afferent nerve terminals releasing ATP (i.e. sprouting) and/or because of an increase in the amount of ATP released from individual afferent nerve terminals. This phenomenon may be analogous to the amplifying mechanism of ATP release previously described in peripheral tissues (Vizi et al., 1992). We recognize that even after disruption of supraspinal pathways, multiple sources for release of ATP exist such as spinal afferent nerve terminals, interneuron’s, and even glial cells. However, irrespective of the source of ATP release, our results clearly demonstrate that central purinergic responses are amplified in chronic SCI.
In summary, SCI induces a dramatic increase in pressure and intravesical cholinergic receptor evoked bladder and spinal ATP release. In normal rats, urothelial muscarinic receptors appear to be the predominant mechanism by which cholinergic receptors induce ATP release from primary afferents in the lumbosacral spinal cord. Following SCI, however, nicotinic receptor mechanisms or, alternatively, purinergic mechanisms may become active, as evidenced by the fact that atropine was only partially effective in inhibiting carbachol-induced spinal ATP release.
Acknowledgments
This study was supported by the National Institute of Health RO1 DK 069988 (for GTS) and The Scott Department of Urology Neurourology Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beckel JM, Kanai A, Lee SJ, de Groat WC, Birder LA. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am J Physiol Renal Physiol. 2006;290(1):F103–10. doi: 10.1152/ajprenal.00098.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Barrick SR, Roppolo JR, et al. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol. 2003;285:F423–F429. doi: 10.1152/ajprenal.00056.2003. [DOI] [PubMed] [Google Scholar]
- Birder LA, Nakamura Y, Kiss S, et al. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nature Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- Brady CM, Apostolidis A, Yiangou Y, Baecker PA, et al. P2X3 immunoreactive nerve fibers in neurogenic detrusor overactivity and the effect of intravesical resiniferatoxin. Eur Urol. 2004;46:247–253. doi: 10.1016/j.eururo.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Burnstock G. P2X receptors in sensory neurons. Br J Anaesth. 2000;84:476–488. doi: 10.1093/oxfordjournals.bja.a013473. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Dunn PM, Zhong Y, Rong W, et al. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol. 2005;567:621–39. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat WC, Kawatani M, Hisamitsu T, Cheng CL, Ma CP, Thor K, Steers W, Roppolo JR. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auton Nerv Syst. 1990;30(Suppl):S71–77. doi: 10.1016/0165-1838(90)90105-r. [DOI] [PubMed] [Google Scholar]
- Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes—a possible sensory mechanism? J Physiol. 1997;505 (Pt 2):503–11. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khera M, Somogyi GT, Kiss S, Boone TB, Smith CP. Botulinum toxin A inhibits ATP release from bladder urothelium after chronic spinal cord injury. Neurochem Int. 2004;45:987–93. doi: 10.1016/j.neuint.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Khera M, Somogyi GT, Salas NA, Kiss S, Boone TB, Smith CP. In vivo effects of botulinum toxin A on visceral sensory function in chronic spinal cord-injured rats. Urology. 2005;66(1):208–12. doi: 10.1016/j.urology.2005.01.055. [DOI] [PubMed] [Google Scholar]
- Kim Y, Yoshimura N, Masuda H, de Miguel F, Chancellor MB. Antimuscarinic agents exhibit local inhibitory effects on muscarinic receptors in bladder-afferent pathways. Urology. 2005;65(2):238–42. doi: 10.1016/j.urology.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Knight GE, Bodin P, de Groat WC, Burnstock G. ATP is released from guinea pig ureter epithelium on distension. Am J Physiol, Renal Physiol. 2002;282:281–288. doi: 10.1152/ajprenal.00293.2000. [DOI] [PubMed] [Google Scholar]
- Kruse MN, Noto H, Roppolo JR, de Groat WC. Pontine control of the urinary bladder and external urethral sphincter in the rat. Brain Res. 1990;532:182–90. doi: 10.1016/0006-8993(90)91758-9. [DOI] [PubMed] [Google Scholar]
- Masuda A, Bunkyo-ku Chancellor MB, Kihara K, de Groat WC, Yoshimura N. Evidence for the involvement of spinal endogenous ATP and P2X receptors in detrusor overactivity in the rats. J Urol. 2006;175(4 Suppl):1273A. [Google Scholar]
- McAdoo DJ, Xu GY, Robak G, Hughes MG. Changes in aminoacid concentrations over time and space around an impact injury and their diffusion through the rat spinal cord. Exp Neurol. 1999;159:538–544. doi: 10.1006/exnr.1999.7166. [DOI] [PubMed] [Google Scholar]
- Nakatsuka T, Gu JG. ATP P2X receptor-mediated enhancement of glutamate release and evoked EPSCs in dorsal horn neurons of the rat spinal cord. J Neurosci. 2001;21:6522–31. doi: 10.1523/JNEUROSCI.21-17-06522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SL, Sluka KA, Arnold MA. A novel transverse push-pull microprobe: in vitro characterization and in vivo demonstration of the enzymatic production of adenosine in the spinal cord dorsal horn. J Neurochem. 2001;76(1):234–46. doi: 10.1046/j.1471-4159.2001.00016.x. [DOI] [PubMed] [Google Scholar]
- Rocha JN, Smith CP, Chancellor MB, de Groat WC, Boone TB, Somogyi GT. Changes in spinal cord transmitter release patterns during volume or noxious evoked bladder afferent activity. J Urol. 2003;169(4 Suppl):372A. [Google Scholar]
- Ruggieri MR., Sr Mechanisms of disease: role of purinergic signaling in the pathophysiology of bladder dysfunction. Nat Clin Pract Urol. 2006;3:206–15. doi: 10.1038/ncpuro0456. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Guo L, Schluesener HJ. Spinal cord injury induces early and persistent lesional P2X4 receptor expression. J Neuroimmunol. 2005;163(1–2):185–9. doi: 10.1016/j.jneuroim.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Smith CP, Somogyi GT, Bird ET, Chancellor MB, Boone TB. Neurogenic bladder model for spinal cord injury: spinal cord microdialysis and chronic urodynamics. Brain Res Brain Res Protoc. 2002;9(1):57–64. doi: 10.1016/s1385-299x(01)00137-4. [DOI] [PubMed] [Google Scholar]
- Smith CP, Vemulakonda VM, Kiss S, Boone TB, Somogyi GT. Enhanced ATP release from rat bladder urothelium during chronic bladder inflammation: effect of botulinum toxin A. Neurochem Int. 2005;47(4):291–7. doi: 10.1016/j.neuint.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Sperlagh B, Vizi ES. Neuronal synthesis, storage and release of ATP. Seminars in the Neurosciences. 1996;8:175–186. [Google Scholar]
- Sperlagh B, Vizi ES, Wirkner K, Illes P. P2X(7) receptors in the nervous system. Prog Neurobiol. 2006;78(6):327–46. doi: 10.1016/j.pneurobio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Sun Y, Keay S, De Deyne PG, Chai TC. Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J Urol. 2001;166(5):1951–6. [PubMed] [Google Scholar]
- Vizi ES. Presynaptic modulation of neurochemical transmission. Prog Neurobiol. 1979;12(3–4):181–290. doi: 10.1016/0301-0082(79)90011-x. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Sperlagh B, Baranyi M. Evidence that ATP, released from the postsynaptic site by noradrenaline, is involved in mechanical responses of guinea-pig vas deferens: cascade transmission. Neuroscience. 1992;50:455–465. doi: 10.1016/0306-4522(92)90437-7. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Increased expression of spinal cord Fos protein induced by bladder stimulation after spinal cord injury. Am J Physiol Regul Integr Comp Physiol. 2000;279:R295–305. doi: 10.1152/ajpregu.2000.279.1.R295. [DOI] [PubMed] [Google Scholar]
- Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neuroscience. 2001;21(15):5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, Nedergaard M. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10(8):821–7. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]