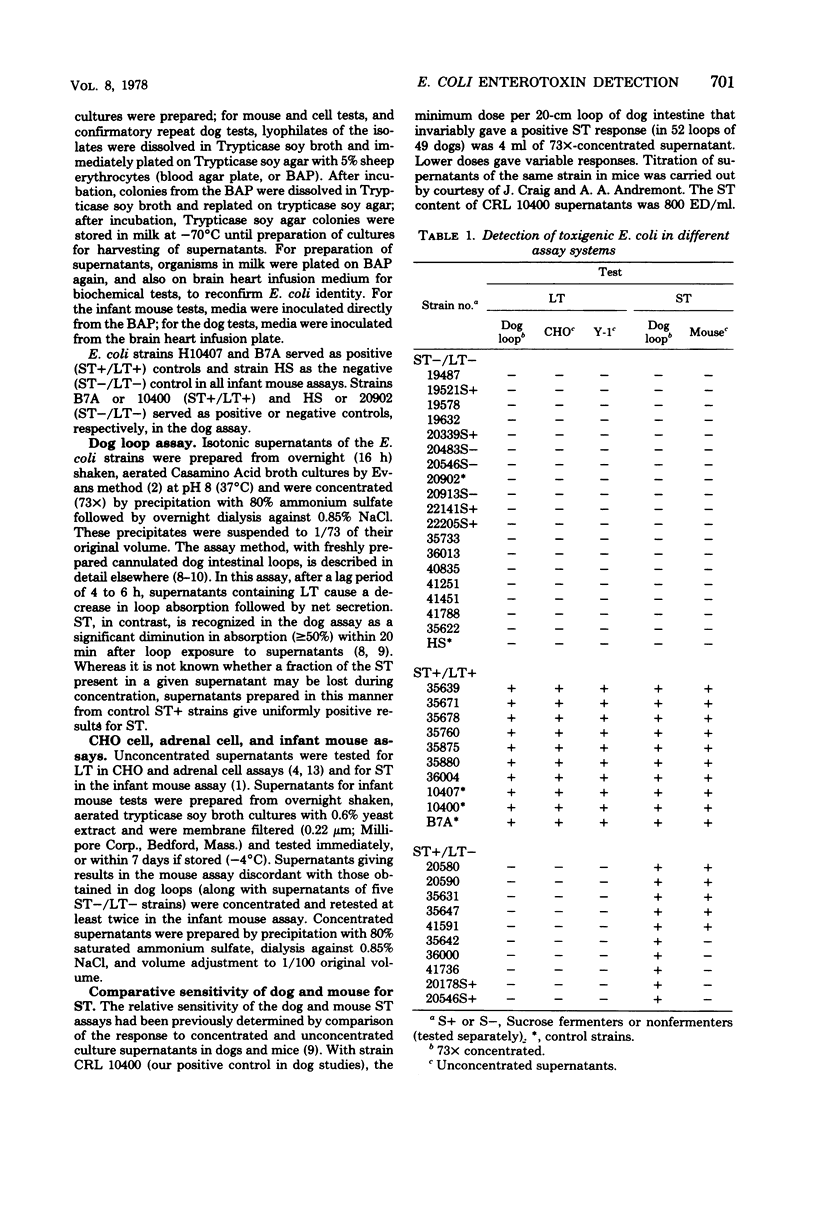
Abstract
The heat-stable enterotoxin (ST) of Escherichia coli can be detected by infant mouse or dog intestinal loop tests. These tests differ in that the dog assay uses concentrated culture supernatants and is based on measurements of net intestinal absorption, whereas the mouse test uses unconcentrated supernatants and depends on gross fluid accumulation. To compare the relative sensitivities of these assays, culture supernatants of randomly selected E. coli isolates from 34 Bangalee diarrhea patients were tested for ST in dog loops and infant mice. Supernatants were also tested for heat-labile enterotoxin (LT) in dog loops, Y-1 adrenal cells, and Chinese hamster ovary cells. E. coli supernatants that produced positive responses for both ST and LT in the dog loop assay (ST+/LT+) also produced positive responses when tested for ST in infant mice and for LT in cell lines. Supernatants of strains negative for ST and LT in dog loop (ST-/LT) were also negative in other assays. Of 10 strains positive for just ST in the dog loop test (ST+/LT-), only 5 were ST positive in the standard infant mouse test. Supernatants of the other five strains (dog loop positive, mouse test negative) were then concentrated 100-fold and retested in mice. Three of these five gave consistently positive results after concentration, and two were only intermittently positive. Concentrated supernatants of negative control strains (ST-/LT-) were all negative in mice. The dog assay detects more strains producing ST than the infant mouse test. The infant mouse test, which detects only gross fluid accumulation, failed to detect approximately half of the 10 strains which produced ST alone (ST+/LT-; P = 0.025). Concentrating supernatants for the mouse assay increases sensitivity for detection of ST, but certain E. coli strains produce a variety of ST to which infant mice do not respond.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dean A. G., Ching Y. C., Williams R. G., Harden L. B. Test for Escherichia coli enterotoxin using infant mice: application in a study of diarrhea in children in Honolulu. J Infect Dis. 1972 Apr;125(4):407–411. doi: 10.1093/infdis/125.4.407. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Gorbach S. L. Production of vascular permeability factor by enterotoxigenic Escherichia coli isolated from man. Infect Immun. 1973 Nov;8(5):725–730. doi: 10.1128/iai.8.5.725-730.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Brunton L. L., Schnaitman T. C., Rebhun L. I., Gilman A. G. Cyclic adenosine monophosphate and alteration of Chinese hamster ovary cell morphology: a rapid, sensitive in vitro assay for the enterotoxins of Vibrio cholerae and Escherichia coli. Infect Immun. 1974 Aug;10(2):320–327. doi: 10.1128/iai.10.2.320-327.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Lee C. S., Engert R. F. Assay of Escherichia coli enterotoxins by in vivo perfusion in the rat jejunum. Infect Immun. 1976 Oct;14(4):1004–1010. doi: 10.1128/iai.14.4.1004-1010.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Bergquist E. J., Nalin D. R., Waterman D. H., Hornick R. B., Young C. R., Sotman S. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet. 1978 May 27;1(8074):1119–1122. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Caplan E. S., Waterman D., Cash R. A., Hornick R. B., Snyder M. J. Diarrhea caused by Escherichia coli that produce only heat-stable enterotoxin. Infect Immun. 1977 Jul;17(1):78–82. doi: 10.1128/iai.17.1.78-82.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalin D. R., Bhattacharjee A. K., Richardson S. H. Cholera-like toxic effect of culture filtrates of Escherichia coli. J Infect Dis. 1974 Dec;130(6):595–607. doi: 10.1093/infdis/130.6.595. [DOI] [PubMed] [Google Scholar]
- Nalin D. R., McLaughlin J. C. Effects of choleragenoid and glucose on the response of dog intestine to escherichia coli enterotoxins. J Med Microbiol. 1978 May;11(2):177–186. doi: 10.1099/00222615-11-2-177. [DOI] [PubMed] [Google Scholar]
- Nalin D. R., McLaughlin J. C., Rahaman M., Yunus M., Curlin G. Enterotoxigenic Escherichia coli and idiopathic diarrhoea in Bangladesh. Lancet. 1975 Dec 6;2(7945):1116–1119. doi: 10.1016/s0140-6736(75)91005-3. [DOI] [PubMed] [Google Scholar]
- Ryder R. W., Sack D. A., Kapikian A. Z., McLaughlin J. C., Chakraborty J., Mizanur Rahman A. S., Merson M. H., Wells J. G. Enterotoxigenic Escherichia coli and Reovirus-like agent in rural Bangladesh. Lancet. 1976 Mar 27;1(7961):659–663. doi: 10.1016/s0140-6736(76)92776-8. [DOI] [PubMed] [Google Scholar]
- Sack D. A., McLaughlin J. C., Sack R. B., Orskov F., Orskov I. Enterotoxigenic Escherichia coli isolated from patients at a hospital in Dacca. J Infect Dis. 1977 Feb;135(2):275–280. doi: 10.1093/infdis/135.2.275. [DOI] [PubMed] [Google Scholar]
- Sack D. A., Sack R. B. Test for enterotoxigenic Escherichia coli using Y-1 adrenal cells in miniculture. Infect Immun. 1975 Feb;11(2):334–336. doi: 10.1128/iai.11.2.334-336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


