Abstract
Objective
Most of the B. burgdorferi genotypes have been isolated from erythema migrans (EM) skin lesions in patients with Lyme disease; outer-surface protein C (OspC) type K strains, which are 16S-23S rRNA intergenic spacer type 2 (RST 2), are most commonly recovered, but a higher percentage of OspC type A strains (RST 1), the next most common type, are detectable in blood. Our goals were to determine the B. burgdorferi genotypes in the joints of patients with Lyme arthritis.
Methods
Joint fluid samples from 124 patients seen over a 30-year period were analyzed for OspC types by semi-nested PCR and sequencing, and for RST by nested PCR and RFLP techniques. This information was correlated with clinical outcome.
Results
OspC and RST genotypes could be determined in 49 of the 124 joint fluid samples (40%). Of the 49 samples, 21 (43%) were OspC type K (RST 2), 11 (22%) were type A (RST 1), and 17 (35%) were distributed among 8 other OspC types and all 3 RSTs. However, among 17 patients who received current antibiotic regimens, all 7 infected with RST 1 strains had antibiotic-refractory arthritis compared with 4 of 6 patients infected with RST 2 strains and only 1 of 4 infected with RST 3 strains (P=0.03).
Conclusions
Most of the B. burgdorferi genotypes infected the joints of patients with Lyme arthritis, particularly OspC type K (RST 2); and genotype frequencies reflected those in EM skin lesions. However, RST 1 strains were most frequent in patients with antibiotic-refractory arthritis.
Lyme disease in the United States, which is caused by the tick-borne spirochete Borrelia burgdorferi, usually begins with an expanding skin lesion, called erythema migrans (EM) (1). Within days to weeks, spirochetes often disseminate, particularly to the nervous system, heart or joints. Within months, about 60% of untreated individuals develop intermittent or persistent arthritis, particularly affecting the knees (2). All manifestations of Lyme disease usually respond well to appropriate oral or intravenous (IV) antibiotic therapy (3). In rare cases, however, Lyme arthritis persists for months or even several years despite 2-3 months of oral or IV antibiotic therapy, or both, which has been termed antibiotic-refractory Lyme arthritis (1,4). After such therapy, PCR testing for B. burgdorferi DNA in joint fluid is usually negative (4), which suggests that joint inflammation may persist after spirochetal eradication.
Two genetic markers of B. burgdorferi, the outer-surface protein C (OspC) type and the 16S-23S rRNA intergenic spacer type (RST), have been used to correlate spirochetal strain variation with clinical outcomes (5-18). OspC typing divides B. burgdorferi strains into 21 genetically distinct types, 16 of which have been identified in the northeastern United States (5). This system allows detailed classification, but sometimes suffers from small groups (6). In contrast, the RST divides B. burgdorferi into only 3 groups (7), which allows larger numbers in individual groups, but may miss differences within groups (8). Therefore, an analysis of strains using both typing systems may give more information.
Among B. burgdorferi strains in the northeastern United States, the plasmid-encoded gene encoding OspC is in strong linkage disequilibrium with the chromosomal gene encoding the 16S-23S rRNA intergenic spacer region (rrs-rrlA), suggesting a clonal structure of strains in this geographic region (13,17,19). In previous analyses, RST 1 corresponded uniquely to OspC genotypes A and B; RST 2 only to OspC genotypes F, H, K, and N, and RST 3 to the remaining 10 OspC genotypes evaluated, including D, E, G, and I (17,18). Thus, in samples from this geographic region, the RST can be inferred accurately from the OspC sequence (18).
Previously, the 2 typing systems have been used primarily to determine the frequencies of isolates from EM skin lesions and to delineate strains associated with hematogenous dissemination. In an initial study of 132 isolates of skin, blood or CSF (5), 15 of the 21 OspC types were identified in EM skin lesions, most commonly types K and A. However, only 4 types (A, B, I, and K), which represent all 3 RSTs, were found at sites of dissemination in blood or CSF. In a later study of 6 blood isolates, dissemination occurred not only with OspC types A, B, I, and K, but also with types H and N (16), suggesting that a wider range of strains may cause disseminated disease.
Recent studies, which employed both typing systems, reported that most of the B. burgdorferi genotypes characterized by either typing system could be found in EM skin lesions, and all of the genotypes found in skin were sometimes detectable in blood (17,18). As with previous reports, OspC type K (RST 2) and OspC type A (RST 1) strains were most common. However, RST 1 strains were over-represented in blood, and RST 3 strains were under-represented (18), suggesting that B. burgdorferi genotypes may have differential pathogenicity.
Only limited information is available regarding spirochetal strains that cause infection at sites of dissemination, where culture has been difficult. Therefore, in this study, we determined the B. burgdorferi OspC and RST types directly, using PCR, RFLP, and sequencing techniques, in a unique collection of joint fluid samples from 124 patients with Lyme arthritis seen during a 30-year period.
PATIENTS AND METHODS
Patients
Joint fluid samples were available from 124 patients with Lyme arthritis who participated in studies of Lyme disease at Yale-New Haven Hospital (1975-1987), Tufts Medical Center (1987-2002), or Massachusetts General Hospital (2002-2006). The Human Investigation Committees at each of these institutions approved the protocols, and all patients (or parents of patients who were minors) provided written informed consent. All 124 patients met the criteria of the Centers for Disease Control and Prevention for the surveillance of Lyme disease (20). Patient with Lyme arthritis had intermittent or persistent episodes of monoarticular or oligoarticular arthritis, and a positive antibody response to B. burgdorferi sonicate by ELISA and Western blot, interpreted according to the CDC criteria (21). For clinical correlations, data from medical records were available in 119 of the 124 patients. The size of joint effusions was determined by joint aspiration, and the joint fluid white cell counts were determined in the hospitals' clinical laboratories.
During the initial study years (1975-1980), patients with Lyme arthritis were not treated with antibiotics (2); during the 1980s, various antibiotic regimens were tested (22-24), and by the 1990s, patients were given oral or intravenous antibiotic regimens that are now recommended by the Infectious Diseases Society of America (IDSA) (3). As physicians became more familiar with Lyme arthritis, patients were referred to us primarily because of incomplete responses to recommended antibiotic regimens. Therefore, our clinic population consisted of more patients with antibiotic-refractory arthritis, a rare manifestation of the disorder, than patients with antibiotic-responsive arthritis (4). For analysis here, as in previous studies (4,25,26), antibiotic-responsive Lyme arthritis was defined as the resolution of arthritis within 3 months after the start of no more than 4 weeks of IV antibiotics or 8 weeks of oral antibiotics. Antibiotic-refractory arthritis was defined as persistent joint swelling for >3 months after the start of >4 weeks of IV antibiotics, >8 weeks of oral antibiotics, or both.
For the comparison of OspC and RST types in patients with joint or skin manifestations of the infection, we used data from previous studies of skin isolates from EM skin lesions (5,17,18). However, with the data from our previous study (17), we recalculated the frequency of hematogenous dissemination based only on PCR evidence of B. burgdorferi DNA in blood and not on clinical signs and symptoms, since culture or PCR testing of blood provides the most direct evidence for hematogenous dissemination.
Identification of OspC types and RST in joint fluid samples
All 124 joint fluid samples were stored at -80°C. DNA isolation and extraction were carried out in a clean room that was not used for other purposes. After rapid thawing, DNA was isolated from 100μl of joint fluid diluted 1:1 with phosphate buffered saline (pH 7.4; Fisher Scientific, Pittsburgh, PA) to reduce viscosity. The extraction was done using a QIAamp DNA Mini Kit (Qiagen, Valencia, CA) according to the Blood and Body Fluids protocol, and DNA was eluted into a final volume of 50μl of sterile water.
Joint fluid samples from all 124 patients were analyzed for the B. burgdorferi ospC gene using semi-nested PCR and sequencing techniques, and for the rrs-rrlA (RST) gene using nested PCR and restriction fragment length polymorphism (RFLP) techniques. The PCR amplifications for both genes used previously described primers (6,8,17). Each 200μl PCR reaction mixture contained from 5-20μl of DNA extracted directly from joint fluid, 1X Qiagen PCR buffer (Qiagen), 0.2mM dNTPs, 2.5mM MgCl2, 0.25μM each of forward and reverse primer, and 5U of Qiagen HotStar Taq polymerase (Qiagen). A 1:10 dilution of the product from the first amplification was used in the second amplification. The PCR cycling conditions for each PCR were identical: a polymerase activation step at 95°C for 15 min, followed by 42 cycles of denaturation at 94°C for 30 sec, primer annealing at 45°C for 30 sec, and extension at 72°C. PCR products were visualized on 2% agarose gels (Invitrogen, Carlsbad, CA) with ethidium bromide. The ospC PCR yields a ~300bp band (6) and the RST PCR yields a ~941bp band (8). When a PCR product of the correct size was present, 100μl of each PCR product were eluted in 50μ1 of distilled water following removal of dNTPs, enzymes and PCR buffers using the QIAquick PCR Purification Kit (Qiagen). For each assay, a negative control sample (dH2O) and a positive control sample (20μl of DNA from an OspC type A, K, or E isolate of B. burgdorferi) were placed side-by-side to the left of the molecular weight ladder and unknown samples.
In the 67 joint fluid samples with a ~300 bp band, 10 μl of purified ospC second-round PCR product was then combined with 10μl of the external (+) primer and submitted for sequencing to the Massachusetts General Hospital (MGH) DNA Core Facility. The OspC type was identified from these sequences in 49 of the samples by nucleotide-nucleotide BLAST (27,28). In the 46 samples with a ~941bp band, the RST was determined in 19 using previously described RFLP techniques (8,17). In addition, 3 samples, 2 identified as RST 1 (OspC types A and B) and one identified as RST 2 (OspC type K), were sequenced at the MGH DNA Core Facility, which confirmed the RFLP typing results. In the 30 samples with positive OspC, but negative RST determinations, DNA was extracted again from a second aliquot of joint fluid (100 μl), and reassessed by PCR and RFLP techniques in a second effort to determine the RST directly, but an RST could be determined in only 4 additional samples. If the result was still negative, the RST type was inferred from the OspC sequence (17-19) for clinical correlations here.
Since the RST type of OspC type J strains has not been reported previously, the sequences of type J strains (GenBank accession nos. DQ437444, EF537359, EF537360, EF53736, and EF537366) were determined by BLAST (24), and these sequences were used to probe GenBank (25) for the type J RST sequences. The RST sequences were then analyzed with the NEBcutter V2.0 shareware program (28), which predicts restriction digestion patterns. The predicted RFLP pattern for the 5 OspC type J strains most closely resembled those of RST 3 strains.
Statistical Analysis
Categorical variables were compared by chi-square test. The distribution of values between groups was analyzed first by 3-way ANOVA, and if significant differences were shown, differences were assessed between the groups by the Mann-Whitney rank sum test. All P values are two-tailed. P-values less than or equal to 0.05 were considered to be statistically significant.
RESULTS
B. burgdorferi OspC and RST types in joint fluid
As determined by PCR, 74 of the 124 joint fluid samples (60%) had an OspC or RST band, or both, on agarose gels. An OspC type was then identified by sequencing in 49 samples (40%), an RST was identified by RFLP analysis in 23 samples (19%), and both an OspC type and RST were determined directly in 22 samples (18%). In each of these 22 samples, the RST matched that predicted from the ospC gene sequence, as shown previously with B. burgdorferi isolates from the northeastern United States (17,18). For the remaining 27 samples in which it was possible to determine the OspC type, but not the RST, the RST was inferred based on the ospC gene sequence. In the one sample in which only the RST could be determined, the OspC type was not known because a single RST includes multiple OspC types. Altogether, the OspC and RST types were determined in 49 samples (40%), and the RST alone was identified in 1 additional sample.
Among the 49 joint fluid samples in which both the OspC type and RST were known, 10 of the 16 OspC types found in the northeastern United States and all 3 RSTs were identified. Of the 49 samples, 21 (43%) were OspC type K (RST 2), 11 (22%) were type A (RST 1), and 17 (35%) were distributed among 8 other OspC types and all 3 RSTs (Table 1). The greater frequency of OspC type K strains than type A strains was statistically significant (P = 0.05). The distribution of RSTs was similar in the samples in which the RST type was determined directly or inferred from the OspC gene sequence (data not shown). Infection with mixed OspC or RST types was not observed. Altogether, nearly half of the patients with Lyme arthritis in whom B. burgdorferi genotypes could be determined were infected with OspC type K (RST 2) strains, and nearly one-quarter had OspC type A (RST 1) strains.
Table 1.
OspC and RST types in joint fluid samples of patients with Lyme arthritis compared with previous data from EM skin isolates.
| -- Previous Studies of EM Skin Isolates -- | Current Study Joint Fluid 1976-2006 N=50(%) | ||||
|---|---|---|---|---|---|
| Study A* | Study B* | Study C* | |||
| RST Type† | OspC Type† | Pre- 1999 N=118(%) | 1991-2005 N=290(%) | 1998-2001 N=90(%) | |
| 1 | |||||
| A | 23 (19) | 46 (16) | 27 (30) | 11 (22)‡ | |
| B | 19 (16) | 37 (13) | 11 (12) | 5 (10) | |
| Total | 42 (36) | 83 (29) | 38 (42) | 16 (33) | |
| 2 | |||||
| F | 0 | 9 (3) | 1 (1) | 1 (2) | |
| H | 6 (5) | 13 (5) | 4 (4) | 5 (10) | |
| K | 32 (27) | 86 (30) | 25 (27) | 21 (43)‡ | |
| N | 3 (3) | 17 (6) | 9 (10) | 1 (2) | |
| Total | 41 (35) | 125 (48) | 39 (43) | 28 (57) | |
| 3 | |||||
| C | 3 (3) | 2 (0.7) | 0 | 1 (2) | |
| D | 1 (1) | 4 (1.4) | 1 (1) | 0 | |
| E | 1 (1) | 14 (5) | 3 (3) | 1 (2) | |
| G | 7 (6) | 14 (5) | 2 (2) | 2 (4) | |
| I | 9 (7) | 20 (7) | 7 (8) | 0 | |
| J | 7 (6) | 3 (1) | 0 | 1 (2) | |
| M | 3 (3) | 11 (4) | 0 | 0 | |
| O | 1 (1) | 1 (0.3) | 0 | 0 | |
| T | 1 (1) | 2 (0.7) | 0 | 0 | |
| U | 2 (2) | 11 (4) | 0 | 0 | |
| Total | 35 (30) | 82 (28) | 13 (14) | 6 (12)§ | |
Study A was done by Seinost et al. (5), study B by Wormser et al. (18), and study C by Jones et al. (17). In Study A, only OspC typing was done, and the RST was inferred here from the OspC sequence.
There were no significant differences among the 4 study groups in the frequencies of any OspC type, as calculated in 2 × 4 tables by chi-square analysis. However, among the 4 groups, the frequency of RST 3 strains was significantly different (P = 0.005).
For comparison of OspC types K and A in joint fluid, P=0.05 by chi-square test.
One RST 3 strain yielded no usable OspC sequence.
Genotype frequencies in joint fluid were similar to those reported in 3 previous studies of EM skin lesions (Table 1) (5,17,18). When the frequencies of OspC types in these previous studies and in the current study were compared, there were no significant differences among the groups. Because there are only 3 RSTs, which stratifies the patients into larger groups, the frequencies of RST 3 strains was less in joint fluid than in EM skin isolates (P = 0.005), but this difference was seen only in comparison with studies A and B in which larger numbers of unusual OspC types were identified in a few patients each. Thus, we concluded that the frequencies of B. burgdorferi genotypes in joint fluid reflected those in EM skin lesions.
Clinical correlations with positive typing results
Clinical information was available in 119 of the 124 patients, and in 48 of the 119 patients (39%), the OspC type and RST were known. To identify clinical factors associated with positive typing results, clinical data in the 48 patients with positive OspC and RST results were compared with those in the 71 patients with negative OspC and RST results (Table 2). The age, sex ratio, duration of arthritis prior to the sample date, and total duration of arthritis were similar in the 2 groups. However, prior to the sample date, patients with positive results more often received oral or intra-articular steroids and had less often been given antibiotics. In addition, on the sample date, they tended to have larger joint effusions, and they had significantly higher joint fluid white cell counts.
Table 2.
Clinical data in patients with Lyme arthritis according to positive or negative typing results.
| Joint fluid samples | ||
|---|---|---|
| OspC and RST type positive N=48* | OspC and RST type negative N=71* | |
| Demographic Data | ||
| Age (years), median (range) | 35.5 (7.0-68) | 34.0 (9.0-79) |
| Number of males (%) | 36 (75) | 46 (65) |
| Year of Infection, Number of patients (%) | ||
| 1976-1985 | 27 (56) † | 25 (35) |
| 1986-1995 | 4 (8) | 17 (24) † |
| 1996-2006 | 17 (35) | 29 (41) |
| Clinical Characteristics | ||
| Duration of arthritis prior to the sample date (months), median (range) | 2.0 (0-19) | 3.0(0-24) |
| Size of joint effusion (mL), median (range) | 50 (15-200) | 40 (4-190) |
| Joint fluid white cell count, median (range) | 21,500 (4550-110,000)† | 12,600 (60-230,000) |
| Total duration of arthritis (months), median (range) | 11 (1.0-71) | 14 (0-102) |
| Treatment | ||
| Oral or intra-articular steroids prior to the sample (%) | 27 (56)† | 24 (34) |
| Antibiotic therapy prior to the sample (%) | 17 (36) | 41 (57)† |
No clinical information was available from 2 of the 50 OspC or RST type-positive patients and 3 of the 74 OspC or RST type-negative patients.
For the comparisons of patients with positive or negative typing results, P≤0.05.
It was primarily during the initial study years (1976-1985), before the cause of the disease was known and before antibiotic trials were completed (22), that patients were treated with intra-articular steroids. During this period, joint fluid samples from the majority of patients had positive typing results, whereas after this period, samples from the majority of patients had negative results (Table 2). Thus, it appeared that larger numbers of spirochetes were present in the joint fluid of patients who received intra-articular steroids, and larger numbers of organisms resulted in larger joint effusions and higher white blood cell counts.
B. burgdorferi genotypes and clinical course
To determine whether the B. burgdorferi genotype influenced clinical features of the arthritis, the 48 patients were stratified into 3 groups according to RST results (Table 3). During the first 10-year period (1976-1985), OspC type K (RST 2) strains were most often identified, whereas during the most recent 10-year period (1996-2006), there was a more equal distribution among the 3 RSTs. The age, sex ratio, duration of arthritis prior to the sample date, and treatment prior to that date were similar among the 3 groups. However, the size of the joint effusion tended to be larger and the total duration of arthritis tended to be longer in the 42 patients infected with RST1 or RST2 strains than in the 6 patients infected with RST3 strains; and the total duration of arthritis was significantly longer in those infected with RST 1 strains than in those infected with RST 3 strains (P=0.05).
Table 3.
Clinical data in patients with Lyme arthritis according to B. burgdorferi genotypes.
| Lyme arthritis patients with | |||
|---|---|---|---|
| RST1 (OspC AB) N=16 | RST 2 (OspC FHKN) N=26* | RST 3 (OspC CEGJ) N=6 | |
| Demographic Data | |||
| Age (years), median (range) | 34 (7-67) | 31 (9-68) | 47 (37-57) |
| Number of males (%) | 11 (69) | 21 (81) | 4 (67) |
| Year of Infection, Number of patients (%) | |||
| 1976-1985 | 8 | 17 | 2 |
| 1986-1995 | 1 | 3 | 0 |
| 1996-2006 | 7 | 6 | 4 |
| Clinical Characteristics | |||
| Duration of arthritis prior to the sample date (months), median (range) | 3.0 (0-18) | 2.0 (0-19) | 1.0 (0-4.0) |
| Size of joint effusion (mL), median (range) | 53 (15-100) | 48 (15-200) | 30 (15-80) |
| Total duration of arthritis (months), median (range) | 13 (2.0-64) | 12 (1.0-71) | 5.25 (1.5-14)† |
| Laboratory Data | |||
| Joint fluid white cell count, median (range) | 17,446 (6877-110,000) | 23,250 (4550-98,000) | 24,600 (17,000-40,556) |
| Treatment | |||
| Oral or intra-articular steroids prior to the sample (%) | 6 (38) | 17 (65) | 4 (67) |
| Antibiotic therapy prior to the sample (%) | 7 (44) | 7 (27) | 3 (50) |
Clinical information was not available in 2 of the 21 patients with OspC type K or RST 2.
For the comparison of groups 1 and 3, P=0.05. There were no other significant differences among the groups.
B. burgdorferi genotypes and antibiotic-refractory arthritis
Of the 48 patients, 17 seen after 1986 received antibiotic therapy according to the current guidelines of the IDSA (3), which allowed categorization in antibiotic-responsive and antibiotic-refractory groups (Table 4). Among the 17 patients, all 7 infected with RST 1 strains had antibiotic-refractory arthritis compared with 4 of 6 patients infected with RST 2 strains and only 1 of 4 infected with RST 3 strains (P=0.03). Patients in the 3 groups had arthritis for a median duration of 1-3 months prior to diagnosis. However, the median duration of arthritis after the start of therapy was significantly longer in those with RST 1 strains (10 months), intermediate in those with RST 2 strains (6 months), and shortest in those with RST 3 strains (2 months) (P=0.03) (Table 4). All 17 patients had positive PCR results for B. burgdorferi DNA in joint fluid prior to or during antibiotic treatment. However, in the 6 patients with antibiotic-refractory arthritis in whom joint fluid was obtained at least once during the post-antibiotic period (5 of whom were infected with RST 1 strains), PCR results were negative.
Table 4.
Clinical data in the 17 patients who received currently recommended antibiotic regimens stratified by OspC and RST types in joint fluid.
| Lyme arthritis patients with | |||
|---|---|---|---|
| RST1 (OspC AB) | RST 2 (OspC FHKN) | RST 3 (OspC CEGJ) | |
| No. of patients with | |||
| Antibiotic-responsive arthritis | 0 | 2 | 3 |
| Antibiotic-refractory arthritis | 7* | 4 | 1 |
| Duration of arthritis (months) median (range) | |||
| Prior to antibiotics | 1 (0.5-12) | 1.5 (0.5-8) | 3 (1-7) |
| After the start of antibiotics | 10 (4-30) † | 6 (1-11) | 2 (1-4) |
For the comparison of all 3 groups, P=0.03; for the comparison of groups 1 and 3, P=0.025.
For the comparison of all 3 groups, P=0.03
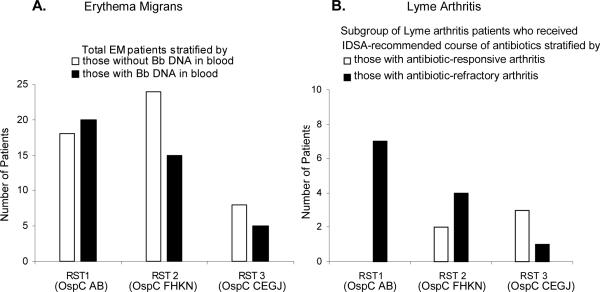
As noted previously (7,17,18), and as shown in Figure 1 using data from our previous study (17), patients with EM who were infected with RST 1 strains were more likely to have evidence of spirochetes in their blood than patients infected with RST 2 or 3 strains (Figure 1, panel A). Similarly, in the current study, a larger number of patients with Lyme arthritis who were infected with RST 1 strains had antibiotic-refractory arthritis than patients infected with RST 2 or 3 strains (Figure 1, panel B). Thus, OspC type K (RST 2) strains were most often found in the joints of patients with Lyme arthritis, but RST 1 strains were most frequent in those with antibiotic-refractory arthritis.
Figure 1. Distribution of RST (OspC) types in patients with EM or Lyme arthritis.
The total number of patients with EM was stratified according to those with (∎) or without (◻) a positive test for B. burgdorferi (Bb) DNA in blood (panel A). The subgroup of Lyme arthritis patients who received currently recommended antibiotic regimens (3), was stratified according to those with antibiotic-responsive (◻) or antibiotic-refractory arthritis (∎) (panel B).
DISCUSSION
In the current study, 10 of the 16 B. burgdorferi OspC types found in the northeastern United Sates and all 3 RST types were identified in 49 of 124 joint fluid samples (40%) from patients with Lyme arthritis seen during a 30-year period. Previous OspC or RST typing was done using culture isolates obtained from EM, blood or CSF samples in which unlimited amounts of spirochetal DNA were available (5,6,11,16). However, it has been difficult to culture B. burgdorferi from joint fluid in patients with Lyme arthritis (30,31), and the OspC type of only one joint fluid isolate (type A) has been reported (5). For this study, B. burgdorferi OspC and RST types were determined directly from joint fluid samples rather than culture isolates, using PCR and sequencing or RFLP techniques. Because only very small amounts of spirochetal DNA were present, nearly half of the samples in which typing could be done were obtained prior to the use of antibiotics for the treatment of Lyme arthritis (1975-1980) when therapy with intra-articular steroids, without antibiotics, presumably increased the spirochetal burden.
In our initial PCR study, published in 1994 (32), B. burgdorferi DNA was detected in joint fluid samples from 75 of 88 patients (85%). In the current study, in which many of the same samples were tested, 74 of 124 patients' samples (60%) had positive PCR results, and in 50 samples (40%), sequencing or RFLP analysis of the PCR product yielded an OspC type or RST. Differences between the 2 studies included that a larger percentage of the samples in the initial study were obtained in the pre-antibiotic period of Lyme arthritis (1975-1980), and the earlier study used more primer sets and a radio-labeled internal probe for the detection of gene sequences. In addition, there may have been some degradation of spirochetal DNA during the 15-year period since the first study was carried out, as suggested by the small number of samples with positive results from the middle years (1986-1995) when currently recommended antibiotic regimens were first used.
Were the results of this study skewed by the fact that it was only possible to determine the B. burgdorferi genotypes in 40% of the 124 joint fluid samples? We think that this result was caused primarily by the fact that very little or no spirochetal DNA was present in many joint fluid samples, particularly those obtained after the start of antibiotic therapy. However, in the samples in which typing could be done, 10 of the 16 OspC types and all 3 RST types were identified, and the distribution of types in joint fluid was similar to that reported in previous studies of EM skin lesions (5,7,14,16,17). Thus, we feel confident that the distribution of B. burgdorferi genotypes in joints reflects that in skin.
In a recent analysis of B. burgdorferi isolates, it was postulated that the spread of a high-virulence OspC type A clone may have contributed to the rise in the incidence of Lyme disease (33). In the current study, OspC type K strains originally comprised a large proportion of the B. burgdorferi genotypes in joints, whereas in recent years, the proportion of OspC type A strains seemed to increase. However, this apparent trend in our study could have also resulted from changes in the referral of patients. Increasingly, we were referred patients with persistent arthritis after antibiotic therapy (4), and these patients were more often infected with RST 1 strains.
The relapsing fever spirochete, Borrelia turicatae, has a structural homologue of OspC called variable surface protein (Vsp), which seems to have a role in tissue tropism (34,35). In a mouse model, VspA strains were neurotropic (34,36), whereas VspB strains were arthritogenic, and they were associated with greater numbers of spirochetes in blood (34,37). Because OspC type A (or RST 1) strains were identified more often in the blood of patients with early, disseminated disease (5,7,16,17) and because OspC type K strains were found slightly more often in CSF isolates from 20 patients with neuroborreliosis (5), we initially hypothesized that OspC type A strains might be found more often in the joints in patients with Lyme arthritis, analogous to observations with B. turicatae (34-37). Instead, OspC type K strains were found more frequently in both skin and joint fluid. Thus, our data do not suggest that OspC plays a role in tissue tropisms at different sites of dissemination in Lyme disease.
Because RST 1 strains are over-represented in blood early in the infection, and RST 3 strains are under-represented (7,18), it is thought that B. burgdorferi genotypes vary in their capacity to disseminate. In support of this observation, mice infected with RST 1 strains by needle inoculation had greater densities of spirochetes in their blood and organs, the spirochetes were present for longer durations, and the mice had more severe organ system involvement than those infected with RST 3 strains (9,10). However, when mice were infected by tick bite, as in the natural infection, RST 1 isolates displayed higher densities in blood, but the number of spirochetes in the heart or bladder was similar with either RST 1 or RST 3 strains (14). This suggests that in the natural infection, smaller numbers of RST 3 organisms, which may not be detectable in blood, are still able to spread to the joints and cause infection there. Consistent with this observation, the frequencies of B. burgdorferi genotypes in human patients in the current study were similar in EM skin lesions and in joints. Thus, it seems that all 3 RSTs have a similar predilection for dissemination, but larger numbers of RST 1 organisms are more often detectable in blood.
Why are RST 1 strains most often associated with antibiotic-refractory Lyme arthritis? We hypothesize that RST 1 strains are more virulent, leading to larger numbers of organisms in blood, but they are also more inflammatory. In a recent study, patients with antibiotic-refractory arthritis had significantly higher levels of pro-inflammatory cytokines and chemokines in joint fluid, including IL-6, TNF-α, IL-1β, IFN-γ, CXCL9 and CXCL10, than patients with antibiotic-responsive arthritis (25). However, the strong immune response induced by RST 1 strains may limit their persistence in the blood or joints. In B. burgdorferi-infected C57/BL6 mice, which were not treated with antibiotics, DNA from an RST 1 strain was initially found in higher numbers in joints than that of an RST 3 strain, but DNA from the RST 3 strain persisted longer than that of the RST 1 strain (personal communication, Dr. Linda Bockenstedt, Yale University School of Medicine). Among the current patients, it was the duration of arthritis after antibiotic therapy when PCR results were negative (4,32,38) that was significantly longer in patients infected with RST 1 strains than in those infected with RST 2 or 3 strains. Thus, a marked inflammatory response, such as that induced by RST 1 strains, may set the stage for joint inflammation that persists after spirochetal killing, particularly in patients with the HLA-DRB1*0401 or 0101 alleles (26) and immune reactivity with outer-surface protein A (39-42).
In summary, this study adds to an emerging literature concerning the differential pathogenicity of strains of B. burgdorferi (5,7,16-18). In the current study, most of the B. burgdorferi genotypes infected the joints of patients with Lyme arthritis, and genotype frequencies reflected those in EM skin lesions. However, RST 1 strains, which appear to be more virulent, were most common in patients with antibiotic-refractory arthritis.
ACKNOWLEDGMENTS
We thank Dr. Linda Bockenstedt for sharing information about RST types in a murine model of Lyme disease, and Colleen Squires for help with preparation of the manuscript.
Supported in part by grant AR-20358 from the National Institutes of Health, the English, Bonter, Mitchell Foundation, the Eshe Fund, and the Lyme/Arthritis Research Fund at Massachusetts General Hospital. Kathryn Jones received support from a scholarship from the Walter J. and Lille A. Berbecker Foundation for the study of Lyme disease, and from the National Institutes of Health training grant AR-007258
REFERENCES
- 1.Steere AC. Lyme disease. N Engl J Med. 2001;345:115–25. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 2.Steere AC, Schoen RT, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987;107:725–31. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- 3.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–34. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 4.Steere AC, Angelis SM. Therapy for Lyme arthritis: strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum. 2006;54:3079–86. doi: 10.1002/art.22131. [DOI] [PubMed] [Google Scholar]
- 5.Seinost G, Dykhuizen DE, Dattwyler RJ, Golde WT, Dunn JJ, Wang IN, et al. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect Immun. 1999;67:3518–24. doi: 10.1128/iai.67.7.3518-3524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang IN, Dykhuizen DE, Qiu W, Dunn JJ, Bosler EM, Luft BJ. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics. 1999;151:15–30. doi: 10.1093/genetics/151.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wormser GP, Liveris D, Nowakowski J, Nadelman RB, Cavaliere LF, McKenna D, et al. Association of specific subtypes of Borrelia burgdorferi with hematogenous dissemination in early Lyme disease. J Infect Dis. 1999;180:720–25. doi: 10.1086/314922. [DOI] [PubMed] [Google Scholar]
- 8.Liveris D, Varde S, Iyer R, Koenig S, Bittker S, Cooper D, et al. Genetic diversity of Borrelia burgdorferi in Lyme disease patients as determined by culture versus direct PCR with clinical specimens. J Clin Microbiol. 1999;37:565–9. doi: 10.1128/jcm.37.3.565-569.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang G, Ojaimi C, Iyer R, Saksenberg V, McClain SA, Wormser GP, et al. Impact of genotypic variation of Borrelia burgdorferi sensu stricto on kinetics of dissemination and severity of disease in C3H/HeJ mice. Infect Immun. 2001;69:4303–12. doi: 10.1128/IAI.69.7.4303-4312.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang G, Ojaimi C, Wu H, Saksenberg V, Iyer R, Liveris D, et al. Disease severity in a murine model of Lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strains. J Infect Dis. 2002;186:782–91. doi: 10.1086/343043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagal V, Postic D, Ruzic-Sabljic E, Baranton G. Genetic diversity among Borrelia strains determined by single-strand conformation polymorphism analysis of the ospC gene and its association with invasiveness. J Clin Microbiol. 2003;41:5059–65. doi: 10.1128/JCM.41.11.5059-5065.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagal V, Portnoi D, Faure G, Postic D, Baranton G. Borrelia burgdorferi sensu stricto invasiveness is correlated with OspC-plasminogen affinity. Microbes Infect. 2006;8:645–52. doi: 10.1016/j.micinf.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Bunikis J, Garpmo U, Tsao J, Berglund J, Fish D, Barbour AG. Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology. 2004;150:1741–55. doi: 10.1099/mic.0.26944-0. [DOI] [PubMed] [Google Scholar]
- 14.Dolan MC, Piesman J, Schneider BS, Schriefer M, Brandt K, Zeidner NS. Comparison of disseminated and nondisseminated strains of Borrelia burgdorferi sensu stricto in mice naturally infected by tick bite. Infect Immun. 2004;72:5262–66. doi: 10.1128/IAI.72.9.5262-5266.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ojaimi C, Mulay V, Liveris D, Iyer R, Schwartz I. Comparative transcriptional profiling of Borrelia burgdorferi clinical isolates differing in capacities for hematogenous dissemination. Infect Immun. 2005;73:6791–802. doi: 10.1128/IAI.73.10.6791-6802.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alghaferi MY, Anderson JM, Park J, Auwaerter PG, Aucott JN, Norris DE, et al. Borrelia burgdorferi ospC heterogeneity among human and murine isolates from a defined region of northern Maryland and southern Pennsylvania: lack of correlation with invasive and noninvasive genotypes. J Clin Microbiol. 2005;43:1879–84. doi: 10.1128/JCM.43.4.1879-1884.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones KL, Glickstein LJ, Damle N, Sikand VK, McHugh G, Steere AC. Borrelia burgdorferi genetic markers and disseminated disease in patients with early Lyme disease. J Clin Microbiol. 2006;44:4407–13. doi: 10.1128/JCM.01077-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wormser GP, Brisson D, Liveris D, Hanincova K, Sandigursky S, Nowakowski J, et al. Borrelia burgdorferi genotype predicts the capacity for hematogenous dissemination during early Lyme disease. J Infect Dis. 2008;198:1358–64. doi: 10.1086/592279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanincova K, Liveris D, Sandigursky S, Wormser GP, Schwartz I. Borrelia burgdorferi sensu stricto is clonal in patients with early Lyme borreliosis. Appl Environ Microbiol. 2008;74:5008–14. doi: 10.1128/AEM.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) Case definitions for infectious conditions under public health surveillance. MMWR Morb Mortal Rep Wkly. 1997;46:1–55. [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC) Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep. 1995;44:590–1. [PubMed] [Google Scholar]
- 22.Steere AC, Green J, Schoen RT, Taylor E, Hutchinson GJ, Rahn DW, et al. Successful parenteral penicillin therapy of established Lyme arthritis. N Engl J Med. 1985;312:869–74. doi: 10.1056/NEJM198504043121401. [DOI] [PubMed] [Google Scholar]
- 23.Dattwyler RJ, Halperin JJ, Volkman DJ, Luft BJ. Treatment of late Lyme borreliosis; randomized comparison of ceftriaxone and penicillin. Lancet. 1998:1191–4. doi: 10.1016/s0140-6736(88)92011-9. [DOI] [PubMed] [Google Scholar]
- 24.Steere AC, Levin RE, Molloy PJ, Kalish RA, Abraham JH, III, Liu NY, et al. Treatment of Lyme arthritis. Arthritis Rheum. 1994;37:878–88. doi: 10.1002/art.1780370616. [DOI] [PubMed] [Google Scholar]
- 25.Shin JJ, Glickstein LJ, Steere AC. High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2007;56:1325–35. doi: 10.1002/art.22441. [DOI] [PubMed] [Google Scholar]
- 26.Steere AC, Klitz W, Drouin EE, Falk BA, Kwok WW, Nepom GT, et al. Antibiotic-refractory Lyme arthritis is associated with HLA-DR molecules that bind a Borrelia burgdorferi peptide. J Exp Med. 2006;203:961–71. doi: 10.1084/jem.20052471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. Available at http://www.ncbi.nlm.nih.gov/blast/Blast.cgi. Last accessed 1 February 2008. [DOI] [PubMed] [Google Scholar]
- 28.Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL. GenBank. Nucleic Acids Res. 2006;34:D16–20. doi: 10.1093/nar/gkj157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vincze T, Posfai J, Roberts RJ. NEBcutter: A program to cleave DNA with restriction enzymes. Nucleic Acids Res. 2003;31:3688–91. doi: 10.1093/nar/gkg526. Available at http://tools.neb.com/NEBcutter2/index.php. Last accessed 1 February 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snydman DR, Schenkein DP, Berardi VP, Lastavica CC, Pariser KM. Borrelia burgdorferi in joint fluid in chronic Lyme arthritis. Ann Intern Med. 1986;104:798–800. doi: 10.7326/0003-4819-104-6-798. [DOI] [PubMed] [Google Scholar]
- 31.Schmidli J, Hunziker T, Moesli P, Schaad UB. Cultivation of Borrelia burgdorferi from joint fluid three months after treatment of facial palsy due to Lyme borreliosis. J Infect Dis. 1988;158:905–6. doi: 10.1093/infdis/158.4.905. [DOI] [PubMed] [Google Scholar]
- 32.Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330:229–34. doi: 10.1056/NEJM199401273300401. [DOI] [PubMed] [Google Scholar]
- 33.Qiu W-G, Bruno JF, McCaig WD, Xu Y, Livey I, Schriefer ME, et al. Wide distribution of a high-virulence Borrelia burgdorferi clone in Europe and North America. Emerg Infect Dis. 2008;14:1097–1104. doi: 10.3201/eid1407.070880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cadavid D, Pachner AR, Estanislao L, Patalapati R, Barbour AG. Isogenic serotypes of Borrelia turicatae show different localization in the brain and skin of mice. Infect Immun. 2001;69:3389–97. doi: 10.1128/IAI.69.5.3389-3397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cadavid D, Pennington PM, Kerentseva TA, Bergstrom S, Barbour AG. Immunologic and genetic analyses of VmpA of a neurotropic strain of Borrelia turicatae. Infect Immun. 1997;65:3352–60. doi: 10.1128/iai.65.8.3352-3360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pennington PM, Allred CD, West CS, Alvarez R, Barbour AG. Arthritis severity and spirochete burden are determined by serotype in the Borrelia turicatae-mouse model of Lyme disease. Infect Immun. 1997;65:285–92. doi: 10.1128/iai.65.1.285-292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pennington PM, Cadavid D, Barbour AG. Characterization of VspB of Borrelia turicatae, a major outer membrane protein expressed in blood and tissues of mice. Infect Immun. 1999;67:4637–45. doi: 10.1128/iai.67.9.4637-4645.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carlson D, Hernandez J, Bloom BJ, Coburn J, Aversa JM, Steere AC. Lack of Borrelia burgdorferi DNA in synovial samples from patients with antibiotic treatment-resistant Lyme arthritis. Arthritis Rheum. 1999;42:2705–9. doi: 10.1002/1529-0131(199912)42:12<2705::AID-ANR29>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 39.Drouin EE, Glickstein LJ, Steere AC. Molecular characterization of the OspA(161-175) T cell epitope associated with treatment-resistant Lyme arthritis: differences among the three pathogenic species of Borrelia burgdorferi sensu lato. J Autoimmun. 2004;23:281–92. doi: 10.1016/j.jaut.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Chen J, Field JA, Glickstein L, Molloy PJ, Huber BT, Steere AC. Association of antibiotic treatment-resistant Lyme arthritis with T cell responses to dominant epitopes of outer surface protein A of Borrelia burgdorferi. Arthritis Rheum. 1999;42:1813–22. doi: 10.1002/1529-0131(199909)42:9<1813::AID-ANR4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 41.Gross DM, Forsthuber T, Tary-Lehmann M, Etling C, Ito K, Nagy ZA, et al. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 1998;281:703–6. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 42.Drouin EE, Glickstein L, Kwok WW, Nepom GT, Steere AC. Searching for borrelial T cell epitopes associated with antibiotic-refractory Lyme arthritis. Mol Immunol. 2008;45:2323–32. doi: 10.1016/j.molimm.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]