Abstract
Purpose
Ocular pulse decreases outflow facility of perfused anterior segments. However, the mechanism by which conventional outflow tissues respond to cyclic intraocular pressure oscillations is unknown. The purpose of the present study was to examine responses of trabecular meshwork (TM) cells to cyclic biomechanical stress in the presence and absence of compounds known to affect cell contractility.
Methods
To model flow in the juxtacanalicular region of the TM and to measure changes in transendothelial flow, human TM cell monolayers on permeable filters were perfused at a constant flow rate until reaching a stable baseline pressure and then were exposed to cyclic stress with an average amplitude of 2.7 mm Hg peak to peak at a 1-Hz frequency for 2 hours in the presence or absence of compounds known to affect cell contractility (isoproterenol, Y27632, pilocarpine, and nifedipine). Pressure was recorded continuously. Immunocytochemistry staining was used to determine filamentous actin stress fiber content, whereas Western blot analysis was used to measure the extent of myosin light chain (p-MLC) phosphorylation and ratio of filamentous to globular actin.
Results
Human TM cells respond to cyclic pressure oscillations by increasing mean intrachamber pressure (decreasing hydraulic conductivity) (126.13% ± 2.4%; P < 0.05), a response blocked in the presence of Y27632, a rho-kinase inhibitor (101.35 ± 0.59; P = 0.234), but not isoproterenol, pilocarpine, or nifedipine. Although mechanical stress appeared to have no effect, Y27632 decreased phosphorylated myosin light chain, filamentous/globular actin ratio, and stress fiber formation in TM cells.
Conclusions
Human TM cells respond to cyclic mechanical stress by increasing intrachamber pressure. Pulse-mediated effects are blocked by Y27632, implicating a role for Rho-kinase-mediated signaling and cellular contractility in ocular pulse-associated changes in outflow facility.
The conventional outflow pathway is pressure sensitive, actively regulating resistance to fluid flow out of the eye.1,2 The exact mechanism by which trabecular outflow tissues sense and respond to different types of mechanical stimuli (i.e., elevated intraocular pressure, circadian rhythm, ocular pulse, shear flow) is not well understood. Previous research has focused primarily on how static changes in intraocular pressure (IOP) affect trabecular outflow tissues; however, little is known about the influence of dynamic stress on tissue regulation.3-7 Cyclic changes in IOP can be observed in vivo as a result of ocular pulse, averaging 2.7 mm Hg/s in human eyes.8,9 Significantly, cyclic oscillations in IOP decrease trabecular outflow facility by approximately 30% in perfused human and porcine anterior segments.10 Although the mechanism(s) responsible for the effect of cyclic biomechanical stress on trabecular tissues is not well understood, contractility has been suggested to play an important role in endothelial cell behavior generally and in outflow facility specifically.11-13 Thus, in the present study, we examined whether a variety of compounds (isoproterenol, a β-adrenergic receptor agonist; pilocarpine, a muscarinic receptor agonist; nifedipine, an L-type calcium channel blocker; Y27632, a rho-kinase inhibitor)14-40 previously known to affect trabecular meshwork (TM) cell contractility modulate cyclic biomechanical stress-mediated effects on TM cells.
Our goal was to determine the cellular mechanism responsible for cyclic biomechanical stress-mediated changes in outflow facility in situ.10 We hypothesized that TM cells react to cyclic oscillations in pressure (i.e., ocular pulse) in a manner similar to responses observed in the intact tissue and that pulse-mediated reactions are sensitive to exogenous modifications of TM cell contractility.
Materials and Methods
Cell Culture
Human TM cells were isolated with a blunt dissection technique followed by extracellular matrix digestion and were characterized and cultured as previously described.41 TM cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, low glucose), with the addition of 10% fetal bovine serum, penicillin (100 U/mL), streptomycin (0.1 mg/mL) and glutamine (0.29 mg/mL). Six different cell strains (TM26, TM83, TM86, TM88, TM90, and TM92) were isolated from nonglaucomatous human eye tissue (ages 15 years, 54 years, 3 months, 25 years, 4 months, and 38 years, respectively) and were used in the present study. Cell lines were chosen based on availability at the time of experiments.
TM Monolayer Perfusions
TM cells were seeded onto permeable filters (Snapwell; Costar, Cambridge, MA) consisting of a 12-mm diameter plastic insert surrounding a polycarbonate filter membrane (1-cm2 surface area; 0.4-μm pore size) at a density of 1 × 105 cells/filter and were cultured for 4 to 30 days. A previously used Ussing chamber (Ussing System CHM5; World Precision Instruments, Sarasota, FL) perfusion system15 was modified to deliver cyclic pressure oscillations while monitoring intrachamber pressure in real time. Cells on filter supports were placed on an Ussing chamber and mounted in a clamp housing. Cells were perfused with 25 mM HEPES-buffered DMEM (serum-free; pH 7.4). Constant flow was delivered to the monolayer (apical to basal) at a rate of 30 μL/min using a syringe-driven constant infusion pump (PHD 2000 Programmable Syringe Pump; Harvard Apparatus, Holliston, MA).
With this setup, we attempted to model the environment that exists in the juxtacanalicular region, where TM cells are in contact with cell neighbors and fluid flow bypasses the apical surface. Thus, we predicted that coordinated contractions of TM cells on filters modify flow pathways (openings) between adjacent cells and change hydraulic conductivity. A pressure transducer (Research Blood Pressure Transducer; Harvard Apparatus) was connected to the apical chamber to record pressure in mm Hg at a frequency of acquisition equaling 100 samples per second (Fig. 1). After a stable baseline was reached, media were exchanged with DMEM or DMEM containing 10 μM isoproterenol, 10 μM pilocarpine, 10 μM nifedipine, or 10 μM Y27632, and perfusion was restarted at a constant flow rate until it reached a new stable baseline. For every experimental condition, a minimum of two different cell lines in at least four individual experiments were perfused for each treatment group to indicate that results were not an artifact of an individual donor or experiment.
Figure 1.
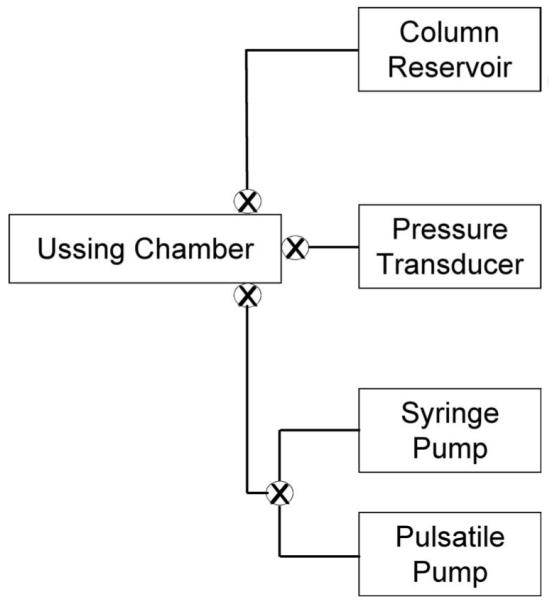
Simplified schematic diagram of the constant flow cell perfusion system modified to introduce intrachamber pressure oscillations.15 Transcellular flow in Ussing chamber occurred in an apical-to-basal direction.
We observed empirically that pressures in the range of 5 to 20 mm Hg were needed to detect control-induced contractions in our model. Thus, experiments having filters with cells that could not maintain pressures within this range were stopped, not tested with drug or pressure oscillations, and excluded from analyses. Empty filters served as negative controls, generating intrachamber pressures of approximately 0.1 mm Hg.
Cell monolayers with stable baseline pressures were exposed to cyclic pressure oscillations with average amplitudes of 2.7 mm Hg peak to peak at a 1-Hz frequency for 2 hours, using a positive piston displacement pump (Pulsatile Blood Pump; Harvard Apparatus) connected in series to the syringe pump. Our delivery system here was the identical setup we used to deliver cyclic pressure oscillations in anterior segment perfusions.10 After the pulsatile period, monolayers either were removed from the chamber or were perfused at a constant flow rate (without pressure oscillations) for 2 hours. After perfusion, cell monolayers were prepared for Western blot analysis or were fixed and DAPI-stained to verify monolayer confluence.
Immunoblot Analysis
Immediately after perfusion, filters were quickly rinsed with PBS at 4°C and were incubated for 30 minutes in Laemmli sample buffer containing 10% β-mercaptoethanol, a Roche protease inhibitor cocktail, and 5 mM NaF. Cell lysates were transferred to microfuge tubes, boiled for 10 minutes, and electrophoresed into 12% polyacrylamide gels separated by SDS-PAGE. Proteins were electrophoretically transferred to nitrocellulose over the course of 1.5 hours. Nitrocellulose membranes were blocked in 5% dry milk in Tris-buffered saline (137 mM NaCl, 25 mM Tris, 2.7 mM KCl) containing 0.2% Tween-20 (TBST) and then were probed with anti-myosin light chain (MLC, 1:1000) or anti-phospho myosin light chain (p-MLC, 1:1000) overnight at 4°C on a rocking platform (Cell Signaling Technology, Beverly, MA). Blots were rinsed three times, 10 minutes each, in TBST and incubated for 1 hour with horseradish peroxidase-conjugated secondary antibodies in 5% milk in TBST (goat anti-rabbit, 1:5000). Membranes were rinsed three more times (10 minutes each) in TBST and then incubated with chemiluminescence substrate (HyGLO; Denville Scientific; Metuchen, NJ) and exposed to x-ray film (Genesee Scientific, San Diego, CA). Film was digitized using a gel-documentation system (EpiChemi II Darkroom; Ultraviolet Products Inc., Upland, CA), and densitometry was performed using imaging software (Laboratory Works, version 4.0.0.8; Ultraviolet Products Inc.). Quantitative comparisons of p-MLC/ MLC protein ratios between some experimental groups (e.g., Y27632 perfusions) were not possible because of the absence of p-MLC signal on x-ray film.
Immunofluorescence Microscopy
Cells used for immunostaining were plated on glass coverslips and grown to confluence for at least 7 days. Monolayers were then organized in five different groups and incubated for 1 hour in HEPES-buffered DMEM (control) or DMEM containing drugs (10 μM isoproterenol, 10 μM pilocarpine, 10 μM nifedipine, or 10 μM Y27632). Isoproterenol and pilocarpine were prepared fresh in a 10 mM stock solution dissolved in HEPES-buffered DMEM; nifedipine was also prepared fresh in a 10 mM stock solution dissolved in ethanol; and Y27632 was dissolved in dH2O for a 1 mM stock solution. Before treatment, all drugs were diluted to a 10 μM solution using HEPES-buffered DMEM.
After the treatment period, cells on coverslips were rinsed twice with dPBS and were fixed using 4% paraformaldehyde for 10 minutes. Cell monolayers were permeabilized in 0.2% nonionic surfactant (Triton X-100; Sigma-Aldrich, St. Louis, MO) in PBS for 5 minutes at room temperature, washed in PBS, then blocked with a solution of PBS containing 10% goat serum (Sigma-Aldrich), 0.2% nonionic surfactant (Triton X-100; Sigma-Aldrich), and 0.5% bovine serum albumin (Sigma) for 30 minutes at 37°C. Cell monolayers were triple-labeled to visualize F-actin, nuclei, and β-catenin (an adapter protein that we used to visualize TM cell borders; data not shown). For β-catenin, coverslips were labeled using rabbit anti-β-catenin IgG (Santa Cruz Biotechnology, Santa Cruz, CA). Cells were incubated in primary antibody diluted 1:400 in blocking solution for 2 hours at 25°C, followed by three 10-minute washes in PBS + 0.2% nonionic surfactant (Triton X-100; Sigma-Aldrich). The coverslips were then incubated with a secondary antibody (fluorescein-goat anti-rabbit IgG; Molecular Probes) diluted 1:1000 in blocking solution for 60 minutes, followed by three 10-minute washes in PBS + 0.2% nonionic surfactant (Triton X-100; Sigma-Aldrich). After washing, cells were incubated for 20 minutes with Alexa Fluor 568 phalloidin (1:40 dilution; Molecular Probes) at 25°C and then were washed using PBS + 0.2% nonionic surfactant (Triton X-100; Sigma-Aldrich) and were incubated in 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) at a 1:1000 dilution for 5 minutes. Labeled cells were visualized by immunofluorescence microscopy using a confocal microscope (Nikon PCM 2000; Biomedical Imaging Facilities, The University of Arizona). DAPI-stained nuclei were visualized at a 400× magnification (IX70 microscope; Olympus, Tokyo, Japan) to evaluate the degree of confluence and stability of monolayers post perfusion. Microscopic field of views represent an approximate area of 0.02 mm2.
Filamentous/Globular Actin
The ratio of filamentous actin (F-actin) to globular actin (G-actin) in TM cells after treatment with pilocarpine or Y27632 was determined using a commercially available kit (BK037; Cytoskeleton). Briefly, confluent and mature TM monolayers were serum starved with HEPES-buffered DMEM for 2 hours and then received 10 μM pilocarpine, 10 μM Y27632, or remained untreated for 30 minutes. Cells were then lysed with F-actin stabilization buffer (50 mM PIPES, pH 6.9, 50 mM KCl, 5 mM MgCl2, 5 mM EGTA, 5% glycerol, 0.1% nonionic surfactant (Nonidet P-40; Affymetrix, Santa Clara, CA), 0.1% nonionic surfactant (Triton X-100; Sigma-Aldrich), 0.1% Tween 20, 0.1% β-mercaptoethanol, 0.001% antifoam C, 1 mM ATP, and protease inhibitor cocktail; Roche, Basel, Switzerland) and centrifuged at 100,000g for 60 minutes at 37°C. Some untreated cell lysates received either 1 μM phalloidin (positive control) or 10 μM cytochalasin-D (negative control) before centrifugation. After removing the supernatant (containing G-actin), the pellet (containing F-actin) was resuspended in an equal volume of ice-cold dH20 containing 10 μM cytochalasin-D and kept on ice for 1 hour with mixing every 15 minutes to dissociate the F-actin. Equal volumes of G-actin and F-actin samples were then mixed with 4× Laemmli buffer containing 10% β-mercaptoethanol, run on a 10% SDS-polyacrylamide gel, and subjected to immunoblot analysis. The blots were incubated with a rabbit anti-actin antibody (1:500) for 1 hour at room temperature. Relative amounts of F-actin and G-actin were determined by densitometry.
LDH Assay
To assess potential cytotoxic effects of pressure oscillations, effluent from perfused cells was monitored for LDH release. For a positive control, TM cells on filters were incubated in 1.5 mL HEPES-buffered DMEM plus 1% nonionic surfactant (Triton X-100; Sigma-Aldrich) for 10 minutes; filters were then scraped, and media were centrifuged and collected. For a negative control, TM cells on filters were incubated for 4 hours in static culture with 1.5 mL HEPES-buffered DMEM at 25°C. Effluent samples (media that bypassed TM cells) were collected from mock-exchanged TM cell perfusions at three different time points (before, during, and after the pulsatile pressure period), and samples were normalized to time, concentrated (to 1.5 mL), and treated as were control samples. Effluent media were analyzed for LDH concentration using a commercial cytotoxicity detection kit (Roche Applied Science). In brief, samples were incubated with tetrazolium salt to obtain a colored formazan, which could be quantified colorimetrically with a precision microplate reader (Emax; Molecular Probes).
Statistical Analysis
Only data in which digitized bands that corresponded to protein of interest falling in the linear range of the x-ray film were used for analyses. Values obtained were analyzed by a two-tailed, paired Student’s t-test assuming unequal variance, and differences were considered significant at P < 0.05.
Results
TM Monolayer Perfusions
Confluent TM cell monolayers were perfused at a constant flow rate of 30 μL/min. After reaching a stable intrachamber pressure (8.51 ± 0.95 mm Hg; mean ± SEM), monolayers were exposed to cyclic pressure oscillations (2.7 mm Hg peak to peak at a frequency of 1 Hz). For each perfusion, mean pressures were normalized to their baseline pressure before pulse. Results in Figure 2 show that human TM cell monolayers respond to cyclic pressure oscillations by significantly increasing mean intrachamber pressure baseline (126.13% ± 8.30%; n = 6; P < 0.05). A significant increase in mean intrachamber pressure (124.31 ± 7.26) was maintained in the 2-hour period after the pulse was stopped.
Figure 2.
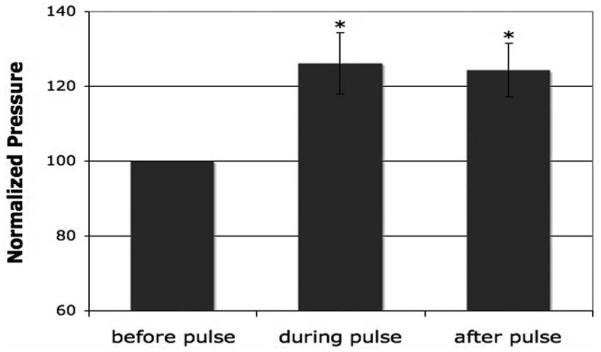
Summary of mean intrachamber pressures of perfused human TM monolayers before, during, and after the introduction of cyclic pressure oscillations. A significant increase in pressure was observed in the presence of cyclic biomechanical stress and was maintained after the pressure oscillations were stopped (*P ≤ 0.05; n = 6). Average perfusion time was 5.29 ± 0.93 hours. Results are presented as mean ± SEM.
Thirty-eight cell monolayers were used to examine the effect of contractility on the pulse-mediated increase in intrachamber pressure. The role of TM cell contractility in pulsatile pressure responses was tested with four different treatments: isoproterenol, pilocarpine, nifedipine, and Y27632. For each perfusion, average pressures were normalized to their baseline pressure before they were exposed to any experimental condition (i.e., pulse or drug exchange). After exchanging intrachamber contents, perfusion was restarted at 30 μL/min in the presence of media (control) or media-containing drug until a new stable baseline was reached. Drug effects were first analyzed for each treatment group by continuously monitoring intrachamber pressure after the exchange, as an inverse indicator of hydraulic conductivity (HC). Results in Figure 3 indicate that only two (pilocarpine and Y27632) of the four compounds affected HC in our system, each of which have been shown previously to affect TM contractility and outflow facility.14,21,35,36,38,42-45 Significant decreases in baseline intrachamber pressure in the presence of Y27632 (75.35% ± 8.32%; P < 0.01) and pilocarpine (83.48% ± 6.72%; P < 0.05) were observed compared to control monolayers (101.75% ± 2.81%).
Figure 3.
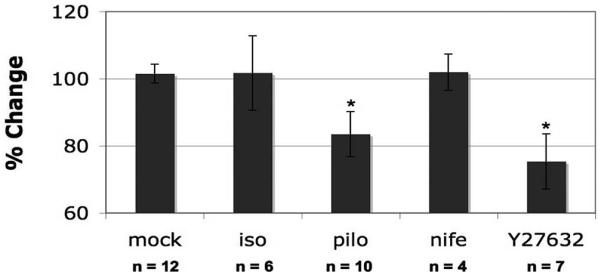
Percentage change in intrachamber pressure in response to drug treatment of human TM monolayers. TM cells were perfused under constant flow. Pressure was recorded and compared before and after the exchange of chamber contents for DMEM (control), isoproterenol (iso), pilocarpine (pilo), nifedipine (nife), or Y27632 (*P ≤ 0.05). Total duration of the perfusion was 10.97 ± 1.13 hours for the control group, 10.11 ± 0.66 hours for the isoproterenol group, 10.5 ± 0.96 hours for the pilocarpine group, 8.71 ± 2.37 hours for the nifedipine group, and 10.14 ± 1.24 hours for the Y27632 group. Results are shown as mean ± SEM.
The effect of each compound on TM monolayer responses to cyclic pressure was studied by exposing the monolayers to a combination of drug and pressure pulse simultaneously. The process of exchanging chamber contents on the pulse-mediated pressure increase was determined by comparing the response observed in the mock exchange group (117.89% ± 3.05%, n = 12) with that in a nonexchange group (126.13% ± 8.30%, n = 6). Shown in Figure 4, chamber exchange (mock) versus no chamber exchange (none) had no effect on pulse-mediated pressure increase (P = 0.269). Next, the effect of pulsatile pressure in the presence of the different cytoskeletally active compounds can also be observed in Figure 4. Results show no significant difference in the response observed between the control group and the groups treated with isoproterenol, pilocarpine, and nifedipine (P = 0.356, P = 0.892, P = 0.231, respectively); however, a statistically significant difference was observed between the control group and the group treated with the rho-kinase inhibitor Y27632 (P = 0.006) in response to pulsatile pressure.
Figure 4.
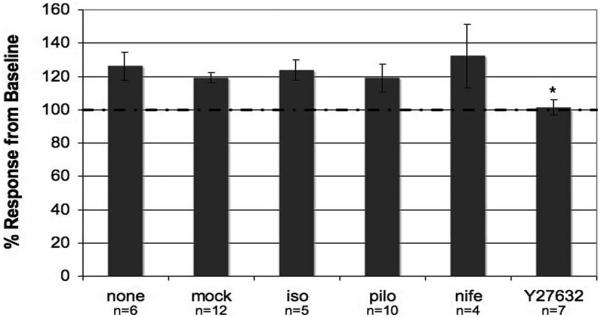
Effect of cyclic pressure oscillations in perfused human TM monolayers in the presence of different compounds. Baseline for each perfusion was normalized to the intrachamber pressure before cyclic biomechanical stress was introduced (*P ≤ 0.01). Total durations of the perfusion in hours were 10.97 ± 1.13 for the control group, 10.11 ± 0.66 for isoproterenol, 10.5 ± 0.96 for pilocarpine, 8.71 ± 2.37 for nifedipine, and 10.14 ± 1.24 for the Y27632 group. Results are shown as mean ± SEM.
In summary, our results show that the HC of TM monolayers increased in the presence of both pilocarpine and Y27632, recorded as a decrease in intrachamber mean pressure after an exchange. Although TM monolayers responded to cyclic pressure oscillations by increasing intrachamber mean pressure, only Y27632 effectively blocked pulse-mediated responses.
Immunoblot Analysis
Rho-kinase-mediated regulation of myosin light chain (MLC) phosphorylation controls cellular contraction.46,47 For this reason, we used immunoblotting to measure changes in MLC phosphorylation as an indirect measurement of contractility.
To compare the effects of the different compounds in the presence of a pulsatile pressure period, a minimum of four perfused filters from each treatment group was selected for Western blot analysis; half the filters were prepared from samples collected immediately at the end of the pulsatile pressure period (during pulse [DP]), and the other half was collected from samples that continued to be perfused for 2 more hours after the pulse was stopped (after pulse [AP]). Thus, each sample corresponded to a different perfusion experiment lasting 6 to 14 hours.
Representative samples of each treatment group are shown in Figure 5. Cells in culture (no perfusion) and cells perfused but not exposed to exchange or pulse were used as controls. No obvious change in myosin light chain phosphorylation (p-MLC) was observed in filters treated with mock, pilocarpine, or nifedipine. In contrast, though isoproterenol appeared to increase p-MLC, groups treated with Y27632 showed a clear decrease in p-MLC.
Figure 5.
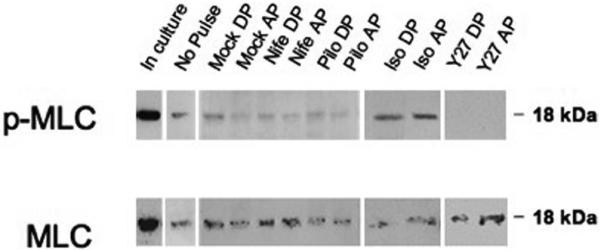
Changes in MLC phosphorylation in perfused human TM monolayer exposed to pulse. Analysis of cell lysates collected from TM monolayers grown on polycarbonate filters before perfusion (in culture or no pulse) or after perfusion. Shown are the results of one representative experiment of two total experiments for each time point in each treatment group. Top: protein levels of phosphorylated myosin light chain (p-MLC). Bottom: levels of total myosin light chain (MLC). Samples were collected after a chamber exchange with only DMEM (mock) or treated DMEM. nife, nifedipine; pilo, pilocarpine; iso, isoproterenol; Y27, Y27632; DP, during pulse; AP, after pulse.
To investigate further the effect of pulsatile pressure on p-MLC status, six TM filters from four different cell lines were perfused in the absence of pressure oscillations and were compared with five filters from three cell lines exposed to pulsatile pressure for 4 hours (data not shown). Similar to results observed when analyzing before pulse versus after pulse (Fig. 5), results in this set of experiments show that there was no difference in the ratio p-MLC/MLC when comparing monolayer perfused in the absence (5.0 ± 2.9) and presence (6.2 ± 5.5) of pulsatile pressure (P = 0.655).
Immunofluorescence
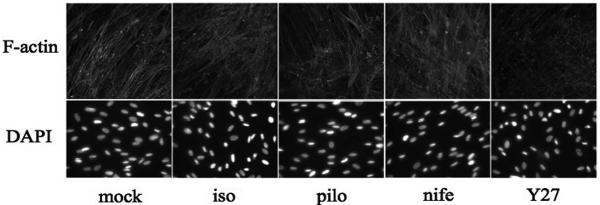
To monitor the effect of different drug treatments on actin polymerization, coverslips containing trabecular meshwork cells were treated and stained for filamentous actin (F-actin) and nuclei (Fig. 6). As a qualitative indicator, F-actin labeling with phalloidin revealed no discernible change in actin polymerization on treatment with nifedipine, isoproterenol, or pilocarpine under the conditions that correspond to drug treatments on filters. In contrast, a decrease in actin polymerization was reproducibly detected after Y27632 treatment, consistent with the decrease in p-MLC observed in the Western blot analysis (Fig. 5) and suggesting that the rho-kinase inhibition of F-actin fiber formation may be responsible for the decreased TM response to pulsatile pressure.
Figure 6.
Visualization of human TM cells on coverslips stained for F-actin (confocal microscopy) and DAPI-stained nuclei (epifluorescence microscopy). Analysis of F-actin stress fiber formation of TM cells in response to 1-hour treatments with DMEM (mock), isoproterenol (iso), pilocarpine (pilo), nifedipine (nife), or Y27632 (Y27). TM cells used for immunostaining showed similar levels of monolayer confluence between treatment groups. Results display representative experiments from a total of three experiments for each treatment group.
Filamentous/Globular Actin Ratio
To quantify effects of Y27632 on human TM cytoskeleton, we measured the relative abundance of filamentous (F)-actin compared with globular (G)-actin. Because of the consequence of pilocarpine treatment on intrachamber pressures observed in experiments shown in Figure 3, we also monitored pilocarpine effects on the F/G-actin ratio in TM cells. Figure 7 shows that treatment with Y27632 resulted in a seven-fold decrease in F/G-actin ratio in TM cell monolayers compared to untreated control (0.076 ± 0.008 vs. 0.531± 0.166, respectively, P = 0.05), consistent with qualitative immunofluorescence results and MLC phosphorylation data. Data observed after pilocarpine treatment (0.551 ± 0.077) were very similar to untreated controls (P = 0.92).
Figure 7.
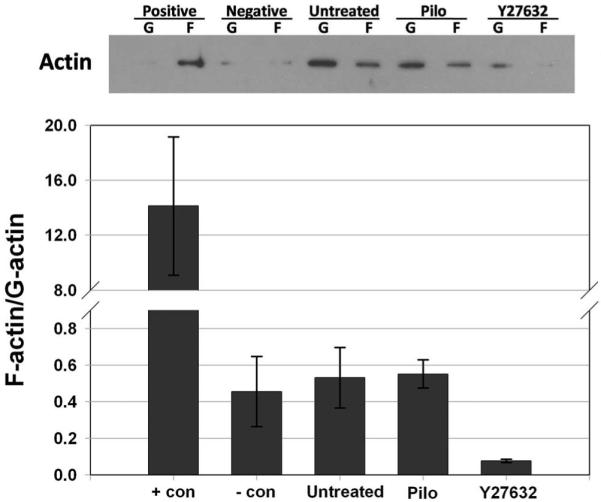
Effects of Y27632 and pilocarpine on actin polymerization in TM cell monolayers. The proportion of filamentous (F)-actin to globular (G)-actin in TM cells was analyzed after 30-minute treatments with 10 μM pilocarpine (pilo), 10 μM Y27632, 1 μM phalloidin (+ con), 10 μM cytochalasin (- con) or an untreated control. Top: actin distribution obtained by immunoblot analysis in one experiment. Bottom: summary of results (mean ± SEM) obtained from three combined experiments.
Lactate Dehydrogenase Release
Appearance of lactate dehydrogenase in the conditioned media (perfusion effluent) from human TM cells on filters was used as an indirect measurement of cell viability. Results showed no significant difference between negative control samples and effluent samples collected from TM monolayer perfusions. Negative control samples displayed 20.14% ± 1.39% absorbance, whereas “before pulse” samples showed 25.31% ± 3.21% (P = 0.16), “during pulse” samples showed 24.51% ± 3.35% (P = 0.23), and “after pulse” samples showed 20.32% ± 3.27% (P = 0.95) absorbance. These data indicate that responses observed as a result of pulsatile pressure were not a consequence of cell damage.
Discussion
The acto-myosin complex has been previously implicated as a regulator of outflow facility. For instance, actin disruptors (latrunculin-A, latrunculin-B) and calmodulin binding protein (caldesmon) alter the conventional outflow pathway, increasing outflow facility in monkey and human eyes.48-53 In addition, changes in actin organization have been observed after a steady state stretch on TM cells grown on silicone membranes.6 However, little is known about the effect that cyclic stress has on actin cytoskeleton and contractility of TM cells. By modifying an Ussing chamber perfusion system, we delivered cyclic biomechanical stress, similar in magnitude and frequency to the ocular pulse measured in vivo. The primary goal of this study was to examine and characterize the effect of cyclic biomechanical stress on human TM monolayers in perfusion culture. The present study reports two major findings: (1) Human TM cell monolayers in cell culture respond to ocular pulse in a fashion and a magnitude similar to those of intact tissue in organ culture, and (2) the rho-kinase pathway appears to mediate TM cell responses to cyclic mechanical stress. Taken together, these results suggest that effects of ocular pulse on outflow facility have a cellular basis.
Our results show that TM monolayers perfused under constant flow conditions were capable of sensing and responding to cyclic biomechanical stress with a reproducible increase in mean intrachamber pressure. Even though this response was not altered in the presence of some compounds known to affect TM cell contractility (isoproterenol, nifedipine, pilocarpine), our observed increase in pressure after exposure to cyclic stress was completely blocked in the presence of Y27632. To further evaluate TM responses to cyclic biomechanical stress, we examined downstream effectors of the Rho-kinase pathway. Similar to reports by others,36,39 our results show a clear decrease in p-MLC plus a resultant decrease in F/G actin ratio and stress fiber formation in confluent TM monolayers treated with Y27632 at the same concentration and time found to block pulse-mediated responses in perfusion experiments. Taken together, our results support the hypothesis that changes in TM cytoskeletal organization affect the response of the monolayers to cyclic biomechanical stress and that the Rho-kinase and downstream effectors are involved in the pulse-mediated response of TM cells.
Although Y27632 was the only compound tested that blocked pulse-mediated effects on intrachamber pressure, Y27632 and pilocarpine significantly decreased mean baseline pressures. Y27632 is a rho-kinase inhibitor that has previously been shown to relax TM cells in vitro35 and to cause cell retraction and rounding of cell bodies.36,37 Y27632 has also been shown to decrease intraocular pressure, therefore increasing conventional outflow facility in rabbit, bovine, porcine, and monkey eyes.36,38-40 Reported effects of pilocarpine on TM cells are more complicated. Pilocarpine binds to muscarinic cholinergic receptors, which are expressed by cells in the trabecular meshwork and the ciliary body. Although pilocarpine increases outflow facility in vivo,14,16,17,42 its effects are generally attributed to ciliary muscle contraction that overpowers a TM contraction. However, after surgical disinsertion of the ciliary muscle from the scleral spur, the direct effect of pilocarpine on TM tissue can be isolated.18,19 Studies using this procedure in monkey eyes reported a minor, and insignificant, response to pilocarpine18,20; however, in human eyes, an increase in outflow facility was observed in the presence of low concentrations of pilocarpine.20 Pilocarpine has been shown to cause contraction27,43 and relaxation20 of TM tissue. Data presented in this manuscript are ambiguous: Pilocarpine decreased intrachamber pressure (indicator of relaxation) but, unlike Y27632, had no effect on filamentous actin content in two assays or phosphorylation status of MLC. Taken together, Y27632 was the only compound tested that showed consistent effects in all five experimental paradigms.
Although Y27632 and pilocarpine result in changes in TM cell contractility in our perfusion model system, only Y27632 blocked pulse-mediated changes in intrachamber pressure, likely the result of their different mechanisms of action. We hypothesize that Y27632 is effective because it inhibits rho-kinase, a protein that resides at a “choke point” of many signaling pathways that affect cell contractility. Thus, activation of cell surface receptors, like muscarinic receptors, may not be sufficient to override signaling induced by cyclical pulsations.
The conventional outflow pathway is pressure sensitive, responding to changes in steady state IOP by altering outflow resistance. Data shown in this study and our previous study suggest an additional regulation of IOP, in which the TM outflow resistance is modified in response to cyclic oscillations in pressure. We have provided evidence showing that the regulation of contractility by Rho-kinase is necessary for trabecular meshwork cell responses to cyclic pressure oscillations. These data strongly suggest that ocular pulse is responsible for modifying a resting level of contractility (tone) in trabecular meshwork cells in vivo. Given that ocular pulse is a repetitive force continuously sensed by trabecular outflow tissues, we hypothesize that the observed decrease in hydraulic conductivity is not a change from baseline but, rather, the reestablishment of a more physiologically relevant state for TM monolayer resistance.
Although this study characterizes the role for Rho-kinase-mediated signaling in ocular pulse-associated changes of hydraulic conductivity in TM cell monolayers, it will be important in the future to extend these observations and to test the role that this pathway plays in conventional outflow tissues in situ. We predict that ocular-pulse-associated decreases in outflow facility of anterior segments will also be blunted in the presence of Y27632, strongly suggesting ocular pulse as one of the physiological forces responsible for setting TM tone in vivo, though such studies are complicated by the effects of Y27632 alone on outflow facility. Regardless, bringing observations of the present study to organ culture or in vivo studies is important to better understand the complicated process of intraocular pressure regulation. We predict in glaucoma that increased resistance to conventional outflow may be, in part, a function of aberrant responses to physiological cues such as ocular pulse.
Acknowledgments
The authors thank Ross Ethier and Darryl Overby for helpful advice during the construction of the experimental approach, Ann Baldwin for loaning the pulsatile blood pump, Kristin Perkumas for help with confocal imaging, and Emely Hoffman for providing the trabecular meshwork cells.
Supported in part by National Eye Institute Grants F31EY016887 (RFR) and EY17007 (WDS), and by the Research to Prevent Blindness Foundation.
Footnotes
Disclosure: R.F. Ramos, None; G.M. Sumida, None; W.D. Stamer, None
References
- 1.Hashimoto JM, Epstein DL. Influence of intraocular pressure on aqueous outflow facility in enucleated eyes of different mammals. Invest Ophthalmol Vis Sci. 1980;19:1483–1489. [PubMed] [Google Scholar]
- 2.Johnstone MA. Pressure-dependent changes in nuclei and the process origins of the endothelial cells lining Schlemm’s canal. Invest Ophthalmol Vis Sci. 1979;18:44–51. [PubMed] [Google Scholar]
- 3.Gonzalez P, Epstein DL, Borras T. Genes upregulated in the human trabecular meshwork in response to elevated intraocular pressure. Invest Ophthalmol Vis Sci. 2000;41:352–361. [PubMed] [Google Scholar]
- 4.Borras T, Rowlette LL, Tamm ER, Gottanka J, Epstein DL. Effects of elevated intraocular pressure on outflow facility and TIGR/MYOC expression in perfused human anterior segments. Invest Ophthalmol Vis Sci. 2002;43:33–40. [PubMed] [Google Scholar]
- 5.Bradley JM, Kelley MJ, Rose A, Acott TS. Signaling pathways used in trabecular matrix metalloproteinase response to mechanical stretch. Invest Ophthalmol Vis Sci. 2003;44:5174–5181. doi: 10.1167/iovs.03-0213. [DOI] [PubMed] [Google Scholar]
- 6.Tumminia SJ, Mitton KP, Arora J, Zelenka P, Epstein DL, Russell P. Mechanical stretch alters the actin cytoskeletal network and signal transduction in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1998;39:1361–1371. [PubMed] [Google Scholar]
- 7.Liton PB, Luna C, Bodman M, Hong A, Epstein DL, Gonzalez P. Induction of IL-6 expression by mechanical stress in the trabecular meshwork. Biochem Biophys Res Commun. 2005;337:1229–1236. doi: 10.1016/j.bbrc.2005.09.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerr J, Nelson P, O’Brien C. Pulsatile ocular blood flow in primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol. 2003;136:1106–1113. doi: 10.1016/s0002-9394(03)00717-7. [DOI] [PubMed] [Google Scholar]
- 9.Coleman DJ, Trokel S. Direct-recorded intraocular pressure variations in a human subject. Arch Ophthalmol. 1969;82:637–640. doi: 10.1001/archopht.1969.00990020633011. [DOI] [PubMed] [Google Scholar]
- 10.Ramos RF, Stamer WD. Effects of cyclic intraocular pressure on conventional outflow facility. Invest Ophthalmol Vis Sci. 2008;49:275–281. doi: 10.1167/iovs.07-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naruse K, Yamada T, Sokabe M. Involvement of SA channels in orienting response of cultured endothelial cells to cyclic stretch. Am J Physiol. 1998;274:H1532–H1538. doi: 10.1152/ajpheart.1998.274.5.H1532. [DOI] [PubMed] [Google Scholar]
- 12.Wang JH, Goldschmidt-Clermont P, Yin FC. Contractility affects stress fiber remodeling and reorientation of endothelial cells subjected to cyclic mechanical stretching. Ann Biomed Eng. 2000;28:1165–1171. doi: 10.1114/1.1317528. [DOI] [PubMed] [Google Scholar]
- 13.Naruse K, Sokabe M. Involvement of stretch-activated ion channels in Ca2+ mobilization to mechanical stretch in endothelial cells. Am J Physiol. 1993;264:C1037–C1044. doi: 10.1152/ajpcell.1993.264.4.C1037. [DOI] [PubMed] [Google Scholar]
- 14.Grierson I, Lee WR, Abraham S. Effects of pilocarpine on the morphology of the human outflow apparatus. Br J Ophthalmol. 1978;62:302–313. doi: 10.1136/bjo.62.5.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stamer WD, Roberts BC, Epstein DL. Hydraulic pressure stimulates adenosine 3′,5′-cyclic monophosphate accumulation in endothelial cells from Schlemm’s canal. Invest Ophthalmol Vis Sci. 1999;40:1983–1988. [PubMed] [Google Scholar]
- 16.Kiland JA, Hubbard WC, Kaufman PL. Low doses of pilocarpine do not significantly increase outflow facility in the cynomolgus monkey. Exp Eye Res. 2000;70:603–609. doi: 10.1006/exer.1999.0818. [DOI] [PubMed] [Google Scholar]
- 17.Barany EH. The mode of action of pilocarpine on outflow resistance in the eye primate (Cercopithecus ethiops) Invest Ophthalmol Vis Sci. 1962;1:712–727. [PubMed] [Google Scholar]
- 18.Kaufman PL, Barany EH. Loss of acute pilocarpine effect on outflow facility following surgical disinsertion and retrodisplacement of the ciliary muscle from the scleral spur in the cynomolgus monkey. Invest Ophthalmol. 1976;15:793–807. [PubMed] [Google Scholar]
- 19.Lutjen-Drecoll E, Kaufman PL, Barany EH. Light and electron microscopy of the anterior chamber angle structures following surgical disinsertion of the ciliary muscle in the cynomolgus monkey. Invest Ophthalmol Vis Sci. 1977;16:218–225. [PubMed] [Google Scholar]
- 20.Erickson KA, Schroeder A. Direct effects of muscarinic agents on the outflow pathways in human eyes. Invest Ophthalmol Vis Sci. 2000;41:1743–1748. [PubMed] [Google Scholar]
- 21.Wiederholt M, Thieme H, Stumpff F. The regulation of trabecular meshwork and ciliary muscle contractility. Prog Retinal Eye Res. 2000;19:271–295. doi: 10.1016/s1350-9462(99)00015-4. [DOI] [PubMed] [Google Scholar]
- 22.Lepple-Wienhues A, Stahl F, Willner U, Schafer R, Wiederholt M. Endothelin-evoked contractions in bovine ciliary muscle and trabecular meshwork: interaction with calcium, nifedipine and nickel. Curr Eye Res. 1991;10:983–989. doi: 10.3109/02713689109020335. [DOI] [PubMed] [Google Scholar]
- 23.Coroneo MT, Korbmacher C, Flugel C, Stiemer B, Lutjen-Drecoll E, Wiederholt M. Electrical and morphological evidence for heterogeneous populations of cultured bovine trabecular meshwork cells. Exp Eye Res. 1991;52:375–388. doi: 10.1016/0014-4835(91)90032-a. [DOI] [PubMed] [Google Scholar]
- 24.Siegner SW, Netland PA, Schroeder A, Erickson KA. Effect of calcium channel blockers alone and in combination with antiglaucoma medications on intraocular pressure in the primate eye. J Glaucoma. 2000;9:334–339. doi: 10.1097/00061198-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Segarra J, Santafe J, Garrido M, Martinez de Ibarreta MJ. The topical application of verapamil and nifedipine lowers intraocular pressure in conscious rabbits. Gen Pharmacol. 1993;24:1163–1171. doi: 10.1016/0306-3623(93)90364-4. [DOI] [PubMed] [Google Scholar]
- 26.Kelly SP, Walley TJ. Effect of the calcium antagonist nifedipine on intraocular pressure in normal subjects. Br J Ophthalmol. 1988;72:216–218. doi: 10.1136/bjo.72.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiederholt M, Schafer R, Wagner U, Lepple-Wienhues A. Contractile response of the isolated trabecular meshwork and ciliary muscle to cholinergic and adrenergic agents. Ger J Ophthalmol. 1996;5:146–153. [PubMed] [Google Scholar]
- 28.Alvarado JA, Murphy CG, Franse-Carman L, Chen J, Underwood JL. Effect of beta-adrenergic agonists on paracellular width and fluid flow across outflow pathway cells. Invest Ophthalmol Vis Sci. 1998;39:1813–1822. [PubMed] [Google Scholar]
- 29.Busch MJ, Kobayashi K, Hoyng PF, Mittag TW. Adenylyl cyclase in human and bovine trabecular meshwork. Invest Ophthalmol Vis Sci. 1993;34:3028–3034. [PubMed] [Google Scholar]
- 30.Lepple-Wienhues A, Rauch R, Clark AF, Grassmann A, Berweck S, Wiederholt M. Electrophysiological properties of cultured human trabecular meshwork cells. Exp Eye Res. 1994;59:305–311. doi: 10.1006/exer.1994.1112. [DOI] [PubMed] [Google Scholar]
- 31.Srinivas SP, Maertens C, Goon LH, et al. Cell volume response to hyposmotic shock and elevated cAMP in bovine trabecular meshwork cells. Exp Eye Res. 2004;78:15–26. doi: 10.1016/j.exer.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki R, Fujikura Y, Anderson PJ. Effects of beta-adrenergic agonists and phosphodiesterase inhibitors on the outflow facility of the eye. Nippon Ganka Gakkai Zasshi. 1997;101:551–557. [PubMed] [Google Scholar]
- 33.Kaufman PL. Epinephrine, norepinephrine, and isoproterenol dose-outflow facility response relationships in cynomolgus monkey eyes with and without ciliary muscle retrodisplacement. Acta Ophthalmol. 1986;64:356–363. doi: 10.1111/j.1755-3768.1986.tb06933.x. [DOI] [PubMed] [Google Scholar]
- 34.Kass MA, Reid TW, Neufeld AH, Bausher LP, Sears ML. The effect of d-isoproterenol on intraocular pressure of the rabbit, monkey, and man. Invest Ophthalmol. 1976;15:113–118. [PubMed] [Google Scholar]
- 35.Rosenthal R, Choritz L, Schlott S, et al. Effects of ML-7 and Y-27632 on carbachol- and endothelin-1-induced contraction of bovine trabecular meshwork. Exp Eye Res. 2005;80:837–845. doi: 10.1016/j.exer.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Honjo M, Tanihara H, Inatani M, et al. Effects of Rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci. 2001;42:137–144. [PubMed] [Google Scholar]
- 37.Koga T, Koga T, Awai M, Tsutsui J, Yue BY, Tanihara H. Rho-associated protein kinase inhibitor, Y-27632, induces alterations in adhesion, contraction and motility in cultured human trabecular meshwork cells. Exp Eye Res. 2006;82:362–370. doi: 10.1016/j.exer.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Lu Z, Overby DR, Scott PA, Freddo TF, Gong H. The mechanism of increasing outflow facility by rho-kinase inhibition with Y-27632 in bovine eyes. Exp Eye Res. 2008;86:271–281. doi: 10.1016/j.exer.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42:1029–1037. [PubMed] [Google Scholar]
- 40.Tian B, Kaufman PL. Effects of the Rho kinase inhibitor Y-27632 and the phosphatase inhibitor calyculin A on outflow facility in monkeys. Exp Eye Res. 2005;80:215–225. doi: 10.1016/j.exer.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Stamer WD, Seftor RE, Williams SK, Samaha HA, Snyder RW. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Curr Eye Res. 1995;14:611–617. doi: 10.3109/02713689508998409. [DOI] [PubMed] [Google Scholar]
- 42.Lutjen-Drecoll E, Wiendl H, Kaufman PL. Acute and chronic structural effects of pilocarpine on monkey outflow tissues. Trans Am Ophthalmol Soc. 1998;96:171–191. discussion 192-175. [PMC free article] [PubMed] [Google Scholar]
- 43.Lepple-Wienhues A, Stahl F, Wiederholt M. Differential smooth muscle-like contractile properties of trabecular meshwork and ciliary muscle. Exp Eye Res. 1991;53:33–38. doi: 10.1016/0014-4835(91)90141-z. [DOI] [PubMed] [Google Scholar]
- 44.Lutjen-Drecoll E. Structural factors influencing outflow facility and its changeability under drugs: a study in Macaca arctoides. Invest Ophthalmol. 1973;12:280–294. [PubMed] [Google Scholar]
- 45.Wiederholt M. Direct involvement of trabecular meshwork in the regulation of aqueous humor outflow. Curr Opin Ophthalmol. 1998;9:46–49. doi: 10.1097/00055735-199804000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Kimura K, Ito M, Amano M, et al. Regulation of myosin phosphatase by rho and rho-associated kinase (rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 47.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, Rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522:177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peterson JA, Tian B, Bershadsky AD, et al. Latrunculin-A increases outflow facility in the monkey. Invest Ophthalmol Vis Sci. 1999;40:931–941. [PubMed] [Google Scholar]
- 49.Ethier CR, Read AT, Chan DW. Effects of latrunculin-B on outflow facility and trabecular meshwork structure in human eyes. Invest Ophthalmol Vis Sci. 2006;47:1991–1998. doi: 10.1167/iovs.05-0327. [DOI] [PubMed] [Google Scholar]
- 50.Peterson JA, Tian B, Geiger B, Kaufman PL. Effect of latrunculin-B on outflow facility in monkeys. Exp Eye Res. 2000;70:307–313. doi: 10.1006/exer.1999.0797. [DOI] [PubMed] [Google Scholar]
- 51.Cai S, Liu X, Glasser A, et al. Effect of latrunculin-A on morphology and actin-associated adhesions of cultured human trabecular meshwork cells. Mol Vis. 2000;6:132–143. [PubMed] [Google Scholar]
- 52.Epstein DL, Rowlette LL, Roberts BC. Acto-myosin drug effects and aqueous outflow function. Invest Ophthalmol Vis Sci. 1999;40:74–81. [PubMed] [Google Scholar]
- 53.Gabelt BT, Hu Y, Vittitow JL, et al. Caldesmon transgene expression disrupts focal adhesions in HTM cells and increases outflow facility in organ-cultured human and monkey anterior segments. Exp Eye Res. 2006;82:935–944. doi: 10.1016/j.exer.2005.12.002. [DOI] [PubMed] [Google Scholar]