Abstract
Objective
Examine how meal patterns are associated with nutrient intakes, lifestyle and socioeconomic factors, and energy misreporting.
Design
A cross-sectional study within the Malmö Diet and Cancer (MDC) cohort. Participants reported on the overall types and frequency of meals consumed, and completed a modified dietary history, a lifestyle and socioeconomic questionnaire, and anthropometric measurements. Based on the reported intake of six different meal types, meal pattern groups were distinguished using Ward's cluster analysis. Associations between meal patterns and nutrient intakes, anthropometric, lifestyle and socioeconomic variables were examined using the χ2-method and analysis of variance.
Subjects
A sub-sample of the MDC study cohort (n=28,098), consisting of 1,355 men and 1,654 women.
Results
Cluster analysis identified five groups of subjects with different meal patterns in both men and women. These meal pattern groups differed regarding nutrient intakes, lifestyle and socioeconomic factors. Subjects reporting frequent coffee meals were more likely to report an ‘unhealthy’ lifestyle, e.g. smoking, high alcohol consumption and low physical activity, while those with a fruit pattern reported a more ‘healthy’ lifestyle. Women were more likely to underreport their energy intake than men, and the degree of underreporting varied between the meal pattern groups.
Conclusions
The meal pattern groups showed significant differences in dietary quality and socioeconomic and lifestyle variables. This supports previous research suggesting that diet is part of a multifaceted phenomenon. Incorporation of aspects on how foods are combined and eaten into public health advices might improve their efficiency.
Keywords: meal patterns, lifestyle, energy misreporting, cluster analysis
Health-related behaviours such as dietary habits, physical activity, and tobacco and alcohol use, are major factors influencing risks of morbidity and mortality in developed countries (1–6). The traditional approach in nutrition epidemiology has focused on the relationship between disease and specific food items or nutrients (7–9). Because the human diet does not consist of single food items or nutrients, patterns of food intake may provide important information, clarifying the association between diet and disease (7, 8, 10–12).
Meal and snack patterns are thought to affect the development of several chronic diseases (13–17), but there is no consensus on how meal patterns influence nutritional status and health. Studies investigating dietary patterns have used a wide array of approaches; including self-reported meal and snack intake (18–22), and statistical methods including factor and cluster analysis (7–9). However, most attention has been given to the eating frequency and temporal distribution of meals (23–25). There is little data on the meal and snack patterns of Swedish populations (26).
Eating patterns are related to several socio-demographic, cultural and behavioural characteristics (27–29), but data on the relationship to macronutrient or micronutrient intakes is scarce. Accurate descriptions of meals and snacks will assist in designing appropriate intervention studies and aid in understanding both the metabolic effects of different meal and snack patterns and their associations to health-related behaviours.
Energy intake (EI) plausibility can be estimated by comparing self-reported EI with estimated total energy expenditure (30). Several psychosocial and behavioural characteristics are related to energy misreporting, including incomplete recordkeeping by subjects, eating restraint, overeating and social desirability (31). Previous studies from the Malmö Diet and Cancer (MDC) cohort investigating diet–disease relationships have shown that energy misreporting and reporting of past food habit change are important in sensitivity analysis (30, 32, 33).
The purpose of this study was to investigate how nutrient intakes and anthropometric, socioeconomic and lifestyle variables varied between groups of individuals with different meal patterns defined by cluster analysis in a sub-sample of the MDC cohort. In addition, we evaluated energy misreporting and past food habit change in relation to reported meal patterns.
Subjects and methods
Study design and population
The MDC study is a population-based prospective cohort study set in Malmö, the third largest city in Sweden with about 250,000 inhabitants. In 1991, the MDC source population was defined as all persons living in the city of Malmö and born between 1926 and 1945. In May 1995, the cohort was extended to include all women born between 1923 and 1950 and all men born between 1923 and 1945. With this extension, 74,138 persons constituted the source population. Inadequate Swedish language skills and mental incapacity were the only exclusion criteria. The MDC study was approved by the Ethical Committee at the Medical Faculty, Lund University (LU 51-90). Details of the recruitment procedures and the cohort are described elsewhere (34, 35). Briefly, the participants were invited by personal letters or came spontaneously after invitation by advertisements in local newspapers, public places, or primary health care centres. The participants visited the MDC screening centre twice. During the first visit, participants were instructed about the dietary data collection, and how to fill out the extensive questionnaire covering socioeconomic and lifestyle factors. Anthropometric measurements and blood collection were conducted on site by nurses. All questionnaires were completed at home. During the second visit the socioeconomic questionnaire was checked and a dietary interview was conducted by trained interviewers. In October 1996, when recruitment was closed, 28,098 participants had completed all baseline examinations.
Individuals (n=3,037) that joined the MDC study from January 1993 until March 1994 were in addition (separately from the other diet history information) requested to give a more detailed overview of the types and number of meals consumed during an ordinary day. The present study is a descriptive, cross-sectional study of this sub-sample of the MDC cohort. After exclusion of 28 subjects on the basis of missing information on total physical activity level (PAL), this study included 3,009 subjects (1,355 men and 1,654 women) aged between 47 and 68 y.
Dietary assessment
The MDC study used an interview-based modified diet history method that combined three methods: (1) a seven-day menu-book collected descriptions of cooked meals, nutrient supplements and cold beverages (including alcoholic beverages); (2) a 168-item dietary questionnaire covering regularly eaten foods other than cooked meals during the past year; and (3) a 45-min interview. The consistency of the information provided was carefully checked so that the questionnaire and menu-book did not overlap. Energy and nutrient intakes were computed from the reported food intake using the MDC Food and Nutrient Database, originating from the Food and Nutrient Database PC Kost2-93 from the National Food Administration in Uppsala, Sweden (36). Data on the validity (37, 38) and reproducibility (39) of the method have been published.
Dietary variables
Meal types
The sub-sample of participants examined in this study provided an overview of the types of meals and snacks they usually consumed during an ordinary day, and how many times per week these meals usually were eaten. If weekends differed significantly from weekdays, the participants were requested to describe two different days (see Appendix A for further detail on registration of meals). Trained nutritionists classified the information provided by the subjects based on dietary and nutrient quality using a structured format. The meals were classified into six categories: (1) cooked/lightly cooked meals (including salads and hot sandwiches); (2) cereal meals (e.g. porridge, sandwiches and muesli); (3) cakes and biscuit meals; (4) fruits, juice or vegetables; (5) coffee or tea (only); and (6) miscellaneous snacks (e.g. sweets, energy-providing drinks). Meals could be either inclusive or non-inclusive of drinks. Meals consisting of more than one of the six categories were prioritised in numerical order (1–6), and coded as a single meal type (e.g. if a cooked meal, Type 1, also included a sandwich, Type 2, or a fruit, Type 4, it was coded as a cooked meal, Type 1, only). Intake of water was not registered.
Nutrient intakes
The nutrient variables examined in this study were: total daily EI as kJ/day coming from all major dietary energy sources (fat, carbohydrates, protein and alcohol), fibre (g/MJ) and percentage of non-alcohol energy (E%) from fat, carbohydrates and protein. For further evaluation of the diet quality, the following micronutrient intakes (per MJ) were examined: iron, calcium, magnesium, zinc, β-carotene equivalents, ascorbic acid, folic acid and vitamin E.
Socioeconomic and lifestyle variables
Information on socioeconomic and lifestyle factors was collected from the lifestyle multiple-choice questionnaire. The participants were divided into four categories to describe their educational level according to the number of years of education completed (i.e. ≤8, 9–10, 11–13, or >13 y). Cohabiting status was dichotomised as living in a single or a cohabiting household. Socioeconomic status was classified according to the Swedish population census as ‘blue-collar workers’, ‘white-collar workers’ and ‘employers/self-employed’ (in this study including high positioned white-collar workers) (40). Physical activity at work was self-rated as very light, light, medium, heavy, or very heavy (ranging from sedentary to extremely strenuous work). Three categories were formed to ensure adequate number of individuals in each category: ‘very light’, ‘light/medium’ and ‘heavy/very heavy’. An overall physical activity score was categorised into quartiles of leisure-time physical activity based on questions adapted from the Minnesota Leisure Time Physical Activity Questionnaire (41, 42). The smoking habits of the participants were defined as current smokers (including irregular smokers), former smokers or never-smokers. Alcohol consumption was classified as zero, low, medium or high. The zero alcohol consumers were distinguished by reporting no alcohol intake in the seven-day menu-book and reporting not to have consumed any alcohol during the last year. Low, medium and high alcohol consumption level was set at alcohol intakes of <15, 15–30, >30 g/day for women, and <20, 20–40, >40 g/day for men.
Evaluation of energy misreporting and past food habit change
Mattisson et al. (30) have previously defined low, adequate and high-energy reporters in the MDC cohort using the procedures described by Goldberg et al. (43) and later refined by Black (44). Energy misreporting was defined as having a ratio of EI to basal metabolic rate (BMR) outside the 95% confidence limits of the calculated PAL. The PAL level of each individual was carefully calculated using all available information in the MDC cohort. The estimation used information from the questionnaire concerning hours and intensity of leisure-time physical activity, hours of household work and hours and intensity of occupational activities. Hours of sleeping, time for self-care and ‘passive’ time were estimated (30). Individuals with EI:BMR below the lower 95% confidence limit were classified as under-reporters of EI. Individuals with EI:BMR within the confidence limits were classified as adequate-energy reporters and individuals with EI:BMR above the upper 95% confidence limit were classified as over-reporters of EI.
Past food habit change was based on the questionnaire item ‘Have you substantially changed your dietary habits because of illness or another reason?’ which was dichotomised into a ‘Yes’ and ‘No’ variable.
Anthropometric variables
Trained nurses measured weight (kg) and height (m). Body mass index (BMI) was calculated from weight (kg) over the square of height (m2). Relative weight categories (i.e. BMI < 18.5; ≥18.5– < 25; ≥25– < 30; ≥30) were used to classify individuals as underweight, normal weight, overweight, or obese, according to the World Health Organizations’ (WHO) recommendations (45).
Statistical analysis
Ward's cluster analysis was used to group the subjects based on their report of usual consumption of six different meal types. The hierarchical tree structure of the Ward's methodology provided information on the order clusters emerged. Cluster solutions of two to eight clusters were evaluated for men and women separately. Five meaningful, stable and well-separated clusters were identified in both genders. The distribution of energy and relative nutrient intakes in the population across meal pattern clusters were expressed as mean±standard deviation (SD) and analysis of variance (ANOVA) examined mean differences in relative nutrient and EIs. Nutrient variables not normally distributed were log transformed before analysis. The associations between meal pattern clusters and gender, socioeconomic and lifestyle variables were assessed using the χ2-test. Significance level was set at p<0.05. The SPSS statistical computer package (version 14; SPSS Inc, Chicago, IL) was used for all statistical analyses.
Results
Study population
The mean age of the population was 57 y (range 47–68). There was no significant age difference between men and women. In general, large percentages of the population (44%) had low educational level (≤8 y), sedentary work (47%), low alcohol consumption (79%) and were non-smoking (69%). The educational level and socioeconomic status of men was higher compared to women (p<0.001). A significantly higher proportion of women (43%) were never-smokers compared to men (28%), however the proportion of current smokers was the same (31%). Men were more likely to have medium or high alcohol consumption (31%) than women (14%). There was no difference between men and women in regards to leisure-time physical activity, however men had a significantly higher degree of occupational physical activity (p<0.001) while women spent more hours per week doing household work (p<0.001). Based on BMI, about 50% of the women were in the normal weight range; 36% overweight and 13% obese. Among men, 37% were in the normal weight range; 48% overweight and 14% obese.
Energy misreporting and past food habit change
Energy underreporting was significantly more common among women (16%) than men (10%); however 80–85% of the study population reported EI on an adequate level. Approximately 25% of the study population claimed to have changed their food habits in the past, due to illness or other reason. There was no significant difference between men and women.
Meal patterns
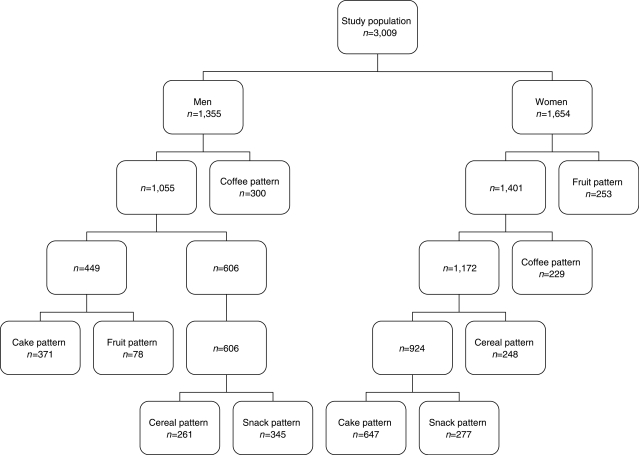
From the information on six meal types, cluster analysis identified five groups of individuals with different meal patterns separately in men and women. The groups differed not only in terms of reported meal frequencies, but also with respect to nutrient intakes and social and lifestyle characteristics. Fig. 1 illustrates the order in which the different meal patterns emerged in the male and female population. The meal pattern groups were named according to the most frequent meal type (see Table 1a and b).
Fig. 1.
The order in which the meal type patterns emerge from the male and the female population, respectively.
Table 1a.
Mean weekly frequency (95% confidence interval) of six meal types for five meal pattern groups in men (n=1,355)
| Cereal pattern | Cake pattern | Fruit pattern | Coffee pattern | Snack pattern | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| Meal type | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | P-value |
| Cooked or lightly cooked | 7.8 | 7.5–8.1 | 8.9 | 8.6–9.2 | 8.1 | 7.4–8.7 | 8.1 | 7.8–8.4 | 7.7 | 7.5–7.8 | <0.0001 |
| Cereal | 22.3 | 21.8–22.8 | 11.8 | 11.5–12.1 | 14.2 | 12.9–15.4 | 13.2 | 12.7–13.7 | 16.7 | 16.3–17.0 | <0.0001 |
| Cake or biscuits | 6.0 | 5.5–6.5 | 8.0 | 7.5–8.5 | 2.4 | 1.8–3.1 | 3.7 | 3.3–4.2 | 1.5 | 1.3–1.7 | <0.0001 |
| Fruits or vegetables | 3.3 | 2.9–3.6 | 4.1 | 3.8–4.5 | 15.3 | 13.6–17.0 | 3.2 | 2.7–3.7 | 2.7 | 2.3–3.0 | <0.0001 |
| Coffee or tea | 1.2 | 0.9–1.5 | 0.5 | 0.4–0.7 | 0.6 | 0.3–1.0 | 10.3 | 9.7–10.9 | 1.4 | 1.1–1.7 | <0.0001 |
| Miscellaneous snacks | 0.9 | 0.7–1.2 | 1.0 | 0.8–1.3 | 1.0 | 0.5–1.5 | 0.9 | 0.7–1.2 | 1.3 | 1.0–1.6 | N.S. |
Table 1b.
Mean weekly frequency (95% confidence interval) of six meal types for five meal pattern groups in women (n=1,654)
| Cereal pattern | Cake pattern | Fruit pattern | Coffee pattern | Snack pattern | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| Meal type | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | P-value |
| Cooked or lightly cooked | 7.6 | 7.3–7.9 | 7.8 | 7.7–8.0 | 8.6 | 8.3–8.9 | 9.5 | 9.1–9.9 | 8.4 | 8.1–8.7 | <0.0001 |
| Cereal | 19.5 | 18.9–20.1 | 15.4 | 15.2–15.7 | 13.4 | 13.0–13.9 | 10.7 | 10.3–11.2 | 11.7 | 11.3–12.2 | <0.0001 |
| Cakes or biscuits | 1.7 | 1.4–1.9 | 9.3 | 9.0–9.6 | 4.0 | 3.6–4.5 | 2.5 | 2.2–2.9 | 3.9 | 3.5–4.3 | <0.0001 |
| Fruits or vegetables | 2.9 | 2.5–3.3 | 4.2 | 3.9–4.5 | 14.0 | 13.5–14.4 | 3.9 | 3.5–4.3 | 5.6 | 5.3–5.9 | <0.0001 |
| Coffee or tea | 1.8 | 1.5–2.2 | 1.0 | 0.8–1.1 | 1.4 | 1.0–1.7 | 9.6 | 8.9–10.2 | 0.8 | 0.5–1.0 | <0.0001 |
| Miscellaneous snacks | 0.5 | 0.3–0.6 | 0.5 | 0.4–0.6 | 0.9 | 0.7–1.1 | 0.8 | 0.5–1.0 | 3.3 | 2.7–3.8 | <0.0001 |
Meal patterns in men
The characteristics of men in the five meal pattern groups, i.e. lifestyle factors and relative nutrient intakes, are described in Tables 2a and 3a. The cereal pattern was associated with a low educational level and alcohol consumption, and a high level of physical activity at work. The mean daily EI for men with the cereal meal pattern was high compared to the other meal patterns. The relative intake of calcium was high, while the relative intake of vitamin C was low. The cake pattern was associated with high age and socioeconomic status, cohabiting, former smoking and zero/low alcohol consumption. Mean E% from protein was lower among these individuals compared to those of other meal patterns and the relative proportion of minerals was low. The fruit pattern was associated with low socioeconomic status, living in a single household, non-smoking, low alcohol consumption and medium PAL at work. Men with the fruit meal pattern had a significantly lower daily EI and E% from fat, and higher relative micronutrient intakes and higher E% from carbohydrates (including relative fibre intake). The coffee pattern was associated with low age, high educational level and socioeconomic status, smoking, high alcohol consumption and low physical activity at work. Men in the coffee pattern group also had higher E% from fat and protein. The snack pattern was associated with living in single households and medium alcohol consumption. Relative intakes of macro- and micronutrients were similar to the other meal patterns.
Table 2a.
Characteristics (%) of the male population (n=1,355) in five meal pattern groups. Significance tested using the χ2-method
| Cereal pattern (n=261) | Cake pattern (n=371) | Fruit pattern (n=78) | Coffee pattern (n=300) | Snack pattern (n=345) | Total (n=1,355) | P-value | |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| ≤55 | 34.5 | 26.7 | 35.9 | 51.7 | 38.0 | 37.1 | <0.0001 |
| 56–64 | 47.1 | 43.7 | 47.4 | 42.3 | 43.2 | 44.1 | |
| ≥65 | 18.4 | 29.6 | 16.7 | 6.0 | 18.8 | 18.8 | |
| Education | |||||||
| ≤8 y | 55.4 | 48.2 | 50.0 | 36.0 | 47.5 | 46.8 | 0.002 |
| 9–10 y | 18.1 | 18.1 | 21.8 | 19.3 | 21.2 | 19.3 | |
| 11–13 y | 8.8 | 11.1 | 10.3 | 17.3 | 12.2 | 12.3 | |
| >13 y | 17.7 | 22.6 | 17.9 | 27.4 | 19.1 | 21.6 | |
| Socioeconomic status | |||||||
| Blue-collar | 42.2 | 32.8 | 47.4 | 27.3 | 41.7 | 36.5 | <0.0001 |
| White-collar (low/med) | 35.2 | 32.8 | 26.9 | 37.7 | 30.2 | 33.3 | |
| White-collar (high) and self-employed | 22.6 | 34.4 | 25.7 | 35.0 | 28.1 | 30.2 | |
| Cohabiting status | |||||||
| Single household | 14.9 | 14.8 | 26.9 | 17.3 | 25.2 | 18.7 | 0.001 |
| Cohabiting household | 85.1 | 85.2 | 73.1 | 82.7 | 74.8 | 81.3 | |
| Smoking status | |||||||
| Current | 31.4 | 27.8 | 19.2 | 38.7 | 32.2 | 31.5 | 0.005 |
| Former | 38.3 | 43.0 | 38.5 | 39.7 | 41.1 | 40.6 | |
| Never | 30.3 | 29.2 | 42.3 | 21.6 | 26.7 | 27.9 | |
| Alcohol consumption | |||||||
| Zero | 6.9 | 2.7 | 10.3 | 4.7 | 4.3 | 4.8 | 0.001 |
| Low | 70.5 | 68.4 | 67.9 | 59.7 | 58.6 | 64.4 | |
| Medium | 16.1 | 22.4 | 16.7 | 25.6 | 29.0 | 23.2 | |
| High | 6.5 | 6.5 | 5.1 | 10.0 | 8.1 | 7.6 | |
| Physical activity at work | |||||||
| Very light | 40.9 | 51.2 | 37.2 | 56.5 | 42.3 | 47.3 | 0.001 |
| Light/medium | 29.7 | 27.7 | 37.2 | 26.4 | 33.1 | 29.7 | |
| Heavy/very heavy | 29.4 | 21.1 | 25.6 | 17.1 | 24.6 | 23.0 | |
| Leisure time physical activity (quartiles of score) | |||||||
| q1 | 24.9 | 20.4 | 25.6 | 30.8 | 26.7 | 25.5 | n.s.* |
| q2 | 24.9 | 22.9 | 19.2 | 24.2 | 24.3 | 23.8 | |
| q3 | 23.0 | 24.0 | 24.4 | 22.5 | 25.8 | 23.9 | |
| q4 | 27.2 | 32.7 | 30.8 | 22.5 | 23.2 | 26.8 | |
| Past food habit change | |||||||
| Yes | 26.9 | 24.6 | 33.3 | 18.3 | 22.3 | 23.6 | 0.029 |
| No | 73.1 | 75.4 | 66.7 | 81.7 | 77.7 | 76.4 | |
| Energy reporting (based on physical activity level, PAL) | |||||||
| Under | 9.6 | 6.5 | 16.7 | 13.0 | 11.0 | 10.3 | 0.005 |
| Adequate | 84.3 | 91.1 | 83.3 | 83.3 | 86.1 | 86.3 | |
| Over | 6.1 | 2.4 | 0.0 | 3.7 | 2.9 | 3.4 | |
| BMI | |||||||
| Underweight (<18.5) | 0.0 | 1.1 | 0.0 | 0.7 | 0.9 | 0.7 | n.s.* |
| Normal (18.5–<25) | 38.7 | 37.2 | 37.7 | 36.7 | 36.2 | 37.1 | |
| Overweight (25–<30) | 52.5 | 46.6 | 50.6 | 46.3 | 46.4 | 47.9 | |
| Obese (303–) | 8.8 | 15.1 | 11.7 | 16.3 | 16.5 | 14.3 |
*Non-significant p ≥ 0.05.
Table 3a.
Mean nutrient intakes (standard deviation, SD) per day for men (n=1,355) in five different meal pattern groups. Significance tested with analysis of variance (ANOVA)
| Cereal pattern | Cake pattern | Fruit pattern | Coffee pattern | Snack pattern | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | P-value | |
| Energy intake (kJ) | 12,095 | 2,983 | 11,153 | 2,673 | 10,746 | 3,064 | 11,041 | 2,840 | 11,184 | 2,892 | <0.0001 |
| Total non-alcoholic energy intake (kJ) | 11,696 | 2,922 | 10,720 | 2,668 | 10,372 | 3,074 | 10,512 | 2,819 | 10,688 | 2,787 | <0.0001 |
| Fat intake (% of total non-alcoholic energy intake) | 39.8 | 6.0 | 39.8 | 6.1 | 35.2 | 6.2 | 40.8 | 6.4 | 40.2 | 6.5 | <0.0001 |
| Carbohydrate intake (% of total non-alcoholic energy intake) | 44.9 | 5.8 | 45.0 | 6.1 | 49.4 | 5.9 | 43.5 | 6.5 | 44.2 | 6.4 | <0.0001 |
| Protein intake (% of total non-alcoholic energy intake) | 15.3 | 2.3 | 15.1 | 2.4 | 15.4 | 2.8 | 15.7 | 2.3 | 15.7 | 2.3 | 0.009 |
| Fibre (g/MJ) | 2.00 | 0.54 | 1.98 | 0.56 | 2.59 | 0.69 | 1.92 | 0.59 | 1.93 | 0.58 | <0.0001* |
| Iron (mg/MJ) | 1.72 | 0.30 | 1.67 | 0.33 | 1.72 | 0.42 | 1.67 | 0.36 | 1.72 | 0.35 | N.S. |
| Calcium (mg/MJ) | 114 | 37 | 106 | 29 | 114 | 43 | 107 | 35 | 110 | 35 | p=0.027 |
| Magnesium (mg/MJ) | 35.3 | 5.6 | 34.7 | 5.4 | 39.6 | 6.3 | 36.5 | 5.9 | 35.1 | 5.6 | <0.0001 |
| Zinc (mg/MJ) | 1.21 | 0.19 | 1.15 | 0.18 | 1.21 | 0.22 | 1.20 | 0.20 | 1.22 | 0.22 | <0.0001 |
| β-carotene equivalents (mg/MJ) | 0.27 | 0.23 | 0.30 | 0.29 | 0.38 | 0.37 | 0.30 | 0.68 | 0.25 | 0.24 | <0.0001* |
| Ascorbic acid (mg/MJ) | 7.8 | 4.7 | 9.0 | 5.1 | 13.6 | 6.0 | 8.3 | 5.4 | 8.4 | 5.8 | <0.0001* |
| Folic acid (µg/MJ) | 23.6 | 6.1 | 23.4 | 6.1 | 28.1 | 6.4 | 23.7 | 7.2 | 23.8 | 5.8 | <0.0001* |
| Vitamin E (mg/MJ) | 1.04 | 0.33 | 1.06 | 0.28 | 1.16 | 0.29 | 1.07 | 0.34 | 1.05 | 0.37 | <0.0001* |
*Significance level tested with log transformed variables.
Meal patterns in women
The characteristics of women in the five meal pattern groups, i.e. lifestyle factors and relative nutrient intakes, are described in Tables 2b and 3b. The cereal pattern was associated with low educational level and socioeconomic status, living in a single household, smoking, zero/low alcohol consumption and low physical activity. Women with the cereal meal pattern had high E% from fat and high relative intakes of minerals, but low relative intakes of the examined vitamins. The cake pattern was associated with high age, low educational level, living in a cohabiting household, low alcohol consumption, medium physical activity and high daily EI with a large percentage of energy coming from fat and carbohydrates. The relative micronutrient intake was low. The fruit pattern was associated with non-smoking and high physical activity, low E% from fat, high E% from carbohydrates and high relative intakes of fibre and micronutrients. The coffee pattern was associated with low age, high educational level and socioeconomic status, living in cohabiting households, smoking, and medium/high alcohol consumption, low daily EI and high E% from protein. The snack pattern was associated with higher age, low socioeconomic status and low physical activity. Relative intakes of macro- and micronutrients were similar to the other meal patterns.
Table 2b.
Characteristics (%) of the female population (n=1,654) in five meal pattern groups. Significance tested using the χ2-method
| Cereal pattern (n=248) | Cake pattern (n=647) | Fruit pattern (n=253) | Coffee pattern (n=229) | Snack pattern (n=277) | Total (n=1,654) | P-value | |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| ≤55 | 41.9 | 31.5 | 41.9 | 64.6 | 36.1 | 40.0 | <0.0001 |
| 56–64 | 43.2 | 48.1 | 42.7 | 31.5 | 46.2 | 43.9 | |
| ≥65 | 14.9 | 20.4 | 15.4 | 3.9 | 17.7 | 16.1 | |
| Education | |||||||
| ≤8 y | 45.6 | 46.9 | 40.7 | 28.1 | 41.2 | 42.2 | <0.0001 |
| 9–10 y | 25.8 | 33.1 | 27.7 | 31.1 | 30.3 | 30.4 | |
| 11–13 y | 10.1 | 5.7 | 8.3 | 8.8 | 9.7 | 7.9 | |
| >13 y | 18.5 | 14.3 | 23.3 | 32.0 | 18.8 | 19.5 | |
| Socioeconomic status | |||||||
| Blue-collar | 43.5 | 41.5 | 40.6 | 30.0 | 42.7 | 40.2 | 0.002 |
| White-collar (low/med) | 46.3 | 47.0 | 47.0 | 48.0 | 43.0 | 46.4 | |
| White-collar (high) and self-employed | 10.2 | 11.5 | 12.4 | 22.0 | 14.3 | 13.4 | |
| Cohabiting status | |||||||
| Single household | 35.1 | 23.0 | 32.0 | 23.6 | 32.2 | 27.8 | <0.0001 |
| Cohabiting household | 64.9 | 77.0 | 68.0 | 76.4 | 67.8 | 72.2 | |
| Smoking status | |||||||
| Current | 40.1 | 26.0 | 22.9 | 44.3 | 33.6 | 31.4 | <0.0001 |
| Former | 26.3 | 24.9 | 28.1 | 25.9 | 22.7 | 25.4 | |
| Never | 33.6 | 49.1 | 49.0 | 29.8 | 43.7 | 43.2 | |
| Alcohol consumption | |||||||
| Zero | 10.1 | 8.0 | 8.7 | 5.7 | 7.6 | 8.0 | <0.0001 |
| Low | 74.6 | 82.4 | 77.1 | 70.7 | 77.6 | 78.0 | |
| Medium | 11.3 | 8.8 | 12.6 | 19.2 | 11.6 | 11.7 | |
| High | 4.0 | 0.8 | 1.6 | 4.4 | 3.2 | 2.3 | |
| Physical activity at work | |||||||
| Very light | 39.9 | 46.8 | 46.2 | 52.7 | 45.5 | 46.3 | n.s.* |
| Light/medium | 50.2 | 40.9 | 41.4 | 37.4 | 42.9 | 42.2 | |
| Heavy/very heavy | 9.9 | 12.3 | 12.4 | 9.9 | 11.6 | 11.5 | |
| Leisure time physical activity (quartiles of score) | |||||||
| q1 | 26.2 | 24.0 | 20.6 | 25.8 | 26.0 | 24.4 | 0.017 |
| q2 | 27.5 | 26.4 | 25.4 | 21.7 | 27.1 | 25.9 | |
| q3 | 25.8 | 30.2 | 23.4 | 24.9 | 21.3 | 26.2 | |
| q4 | 20.5 | 19.4 | 30.6 | 27.6 | 25.6 | 23.5 | |
| Past food habit change? | |||||||
| Yes | 31.5 | 23.2 | 24.9 | 18.8 | 25.7 | 24.5 | 0.023 |
| No | 68.5 | 76.8 | 75.1 | 81.2 | 74.3 | 75.5 | |
| Energy reporting (based on physical activity level, PAL) | |||||||
| Under | 21.4 | 9.7 | 17.8 | 26.2 | 16.2 | 16.1 | <0.0001 |
| Adequate | 74.6 | 87.5 | 81.0 | 71.2 | 79.8 | 81.0 | |
| Over | 4.0 | 2.8 | 1.2 | 2.6 | 4.0 | 2.9 | |
| BMI | |||||||
| Underweight (<18.5) | 0.4 | 1.7 | 0.8 | 1.3 | 1.1 | 1.2 | 0.025 |
| Normal (18.5– <25) | 51.8 | 52.1 | 40.0 | 53.7 | 46.4 | 49.5 | |
| Overweight (25– <30) | 35.2 | 33.6 | 45.2 | 29.3 | 40.5 | 36.2 | |
| Obese (≥30) | 12.6 | 12.6 | 14.0 | 15.7 | 12.0 | 13.1 | |
*Non-significant p ≥ 0.05.
Table 3b.
Mean nutrient intakes (standard deviation, SD) per day for women (n=1,654) in five different meal pattern groups. Significance level tested with analysis of variance (ANOVA)
| Cereal pattern | Cake pattern | Fruit pattern | Coffee pattern | Snack pattern | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | P-value | |
| Energy intake (kJ) | 8,453 | 2,078 | 8,745 | 1,999 | 8,556 | 2,026 | 7,962 | 2,232 | 8,351 | 2,333 | 0.002 |
| Total non-alcoholic energy intake (kJ) | 8,288 | 2,099 | 8,572 | 1,986 | 8,351 | 1,995 | 7,675 | 2,172 | 8,116 | 2,304 | <0.0001 |
| Fat intake (% of total non-alcoholic energy intake) | 39.5 | 6.9 | 39.0 | 5.9 | 35.8 | 5.7 | 39.2 | 6.6 | 38.5 | 6.1 | <0.0001 |
| Carbohydrate intake (% of total non-alcoholic energy intake) | 44.3 | 6.6 | 45.6 | 5.5 | 48.3 | 6.0 | 44.0 | 6.6 | 45.6 | 6.0 | <0.0001 |
| Protein intake (% of total non-alcoholic energy intake) | 16.2 | 2.8 | 15.5 | 2.3 | 15.9 | 2.6 | 16.8 | 2.6 | 15.9 | 2.9 | <0.0001 |
| Fibre (g/MJ) | 2.19 | 0.69 | 2.23 | 0.61 | 2.71 | 0.71 | 2.25 | 0.69 | 2.25 | 0.76 | <0.0001* |
| Iron (mg/MJ) | 1.64 | 0.31 | 1.64 | 0.30 | 1.64 | 0.34 | 1.65 | 0.32 | 1.59 | 0.33 | N.S. |
| Calcium (mg/MJ) | 136 | 42 | 126 | 34 | 131 | 35 | 129 | 40 | 132 | 37 | 0.003 |
| Magnesium (mg/MJ) | 38.2 | 6.6 | 36.7 | 5.9 | 40.8 | 6.2 | 39.7 | 7.0 | 38.5 | 8.1 | <0.0001 |
| Zinc (mg/MJ) | 1.25 | 0.22 | 1.20 | 0.20 | 1.23 | 0.20 | 1.27 | 0.23 | 1.21 | 0.22 | <0.0001 |
| β-carotene equivalents (mg/MJ) | 0.43 | 0.44 | 0.40 | 0.36 | 0.58 | 0.45 | 0.46 | 0.44 | 0.45 | 0.45 | <0.0001* |
| Ascorbic acid (mg/MJ) | 11.0 | 6.5 | 11.4 | 5.8 | 16.8 | 7.2 | 13.6 | 8.0 | 13.6 | 8.3 | <0.0001* |
| Folic acid (µg/MJ) | 28.1 | 7.6 | 26.4 | 6.8 | 31.5 | 8.1 | 29.2 | 9.9 | 27.8 | 8.9 | <0.0001* |
| Vitamin E (mg/MJ) | 1.13 | 0.40 | 1.14 | 0.34 | 1.25 | 0.35 | 1.21 | 0.31 | 1.14 | 0.36 | <0.0001* |
*Significance level tested with log transformed variables.
Energy misreporting and past food habit change
Underreporting among men was highest in the fruit pattern group (16.7%) and among women in the coffee pattern group (26.2%). Underreporting was lowest in the cake pattern groups for both men (6.5%) and women (9.7%). Over-reporting of energy was most common for men with the cereal pattern (6.1%) and women with the cereal and snack patterns (4.0%). Reporting a change in food habits in the past was most common for men with the fruit pattern (33.3%) and women with the cereal pattern (31.5%). Men and women in the coffee pattern groups were the least likely to have changed their food habits in the past (18.3% and 18.8%, respectively).
Discussion
This study shows that specific meal patterns are associated with differences in reported energy and selected nutrient intakes in this sample from a Swedish population. The socioeconomic, lifestyle and anthropometric profile of men and women in our study population differed significantly, which strongly supports that examination of dietary habits should be carried out separately for men and women. Further, results also showed that men and women with apparently similar meal patterns sometimes differed in regards to nutrient intakes as well as behavioural and social profiles. This suggests that men and women may choose different foods and/or food combinations even when they report similar intake of different meal types. Energy misreporting and past food habit change was associated with reported meal types.
In population-based research, it is important to recognise that subjects who agree to participate in a study are likely to be different from those who choose not to. The differences between participants and non-participants in the MDC study has been described elsewhere (34), and it was concluded that the proportion of participants reporting good health is higher in the MDC study than in non-participants, but the socio-demographic structure was similar.
An interesting result is the order in which the meal patterns emerge among men and women (see Fig. 1). The clusters that emerge first may be considered more stable. For men the most distinct behaviour was the coffee pattern, while among women it was the fruit pattern. The snack pattern emerged last among both men and women, with less distinct characteristics, suggesting that this aggregate represent a less stable pattern (i.e. a mix of several meal patterns). A limitation with cluster analysis is that there is no standard for determining the number of clusters. However, the five-cluster solution that was used consisted of at least four stable, meaningful clusters that were well separated in both genders. Another problem is that the within cluster range is often wide and may contain null intakes of one or more meals chosen to identify the clusters (i.e. less distinct clusters). Together with misreporting, this may produce clusters with less distinct characteristics (16).
Our findings also support previous studies that dietary patterns are likely to vary according to sex, socioeconomic status, culture and health-behaviours (8–10, 17, 27–29, 46, 47). The interaction among physiological, psychological and socio-cultural forces are associated with most health-related behaviours and all affect food choices and may in themselves also play an important role in the aetiologies of chronic diseases (10, 48). The relation of a specific meal pattern to characteristics that are often associated with ‘healthy’ or ‘un-healthy’ lifestyles is best illustrated in this study by the coffee pattern which was closely related to a sedentary behaviour, high alcohol consumption and smoking, which are established risk factors for disease (47–49). On the other hand, the fruit pattern was associated with non-smoking, low alcohol consumption and higher level of physical activity (see Table 2a and b). However, it is important to note that the associations seen between e.g. the fruit pattern and lifestyle factors cannot be attributed to fruit per se.
An important finding of this study is that meal patterns are clearly linked to differences in dietary quality (Table 3a and 3b). Individuals with the coffee pattern consumed diets with high E% from fat and protein, and the relative intake of micronutrients was high compared to the other patterns; suggesting an overall diet with meat and cooked foods but maybe less plant foods. Individuals of the fruit pattern consumed diets with low E% from fat and high relative intakes of fibre and micronutrients, especially vitamins, suggesting an overall diet high in plant foods. However, this group had a large proportion of under-reporters and overweight individuals. It is possible that some individuals in this group unconsciously change their diet during the registration period and/or they over-report ‘healthy’ foods, because of social desirability. The cake pattern was associated with low relative micronutrient intakes and high E% from fat and carbohydrates. The cereal pattern was associated with high relative intakes of minerals but low relative intakes of vitamins; this could be due to replacing cooked foods, vegetables and fruits with cereal and sandwich meals. Nutrient intake characteristics of individuals of the snack pattern group were less distinct compared to the other meal patterns.
A common source of bias for studies on diet is energy misreporting. EI is commonly underestimated and ‘social desirability’ may further influence both what subjects actually eat and what they report eating. Obese individuals tend to underreport their dietary intake (50–53), but whether this is a consequence of selective underreporting of certain foods or general underreporting of all foods is not known (54). Consistent with previous studies (50, 54), women in this study were more likely to underreport their EI than men. Also, the degree of underreporting was different for specific meal patterns. A large proportion of men with the fruit pattern were classified as under-reporters, and for women underreporting was more common in the coffee pattern group. It is possible that some women report having only coffee, and neglect to report an accompanying snack or cake, leading to misclassification of this meal type. For both men and women, the cake pattern had the lowest proportion of under-reporters; suggesting that individuals reporting a high frequency of cake and biscuit meals do not mind report eating ‘unhealthy’ foods. Also, a comparatively large proportion (~25%) of the study population claimed to have changed their dietary habits in the past, suggesting less stable food habits. Men with the fruit pattern and women with the cereal pattern reported past food habit change to a larger extent than men and women with the other meal patterns. Men and women in the coffee pattern groups were the least likely to have changed their food habits in the past. The importance of accounting for energy misreporting and past food habit change in studies investigating diet–disease associations has previously been shown (30, 33).
The definition of an eating occasion varies in the literature depending on the purpose of the investigation. While some studies have separated eating occasions into ‘meals’ and ‘snacks’ based on energy content and time constraint (21, 55), other studies have allowed subjects to classify their meals into categories themselves (20, 22). Some studies have also looked at a ‘nibbling’ versus a ‘gorging’ eating behaviour, and inconsistencies in the determination of these two behaviours have led to conflicting results regarding the health effects of the two behaviours (56–59). A productive scientific dialogue regarding meal patterns in relation to health outcomes requires a common meal pattern typology. In this study, meal types were classified by trained nutritionists using the same structured classification scheme to minimise inconsistencies. Also, the description provided by participants of meals consumed during a typical day included no information on energy or nutrient content. This may have reduced energy underreporting associated with meal types, but still some misclassification may be present due to misreporting by the subjects.
This study suggests that the type of meal (i.e. how foods are combined and eaten) may determine the overall nutrient intake. This may appear self-evident, but further studies are required on food combinations and the context of eating, and disease. In fact, it is plausible that public health advice and strategies to change nutrient intakes may need to focus on meal types to a much larger extent in order to be understandable and easily adaptable by the public. Analysis of meal patterns should however consider the complexity of dietary habits, including the importance of temporal distribution of meal, and when and how meals are consumed.
In conclusion, groups with specific meal patterns show differences in nutrient intakes, lifestyle and socioeconomic factors, suggesting that exploration of the association between health-related behaviours and patterns of eating may be important in public health nutrition. Nutrition epidemiology is often hindered by misreporting of subjects as well as the change in food habits over time. These and several other sources of bias must always be taken into consideration when examining and interpreting dietary patterns. Our findings indicate the importance of evaluating how lifestyle and life circumstances interact with diet. However, in order to compare eating styles and meal patterns across studies, there is a need for uniform methods for identifying and assessing meal patterns within and between populations.
Conflict of interest and funding
The authors have not received any funding or benefits from industry to conduct this study.
Appendix A
Example of how the meal intake during a typical week was self-reported by the participants in the Malmö Diet and Cancer study, including instructions to participants.
Meal Intake
Describe in broad terms the meals including snacks that you usually eat/drink during one day. Note the name of the meal, the time of the meal, what it consist of, and how many times per week you usually eat this meal. If Saturday and Sunday are significantly different from weekdays, you can report two meal intakes. See example.
| Monday–Friday | Meal name | Time (approx.) | What it consist of? | How many times per week? |
|---|---|---|---|---|
| Breakfast | 6.30 | Coffee + 2 sandwiches | 5 | |
| Before lunch | Approx. 10 | Fruit or biscuit with tea | 5 | |
| Lunch | 12 | Cooked meal, bread, beer | 5 | |
| Afternoon coffee | 14 | Coffee + sweet roll | 5 | |
| Dinner | 18 | 3–4 sandwiches with different spreads | 5 | |
| Evening tea | 20 | Tea + small cake (Friday night drink, cheese, or shrimps) | 4 + 1 | |
|
| ||||
| Saturday–Sunday | Meal name | Time (approx.) | What it consist of? | How many times per week? |
|
| ||||
| Breakfast | 8 | Coffee, porridge, sandwich, juice | 2 | |
| Morning coffee | 10.30 | Coffee + cake | 2 | |
| Lunch | 12 | Sandwiches + beer | 2 | |
| Afternoon coffee | 15 | Coffee + pastry | 2 | |
| Dinner | 18 | Cooked meal, dessert, wine on Saturdays | 2 | |
| Evening tea | 20 | Tea + biscuits | 2 | |
References
- 1.Sugiyama T, Xie D, Graham-Maar R, Inoue K, Kobayashi Y, Stettler N. Dietary and lifestyle factors associated with blood pressure among U.S. adolescents. J Adolesc Health. 2007;40:166–72. doi: 10.1016/j.jadohealth.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Hu F. Overweight and obesity in women: health risks and consequences. J Womens Health (Larchmt) 2003;12:163–72. doi: 10.1089/154099903321576565. [DOI] [PubMed] [Google Scholar]
- 3.Carlsson S, Andersson T, Lichtenstein P, Michaëlsson K, Ahlbom A. Physical activity and mortality: Is the association explained by genetic selection? Am J Epidemiol. 2007;166:255–9. doi: 10.1093/aje/kwm132. [DOI] [PubMed] [Google Scholar]
- 4.Ekberg-Aronsson M, Nilsson P, Nilsson J-Å, Löfdahl C-G, Löfdahl K. Mortality risks among heavy smokers with special reference to women: a long-term follow-up of an urban population. Eur J Epidemiol. 2007;22:301–9. doi: 10.1007/s10654-007-9120-7. [DOI] [PubMed] [Google Scholar]
- 5.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. Br Med J. 2004;328:1519–28. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thun M, Peto R, Lopez A, Monaco J, Henley S, Heath CJ, et al. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. N Engl J Med. 1997;337:1705–14. doi: 10.1056/NEJM199712113372401. [DOI] [PubMed] [Google Scholar]
- 7.Hu F. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Villegas R, Salim A, Collins M, Flynn A, Perry U. Dietary patterns in middle-aged Irish men and women defined by cluster analysis. Pub Health Nutr. 2004;7:1017–24. doi: 10.1079/PHN2004638. [DOI] [PubMed] [Google Scholar]
- 9.Kant A. Dietary patterns and health outcomes. J Am Diet Assoc. 2004;104:615–35. doi: 10.1016/j.jada.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Randall E, Marshall J, Graham S, Brasure J. Patterns in food use and their associations with nutrient intakes. Am J Clin Nutr. 1990;52:739–45. doi: 10.1093/ajcn/52.4.739. [DOI] [PubMed] [Google Scholar]
- 11.Fung T, Rimm E, Spiegelman D, Rifai N, Tofler GH, Willett WC, et al. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr. 2001;73:61–7. doi: 10.1093/ajcn/73.1.61. [DOI] [PubMed] [Google Scholar]
- 12.Jacques P, Tucker K. Are dietary patterns useful for understanding the role of diet in chronic disease? Am J Clin Nutr. 2001;73:1–2. doi: 10.1093/ajcn/73.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Fábry P, Fodor J, Geizerová H, Hejl Z, Balcarová O, Zvolánková K. Meal frequency and ischaemic heart-disease. Lancet. 1968;2:190–1. doi: 10.1016/s0140-6736(68)92622-6. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins D, Jenkins A, Wolever T, Vuksan V, Rao AV, Thompson LU, et al. Low glycemic index: lente carbohydrates and physiological effects of altered food frequency. Am J Clin Nutr. 1994;59:706S–9S. doi: 10.1093/ajcn/59.3.706S. [DOI] [PubMed] [Google Scholar]
- 15.Ma Y, Bertone E, Stanek EJ, III, Reed GW, Hebert JR, Cohen NL, et al. Association between eating patterns and obesity in a free-living US adult population. Am J Epidemiol. 2003;158:85–92. doi: 10.1093/aje/kwg117. [DOI] [PubMed] [Google Scholar]
- 16.Togo P, Osler M, Sorensen T, Heitmann B. Food intake patterns and body mass index in observational studies. Int J Obes. 2001;25:1741–51. doi: 10.1038/sj.ijo.0801819. [DOI] [PubMed] [Google Scholar]
- 17.Wirfält E, Hedblad B, Gullberg B, Mattisson I, Andrén C, Rosander U, et al. Food patterns and components of the metabolic syndrome in men and women: a cross-sectional study within the Malmö Diet and Cancer cohort. Am J Epidemiol. 2001;154:1150–9. doi: 10.1093/aje/154.12.1150. [DOI] [PubMed] [Google Scholar]
- 18.Lennernäs M, Andersson I. Food-based classification of eating episodes (FBCE) Appetite. 1999;32:53–65. doi: 10.1006/appe.1998.0196. [DOI] [PubMed] [Google Scholar]
- 19.Kerver J, Yang E, Obayashi S, Bianchi L, Song W. Meal and snack patterns are associated with dietary intake of energy and nutrients in US adults. J Am Diet Assoc. 2006;106:46–53. doi: 10.1016/j.jada.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 20.Roos E, Prättälä R. Meal patterns and nutrient intake among adult Finns. Appetite. 1997;29:11–24. doi: 10.1006/appe.1996.0095. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein I, Zimmerman J, Czeisler C, Weitzman E. Meal patterns in “free-running” humans. Physiol Behav. 1981;4:621–3. doi: 10.1016/0031-9384(81)90232-8. [DOI] [PubMed] [Google Scholar]
- 22.Bellisle F, Dalix A, Mennen L, Galan P, Hercberg S, de Castro J, et al. Contribution of snacks and meals in the diet of French adults: a diet-diary study. Physiol Behav. 2003;79:183–9. doi: 10.1016/s0031-9384(03)00088-x. [DOI] [PubMed] [Google Scholar]
- 23.Toschke A, Küchenhoff H, Koletzko B, von Kries R. Meal frequency and childhood obesity. Obes Res. 2005;13:1932–8. doi: 10.1038/oby.2005.238. [DOI] [PubMed] [Google Scholar]
- 24.Mann J. Meal frequency and plasma lipids and lipoproteins. Br J Nutr. 1997;77:S83–90. doi: 10.1079/bjn19970106. [DOI] [PubMed] [Google Scholar]
- 25.Bertéus Forslund H, Torgerson J, Sjöström L, Lindroos A. Snacking frequency in relation to energy intake and food choices in obese men and women compared to a reference population. Int J Obes. 2005;29:711–9. doi: 10.1038/sj.ijo.0802950. [DOI] [PubMed] [Google Scholar]
- 26.Gatenby S. Eating frequency: methodological and dietary aspects. Br J Nutr. 1997;77:S7–20. doi: 10.1079/bjn19970100. [DOI] [PubMed] [Google Scholar]
- 27.Engeset D, Alsaker E, Ciampi A, Lund E. Dietary patterns and lifestyle factors in the Norwegian EPIC cohort: the Norwegian Women and Cancer (NOWAC) study. Eur J Clin Nutr. 2005;59:675–84. doi: 10.1038/sj.ejcn.1602129. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez-Villegas A, Delgado-Rodríguez M, Martínez-González M, de Irala-Estévez J. Gender, age, socio-demographic and lifestyle factors associated with major dietary patterns in the Spanish Project SUN (Seguimiento Universidad de Navarra) Eur J Clin Nutr. 2003;57:285–92. doi: 10.1038/sj.ejcn.1601528. [DOI] [PubMed] [Google Scholar]
- 29.James W, Nelson M, Ralph A, Leather S. Socioeconomic determinants of health: the contribution of nutrition to inequalities in health. Br Med J. 1997;314:1545–49. doi: 10.1136/bmj.314.7093.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattisson I, Wirfält E, Aronsson C, Wallström P, Sonestedt E, Gullberg B, et al. Misreporting of energy: prevalence, characteristics of misreporters and influence on observed risk estimates in the Malmö Diet and Cancer cohort. Br J Nutr. 2005;94:832–42. doi: 10.1079/bjn20051573. [DOI] [PubMed] [Google Scholar]
- 31.Maurer J, Taren DL, Teixeira PJ, Thomson CA, Lohman TG, Going SB, et al. The psychosocial and behavioral characteristics related to energy misreporting. Nutrition reviews. 2006;64:53–66. doi: 10.1111/j.1753-4887.2006.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 32.Sonestedt E, Wirfält E, Gullberg B, Berglund G. Past food habit change is related to obesity, lifestyle and socio-economic factors in the Malmo Diet and Cancer Cohort. Pub Health Nutr. 2005;8:876–85. doi: 10.1079/phn2005736. [DOI] [PubMed] [Google Scholar]
- 33.Sonestedt E, Gullberg B, Wirfält E. Both food habit change in the past and obesity status may influence the association between dietary factors and postmenopausal breast cancer. Pub Health Nutr. 2007;10:769–79. doi: 10.1017/S1368980007246646. [DOI] [PubMed] [Google Scholar]
- 34.Manjer J, Carlsson S, Elmstahl S, Gullberg B, Janzon L, Lindström M, et al. The Malmo Diet and Cancer study: representativity, cancer incidence and mortality in participants and non-participants. Eur J Cancer Prevent. 2001;10:489–99. doi: 10.1097/00008469-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Berglund G, Elmståhl S, Janzon L, Larsson S. The Malmö Diet and Cancer study. Design and feasibility. J Intern Med. 1993;233:53–7. doi: 10.1111/j.1365-2796.1993.tb00647.x. [DOI] [PubMed] [Google Scholar]
- 36.Callmer E, Riboli E, Saracci R, Akesson B, Lindegärde F. Dietary assessment methods evaluated in the Malmo food study. J Intern Med. 1993;233:53–7. doi: 10.1111/j.1365-2796.1993.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 37.Elmståhl S, Riboli E, Lindegärde F, Gullberg B, Saracci R. The Malmö food study: the relative validity of a modified diet history method and an extensive food frequency questionnaire for measuring food intake. Eur J Clin Nutr. 1996;50:143–51. [PubMed] [Google Scholar]
- 38.Riboli E, Elmståhl S, Saracci R, Gullberg B, Lindegärde F. The Malmö food study: validity of two dietary assessment methods for measuring nutrient intake. Int J Epidemiol. 1997;26:S161–73. doi: 10.1093/ije/26.suppl_1.s161. [DOI] [PubMed] [Google Scholar]
- 39.Elmståhl S, Gullberg B, Riboli E, Saracci R, Lindegärde F. The Malmö food study: the reproducibility of a novel diet history method and an extensive food frequency questionnaire. Eur J Clin Nutr. 1996;50:134–42. [PubMed] [Google Scholar]
- 40.National Bureau of Statistics. Stockholm, Sweden: Statistics Sweden (in Swedish); 1989. Occupations in population and housing census 1985 (FoB 85) according to Nordic standard occupational classification (Nordisk yrkesklassificering NYK) and Swedish socioeconomic classification (Socioekonomisk indelning SEI) [Google Scholar]
- 41.Taylor H, Jacobs DJ, Schucker B, Knudsen J, Leon A, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–55. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 42.Richardson M, Leon A, Jacobs DJ, Ainsworth B, Serfass R. Comprehensive evaluation of the Minnesota Leisure Time Physical Activity Questionnaire. J Clin Epidemiol. 1994;47:271–81. doi: 10.1016/0895-4356(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 43.Goldberg GR, Black AE, Jebb SA, Cole TJ, Murgatroyd PR, Coward WA, et al. Critical evaluation of energy intake using fundamental principles of energy physiology: 1 derivation of cut-off limits to identify under-recording. Eur J Clin Nutr. 1991;45:569–81. [PubMed] [Google Scholar]
- 44.Black AE. Critical evaluation of energy intake using the Goldberg cut-off for energy intake: basal metabolic rate. A practical guide to its calculation, use and limitations. Int J Obes. 2000;24:1119–30. doi: 10.1038/sj.ijo.0801376. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organisation. WHO Technical Report Series no. 894. Geneva: WHO; 2000. Obesity: preventing and managing the global epidemic. [PubMed] [Google Scholar]
- 46.Eiben G, Dey D, Rothenberg E, Steen B, Björkelund C, Bengtsson C, et al. Obesity in 70-year-old Swedes: secular changes over 30 years. Int J Obes. 2005;29:810–7. doi: 10.1038/sj.ijo.0802940. [DOI] [PubMed] [Google Scholar]
- 47.Berrigan D, Dodd K, Troiano R, Krebs-Smith S, Barbash R. Patterns of health behavior in U.S. adults. Prevent Med. 2003;36:615–23. doi: 10.1016/s0091-7435(02)00067-1. [DOI] [PubMed] [Google Scholar]
- 48.Patterson R, Haines P, Popkin B. Health lifestyle patterns of U.S. adults. Prevent Med. 1994;23:453–60. doi: 10.1006/pmed.1994.1062. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez A, Norman G, Sallis J, Calfas K, Rock C, Patrick K. Patterns and correlates of multiple risk behaviours in overweight women. Prevent Med. 2008;46:196–202. doi: 10.1016/j.ypmed.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bazelmans C, Matthys C, De Henauw S, Dramaix M, Kornitzer M, De Backer G, et al. Predictors of misreporting in an elderly population: the ‘Quality of life after 65’ study. Pub Health Nutr. 2007;10:185–91. doi: 10.1017/S1368980007246774. [DOI] [PubMed] [Google Scholar]
- 51.Lafay L, Basdevant A, Charles M, Vray M, Balkau B, Borys JM, et al. Determinants and nature of dietary underreporting in a free-living population: the Fleurbaix Laventie Ville Santé (FLVS) study. Int J Obes Relat Metab Disord. 1997;7:567–73. doi: 10.1038/sj.ijo.0800443. [DOI] [PubMed] [Google Scholar]
- 52.Braam L, Ocké M, Bueno-de-Mesquita H, Seidell J. Determinants of obesity-related underreporting of energy intake. Am J Epidemiol. 1998;147:1081–6. doi: 10.1093/oxfordjournals.aje.a009402. [DOI] [PubMed] [Google Scholar]
- 53.Voss S, Kroke A, Klipstein-Grobusch K, Boeing H. Obesity as a major determinant of underreporting in a self-administered food frequency questionnaire: results from the EPIC-Potsdam study. Z Ernahrungswiss. 1997;36:229–36. doi: 10.1007/BF01623369. [DOI] [PubMed] [Google Scholar]
- 54.Heitmann B, Lissner L. Dietary underreporting by obese individuals – is it specific or non-specific? Br Med J. 1995;311:986–9. doi: 10.1136/bmj.311.7011.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Castro J. Genetic influences on daily intake and meal patterns of humans. Physiol Behav. 1993;4:777–82. doi: 10.1016/0031-9384(93)90188-l. [DOI] [PubMed] [Google Scholar]
- 56.Summerbell C, Moody R, Shanks J, Stock M, Geissler C. Relationship between feeding pattern and body mass index in 220 free-living people in four age groups. Eur J Clin Nutr. 1996;50:513–9. [PubMed] [Google Scholar]
- 57.Bellisle F, McDevitt R, Prentice A. Meal frequency and energy balance. Br J Nutr. 1997;77:S57–70. doi: 10.1079/bjn19970104. [DOI] [PubMed] [Google Scholar]
- 58.Taylor M, Garrow J. Compared with nibbling, neither gorging nor a morning fast affect short-term energy balance in obese patients in a chamber calorimeter. Int J Obes Relat Metab Disord. 2001;25:519–28. doi: 10.1038/sj.ijo.0801572. [DOI] [PubMed] [Google Scholar]
- 59.Drummond S, Crombie N, Kirk T. A critique of the effects of snacking on body weight status. Eur J Clin Nutr. 1996;12:779–83. [PubMed] [Google Scholar]