Abstract
Schistosoma japonicum is the most pathogenic agent of hepatosplenic schistosomiasis that has killed millions of people in China. It causes extended fibrosis in the periportal space (PPF) and in the hepatic parenchyma (ParF). The deposition of the extracellular matrix proteins composing the fibrotic scar is regulated by cytokines and chemokines, the production of which may play a key role in disease progression. We investigated whether fibrosis and splenomegaly in fishermen living in an endemic region for S. japonicum were associated with abnormal cytokine/chemokine production by blood mononuclear cells. PPF and ParF were considered as separate fibrosis phenotypes. PPF was associated (by univariate analysis) with low levels of IL-10 (p=4 × 10-4), RANTES (p=4 × 10-4) and MIP-1α (p=7 × 10-3) production in cultures of blood leukocytes stimulated with schistosome egg antigens. Logistic regression that included exposure, anti-schistosome treatments and drinking habits as covariates showed that IL-10 exhibited the strongest association with PPF (p=1 × 10-4, OR=10.8, CI=3.2-38). Splenomegaly was associated with low levels of IL-10 production (p=4 × 10-3) even in the presence of PPF as covariate (p=0.01, OR=3.5, 1.3-8.9), indicating a probable direct relationship between IL-10 and splenomegaly. Furthermore, ParF was associated with low levels of production for IFN-γ (p=3.5 10-3; OR= 8.2; 2-33) but not for IL-10 or RANTES. These data are consistent with IL-10 playing a key role in the development of severe hepatic and spleen disease and differences in the cytokine-mediated control of PPF and ParF in humans infected with S. japonicum.
Keywords: Human, Parasitic helminths, Cytokines, Inflammation
Introduction
Schistosomiasis is the second most important parasitic disease worldwide after malaria. It comprises a group of chronic diseases caused by helminth digenic trematodes of the Schistosoma genus. Schistosoma japonicum and S. mansoni are the principal agents of hepatosplenic schistosomiasis. They cause severe hepatic inflammation, which, in some subjects, progresses to massive periportal fibrosis (PPF), portal blood hypertension, varicose veins, ascites and death. S. japonicum is by far the most pathogenic of these species. It has caused millions of deaths in China, where it remains uncontrolled in certain regions. This pathogenicity is linked to more extensive deposition of the fibrotic mesh in the periportal space and hepatic parenchyma. Parenchymal fibrosis (ParF) is not observed in subjects infected with S. mansoni. The pathogenicity of S. japonicum probably results from the capacity of this pathogen to infect various mammalian hosts, including buffaloes, which are likely responsible for most human infections in China, whereas humans are the principal vertebrate host of S. mansoni. This may have slowed the co-evolution of S. japonicum with the human immune system, potentially accounting for the strong human immune reactions to this schistosome.
The pathology of chronic schistosomiasis results from the egg-induced immune response organised as granuloma causing tissue damage and associated fibrotic changes. Inflammation products and molecules released by damaged hepatocytes stimulate the differentiation of hepatic stellate cells into myofibroblasts, which secrete extracellular matrix proteins (ECMP) into the perisinusoidal space (1). Periportal fibrosis (PPF) results from the excessive accumulation of ECMP in the periportal space, close to granulomas. However, it remains unclear why fibrotic deposits occur in the liver parenchyma (ParF), at some distance from the perisinusoidal space. PPF leads to portal hypertension, varicose veins and ascites. Severe disease develops in 5 to 20% of patients and the annual death rate due to S. japonicum has been estimated at 0.27% in the Dong Ting Lake region — the home of our study population (2).
The egg-induced inflammation and fibrotic response are regulated by cytokines and chemokines. Th2 cytokines (IL-4 and IL-13) are fibrogenic (3-5), whereas IFN-γ inhibits the production of ECMP and increases collagenase activity by stimulating matrix metalloproteases (MMP) and inhibiting tissue inhibitors of MMP (TIMP) (6-8). TNF-α, TGF-β and IL-1 stimulate the differentiation of stellate cells into myofibroblasts (9). IL-10 may play an important role in this process, regulating Th1 and Th2 responses (10). Chemokines are also involved in granuloma formation and fibrosis. Monocyte chemotactic protein 1 (MCP-1) both enhances fibroblast collagen production by up-regulating TGF-β and increases MMP synthesis, thereby modulating the balance between collagen deposition and turnover (11). Macrophage inflammatory proteins (MIP) are key players in the pathogenesis of many inflammatory conditions and diseases, including granuloma formation and wound healing (12). Work on humans infected with S. mansoni in Sudan has demonstrated that PPF results, at least partly, from low levels of IFN-γ production, linked with mutations in the IFN-γ gene (13, 14). TNF-α production is also associated with the aggravation of PPF (14). These observations were confirmed by a study in Uganda, showing that low IFN-γ and high TNF levels are associated with PPF. This study also reported high RANTES and low IL-10 levels in affected subjects, as a function of sex and age (15). Other studies have shown that high levels of IL-4, IL-5 and IL-13 production are associated with the aggravation of hepatic fibrosis in humans infected with S. mansoni (16, 17).
In endemic regions of S.japonicum in China, it was frequent that half of the village population was killed by schistosomes, nevertheless some subjects survived well the infection and resisted to disease. Human resistance to infection with S. mansoni depends on the genetics of the host and major susceptibility loci and genes have been identified (18-23). Furthermore, disease is controlled by genetic loci other than those controlling infection (13, 24, 25). The extent to which these genetic and immunological observations for S. mansoni infections can be extended to S. japonicum remains unclear, as S. japonicum is considerably more pathogenic. We evaluated this issue and determined whether the cytokines shown to control disease in animals and humans infected with S. mansoni could account for the complex pattern of hepatic fibrosis induced by S. japonicum. Our data are consistent with IL-10 playing a key regulatory role in the development of severe liver and spleen disease and with differences in the control of PPF and ParF by cytokines.
Materials and Methods
Study area and subjects
We studied fishermen from the Dongting Lake region, Hunan Province, China. This region is endemic for S. japonicum. We selected subjects from the population of fishermen who had been fishing for at least fifteen years and had a fishing activity (mostly net and electric fishing) resulting in a high degree of contact with the lake. We evaluated various factors that might affect the development of hepatic fibrosis and have included them as covariates in the multivariate analysis: age, sex, drinking habits, number of praziquantel treatments in the last 20 years, number of years of fishing activity.
Hepatic fibrosis was evaluated by ultrasound, using the WHO grading scale (26) modified as previously described (Li.J et al., personal communication). The WHO scale grades parenchymal (ParF) and periportal fibrosis (PPF) separately. PPF is graded A, B, C, CL, D, E or F. The C linear thickening pattern (CL) of PPF represents an uninterrupted linear wall thickness of portal vein extending from portal vein to its branches. Such uninterrupted feature distinguished it from grade C (discontinuous thickness) of PPF and the linear pattern was different from the patches observed in grades D, E, F. As more than 60 % of the fishermen in this heavily exposed population had grade CL, we refined the grading of CL into CLL (CL light), CLM (CL medium) and CLH (CL heavy): CLL PPF was only observed in left liver lobe, CLM or CLH PPF were observed in both right and left liver lobes. Subjects with limited right liver lobe fibrosis (extending to second order branches) were classified as CLM and those PPF extended further down the second order branches were classified as CLH. Only grade CLH was associated with evidence of portal hypertension. It was therefore grouped with grades D, E and F to define a severe PPF phenotype; CLM was considered to correspond to advanced PPF. Conversely, CLL was grouped with grades A, B and C. The network parenchymal fibrosis (also known as network fibrosis) was graded according to the WHO scale as narrow mesh (GN) when the lumen diameter of net is no more than 12mm and wide mesh (GW) if more than 12mm. Depending on the thickness of mesh streak or band in our study, we classified it as GNL (or GWL) if mesh streak (or band) is less than 2mm thick (<2mm), GNM (or GWM) if 2-4mm and GNH (or GWH) if more than 4mm. Since very few fishermen were GW, subjects with GW grade were not included in the present immunological study.
Ultrasound data for the study subjects are presented in Table I. Note that the PV is almost normal in all PPF classes, probably due to the treatment of hypertension by local doctors, but that subjects with advanced PPF have enlarged splenic veins and spleen. Eight of the study subjects had undergone splenectomy; their ultrasound data are not included in Table I, and immunological data from these subjects are presented only in table VII.
Table I. Portal vein (PV) and splenic vein (SV) diameter, spleen size and ParF (GNH) in Study subjects with various grades of PPF.
Measurements are arithmetic means (SEM).
| Sex | PPF grade | N |
PV(mm)
(SEM) |
SV(mm)
(SEM) |
Spleen (SEM) |
ParF
(% in PPF grade) |
|---|---|---|---|---|---|---|
| Male | A,B,C,CLL | 42 | 11.5 (.23) | 5.4 (.23) | 98 (2.4) | 14 (33.3%) |
| Male | CLM | 32 | 11.5 (.21) | 5.8 (.25) | 101 (3) | 5 (15.6%) |
| Male | CLH,D,E,F | 21 | 11.6 (.24) | 6.5 (.4) | 114.7 (5.9) | 5 (23.8%) |
| Female | ABC,CLL | 16 | 10.4 (.19) | 4.8 (.18) | 92.9 (2.6) | 0 (0%) |
| Female | CLM | 3 | 10 (1) | 5 (1) | 98 (2.4) | 0 (0%) |
| Female | CLH,D,E,F | 6 | 10.9 (.24) | 6.6 (.8) | 111.4 (14.4) | 1 (16.6%) |
Table VII. Effects of splenectomy on cytokines production in cultures of SEA-stimulated blood mononuclear cells from subjects with severe PPF.
*p=0.03. In the severe PPF group, PBMCs from 23 and 17 patients were stimulated for 20 and 72 hours, respectively. The splenectomised group contained eight patients.
| Severe PPF grade CLH, D, E |
Severe PPF After splenectomy |
|||||
|---|---|---|---|---|---|---|
| Culture conditions | Detection limit | Median values (pg/ml) | 75% upper percentile value | Median values (pg/ml) | 75% upper percentile value | |
| TNF-α | SEA 20h | 3.9 | 58.9 | 386 | 109.5 | 572.1 |
| IL-10** | SEA 20h | 3.9 | 34.7 | 145 | 125.2 | 232.7 |
| IL-4 | SEA 72h | 3.9 | 2.4 | 13 | 5.9 | - |
| IL-13 | SEA 72h | 0.78 | 16.1 | 142 | 175.5 | - |
| IL-1α | SEA 20h | 3.9 | 7.7 | 49 | 41.8 | 86.4 |
| IL-5 | SEA 72h | 3.9 | 5 | 95 | 154.2 | - |
| IL-6 | SEA 72h | 1.5 | 2917.2 | 7592 | 3858.6 | - |
| IFN-γ | SEA 72h | 0.78 | 19.8 | 46 | 26.7 | - |
| RANTES** | SEA 20h | 7.8 | 3331.4 | 5127 | 4728.7 | 7952 |
| MIP-1α* | SEA 72h | 3.9 | 1802.3 | 8421 | 11143.8 | 27067 |
| MCP-1 | SEA 20h | 3.9 | 32889 | 59146 | 40403.2 | 63078 |
This study was reviewed and approved by the WHO and the Hunan Institute of Parasitic Disease, China. Written informed consent was obtained from each subject and from the local administration before the study.
Antigen (Ag) preparation
Frozen pellets of S. japonicum eggs were suspended in PBS and sonicated twice, for 10 minutes each, in PBS on ice. Insoluble material was removed by ultracentrifugation at 5000g for 30 minutes at 4°C. Supernatants (soluble egg antigens or SEA) containing 3 mg/ml of protein were stored at −80°C.
Cytokine production and titration
PBMC were isolated from venous blood collected into heparin-treated tubes, by Ficoll-Hypaque gradient sedimentation (400g for 30 min at 18°C). They were washed three times in 2 mM L-glutamine RPMI and suspended in RPMI supplemented with 10 μg/ml penicillin/streptomycin, 50 μM 2-mercaptoethanol, 10% FBS, 10 mM HEPES and 100 μg/ml sodium pyruvate and dispensed at a density of 2 × 106 cells/well in 24-well culture plates. We added 10 μg/ml SEA or PHA to the wells and incubated the plates at 37°C under an atmosphere containing 5% CO2, in a humid incubator. Supernatants were removed after 20, 48 or 72 hours of incubation and cytokines were titrated by ELISA, according to the kit manufacturers’ instructions. IL-4, IL-5, IL-10, TNF-α and MCP-1 were determined with BD OptEIA ELISA sets (Becton Dickinson, detection limit 4 pg/ml). IL-6, IL-13 and IFN-γ were titrated with Diaclone ELI-pair (Diaclone, detection limit 1.56 pg/ml for IL-6 and 0.78 pg/ml for IL-13 and IFN-γ). IL-1α, MIP-1α and RANTES were determined with R&D systems Duoset (R&D systems, detection limit 3.9 pg/ml for IL-1α and MIP-1α and 7.8 pg/ml for RANTES).
Statistical analysis
We first tested the association of each cytokine with the clinical phenotype (PPF, ParF or splenomegaly, as defined above), using non-parametric Mann-Whitney tests. Fibrosis depends on covariates that might confound the effects of cytokines. Furthermore, cytokines are not independent from each others.Thus, the various covariates and cytokines must be evaluated simultaneously, by multivariate analysis. We carried out logistic regression to determine the regression relationship between the probability of an individual to develop advanced disease and various covariates. Cytokine levels were assigned to three classes of equal size (low, medium and high). Age and the number of years of fishing were treated as quantitative variables, whereas sex (male, female), drinking rice alcohol (5 classes: no drinking, occasional drinking, 50 ml /day, 100-150 /day, >200/day) and number of praziquantel treatments in the last 20 years (no treatment, 1-2, 3-6, 7-10, >10) were analyzed as qualitative variables. Binary clinical phenotypes were defined as indicated (see ultrasound evaluation and tables). The analysis was performed including age, sex, number of years of fishing, drinking habits, number of treatments and cytokines showing an association (p<10-2) or a trend towards association (p=0.1) with the clinical phenotype in univariate analysis. Variables with more than two classes were tested as binary variables in the regression models. For example, drinking habits were tested as four binary variables: a) 0 versus “occasionally”, up to > 200 ml; b) 0 + “occasionally” versus 50 to > 200 ml; c) 0 to 50 ml versus 150 to > 200 ml; d) 0 to 150 ml versus > 200 ml. Odds ratios (ORs) were calculated to assess the strength of the association of the covariates with fibrosis, and provided a good approximation of the relative risk associated with the covariates. OR data are expressed with confidence intervals (CIs).
Results
Non-immunological factors that may affect the risk of hepatic disease in the selected study subjects
Study subjects were selected from a population of individuals with a high level of contact with the lake. They were fishermen who had been fishing for at least 15 years. Most were born on a boat or on a small island in the middle of the lake and most carried out net or electric fishing, requiring frequent immersion in the lake to set up the nets or to catch the paralysed fish. There was little difference in exposure between the men and women in the selected study cohort. There are more men than women in the cohort because PPF was more frequent in men than in women in the population as a whole. We took care to ensure that the women selected for this study had a high degree of contact with the water of the lake. Ultrasound evaluations were carried out for the group of patients selected (Table I). Patients without PPF or with mild PPF were graded A,B,C or CLL, as indicated in methods. Patients with advanced PPF were graded CLM, and those with severe PPF were graded CLH, D, E or F. Only patients with PPF of grades CLH, D,E or F presented evidence of portal hypertension (increase in splenic vein diameter). Little increase on portal vein diameter was observed in this group, as in the general population. The reason for this is unclear, but may be related to successful medical treatment of hypertension. Subjects with severe PPF, in both the study and general populations, had enlarged spleens. Severe network fibrosis (ParF) was observed in all PPF groups, indicating a lack of correlation between PPF and ParF. Indeed, some subjects with severe PPF presented no ParF, whereas some patients with no or mild PPF presented evidence of ParF. This is consistent with our results for the total population of fishermen (Luo.X et al., personal communication).
If we considered male and female subjects separately (Table II), the three disease groups — mild, advanced and severe PPF — did not differ for most of the covariates measuring high levels of contact with the lake (living on a boat or island, type of fishing). Subjects with severe PPF were of similar age to the subjects in the other groups. However, they had been fishing for longer periods of time, suggesting that some subjects with CLM disease may progress to CLH if they continue fishing. As the number of treatments and drinking habits may affect fibrosis grade, we also controlled for these variables in the study groups and no major differences can be observed between PPF grade groups for these two variables, in men or women. However, women tended to have had fewer treatments and to drink less than men, and this was true of the entire population of fishermen.
Table II. Epidemiological covariates in the various PPF groups.
a) Arithmetic means (SEM); b) Alcohol intake: 6 classes were defined for drinking behaviour: no drinking, occasionally drinking, 50 ml/day, 100-150 ml/day, 200 ml/day or more; c) Praziquantel treatments in the last twenty years: none, 1-2, 3-6, 7-9, >10 treatments.
| Sex | Epidemiological covariates |
PPF
A,B,C,CLL |
PPF
CLM |
PPF
CLH,D,E,F |
|---|---|---|---|---|
| Male subjects | N | 42 | 32 | 21 |
| Age | 48.5 (1.8) | 48.4 (2) | 45.5 (2.6) | |
| Living on a boat (%) | 13.5 | 31 | 11.1 | |
| Living on island (%) | 81.1 | 62 | 77.8 | |
| Years of fishing | 33.6 (1.9) | 32.6 (1.9) | 41.5 (2.6) | |
| Net fishing (%) | 90.2 | 70.7 | 67.8 | |
| Electric fishing (%) | 7.3 | 58.5 | 32.1 | |
| Drinking (median) | Occasionally | Occasionally/50 ml | Occasionally | |
| Praziquantel treatments (median) | 1-2 | 1-2 | 3-6 | |
| Female subjects | N | 16 | 3 | 6 |
| Age | 45.5 (2.6) | 48.5 (1.8) | 44 (6.4) | |
| Living on a boat (%) | 27.2 | 100 | 100 | |
| Living on island (%) | 72.7 | 0 | 0 | |
| Years of fishing | 26.1 (1.9) | 33.6 (1.8) | 29.9 (5.2) | |
| Net fishing (%) | 76.4 | 100 | 85.7 | |
| Electric fishing (%) | 23.5 | 0 | 14.3 | |
| Drinking (Median) | Occasionnally | no | no | |
| Praziquantel treatments (median) | 3-6 | 1-2 | 1-2 |
Cytokines produced by the PBMCs of patients with mild, intermediate or severe PPF
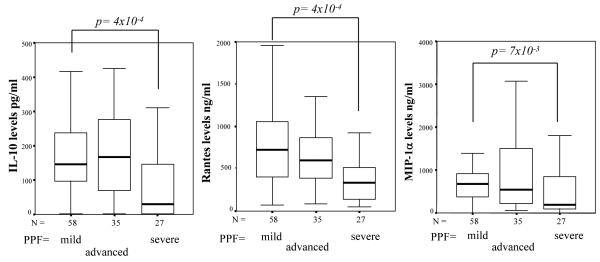
The cytokines produced by the SEA-stimulated PBMC of 127 patients with little or no (A, B) or mild (C, CLL) PPF, advanced (CLM) PPF and severe (CLH, D, E, F) PPF are shown in table III. For each PPF group, median values and the upper limits of the 95% confidence interval are reported for cytokine levels. Subjects with severe fibrosis produced less IL-10, RANTES and MIP-1α than subjects with no fibrosis or mild fibrosis. Median IL-10 levels in the subjects with no or mild PPF was 151.6 pg/ml whereas they were 34.7 pg/ml in the severe PPF group (p=4 × 10-4). Similarly, median RANTES levels in the no or mild PPF group reached 7097 pg/ml, versus only 3331 pg/ml in the severe PPF group (p=4 × 10-4). Median MIP-1α levels were 7053 pg/ml in the no or mild PPF group and 1802 pg/ml in the severe PPF group (p=7 × 10-3). These results are illustrated in figure 1. Note that IL-10 (p=9 × 10-3), RANTES (p=10-3) and MIP-1α (p=10-2) levels also differed significantly between the clinical groups if we tested all fishermen in the analysis, including those who had been fishing for less than 15 years.
Table III. Cytokines produced in cultures of SEA-stimulated blood mononuclear cells from study subjects: association of disease with IL-10 and RANTES.
The number of subjects in each group is indicated in Table II. Cytokines were titrated after 20 h (TNF-α, IL-10, IL-1α, RANTES, and MCP-1) or 72 hours (IL-4, IL-13, IL-5, IL-6 and MIP-1α) of culture. Cytokine levels were compared between the clinical groups, using the non-parametric Mann-Whitney test; statistically significant differences were observed for IL-10 and RANTES (**p=4 × 10-4) and MIP-1α (*p=7 × 10-3) when comparing patients with no or mild PPF with the group with severe PPF.
|
No or Mild PPF
grade A, B, C,CLL |
Intermediate PPF
grade CLM |
Severe PPF
grade CLH, D, E |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Culture conditions | Detection limit | % >limit | Median values (pg/ml) | 75% upper percentile value | % >limit | Median values (pg/ml) | 75% upper percentile value | % >limit | Median values (pg/ml) | 75% upper percentile value | |
| TNF-α | SEA 20h | 3.9 | 42 | 19.4 | 432 | 17 | 148.5 | 476 | 8 | 58.9 | 386 |
| IL-10** | SEA 20h | 3.9 | 95 | 151.6 | 240 | 91 | 166.6 | 279 | 67 | 34.7 | 145 |
| IL-4 | SEA 72h | 3.9 | 32 | 2 | 6 | 33 | 2 | 9 | 39 | 2.4 | 13 |
| IL-13 | SEA 72h | 0.78 | 94 | 14.2 | 98 | 96 | 12.3 | 165 | 78 | 16.1 | 142 |
| IL-1α | SEA 20h | 3.9 | 78 | 20.1 | 35 | 80 | 16.9 | 36.3 | 59 | 7.7 | 49 |
| IL-5 | SEA 72h | 3.9 | 42 | 4 | 84 | 43 | 4 | 77 | 50 | 5 | 95 |
| IL-6 | SEA 72h | 1.5 | 100 | 4106.2 | 9081 | 100 | 5526.3 | 11363.5 | 100 | 2917.2 | 7592 |
| IFN-γ | SEA 72h | 0.78 | 84 | 19.7 | 72 | 75 | 18.3 | 52 | 83 | 19.8 | 46 |
| RANTES** | SEA 20h | 7.8 | 100 | 7097.1 | 10472 | 100 | 5953.4 | 8765 | 100 | 3331.4 | 5127 |
| MIP-1a* | SEA 72h | 3.9 | 100 | 7053.7 | 9505 | 100 | 5432 | 15762 | 100 | 1802.3 | 8421 |
| MCP-1 | SEA 20h | 3.9 | 100 | 22799 | 37093 | 100 | 38499 | 68852 | 100 | 32889 | 59146 |
Fig. 1. Production of IL-10, RANTES and MIP-1α in SEA-stimulated cultures of PBMC from subjects with no or mild (A, B, CLL), advanced (CLM) or severe fibrosis (CLH, D, E, F).
The horizontal black line within the boxes represents the median value. The boxes indicate the 25th to 75th percentile range and the error bars indicate standard deviation. The association of each cytokine (univariate analysis) with clinical phenotype was assessed with the Mann-Whitney test.
Association of low levels of IL-10 production with advanced and severe PPF
As several covariates could potentially act as confounding factors, we simultaneously tested all covariates (age, sex, exposure, number of praziquantel treatments, alcohol intake and ParF severity) for association with the clinical phenotypes. Severe PPF was associated with higher exposure (number of years of fishing) and larger numbers of praziquantel treatments (> 10 treatments in the last 20 years), as expected for patients treated by local hospitals. There was no evidence in this study sample (in which clinical groups were matched as close as possible) of an association between severe PPF and age or sex, and no association with ParF.
We then assessed the association of covariates and cytokines with PPF (Table IV). IL-10 showed the strongest association with severe PPF in such an analysis and excluded all other cytokines from the regression model. Low levels of IL-10 were associated with a high risk of developing advanced or severe PPF (p=1 × 6 10-3, OR=4, confidence interval: CI = 1.4-11) and “treatment” was the only covariate retained in the model (p= 8 × 10-3). Severe PPF was associated with low levels of IL-10 (p=1 × 10-4, OR=10.8, CI= 3.2-38), with exposure (p=0.03) and number of treatments (p=8 × 10-3) also present in the best model. Then, risk of developing severe PPF was found to be about 10 times higher in subjects producing low levels of IL-10 than in those producing large amounts of this cytokine. If we excluded IL-10 levels from the analysis, low RANTES levels and low MIP-1α levels were associated with a higher risk of developing severe PPF (p=0.03, OR=4, CI=1.2-14 and p=8 × 10-4, OR=8, CI=2.4-27.5, respectively) in the presence of exposure (p=2 × 10-3) and treatments (p=3 × 10-3).
Table IV. Logistic regression analysis confirming the strong association between PPF and IL-10 levels.
Stepwise regression analysis was performed as described in the methods. Praziquantel classes were defined as indicated in the legend to table II; cytokine levels were assigned to three classes of equal size. We compared the (CLM, CLH, D, E, F) PPF subjects or the (CLH, D, E, F) PPF subjects with the group of patients with no or mild fibrosis (A,B,C,CLL PPF subjects).
| Phenotype | Covariates | Odds Ratio | CI | p |
|---|---|---|---|---|
|
No or Mild PPF / Advanced or severe PPF (A, B, C, CLL ) / (CLM ,CLH, D, E,F) |
IL-10 >50 / <50 pg/ml |
4 | 1.4-11 | 1.6 × 10-3 |
| Praziquantel T >10T / <10T |
3.9 | 1.7-9 | 8 × 10-3 | |
|
No or Mild PPF / Severe PPF (A, B, C, CLL ) / (CLH, D, E, F) |
IL-10 >50 / <50 pg/ml |
10.8 | 3.2-38 | 1 × 10-4* |
| Years of fishing | 1.05 | 1.003-1.1 | 0.03 | |
| Praziquantel T >10T / <10T |
7.3 | 1.7-33 | 8 × 10-3 |
When “treatments” was excluded from the analysis, low IL-10 levels remained associated with phenotype (p=6 × 10-4, OR=6 and CI=2.1-16.6)
Association of cytokine production by PBMCs with severe parenchymal fibrosis
We determined cytokine levels in cultures of SEA-stimulated PBMC from two clinical groups for ParF (table V): no (G0) versus intermediate (GNM) or severe (GNH) ParF. An univariate analysis detected no clear associations (after correction for multiple comparisons) between cytokine levels and ParF severity. However, there was a trend towards an association of ParF with low levels of IFN-γ (p=0.04) and high levels of RANTES (p=0.03) production.
Table V. Cytokines produced in culture of SEA stimulated PBMC from subjects with various parenchymal fibrosis grades.
Cultures were performed as indicated in Table III. There were 45 subjects in the G0 group, 41 subjects in the GNM group and 29 subjects in the GNH group. Cultures conditions are as indicated in Table III. The statistical analysis was performed as in Table III.
| Low ParF Grade 0 |
Intermediate ParF Grade GNM |
Severe ParF Grade GNH |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Culture conditions | % >limit | Median values (pg/ml) |
75% upper percentile value | % >limit | Median values (pg/ml) |
75% upper percentile value | % >limit | Median values (pg/ml) |
75% upper percentile value | |
| TNF-α | SEA 20h | 81 | 180.3 | 631.4 | 73 | 71.7 | 2392 | 72 | 66.6 | 534 |
| IL-10 | SEA 20h | 86 | 160.9 | 277 | 90 | 159.4 | 240 | 93 | 139 | 186 |
| IL-4 | SEA 72h | 43 | 2.6 | 9.5 | 20 | 2 | 3.4 | 18 | 2 | 3.7 |
| IL-13 | SEA 72h | 89 | 44.9 | 176.4 | 90 | 10.4 | 59.5 | 94 | 6.7 | 61.5 |
| IL-1α | SEA 20h | 72 | 20.7 | 42 | 75 | 17 | 40 | 81 | 18 | 37.8 |
| IL-5 | SEA 72h | 51 | 10.2 | 131.2 | 45 | 4 | 684 | 27 | 4 | 43 |
| IL-6 | SEA 72h | 100 | 5273.3 | 10064 | 100 | 3692.5 | 15211 | 100 | 3706.4 | 8365 |
| IFN-γ | SEA 72h | 78 | 26.7 | 119 | 80 | 10.6 | 35.1 | 74 | 18.1 | 28.9 |
| RANTES | SEA 20h | 100 | 5124.5 | 7850.9 | 100 | 8292.2 | 11879 | 100 | 7129.9 | 9994 |
| MIP-1α | SEA 72h | 100 | 7715 | 17983 | 100 | 7302.1 | 14794 | 100 | 5047 | 9505 |
| MCP-1 | SEA 20h | 100 | 35471 | 57148 | 100 | 24484.8 | 65284 | 100 | 21708.4 | 32225 |
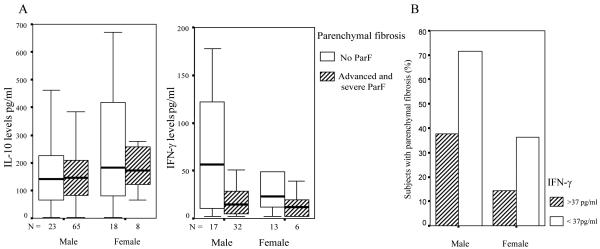
We used logistic regression to assess the associations between, sex, exposure, number of treatments, drinking habits, PPF severity and ParF phenotype. Age and sex were associated with ParF (p=0.01). The relative risk of developing ParF was 14 times higher in men than in women and increased with age. We then tested both covariates and cytokines for an association with ParF and found an association between low levels of IFN-γ and (GNM + GNH) ParF (Table VI). The risk of developing severe ParF was 8.2 times higher in subjects producing low levels of IFN-γ than in subjects producing large amounts of this cytokine (p=3.5 × 10-3; OR= 8.2; CI =2-33). This result, and the lack of correlation of ParF with IL-10, are illustrated in Fig. 2, which shows IL-10 and IFN-γ production in PBMC cultures from subjects with different ParF phenotypes and the proportion of subjects with GNM + GNH disease in IFN-γ classes.
Table VI. Stepwise regression analysis showing that parenchymal fibrosis is associated with low levels of IFN-γ production.
We compared subjects without ParF (GN0) with GNM or H subjects. As covariates, we included other cytokines and chemokines showing a trend towards association in univariate analysis (p<0.2), age, sex, exposure (years of fishing), alcohol intake and number of praziquantel treatments.
| Covariate | Odds ratio | Confidence interval | p |
|---|---|---|---|
| Sex (female versus male) | 5.8 | 1.4-24 | 0.013 |
| Age | 1.17 | 1.03-1.33 | 0.012 |
| Exposure | 0.87 | 0.7-0.99 | 0.04 |
| IFN-γ (>37 versus <37pg/ml) | 8.2 | 2-33 | 3.5 × 10-3* |
Fig. 2.
A. Production of IL-10 and IFN-γ in SEA-stimulated cultures of PBMC from male and female subjects with no parenchymal fibrosis (G0) and male and female subjects with advanced or severe parenchymal fibrosis (GNM+GNH).
White boxes represent subjects without parenchymal fibrosis. Hatched boxes represent subjects with advanced and severe ParF. The horizontal black line within the boxes represents the median value. The boxes indicate the 25th to 75th percentile range and the errors bars indicate the standard deviation.
B. Proportion of male and female subjects with parenchymal fibrosis in (GNM+GNH) groups with high (> 37 pg/ml) and low/intermediate (<37 pg/ml) levels of IFN-γ production.
White and hatched bars represent the percentages of male and female subjects, respectively, with parenchymal fibrosis. IFN-γ levels from 72 h SEA-stimulated PBMC cultures were assigned to three classes of equal size (low <17 pg/ml, intermediate 17-37 pg/ml, high>37 pg/ml).
Splenomegaly is associated with low IL-10 levels
Splenomegaly and hypersplenism are serious clinical consequences of schistosome infections. Splenomegaly is caused by both spleen cell proliferation and by congestion due to portal hypertension. We evaluated whether cytokines were associated with splenomegaly and found that the production of low levels of IL-10 in vitro was significantly associated (p=4 × 10-3) with an enlarged spleen. We compared spleens with sizes in the upper quartile (size >110 cm) with spleens in the two lowest quartiles (size < 98.5 cm); (note that mean spleen size in the subjects with severe fibrosis was 129 +/−24 cm and that in the subjects with no or mild fibrosis was 98 +/− 14 cm). None of the other cytokines tested was associated with spleen size. Age, sex and number of praziquantel treatments were not significant covariates in this association. In a multivariate analysis simultaneously assessing the contributions of PPF and IL-10 to splenomegaly, IL-10 (p=0.01, OR=3.5, CI=1.3-8.9) and PPF (p=8 × 10-3) were found to be independently associated with splenomegaly, indicating that a significant part of the association of IL-10 with splenomegaly is not just a consequence of the association of IL-10 with PPF. This result is illustrated in Fig. 3, which shows the proportion of subjects with splenomegaly in IL-10 classes separately for subjects with and without PPF in males and females.
Fig. 3. Proportion of splenomegaly in groups with high and low IL-10 levels in the (A, B, C) and (CLH, D, E, F) PPF groups.

IL-10 levels from 20 h SEA-stimulated PBMC cultures were assigned to two groups of equal size (<130 pg/ml and >130 pg/ml).
Splenectomy may restore higher levels of IL-1α and MIP-1α
Cytokines produced by SEA-stimulated PBMCs from subjects with severe PPF and subjects with severe PPF after splenectomy indicated a trend towards an increase in the production of all cytokines after splenectomy (Table VII). The differences were clearer, but not statistically significant (p=0.03), for IL-1α and MIP-1α. The spleen may therefore be involved in the regulation of cytokine production in subjects with advanced disease. However, this requires confirmation in a larger sample of subjects.
Discussion
The aim of this study was to determine whether PPF and ParF in human S. japonicum infections were associated with a characteristic pattern of cytokines. Studies in Sudan (14) and Uganda (15), on subjects infected with S. mansoni have reported an association of low levels of IFN-γ production and high levels of TNF production with a high risk of PPF. Low levels of RANTES production have also been associated with PPF, as a function of age and sex (15). Animal studies have provided strong evidence that IL-13 and IL-10 contribute to fibrosis, and high levels of IL-13 and low levels of IL-10 have been associated with PPF in humans infected with S. mansoni (15, 27, 28) (see also Table VIII). Studies on the immunology of hepatic fibrosis in humans infected with S. japonicum are scarce; a recent study showed that hepatic fibrosis was associated with TH2 cytokines. However, the authors did not deal with ParF and PPF separately and did not evaluate epidemiological covariates (29). Our evaluation of several hundred fishermen infected with S. japonicum indicated that ParF and PPF should be considered independently, as they are not well correlated in subjects from endemic areas: severe PPF may occur without ParF and, conversely, advanced ParF may be observed in subjects with no PPF. We also found that PPF was correlated with evidence of portal hypertension, splenomegaly and left liver lobe regression, whereas ParF was not (Luo.X et al., personal communication). The molecular causes of ParF remain unclear. ParF may result from the diffusion of toxic substances emitted by eggs, not arrested by the granuloma. It may also reflect a strong inflammatory response extending beyond the main portal branches. In any case, in this study population, ParF occurred only in subjects infected with schistosomes. It was not seen in cases of liver damage unrelated to schistosomiasis.
Table VIII. Previous studies on cytokines associated with severe fibrosis due to S. mansoni.
| Population | Year | Authors | Parasite | Cytokines tested | Cytokines associated with protection | Cytokines associated with severe disease |
|---|---|---|---|---|---|---|
| Sudan | 2002 | Henri et al. | S.mansoni | IL-1β, IL-4, IL-5, IL-6, IL-10, IFN-γ, TNF-α | IFN-γ | TNF-α |
| Brazil | 2004 | De Jesus et al. | S.mansoni | IL-5, IL-10, IL-13, IFN-γ, TNF-α, TGF-β | IL-5, IL-10, IL-13 | |
| Uganda | 2004 | Booth et al. | S.mansoni | IL-3, IL-4, IL-5, IL-10, IL-13, IFN-γ, TNF-α, IL-1β, RANTES | IL-10, IFN-γ or RANTES depending on age and gender | TNF-α |
| Brazil | 2006 | Oliveira et al. | S.mansoni | IFN-γ, TNF-α, TGF-β, IL-4, IL-10, IL-13 | IL-10 (low association) | IL-13 |
We have shown here that IL-10, RANTES and MIP-1α levels are lower in cultures of leukocytes from subjects with severe PPF than in those of PBMC from subjects with no or mild fibrosis. The strong association between low levels of IL-10 and severe PPF was confirmed after taking into account the effects of various major covariates, such as exposure, alcohol intake and number of anti-schistosome treatments. The association of low levels of IL-10 production with PPF was robust: it was observed with severe PPF and with severe + advanced PPF as disease phenotypes, with or without other covariates. Our findings also show that IL-10 is associated with splenomegaly independently of PPF, indicating that splenomegaly and IL-10 are probably directly related.
The strong association between disease (PPF and splenomegaly) and IL-10, together with observations on IL-10 levels in humans infected with S. mansoni (15, 28), show that IL-10 plays a key regulatory role in pathogenesis as previously observed in experimental models (10), rather than being just one of many cytokines regulating fibrosis. In mice infected with S. mansoni, IL-10 influences the polarisation of the egg-induced Th2 response and IL-10-deficient mice mount a mixed Th1 and Th2 immune response, resulting in severe liver damage and high mortality rates (30, 31). IL-10, by down-regulating the immune response, plays a crucial role in preventing disease caused by the large numbers of schistosome eggs accumulating in the human liver and triggering inflammation. IL-10 may control the balance between egg destruction and tissue damage, thereby preventing the extensive deposition of fibrotic tissue.
IL-10 also has antifibrotic properties, enabling it to modulate extracellular matrix deposition by down-regulating collagen production or up-regulating the production of collagenases, such as matrix metalloproteinase 13 (MMP-13), which degrades extracellular matrix (32-34).
IL-10 was originally thought to be produced by CD4+ Th2 cells (35). However, it is now known to be produced by various cell types: IL-10 mediates the effects of some CD4+ CD25+ T regulatory cells (36, 37). Tregs are also recruited by S. japonicum eggs (38). The pathogenesis of schistosomiasis mansoni in mice is also regulated by IL-10-producing cells (10). Th1 cells have also been shown to produce IL-10 (39, 40). Thus, IL-10 and IFN-γ are not necessarily produced in a mutually exclusive manner by T cells. This is of prime importance if these two cytokines are to act synergistically to prevent severe fibrosis, as suggested here. Our work suggests that splenectomy restores the normal production of certain cytokines, including IL-10, indicating that the weak cytokine response is reversible. The improvement of the cytokine response is associated with a general improvement in the clinical state of the patient. Spleen hyperactivity in subjects with advanced PPF may be related to an imbalance in the regulatory networks that normally keep the inflammatory response under control. Indeed, the massive lymphoproliferation observed in the spleen of these subjects indicates disturbances in regulation of the immune response, consistent with the association of low IL-10 production and splenomegaly.
If IL-10 levels were not included in the multivariate analysis, low levels of RANTES and MIP-1α production were associated with a higher risk of PPF. RANTES is involved in Th2 responses; it attracts leukocytes to the site of inflammation (41) and may be involved in regulating Th1 and Th2 responses and down-regulating SEA-induced Th2-type granuloma (42). Thus, RANTES may complement the action of IL-10 in controlling immune reactions to antigenic and irritating substances produced by eggs and worms.
MIP-1α is produced by many cell types, including macrophages, dendritic cells and lymphocytes. It is released after the embolisation of schistosome eggs into the lungs of mice, and its neutralisation reduces granuloma size (43). Mice deficient in the MIP-1α receptor, CCR1, have egg-induced pulmonary granulomas 40% smaller than those of normal mice (44), suggesting that MIP-1α and CCR1 may promote the granulomatous reaction. MIP-1α and RANTES signal through the same cell surface receptors — CCR1 and CCR5 — and stimulation through CCR1 may increase morbidity in murine schistosomiasis (44). Conversely, the CCR5 receptor has been implicated in the promotion of Th1 immune responses in mice (45), and its activation increases IFN-γ production(46). Souza et al. recently suggested that severe forms of the disease may result from unbalanced chemokine receptor activation, favouring the activation of CCR1 over that of CCR5 (47).
This study was the first to analyse ParF separately from PPF. ParF was correlated with low IFN-γ levels, confirming the role played by this cytokine in the control of fibrosis progression in schistosome-infected subjects (6-8, 14, 19). A key role for IFN-γ was also suggested by the association of two polymorphisms in IFNG (IFNG+2109A/G and IFNG+3810G/A) with severe PPF caused by S .mansoni (13). However, ParF was not associated with IL-10, and we observed no trend for an association of ParF with IL-10 levels, even when IFN-γ was removed from the analysis. This finding confirms that ParF and PPF are regulated differently.
In conclusion, this work provides a clearer indication as to which cytokines may be crucial for preventing disease in patients with massive stimulation due to eggs of S. japonicum. It indicates that IL-10 probably plays a key role in regulation, as observed in experimental models and suggested in studies of humans infected with S. mansoni. The much more apparent role of IL-10 in S. japonicum infections may result from strong stimulation of the host immune system by S. japonicum products, resulting in a need for strong anti-inflammatory control, which can most efficiently be delivered by IL-10.
Finally, this work, together with ongoing epidemiological studies, demonstrates that ParF and PPF are not regulated in the same way. In future studies, we will evaluate the genetic factors selectively regulating each of these two fibrosis phenotypes and determining how rapidly infected fishermen progress towards severe disease.
Acknowledgments
We thank Sandrine Cabantous for excellent technical work and Christophe Chevillard for his advice during the course of the study.
Footnotes
This work was supported by the Institut National de la Santé et de la Recherche Médicale, the World Health Organisation (RIC04001AP), the cohort and collection subvention, INSERM reference 4CH04G and the “Ministry of Science and Technology in China ” ID 2004AA2Z3610.
References
- 1.Grimaud JA, Borojevic R. Chronic human schistosomiasis mansoni. Pathology of the Disse’s space. Lab Invest. 1977;36:268. [PubMed] [Google Scholar]
- 2.Li YS, Sleigh AC, Ross AG, Williams GM, Tanner M, McManus DP. Epidemiology of Schistosoma japonicum in China: morbidity and strategies for control in the Dongting Lake region. Int J Parasitol. 2000;30:273. doi: 10.1016/s0020-7519(99)00201-5. [DOI] [PubMed] [Google Scholar]
- 3.Cheever AW, Finkelman FD, Cox TM. Anti-interleukin-4 treatment diminishes secretion of Th2 cytokines and inhibits hepatic fibrosis in murine schistosomiasis japonica. Parasite Immunol. 1995;17:103. doi: 10.1111/j.1365-3024.1995.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 4.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999;104:777. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiaramonte MG, Schopf LR, Neben TY, Cheever AW, Donaldson DD, Wynn TA. IL-13 is a key regulatory cytokine for Th2 cell-mediated pulmonary granuloma formation and IgE responses induced by Schistosoma mansoni eggs. J Immunol. 1999;162:920. [PubMed] [Google Scholar]
- 6.Duncan MR, Berman B. Gamma interferon is the lymphokine and beta interferon the monokine responsible for inhibition of fibroblast collagen production and late but not early fibroblast proliferation. J Exp Med. 1985;162:516. doi: 10.1084/jem.162.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallat A, Preaux AM, Blazejewski S, Rosenbaum J, Dhumeaux D, Mavier P. Interferon alfa and gamma inhibit proliferation and collagen synthesis of human Ito cells in culture. Hepatology. 1995;21:1003. [PubMed] [Google Scholar]
- 8.Baroni GS, D’Ambrosio L, Curto P, Casini A, Mancini R, Jezequel AM, Benedetti A. Interferon gamma decreases hepatic stellate cell activation and extracellular matrix deposition in rat liver fibrosis. Hepatology. 1996;23:1189. doi: 10.1002/hep.510230538. [DOI] [PubMed] [Google Scholar]
- 9.Gressner AM. The cell biology of liver fibrogenesis - an imbalance of proliferation, growth arrest and apoptosis of myofibroblasts. Cell Tissue Res. 1998;292:447. doi: 10.1007/s004410051073. [DOI] [PubMed] [Google Scholar]
- 10.Hesse M, Piccirillo CA, Belkaid Y, Prufer J, Mentink-Kane M, Leusink M, Cheever AW, Shevach EM, Wynn TA. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J Immunol. 2004;172:3157. doi: 10.4049/jimmunol.172.5.3157. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto T, Eckes B, Mauch C, Hartmann K, Krieg T. Monocyte chemoattractant protein-1 enhances gene expression and synthesis of matrix metalloproteinase-1 in human fibroblasts by an autocrine IL-1 alpha loop. J Immunol. 2000;164:6174. doi: 10.4049/jimmunol.164.12.6174. [DOI] [PubMed] [Google Scholar]
- 12.Maurer M, von Stebut E. Macrophage inflammatory protein-1. Int J Biochem Cell Biol. 2004;36:1882. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Chevillard C, Moukoko CE, Elwali NE, Bream JH, Kouriba B, Argiro L, Rahoud S, Mergani A, Henri S, Gaudart J, Mohamed-Ali Q, Young HA, Dessein AJ. IFN-gamma polymorphisms (IFN-gamma +2109 and IFN-gamma +3810) are associated with severe hepatic fibrosis in human hepatic schistosomiasis (Schistosoma mansoni) J Immunol. 2003;171:5596. doi: 10.4049/jimmunol.171.10.5596. [DOI] [PubMed] [Google Scholar]
- 14.Henri S, Chevillard C, Mergani A, Paris P, Gaudart J, Camilla C, Dessein H, Montero F, Elwali NE, Saeed OK, Magzoub M, Dessein AJ. Cytokine regulation of periportal fibrosis in humans infected with Schistosoma mansoni: IFN-gamma is associated with protection against fibrosis and TNF-alpha with aggravation of disease. J Immunol. 2002;169:929. doi: 10.4049/jimmunol.169.2.929. [DOI] [PubMed] [Google Scholar]
- 15.Booth M, Mwatha JK, Joseph S, Jones FM, Kadzo H, Ireri E, Kazibwe F, Kemijumbi J, Kariuki C, Kimani G, Ouma JH, Kabatereine NB, Vennervald BJ, Dunne DW. Periportal fibrosis in human Schistosoma mansoni infection is associated with low IL-10, low IFN-gamma, high TNF-alpha, or low RANTES, depending on age and gender. J Immunol. 2004;172:1295. doi: 10.4049/jimmunol.172.2.1295. [DOI] [PubMed] [Google Scholar]
- 16.Silva-Teixeira DN, Contigli C, Lambertucci JR, Serufo JC, Rodrigues V., Jr. Gender-related cytokine patterns in sera of schistosomiasis patients with Symmers’ fibrosis. Clin Diagn Lab Immunol. 2004;11:627. doi: 10.1128/CDLI.11.3.627-630.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jesus AR, Silva A, Santana LB, Magalhaes A, de Jesus AA, de Almeida RP, Rego MA, Burattini MN, Pearce EJ, Carvalho EM. Clinical and immunologic evaluation of 31 patients with acute schistosomiasis mansoni. J Infect Dis. 2002;185:98. doi: 10.1086/324668. [DOI] [PubMed] [Google Scholar]
- 18.Abel L, Demenais F, Prata A, Souza AE, Dessein A. Evidence for the segregation of a major gene in human susceptibility/resistance to infection by Schistosoma mansoni. Am J Hum Genet. 1991;48:959. [PMC free article] [PubMed] [Google Scholar]
- 19.Dessein A, Kouriba B, Eboumbou C, Dessein H, Argiro L, Marquet S, Elwali NE, Rodrigues V, Li Y, Doumbo O, Chevillard C. Interleukin-13 in the skin and interferon-gamma in the liver are key players in immune protection in human schistosomiasis. Immunol Rev. 2004;201:180. doi: 10.1111/j.0105-2896.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- 20.Marquet S, Abel L, Hillaire D, Dessein H, Kalil J, Feingold J, Weissenbach J, Dessein AJ. Genetic localization of a locus controlling the intensity of infection by Schistosoma mansoni on chromosome 5q31-q33. Nat Genet. 1996;14:181. doi: 10.1038/ng1096-181. [DOI] [PubMed] [Google Scholar]
- 21.Kouriba B, Chevillard C, Bream JH, Argiro L, Dessein H, Arnaud V, Sangare L, Dabo A, Beavogui AH, Arama C, Traore HA, Doumbo O, Dessein A. Analysis of the 5q31-q33 locus shows an association between IL13-1055C/T IL-13-591A/G polymorphisms and Schistosoma haematobium infections. J Immunol. 2005;174:6274. doi: 10.4049/jimmunol.174.10.6274. [DOI] [PubMed] [Google Scholar]
- 22.Campino S, Kwiatkowski D, Dessein A. Mendelian and complex genetics of susceptibility and resistance to parasitic infections. Semin Immunol. 2006;18:411. doi: 10.1016/j.smim.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Muller-Myhsok B, Stelma FF, Guisse-Sow F, Muntau B, Thye T, Burchard GD, Gryseels B, Horstmann RD. Further evidence suggesting the presence of a locus, on human chromosome 5q31-q33, influencing the intensity of infection with Schistosoma mansoni. Am J Hum Genet. 1997;61:452. doi: 10.1016/S0002-9297(07)64073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dessein AJ, Hillaire D, Elwali NE, Marquet S, Mohamed-Ali Q, Mirghani A, Henri S, Abdelhameed AA, Saeed OK, Magzoub MM, Abel L. Severe hepatic fibrosis in Schistosoma mansoni infection is controlled by a major locus that is closely linked to the interferon-gamma receptor gene. Am J Hum Genet. 1999;65:709. doi: 10.1086/302526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanton RE, Salam EA, Ehsan A, King CH, Goddard KA. Schistosomal hepatic fibrosis and the interferon gamma receptor: a linkage analysis using single-nucleotide polymorphic markers. Eur J Hum Genet. 2005;13:660. doi: 10.1038/sj.ejhg.5201388. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. Richter J, Hatz C, Campagne G, Bergquist NR, Jenkins JM. Ultrasound in Schistosomiasis: A Pratical Guide to the Standardized use of Ultrasonography for the assessment of Schistosomiasis-related morbidity. 2000. TDR/STR/SCH.TDR/WHO. [Google Scholar]
- 27.de Jesus AR, Magalhaes A, Miranda DG, Miranda RG, Araujo MI, de Jesus AA, Silva A, Santana LB, Pearce E, Carvalho EM. Association of type 2 cytokines with hepatic fibrosis in human Schistosoma mansoni infection. Infect Immun. 2004;72:3391. doi: 10.1128/IAI.72.6.3391-3397.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alves Oliveira LF, Moreno EC, Gazzinelli G, Martins-Filho OA, Silveira AM, Gazzinelli A, Malaquias LC, LoVerde P, Leite PM, Correa-Oliveira R. Cytokine production associated with periportal fibrosis during chronic schistosomiasis mansoni in humans. Infect Immun. 2006;74:1215. doi: 10.1128/IAI.74.2.1215-1221.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coutinho HM, Acosta LP, Wu HW, McGarvey ST, Su L, Langdon GC, Jiz MA, Jarilla B, Olveda RM, Friedman JF, Kurtis JD. Th2 cytokines are associated with persistent hepatic fibrosis in human Schistosoma japonicum infection. J Infect Dis. 2007;195:288. doi: 10.1086/510313. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164:6406. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- 31.Wynn TA, Morawetz R, Scharton-Kersten T, Hieny S, Morse HC, 3rd, Kuhn R, Muller W, Cheever AW, Sher A. Analysis of granuloma formation in double cytokine-deficient mice reveals a central role for IL-10 in polarizing both T helper cell 1- and T helper cell 2-type cytokine responses in vivo. J Immunol. 1997;159:5014. [PubMed] [Google Scholar]
- 32.Mathurin P, Xiong S, Kharbanda KK, Veal N, Miyahara T, Motomura K, Rippe RA, Bachem MG, Tsukamoto H. IL-10 receptor and coreceptor expression in quiescent and activated hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G981. doi: 10.1152/ajpgi.00293.2001. [DOI] [PubMed] [Google Scholar]
- 33.Thompson K, Maltby J, Fallowfield J, McAulay M, Millward-Sadler H, Sheron N. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis. Hepatology. 1998;28:1597. doi: 10.1002/hep.510280620. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto T, Eckes B, Krieg T. Effect of interleukin-10 on the gene expression of type I collagen, fibronectin, and decorin in human skin fibroblasts: differential regulation by transforming growth factor-beta and monocyte chemoattractant protein-1. Biochem Biophys Res Commun. 2001;281:200. doi: 10.1006/bbrc.2001.4321. [DOI] [PubMed] [Google Scholar]
- 35.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 37.Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa TC, Cumano A, Bandeira A. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol. 2001;166:3008. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Zhao J, Yang Y, Zhang L, Yang X, Zhu X, Ji M, Sun N, Su C. Schistosoma japonicum egg antigens stimulate CD4 CD25 T cells and modulate airway inflammation in a murine model of asthma. Immunology. 2007;120:8. doi: 10.1111/j.1365-2567.2006.02472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25(-)Foxp3(-) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007;204:285. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, Sher A. Conventional T-bet(+)Foxp3(-) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204:273. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bisset LR, Schmid-Grendelmeier P. Chemokines and their receptors in the pathogenesis of allergic asthma: progress and perspective. Curr Opin Pulm Med. 2005;11:35. doi: 10.1097/01.mcp.0000144502.50149.e0. [DOI] [PubMed] [Google Scholar]
- 42.Chensue SW, Warmington KS, Allenspach EJ, Lu B, Gerard C, Kunkel SL, Lukacs NW. Differential expression and cross-regulatory function of RANTES during mycobacterial (type 1) and schistosomal (type 2) antigen-elicited granulomatous inflammation. J Immunol. 1999;163:165. [PubMed] [Google Scholar]
- 43.Lukacs NW, Kunkel SL, Strieter RM, Chensue SW. The role of chemokines in Schistosoma mansoni granuloma formation. Parasitol Today. 1994;10:322. doi: 10.1016/0169-4758(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 44.Gao JL, Wynn TA, Chang Y, Lee EJ, Broxmeyer HE, Cooper S, Tiffany HL, Westphal H, Kwon-Chung J, Murphy PM. Impaired host defense, hematopoiesis, granulomatous inflammation and type 1-type 2 cytokine balance in mice lacking CC chemokine receptor 1. J Exp Med. 1997;185:1959. doi: 10.1084/jem.185.11.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andres PG, Beck PL, Mizoguchi E, Mizoguchi A, Bhan AK, Dawson T, Kuziel WA, Maeda N, MacDermott RP, Podolsky DK, Reinecker HC. Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J Immunol. 2000;164:6303. doi: 10.4049/jimmunol.164.12.6303. [DOI] [PubMed] [Google Scholar]
- 46.Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, Dayer JM. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 47.Souza AL, Sousa-Pereira SR, Teixeira MM, Lambertucci JR, Teixeira AL. The role of chemokines in Schistosoma mansoni infection: insights from human disease and murine models. Mem Inst Oswaldo Cruz. 2006;101(Suppl 1):333. doi: 10.1590/s0074-02762006000900054. [DOI] [PubMed] [Google Scholar]