Abstract
The role of Th17 lymphocytes in immunopathogenic processes has been well established, but little is known about their basic cell features. In this study, we compared polarized Th1 and Th17 for key biological activities related to pathogenicity and trafficking. Th1 and Th17 lineages were derived from TCR-transgenic CD4 murine cells specific against hen egg lysozyme. When adoptively transferred into mice expressing hen egg lysozyme in their eyes, both Th1 and Th17 induced ocular inflammation but with slight differences in histological pathology. PCR analysis revealed selective expression of IFN-γ or IL-17 in eyes of Th1 or Th17 recipients, respectively. Additionally, Th1 and Th17 were found to differ in three other key activities: 1) Th17 cells were inferior to Th1 cells in their capacity to trigger massive lymphoid expansion and splenomegaly; 2) the proportion of Th1 cells among infiltrating cells in inflamed recipient eyes declined rapidly, becoming a minority by day 7, whereas Th17 cells remained in the majority throughout this period; and 3) remarkable differences were noted between Th1 and Th17 cells in their expression of certain surface markers. In particular, reactivated Th1 expressed higher levels of CD49d and α4β7 (mucosal homing) in vitro and higher levels of CXCR3 (Th1 trafficking) in vivo. Reactivated Th17, however, expressed higher levels of αEβ7 (epithelial tissue homing) and CD38 (activation, maturation and trafficking) in vitro, but in vivo Th17 expressed higher levels of α4β7 and CCR6 (lymphocyte trafficking). These data reveal that Th1 and Th17 cells differ in several key biological activities influencing migration and pathogenic behavior during inflammatory disease.
Recent studies have identified a population of T helper cells that produce members of the IL-17 cytokine family and have been designated “Th17” (1–3). Most IL-17 cytokines are strongly proinflammatory, and Th17 cells have been shown to play a critical role in immune-mediated inflammation (1–7) as well as in defense against microbial infection (8–10). Th17 cells were also reported to be superior to Th1 cells by far in their capacity to adoptively transfer experimental autoimmune encephalomyelitis (6) or experimental colitis (11). Yet, highly purified Th1 cells have been shown in numerous studies to be highly immunopathogenic and capable of inducing experimental autoimmune disease at low cell numbers (12–14). It has been proposed, therefore, that both Th1 and Th17 are involved in the pathogenic process, with each cell type playing a certain specific role (2, 3, 15, 16).
Many features of T cell populations, including their immunopathogenic capacity, have been defined by using TCR-transgenic (Tg) 4 mice in which most T cells express the same Ag receptor (12–14). To the best of our knowledge, this approach for defining the basic features of Th17 cells, including their pathogenic potential and other biological capacities, has not yet been reported. The present study used this approach to compare polarized populations of Th1 and Th17 for their capacity to adoptively transfer immune-mediated ocular inflammation as well as for other key biological activities.
In our experimental system, T cells from TCR-Tg mice specific against hen egg lysozyme (HEL) have been shown to induce ocular inflammation when adoptively transferred into Tg mice expressing HEL in their eyes (14, 17, 18). We obtained polarized lineages of Th1 and Th17 by repeated incubation of naive CD4 cells from TCR-Tg mice with HEL and mixtures of cytokines and Abs known to skew activation toward either a Th1 or a Th17 phenoype. Both Th lineages were found to be immunopathogenic, with slight differences in patterns of pathological changes induced in recipient eyes. In addition, the two lineages differed remarkably in their capacity to stimulate massive lymphoid expansion in recipient mice, in their profile of chemokine secretion, as well as in their expression of several surface markers in vitro and in vivo. Interestingly, remarkable differences were also noted between the two subsets in their kinetics of intraocular invasion and host cell recruitment.
Materials and Methods
Mice
“HEL-Tg” mice expressing membrane-associated HEL under control of the αA-crystallin promoter and on an FVB/N background were generated as described (19). HEL-specific TCR-Tg mice (designated “3A9”) on a B10.BR background, were a gift from M. Davis (Stanford University, Stanford, CA). Tg mice from each line were mated to produce (FVB/N × B10.BR) F1 hybrids that express HEL, HEL-specific TCR, or both or neither transgene (“wild type” (WT)). The F1 hybrids containing the different transgenes were used for all experiments. For all adoptive transfer studies, reactivated cells from 3A9 donors were injected into HEL-Tg mice or syngeneic WT mice. All manipulations were performed in compliance with the National Institutes of Health Resolution on the Use of Animals in Research.
Cytokines, Abs, and Ag
IL-6 and TGF-β were provided by R&D Systems, IL-1α was obtained from PeproTech, IL-2 was from Midwest Medical, anti-IFN-γ (clone R4–6A2) was from Harlan Bioproducts for Science, anti-IL-4 (clone 11B11) was from National Cancer Institute-Frederick Biological Resources Branch Preclinical Repository (Frederick, MD), and both IL-12 and HEL were purchased from Sigma-Aldrich. The following reagents were purchased from BD Biosciences: anti-CD4 PerCP, allophycocyanin, or PE-Cy7 (clone RM4–5), anti-CD16/CD32 (clone 2.4G2), anti-CD38 PE (clone 90), anti-CD45RB PE (clone 16A), anti-CD49d PE (clone 9C10 (MFR4.B)), anti-CD62L PE (clone MEL-14), anti-CD103 PE (clone M290), anti-LPAM-1 PE (clone DATK32), anti-IFN-γ allophycocyanin (clone XMG1.2), anti-IL-12 (clone C17.8), anti-IL-17 PE (clone TC11–18H10.1), 7-amino-actinomycin D, and IgG isotype control. Anti-CCR6 PE (clone 140706) and anti-CXCR3 PE (clone 220803) were provided by R&D Systems. Anti-CCR7 PE (clone 4B12) was obtained from eBioscience. A clonotypic mAb specific for the TCR of 3A9 mice, designated “1G12,” a gift from E. Unanue (Washington University, St. Louis, MO), was conjugated with FITC.
Generation of cell lines
Cells were isolated from spleens and lymph nodes of 3A9 mice and then CD4 cells were purified by T cell enrichment (R&D Systems) and MACS microbead separation (Miltenyi Biotec). CD4 cells (5 × 105/2 ml) were incubated for 3 days in RPMI 1640 containing 10% FBS and 50 µM 2-ME with 2 µg/ml HEL and irradiated (30 Gy) syngeneic WT naive splenocytes serving as APCs (5 × 106/2 ml) (14) in either Th1-polarizing conditions (10 ng/ml IL-12 and 10 µg/ml anti-IL-4) or Th17-polarizing conditions (3 ng/ml TGF-β, 10 ng/ml IL-6, 5 ng/ml IL-1α, 10 µg/ml anti-IL-4, 10 µg/ml anti-IL-12, and 20 µg/ml anti-IFN-γ). Polarized cells were expanded in the presence of 40 IU/ml IL-2 for an additional 4 days with daily 1-ml medium changes. Finally, cells were harvested and replated for 3 days of reactivation with 2 µg/ml HEL, WT naive irradiated APCs, and either 10 ng/ml IL-12 plus 40 IU/ml IL-2 for Th1 cells or a cytokine-Ab mixture for Th17 cells (10 ng/ml IL-23, 3 ng/ml TGF-β, 10 ng/ml IL-6, 5 ng/ml IL-1α, 40 IU/ml IL-2, and 20 µg/ml anti-IFN-γ). For the generation of cell lines designated “expanded II,” reactivated cells were reincubated in the presence of 40 IU of IL-2 for an additional 11 days with daily 1-ml medium changes.
Reactivated Th2 cell lines were generated as detailed elsewhere (14).
Surface Ag analysis
Cells were stained according to standard protocol with a set of conjugated Abs including 1G12 FITC, CD4 PerCP, or allophycocyanin, a surface Ag of choice conjugated with PE (see above), plus 7-amino-actinomycin D to exclude dead cells. Stained cells were analyzed by FACSCalibur flow cytometry and analyzed with FlowJo 6.4.7. Before all staining, anti-CD16/CD32 was used to block FcRs.
Cytokine analysis
Release of cytokines and chemokines into the culture medium was measured on day 3 of reactivation by the SearchLight system (Pierce Biotechnology).
For intracellular cytokine analysis, reactivated cultures were stimulated with 20 ng/ml PMA and 1 µM ionomycin (Sigma-Aldrich) plus Golgi-Stop (BD Biosciences) for 5 h. Stimulated cells were processed on a Lympholyte- M gradient (Cedarlane) for dead cell removal. Cells were then stained for surface CD4 FITC, washed, and then fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences). Finally, cells were stained for intracellular IFN-γ allophycocyanin/IL-17 PE and analyzed by FACScali-bur flow cytometry.
For mRNA analysis, TRIzol reagent (Invitrogen) was used to extract total RNA from reactivated cells or from whole eyes of recipients after mice were perfused with PBS on day 5 postadoptive transfer. Total RNA was incubated with DNase (Promega), reverse transcribed into cDNA using oligo primers and SuperScript II RT (Invitrogen), and then analyzed by real-time RT-PCR on an ABI0 Prism 7700 with TaqMan primer/probe sets for IFN-γ and IL-17 (Applied Biosystems).
Adoptive transfer studies
Th1 or Th17 cells were harvested on day 3 of reactivation, resuspended in RPMI 1640, and injected via the tail vein into naive HEL-Tg or WT mice.
For HEL-Tg recipients, eyes were collected at different time points. To perform mRNA analysis (described above), eyes were collected on day 5 postadoptive transfer. For histology, eyes were collected on day 7 and processed by conventional H&E methods. For isolation of inflammatory cells, eyes were collected on days 4 and 7 (see below).
For WT recipients, spleens were collected on day 4 postadoptive transfer, weighed, and splenocyte numbers were determined. Blood was processed on an Isolymph gradient (Budenheim/Gallard-Schlesinger) for mononuclear leukocyte (MNL) isolation and counting.
Isolation of cells from eyes
On days 4 and 7 postadoptive transfer, eyes of Th1 or Th17 recipients were collected, dissected, and digested in RPMI 1640 medium containing 10% FCS, 1 mg/ml collagenase D (Roche), and deoxyribonuclease I (Sigma-Aldrich) for 2 h. Following digestion, eye tissue was mechanically disrupted and filtered through a 40-µm cell strainer. Isolated cells were washed twice in RPMI 1640 and prepared for flow cytometric analysis.
Statistical analyses
Student’s t test was used for statistical analyses. Significance was defined as p < 0.05.
Results
Polarized Th1 and Th17 cells selectively produce either IFN-γ or IL-17
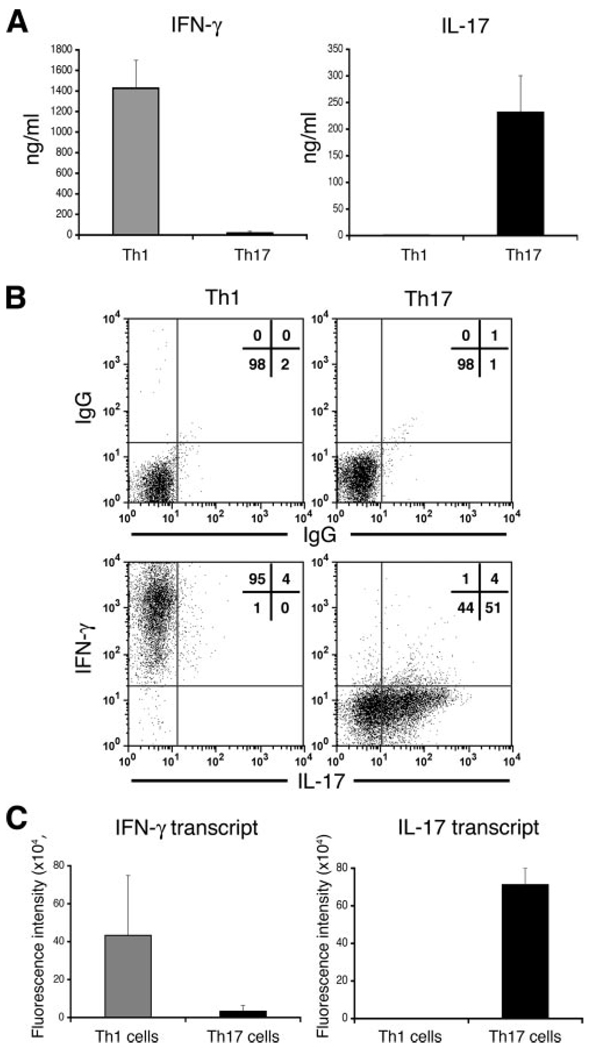
To generate Th1 and Th17 lineages, TCR-Tg CD4 cells specific against HEL were polarized and reactivated using mixtures of cytokines and Abs known to drive polarization toward a Th1 or Th17 phenotype (see Materials and Methods). We examined each lineage for phenotypic purity by measuring the release into culture medium (Fig. 1A), intracellular production (Fig. 1B), and mRNA transcript levels (Fig. 1C) of the two signature cytokines, IFN-γ and IL-17. All assays revealed high levels of polarity, with selective expression of IFN-γ by Th1 or that of IL-17 by Th17.
FIGURE 1.
Polarized and reactivated Th1 and Th17 cells selectively express IFN-γ or IL-17. A, Supernatants of Th1 or Th17 cultures were collected following reactivation and were then tested for levels of IFN-γ or IL-17 by SearchLight technology. The data are expressed as mean values ± SEM of three experiments. B, Intracellular expression of IFN-γ and IL-17 by polarized and reactivated Th1 and Th17 before adoptive transfer into recipient mice. C, Selective expression of IFN-γ or IL-17 transcripts from polarized and reactivated Th1 or Th17, respectively. Total RNA was collected from reactivated cells, pooled, and then analyzed by real time RT-PCR. The data are expressed as mean values ± SEM of two experiments.
Secretion of chemokines and cytokines by Th cell lines
Th cells mediate inflammation mainly by secreting cytokines and chemokines that affect other cell populations. To collect information on the activity of Th1 and Th17 cells, we measured the levels of several major chemokines from culture supernatants following 3 days of reactivation. To obtain an expanded perspective on this issue, we also examined the secretion pattern of reactivated Th2 cell lines. Table I summarizes a representative experiment; similar secretion profiles were obtained in two other experiments. It is notable that the number of Th1 cells per milliliter at the end of restimulation was considerably lower than that of the other Th populations as a result of their high apoptosis. The collected data identify four chemokines that are secreted at relatively high levels by the tested Th populations, with individual patterns of preference by each of the three Th subsets (Table I). These chemokines include MDC/CCL22, MIP-1α/CCL3, RANTES/CCL5 and TARC/CCL17 (where TARC stands for “thymus- and activation-regulated chemokine”). We also show in Table I the levels of IL-10 secreted by the three cell lines, because the production of IL-10 by Th17 was recently reported to determine the immunopathogenicity of Th17 cell preparations (20). Moderate levels of IL-10 were secreted by Th1 and Th17, whereas Th2 secreted an ~10-fold higher level of this cytokine. Levels of all tested molecules were profoundly lower than those of the signature cytokines.
Table I.
Secretion of chemokines and cytokines by reactivated Th line cellsa
| Th Line | MDC/CCL22 | MIP-1a/CCL3 | RANTES/CCL5 | TARC/CCL17 | MIP-2/CXCL1–3 | Eotaxin/CCL11 | MCP-1/CCL2 | IL-10 |
|---|---|---|---|---|---|---|---|---|
| Th1 | 7,100b | 1,750 | 588 | 190 | 58 | 78 | 79 | 4,100 |
| Th17 | 3,700 | 10,100 | 632 | 1,940 | 158 | 98 | 53 | 4,835 |
| Th2 | 13,300 | 67 | 154 | 25,560 | 156 | 418 | 87 | 36,020 |
Supernatants of line cells were collected following 3 days of reactivation and the levels of tested molecules were measured by the SearchLight technology. Levels of the signature cytokines were 1.6 × 106 pg/ml IFN-γ for Th1 and 0.4 × 106 pg/ml IL-17 for Th17; IL-4 secretion could not be measured in Th2 cultures because this cytokine was added to their stimulation mixture. All three lines had only trace levels of the signature cytokines of the other lines.
All values are expressed as pg/ml.
Both Th1 and Th17 are immunopathogenic, with some differences in the histopathological changes induced by each
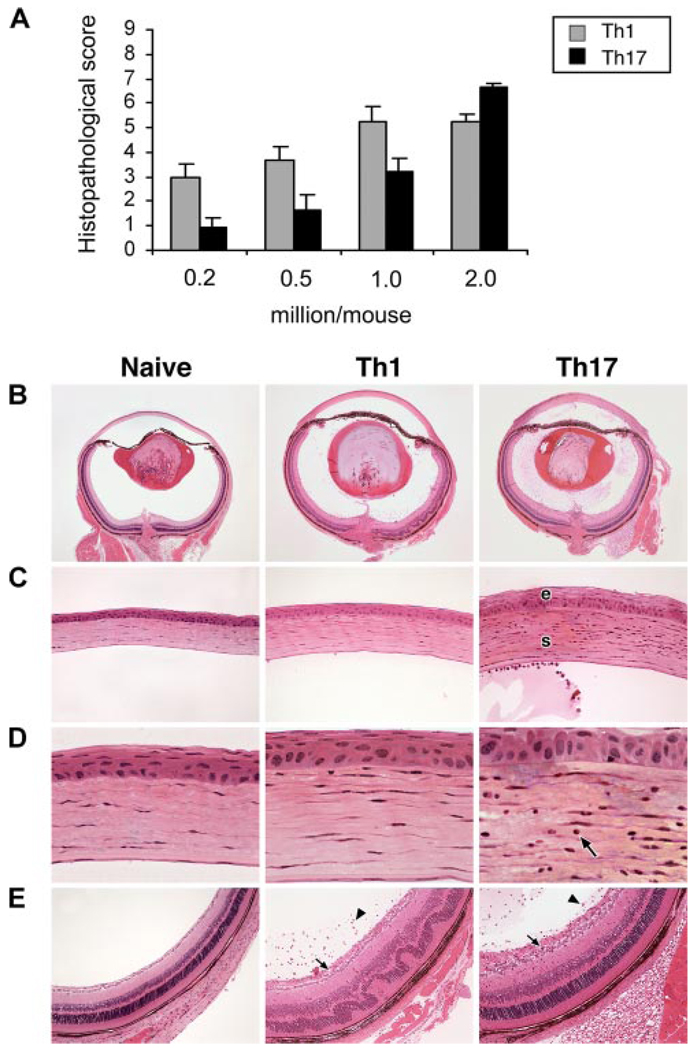
To compare Th1 and Th17 cells of our system for their immunopathogenic capacities, we adoptively transferred different numbers of HEL-specific polarized and reactivated Th1 or Th17 cells into groups of mice that express membrane-associated HEL in their eyes (“HEL-Tg” mice). We examined eyes of recipient mice 7 days later for pathological changes that were scored on a 0–9 scale as detailed elsewhere (14, 17). These data are summarized in Fig. 2A; Th1 was found to be more immunopathogenic at lower cell numbers, but similar levels of severity were induced in mice injected with 2 × 106 cells from both the Th1 and Th17 lineages.
FIGURE 2.
Polarized Th1 and Th17 cells both induce pathological changes in eyes of recipient mice. A, Immunopathogenicity of polarized Th1 and Th17 cells. Different numbers, as indicated, of polarized and reactivated Th1 or Th17 cells were injected into naive HEL-Tg recipients. Eyes were collected on day 7 and histopathological changes were scored on a scale of 0–9, as detailed in Ref 14. B–E, Eye sections of a control naive HEL-Tg mouse eye and typical changes in eyes of recipients injected with 1 × 106 polarized and reactivated Th1 or Th17 cells. B, Whole eyes showing inflammatory changes in both the anterior and posterior segments of recipient eyes consisting of mixed inflammatory cellular infiltrates and proteinaceous exudates. C, Corneas of the same two recipient eyes showing edema of the corneal epithelium (e) and stroma (s) in the Th17 recipient. D, Higher magnification depicting infiltration of inflammatory cells (arrow) into the central portion of the cornea in the Th17 recipient. E, Posterior segments demonstrating inflammatory cells in the vitreous (arrowheads) and inner retinal layers, including the nerve fiber layer and ganglion cell layer (arrows). In addition, the Th1 recipient eye shows retinal folding (H&E staining). Original magnification: ×25 (B); ×200 (C); ×630 (D); ×100 (E).
Fig. 2, B–E depict typical changes in the eyes of recipients injected with 1 × 106 cells of the two lineages. Sections of a naive HEL-Tg mouse, added for comparison, show no signs of inflammation in any ocular tissue. Conversely, both Th1 and Th17 induced inflammation in the anterior and posterior eye segments as well as edema in most tissues, which is apparent when compared with corresponding tissue from the naive control. Interestingly, Th17 differed from Th1 by exhibiting a stronger affinity for the cornea of inflamed eyes; infiltrating cells in Th17 recipient eyes were found to invade into the central, deep corneal stroma (Fig. 2D). Th17 recipient eyes were also noted to have more severe corneal epithelial edema (Fig. 2C). In contrast, Th1 recipient eyes depicted more severe retinal inflammation and folding than Th17 recipient eyes (Fig. 2E).
Selective production of IFN-γ or IL-17 in eyes of Th1 or Th17 recipients
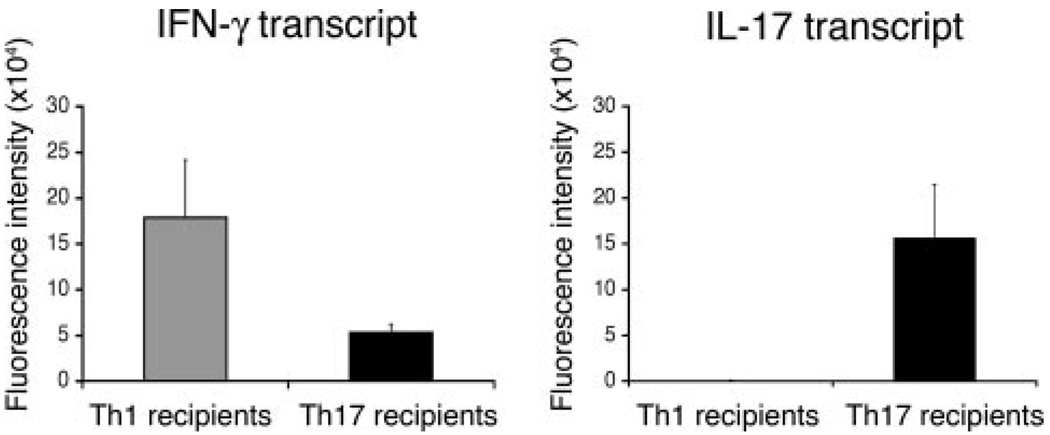
To determine whether adoptively transferred cells maintained lineage specificity, we next examined the expression levels of IFN-γ and IL-17 in inflamed eyes of Th1 and Th17 recipients. We determined expression levels by measuring mRNA transcripts from whole inflamed eyes using quantitative PCR. The data from two experiments, summarized in Fig. 3, show a predominance of IFN-γ in Th1 recipient eyes and a predominance of IL-17 in Th17 recipient eyes. These data confirm the selective expression of either IFN-γ or IL-17 by the Th1 and Th17 lineages, even following adoptive transfer and inflammation in the eye. Of interest was the finding that although IL-17 transcript was essentially absent in the eyes of Th1 recipients, a low level of IFN-γ transcript was expressed in the eyes of Th17 recipients.
FIGURE 3.
IFN-γ and IL-17 transcripts are selectively expressed in inflamed eyes of recipient mice. Eyes of recipient mice were collected on day 5 postadoptive transfer of 2 × 106 reactivated Th1 or Th17 cells. Total RNA was collected, pooled, and analyzed by real time RT-PCR for transcript levels of IFN-γ and IL-17. The data are expressed as mean values ± SEM of two experiments.
Th1 and Th17 differ remarkably in their capacity to stimulate host lymphoid expansion in naive recipients
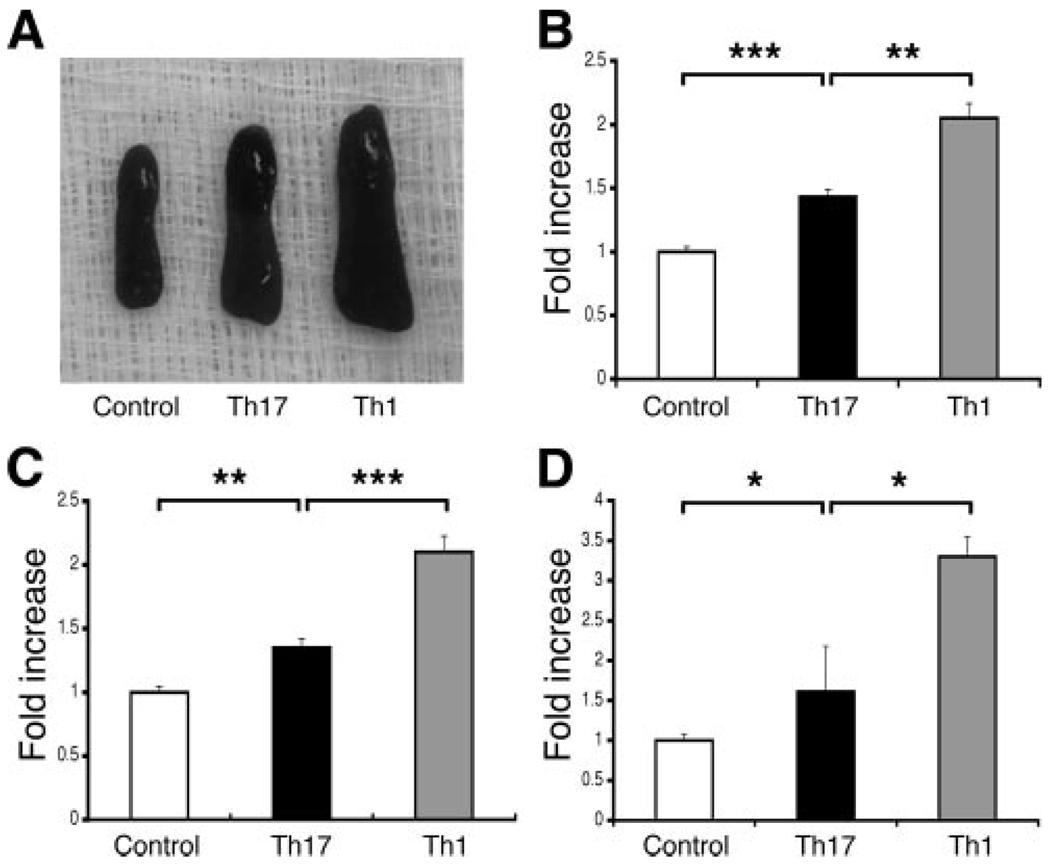
In a previous communication (18) we showed that polarized, reactivated Th1 cells have the capacity to induce transient splenomegaly when adoptively transferred into naive recipient mice. An increase in spleen size and cellularity peaks on day 4 postadoptive transfer, consisting of a mixed host cell population, then leads to a rapid decrease and return to baseline by day 7. Based on these earlier observations, we compared the spleen size and cell number of Th1 and Th17 recipients on day 4 postadoptive transfer (Fig. 4). In line with our previous data (18), spleens in recipients of 10 × 106 Th1 increased in size and cell number to approximately twice the value of control mice. Conversely, significantly smaller changes were noted in spleen size (Figs. 4, A and B) and cell number (Fig. 4C) in Th17 recipients.
FIGURE 4.
Th1 and Th17 cells differ in their capacity to stimulate lymphoid expansion in recipient mice. Groups of two or three syngeneic WT mice were injected with 10 × 106 polarized and reactivated Th1 or Th17 cells. Recipient mice were euthanized 4 days later, representative spleens were chosen for photography (A), and spleens were pooled for weight (B) and cell counts (C). In addition, blood samples were collected and pooled and the number of MNLs was determined (D). All values are expressed as the fold increase in recipient mice compared with noninjected controls; injection of naive CD4 cells from 3A9 donors had no effect on the parameters shown here (Ref. 18 and data not shown). The data are expressed as mean values ± SEM of four or five experiments.*, p < 0.05;**, p < 0.0005;***, p < 0.0001.
In addition, previous adoptive transfer studies of reactivated Th1 showed a sharp increase in circulating MNLs that accompanied changes in the spleen (our unpublished data). We therefore tested peripheral blood from Th1 and Th17 recipients on day 4 postadoptive transfer. Similar to the trends in spleen size and cellularity, Th1 recipients showed a substantially greater increase in circulating MNLs than Th17 recipients (Fig. 4D).
Given that T-regulatory cells could have contributed to the inferior capacity of Th17 to stimulate lymphoid expansion, we used flow cytometric analysis to evaluate our cell preparations. CD4+CD25+Foxp3+ cells were not detected in any of the Th17 cell suspensions tested (data not shown).
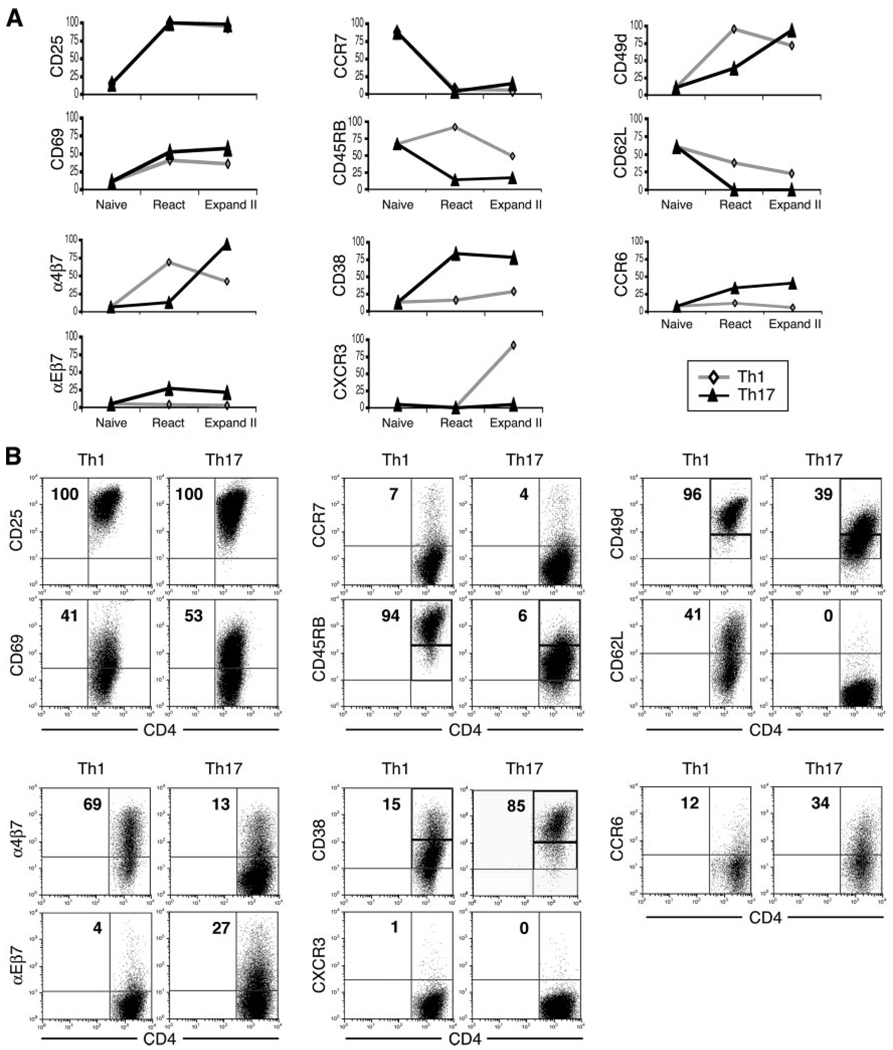
Cultured Th1 and Th17 differ in their expression of surface molecules at two stages in vitro
In view of our observation that Th1 and Th17 can induce distinct changes in the eyes and spleens of recipient mice, we examined two stages of culture for differences in surface molecule expression. We tested the “reactivation” stage just before adoptive transfer and the “expansion II” stage following 11 days of additional incubation with IL-2 (21). Data from a representative experiment are summarized both in graphic form (Fig. 5A) and in flow cytometry plots (Fig. 5B); similar observations were made in two additional experiments. Fig. 5A additionally shows the staining pattern of naive CD4 cells before in vitro activation. As expected, naive cells were negative for a majority of tested surface molecules with the exception of CCR7, CD45RB, and CD62L.
FIGURE 5.
Differential surface marker expression on Th1 and Th17 cells in vitro. A, Suspensions of naive CD4 cells, as well as Th1 or Th17 cells at two different stages in vitro, were immunostained with mAbs against major surface markers. Th1 and Th17 cell surface markers were analyzed at the following culture stages: 1) after polarization and reactivation, just before injection into recipient mice (“React”); and 2) after 11 days of additional culture in the presence of IL-2 (“Expand II”). Numbers represent the percentage of positive or “high” positive cells gated on the CD4+1G12+7AAD− population. Recorded values were obtained in a representative experiment; similar results were obtained in two additional experiments. B, Flow cytometric plots of a representative experiment showing Th1 and Th17 surface marker expression at the “reactivated” stage.
At the reactivation stage, Th1 and Th17 exhibited similar expression levels of the activation markers CD25 and CD69 (22, 23) as well as the chemokine receptor CCR7 (24). Remarkably, however, reactivated Th1 and Th17 showed significantly different expression levels for all other tested markers. The expression level of CD45RB, known to be down-regulated on mature cells (25), was lower on Th17 than on Th1. In contrast, Th1 expressed higher levels of the marker CD49d, while down-regulation of the molecule CD62L was more pronounced on Th17. Increased expression of CD49d and decreased expression of CD62L together characterize the ability of T lymphocytes to invade nonlymphoid tissues (18, 26). Expression levels of α4β7 (LPAM-1) and αEβ7 (CD103) were also significantly different; Th1 cells were found to express higher levels of α4β7, an integrin involved in mucosal tissue homing (27), whereas Th17 expressed moderately higher levels of αEβ7, an integrin involved in epithelial tissue homing (28). Additionally, a noticeable difference was found in the expression patterns of CD38, a multifunctional marker for maturation, activation, and trafficking (29, 30), with Th17 expressing high levels and Th1 expressing low levels. Neither Th lineage at this stage of culture expressed measurable levels of the chemokine receptor CXCR3.
Fewer differences between Th1 and Th17 were observed after an extended incubation with IL-2 (i.e., the “expansion II” stage), as compared with reactivated cells. Exceptions included an increase in levels on Th17 of CD49d and α4β7 with a concomitant decrease on Th1, as well as a sharp increase in CXCR3 expression only on Th1. This pattern regarding CXCR3 up-regulation on Th1 is in line with our previous data (21). Of note, Th17 did not up-regulate CXCR3 but alternatively up-regulated the two other trafficking markers, CD49d and α4β7. As seen with pathological changes in the eye and spleen, this finding emphasizes a distinct difference in the activities of Th1 and Th17 upon exposure to certain environmental stimuli.
A recent publication (31) showed that the chemokine receptor CCR6 is expressed by human Th17 profoundly more than by Th1. Flow cytometric analysis of CD4 cells in our system (Fig. 5) revealed a low level of CCR6 on naive cells, a marginal increase on Th1 following reactivation, and the lowest expression following expansion. In contrast, the level of CCR6 on Th17 increased following reactivation and was further elevated to >40% following expansion II.
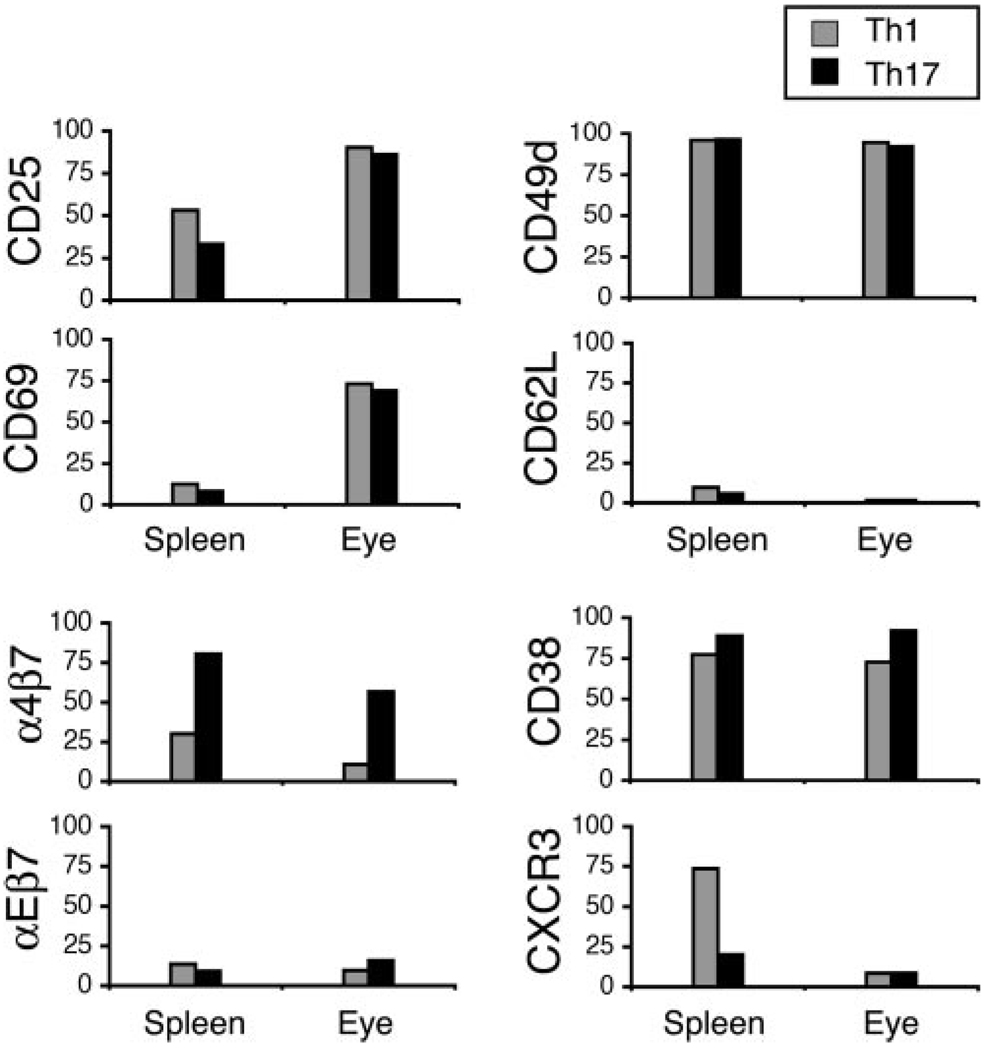
Adoptively transferred Th1 and Th17 express different surface molecules in spleens and eyes of recipients
As shown by other groups and our own (21, 32), adoptively transferred Th cells can undergo significant surface molecule changes upon reaching the spleen or after invading target tissue. We there-fore compared, in the current study, surface marker expression on Th1 and Th17 from the spleens and eyes of recipient mice. Surface marker analysis was performed on day 4 postadoptive transfer because Th1 invasion of the eye has been shown to peak on day 4 with relatively low levels of host cell recruitment (18, 21). We used a clonotypic Ab specific against the 3A9 Tg TCR, designated “1G12”, to detect donor cells in recipient eyes.
Fig. 6 summarizes data from a representative experiment; similar data were collected in an additional experiment. Of particular interest, we found that: 1) unlike the striking difference in culture between Th1 and Th17 (Fig. 5), most surface marker levels in vivo were relatively similar; 2) exceptions to these similarities included a considerably higher level of α4β7 expressed on Th17, both in spleens and eyes of recipients, whereas only Th1 expressed high levels of CXCR3 in recipient spleens; and 3) the activation markers CD25 and CD69, especially the latter, were more highly expressed in recipient eyes compared with recipient spleens. Our in vivo findings further highlight the differences that exist between Th1 and Th17 with regard to their ability to up-regulate distinct trafficking markers. Any surface marker discrepancies between our in vitro and in vivo studies likely result from specific cytokine milieus present in each studied environment.
FIGURE 6.
Differential surface marker expression on donor cells in the spleens and eyes of recipient mice. Cells were isolated on day 4 postadoptive transfer of 10 × 106 reactivated Th1 or Th17 cells into recipient mice. Numbers represent the percentage of positive or “high” positive cells gated on the CD4+1G12+7AAD− population. Recorded values were obtained in a representative experiment; similar data were obtained in an additional experiment.
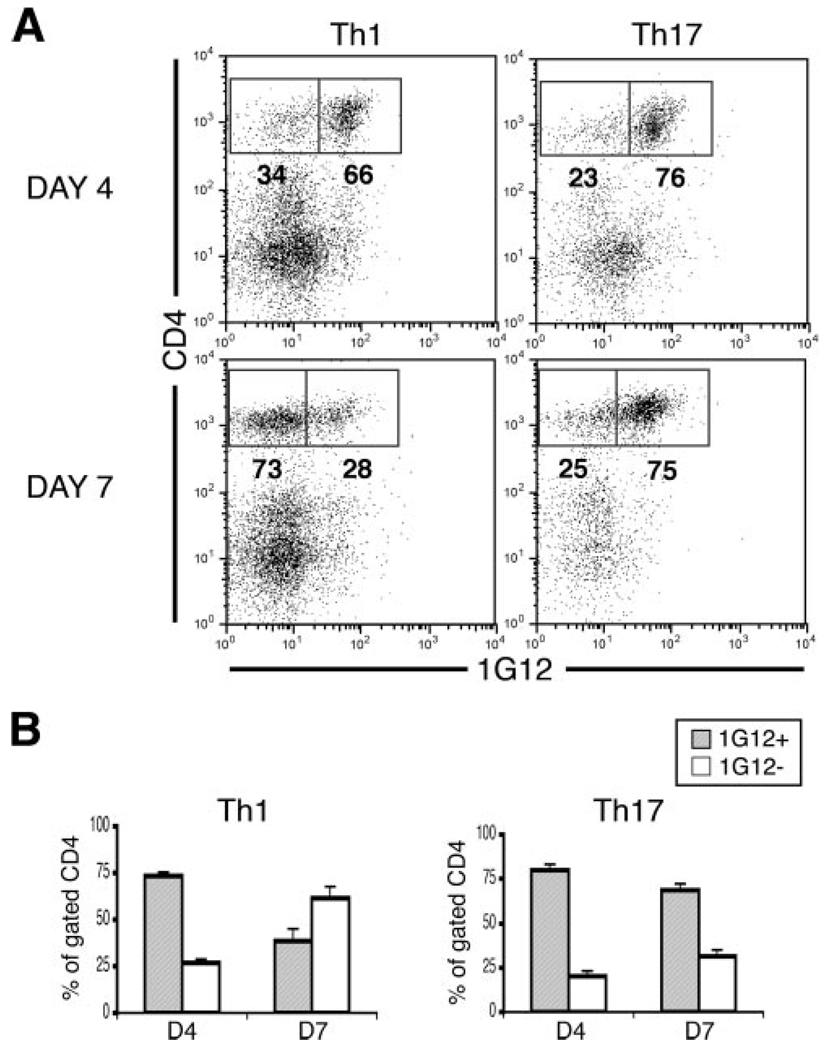
Th1 and Th17 differ in their kinetic invasion and host cell recruitment in recipient eyes
Because Th1 and Th17 were found to up-regulate distinct trafficking markers in vitro and in vivo, we compared each lineage for its unique capacity to invade and recruit host cells in recipient eyes. It has been shown that a major component of immune-mediated inflammation is the recruitment of nonspecific lymphoid cells to inflamed tissue (18, 33, 34). In this study, we isolated cells from inflamed recipient eyes on days 4 and 7 postadoptive transfer of Th1 or Th17 and then analyzed for proportions of donor and host cell populations. According to previous studies, Th1 recruitment of host cells was expected to reach its peak on day 7 (18, 21).
Flow cytometric analysis of donor and host cell populations, isolated on days 4 and 7 postadoptive transfer revealed a striking difference between the two cell lineages. Data from a representative experiment are shown in Fig. 7A, while Fig. 7B summarizes data from this and two other experiments. In line with previous observations (21), the majority of infiltrating cells in Th1 recipient eyes were donor cells (1G12+) on day 4, whereas the majority had shifted to host cells (1G12−) by day 7. In contrast, little change was seen in the ratio between donor and host cells in Th17 recipient eyes from day 4 to day 7; donor cells were the clear majority at both time points in Th17 recipient eyes. These remarkable differences in the kinetics of invasion and host cell recruitment suggest that the basic characteristics of Th1 and Th17 may play a role in establishing disease patterns unique to each lineage.
FIGURE 7.
Th1 and Th17 differ in their kinetics of accumulation in recipient mouse eyes and the recruitment of host cells. Recipient mice were injected with 10 × 106 reactivated Th1 or Th17 cells and eyes were collected on days 4 and 7 postadoptive transfer. Cells were obtained by collagenase treatment of the collected eyes, and flow cytometric analysis was performed to measure the percentages of CD4 and 1G12 expression. 1G12 is a clonotypic Ab that selectively stains donor cells. A, Flow cytometry plots from a representative experiment. B, Summary of mean percentage values ± SEM of three experiments.
Discussion
The important role played by Th17 cells in immunopathogenic processes has been well established (1–7), but until now, little had been described concerning their basic mechanism of action or other biological activities. To collect new data about Th17 cells and to compare their activities with those of the well-described Th1 cells, we generated in vitro lineages of both Th1 and Th17 from naive CD4 cells expressing a TCR specific against HEL. We then examined in vitro and in vivo differences between these two lineages. The purity of each lineage was demonstrated by measuring the production of IFN-γ or IL-17, the signature cytokines for the Th1 and Th17 cell types, respectively.
The culture conditions that we used to generate Th1 and Th17 lineages were in line with previously published methods (9). It is important to note, however, that our Th17 lineage was cultured in the presence of IL-2, both at the “expansion” and “reactivation” stages. Recently, IL-2 was reported to have an inhibitory effect on the activation of Th17 cells from a naive state (35). In our study, we detected no difference in IL-17 expression when Th17 cells were cultured with or without IL-2 following the initial polarization step (data not shown), thus indicating that inhibitory effects of IL-2 are likely restricted to an earlier stage of activation. Indeed, we found in another study (G. Shi, C. A. Cox, B. P. Vistica, C. Tan, E. F. Wawrousek, and I. Gery, “Phenotype switching by inflammation-inducing polarized Th17, but not by Th1 cells,” submitted for publication) that adding anti-IL-2 Ab to the early activation mixture (35) increased considerably the proportion of Th17 cells, from 50–60% to 70–80% following routine reactivation.
When transferred into Tg mice expressing HEL in their eyes, both Th1 and Th17 induced ocular inflammation. Some differences were noted, however, in specific pathological features resulting from each lineage, as depicted in Fig. 2. In addition, flow cytometric analysis of infiltrating cell populations in recipient eyes revealed remarkably higher proportions of neutrophils (Gr-1+) in Th17 eyes when compared with Th1 eyes (11.3 vs 3.7%, respectively). In contrast, macrophages (F4/80+) and CD3 cells were observed in similar proportions in both the Th1 group and the Th17 group. Greater accumulation of neutrophils in Th17 recipients is in accord with reports of IL-17 acting as a stimulator of neutrophil mobilization (36, 37) as well as granulopoiesis (3).
It is of interest that unlike the observed pathogenic similarity between our polarized Th1 and Th17 cells, Langrish et al. (6) and Elson et al. (11) found that Th17 cells are by far superior to Th1 cells when tested for their capacity to induce experimental auto-immune encephalomyelitis or colitis, respectively. The discrepancy that exists between our model and the other two models has not yet been elucidated.
The contribution of Th1 and Th17 cells to pathogenic processes of ocular inflammation has been analyzed mostly in eyes with experimental autoimmune uveitis. Both Th1 and Th17 cells were identified in these eyes (38–40), and Yoshimura et al. (38) suggested that the two cell populations are involved at different phases of the inflammatory process. In addition, we analyzed the Th populations in mouse eyes in which autoimmune-mediated inflammation was triggered by treatment with TLR ligands, using the system described by Fujimoto et al. (41). In this system, naive 3A9 CD4 cells induce ocular inflammation in HEL-Tg recipients following activation in vivo by TLR ligands. Different ratios were found between Th1 and Th17 cells in mice treated with various ligands, with the predominance of Th17 observed in mice treated with pertussis toxin or LPS but with more Th1 than Th17 cells identified in the eyes of mice treated with CpG oligodeoxynucleotide (G. Shi, unpublished data).
Analysis of cytokines expressed in the eyes of Th1 and Th17 recipients revealed high levels of selectivity, with a predominance of IFN-γ or IL-17, respectively. Importantly, essentially no IL-17 was detected in Th1 recipient eyes, whereas a low level of IFN-γ was detected in Th17 recipient eyes. The IFN-γ detected in the eyes of Th17 recipients could be produced by three different cell populations: 1) “double positive” cells, producing both IFN-γ and IL-17 (42, 43); 2) Th17 cells that transformed into Th1 cells; and 3) host cells that were recruited into the inflamed eyes (18) and acquired a Th1 phenotype. Data collected in another study (G. Shi, C. A. Cox, B. P. Vistica, C. Tan, E. F. Wawrousek, and I. Gery, “Phenotype switching by inflammation-inducing polarized Th17, but not by Th1 cells,” submitted for publication) suggested that all three mechanisms do in fact take place in Th17 recipient eyes.
Th1 and Th17 differed considerably in their capacity to induce transient splenomegaly as well as an increase in circulating MNLs in naive recipients. The mechanisms involved in these two processes are not entirely known, but it is conceivable that specific cytokines released by adoptively transferred cells played an important role. Differences in cytokine profiles of Th1 and Th17, therefore, may contribute to a difference in the capacity of each cell lineage to induce certain systemic and tissue-specific phenomena. Our notion of Th1-specific spleen enlargement is supported by a recent paper from Uhlig et al. (15), showing that splenomegaly induced by anti-CD40 Ab is IL-12 dependent but relatively unaffected by a deficiency in IL-23.
Flow cytometric analyses of cell populations in enlarged spleens of both Th1 and Th17 recipient mice revealed that the proportions of host CD4 cells, CD8 cells, B cells (CD19+), and dendritic cells (CD11c+) in recipients were similar to those in naive controls. Spleens of both Th1 and Th17 recipients, however, had approximately twice the amount of macrophages (CD11b+) and about three times the amount of granulocytes (Gr-1+) than naive controls (data not shown).
Because Th1 and Th17 were observed to induce different systemic and tissue-specific effects, we analyzed for the expression of cell surface markers known to be involved in activation, maturation, and trafficking. At two stages of in vitro culture, our data indicated that Th1 and Th17 do in fact show remarkable differences in their ability to up-regulate or down-regulate maturation and trafficking markers. At reactivation, the stage just before adoptive transfer, Th17 expressed a more mature effector phenotype than Th1. Furthermore, after a subsequent period of expansion, Th1 and Th17 were found to up-regulate different trafficking markers. Our data show, therefore, that Th1 and Th17 have the ability to express distinct trafficking markers in parallel culture stages, thus offering a potential theory for why Th1 and Th17 differ in their homing patterns to certain types of tissue (15, 44). It is also of note that Nakae et al. recently reported that Th1 and Th17 differ in the expression of various other cell markers (45).
It has been hypothesized that changes in the expression of adhesion markers in vivo enable lymphocytes to leave the circulation and traffic to inflamed tissue (21, 32). This hypothesis is supported by a recent finding that LFA-1 knockout mice do not develop experimental autoimmune encephalomyelitis, perhaps due to an inability of Th17 to traffic into the CNS (46). We found that Th1 located in recipient spleens postadoptive transfer significantly up-regulated their expression of CXCR3, whereas Th17 up-regulated the integrin α4β7. CXCR3 is a chemokine receptor that has been described to be involved in trafficking to various tissue sites (21, 32, 47), whereas α4β7 is more specialized in trafficking to mucosal tissue (i.e., lamina propria of the gut) (27). In our model of the eye, a major port of entry for inflammatory cells is at the limbus, which comprises mucosal tissue known as conjunctiva-associated lymphoid tissue (48, 49). This observation, therefore, lends support for the role of α4β7 in Th17 trafficking patterns to the eye. Importantly, further analysis of Th17 from the eyes of recipients revealed persistently high α4β7 expression, whereas both Th1 and Th17 significantly down-regulated CXCR3 in the eye.
Furthermore, comparison of Th1 and Th17 surface marker expression at the “reactivated” stage in vitro (Fig. 5) with expression in the recipient eye or spleen (Fig. 6) revealed considerable differences. Of particular interest was the varying expression of two trafficking molecules: 1) α4β7 was more highly expressed by reactivated Th1 in vitro, but Th17 expressed higher levels than Th1 in spleen and eyes; and 2) CXCR3 was nearly absent on reactivated Th1 in vitro but then became highly expressed on Th1 in recipient spleens. These changes in surface marker expression can likely be attributed to different cytokine milieus present in each environment studied.
Interestingly, a drug called natalizumab, which has been used to treat autoimmune diseases such as multiple sclerosis and inflammatory bowel disease (50, 51), is believed to function by blocking the α4 subunit of both CD49d (α4β1) and α4β7. Th17 cells have recently been shown to play a similar or increased role, compared with Th1 cells, in these two diseases (52). Our in vivo findings support the inhibitory effect of natalizumab on autoimmune inflammation, because both Th1 and Th17 were found to express high levels of CD49d in the eye. It is important to note that Th17 also expressed high in vivo levels of α4β7, which could further amplify the inhibitory effect of natalizumab. It is presumable that other blocking Abs, such as an antagonist against the whole dimer α4β7 (53), would be useful in targeting Th17-specific disease.
Finally, Th1 and Th17 also differed remarkably in their recruitment of host cells to the induced site of eye inflammation. Whereas Th1 effectively recruited CD4 host cells and rapidly became a minority among infiltrating cells, Th17 remained in the majority from day 4 to day 7 (Fig. 7). The sharp drop in donor Th1 cells in recipient eyes could be attributed, at least in part, to the high susceptibility of these cells to activation-induced cell death. In another study, we found that Th1 are strikingly more susceptible to apoptosis upon activation when compared with Th2 or Th17 (G. Shi, B. Vistica, M. Ramaswamy, C. Cox, C. Tan, E. Wawrousek, R. Siegel, I. Gery, unpublished data). It is conceivable, therefore, that exposure to HEL in the recipient eye initiates death in a large proportion of Th1 cells.
Unlike donor cells, host cells invade the affected eye by non-Ag-specific mechanisms due to breakdown of the blood-tissue barrier. It is also important to note that T cells of HEL-Tg recipient mice are immunologically tolerant to HEL (19), mainly due to negative selection in the thymus (54).
In summary, our experimental system made it possible to compare polarized lineages of Th1 and Th17 for their basic features and respective biological activities. We found in our system that both lineages were immunopathogenic, with slight differences in certain aspects of pathological change induced by each. Notably, remarkable differences were observed between Th1 and Th17 in their expression of several major trafficking markers, both in vitro and in vivo, as well as in their capacity to induce host lymphoid expansion and recruitment to the inflamed eye.
Acknowledgments
We thank Drs. Charles E. Egwuagu and Cheng-Rong Yu for useful discussions, and the National Eye Institute Histology Core for preparation of histology slides.
Footnotes
This work was supported by the Intramural Research Program of the National Eye Institute, National Institutes of Health.
Abbreviations used in this paper: Tg, transgenic; HEL, hen egg lysozyme; MNL, mononuclear leukocyte; WT, wild type.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Bettelli E, Oukka M, Kuchroo VK. TH-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 2.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 4.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 5.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amadi-Obi A, Yu C-R, Liu X, Mahdi RM, Levy-Clark G, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. Th17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat. Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 8.Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, Cua DJ, Powrie F, Cheever AW, Maloy KJ, Sher A. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J. Exp. Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins SC, Jarnicki AG, Lavelle EC, Mills KH. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J. Immunol. 2006;177:7980–7989. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- 11.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 12.Lafaille JJ, Keere FV, Hsu AL, Baron JL, Haas W, Raine CS, Tonegawa S. Myelin basic protein-specific T helper 2 (Th2) cells cause experimental autoimmune encephalomyelitis in immunodeficient hosts rather than protect them from the disease. J. Exp. Med. 1997;186:307–312. doi: 10.1084/jem.186.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz JD, Benoist C, Mathis D. T helper cell subsets in insulin-dependent diabetes. Science. 1995;268:1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 14.Kim SJ, Zhang M, Vistica BP, Chan CC, Shen DF, Wawrousek EF, Gery I. Induction of ocular inflammation by T-helper lymphocytes type 2. Invest. Ophthalmol. Visual Sci. 2002;43:758–765. [PubMed] [Google Scholar]
- 15.Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, Robinson N, Buonocore S, Tlaskalova-Hogenova H, Cua DJ, Powrie F. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Wraith DC. Anti-cytokine vaccine and the immunotherapy of autoimmune diseases. Eur. J. Immunol. 2006;36:2844–2848. doi: 10.1002/eji.200636760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foxman EF, Zhang M, Hurst SD, Muchamuel T, Shen D, Wawrousek EF, Chan CC, Gery I. Inflammatory mediators in uveitis: differential induction of cytokines and chemokines in Th1-versus Th2-mediated ocular inflammation. J. Immunol. 2002;168:2483–2492. doi: 10.4049/jimmunol.168.5.2483. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Fujimoto C, Vistica BP, Wawrousek EF, Kelsall B, Gery I. Active participation of antigen-nonspecific lymphoid cells in immune-mediated inflammation. J. Immunol. 2006;177:3362–3368. doi: 10.4049/jimmunol.177.5.3362. [DOI] [PubMed] [Google Scholar]
- 19.Lai JC, Fukushima A, Wawrousek EF, Lobanoff MC, Charukamnoetkanok P, Smith-Gall SJ, Vistica BP, Lee RS, Egwuagu CE, Whitcup SM, Gery I. Immunotolerance against a foreign antigen transgenically expressed in the lens. Invest. Ophthalmol. Visual Sci. 1998;39:2049–2057. [PubMed] [Google Scholar]
- 20.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-β and IL-6drive the production of IL-17and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nat. Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Vistica BP, Takase H, Ham DI, Fariss RN, Wawrousek EF, Chan C-C, DeMartino JA, Farber JM, Gery I. A unique pattern of up- and down-regulation of chemokine receptor CXCR3 on inflammation-inducing Th1 cells. Eur. J. Immunol. 2004;34:2885–2894. doi: 10.1002/eji.200425318. [DOI] [PubMed] [Google Scholar]
- 22.Sancho D, Gomez M, Sanchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136–140. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald TT. The role of activated T lymphocytes in gastrointestinal disease. Clin. Exp. Allergy. 1990;20:247–252. doi: 10.1111/j.1365-2222.1990.tb02679.x. [DOI] [PubMed] [Google Scholar]
- 24.Kursar M, Hopken UE, Koch M, Kohler A, Lipp M, Kaufmann SHE, Mittrucker H-W. Differential requirements for the chemokine receptor CCR7 in T cell activation during Listeria monocytogenes infection. J. Exp. Med. 2005;201:1447–1457. doi: 10.1084/jem.20041204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hove T, Olle The F, Berkhout M, Bruggeman JP, Vyeth-Dreese FA, Slors JFM, van Deventer SJH, te Velde AA. Expression of CD45RB functionally distinguishes intestinal T lymphocytes in inflammatory bowel disease. J. Leukocyte Biol. 2004;75:1010–1015. doi: 10.1189/jlb.0803400. [DOI] [PubMed] [Google Scholar]
- 26.Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat. Rev. Immunol. 2004;4:325–335. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 27.Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of α4-integrins in lymphocyte homing to mucosal tissues in vivo. J. Immunol. 1994;152:3282–3293. [PubMed] [Google Scholar]
- 28.Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the αEβ7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 29.Sandoval-Montes C, Santos-Argumedo L. CD38 is expressed selectively during the activation of a subset of mature T cells with reduced proliferation but improved potential to produce cytokines. J. Leukocyte Biol. 2005;77:513–521. doi: 10.1189/jlb.0404262. [DOI] [PubMed] [Google Scholar]
- 30.Frasca L, Fedele G, Deaglio S, Capuano C, Palazzo R, Vaisitti T, Malavasi F, Ausiello CM. CD38 orchestrates migration, survival, and Th1 immune response of human mature dendritic cells. Blood. 107:2392–2399. doi: 10.1182/blood-2005-07-2913. [DOI] [PubMed] [Google Scholar]
- 31.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 32.Flugel A, Berkowicz T, Ritter T, Labeur M, Jenne DE, Li Z, Ellwart JW, Willem M, Lassmann H, Wekerle H. Migratory activity and functional changes of green fluorescent effector cells before and during experimental autoimmune encephalomyelitis. Immunity. 2001;14:547–560. doi: 10.1016/s1074-7613(01)00143-1. [DOI] [PubMed] [Google Scholar]
- 33.Steinman L. A few autoreactive cells in an autoimmune infiltrate control a vast population of nonspecific cells: a tale of smart bombs and the infantry. Proc. Natl. Acad. Sci. USA. 1996;93:2253–2256. doi: 10.1073/pnas.93.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caspi RR, Chan C-C, Fujino Y, Najafian F, Grover S, Hansen CT, Wilder RL. Recruitment of antigen-nonspecific cells plays a pivotal role in the pathogenesis of a T cell-mediated organ-specific autoimmune disease, experimental autoimmune uveoretinitis. J. Neuroimmunol. 1993;47:177–188. doi: 10.1016/0165-5728(93)90028-w. [DOI] [PubMed] [Google Scholar]
- 35.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, et al. Interleukin-signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Laan M, Cui Z-H, Hoshino H, Lötvall J, Sjöstrand M, Gruenert DC, Skoogh B-E, Lindén A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- 37.Witowski J, Pawlaczyk K, Breborowicz A, Scheuren A, Kuzlan-Pawlaczyk M, Wisniewska J, Polubinska A, Friess H, Gahl GM, Frei U, Jörres A. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO chemokine from mesothelial cells. J. Immunol. 2000;165:5814–5821. doi: 10.4049/jimmunol.165.10.5814. [DOI] [PubMed] [Google Scholar]
- 38.Yoshimura T, Sonoda K-H, Miyazaki Y, Iwakura Y, Ishibashi T, Yoshimura A, Yoshida H. Differential roles for IFN-γ and IL-17 in experimental autoimmune uveoretinitis. Int. Immunol. 2008;20:209–214. doi: 10.1093/intimm/dxm135. [DOI] [PubMed] [Google Scholar]
- 39.Liu X, Lee YS, Yu C-R, Egwuagu CE. Loss of STAT3 in CD+T cells prevents development of experimental autoimmune diseases. J. Immunol. 2008;180:6070–6076. doi: 10.4049/jimmunol.180.9.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luger D, Silver PB, Tung J, Cua D, Coen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan C-C, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J. Exp. Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujimoto C, Yu C-R, Shi G, Vistica BP, Wawrousek EF, Klinman DM, Chan C-C, Egwuagu CE, Gery I. Pertussis toxin is superior to TLR ligands in enhancing pathogenic autoimmunity, targeted at a neo-self antigen by triggering robust expansion of Th1 cells and their cytokine production. J. Immunol. 2006;177:6896–6903. doi: 10.4049/jimmunol.177.10.6896. [DOI] [PubMed] [Google Scholar]
- 42.Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J. Exp. Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzhinghi B, Parente E, Fili L, Ferri S, Frosali F, et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGeachy MJ, Cua DJ. T cells doing it for themselves: TGF-γ regulation of Th1 and Th17 cells. Immunity. 2007;26:547–549. doi: 10.1016/j.immuni.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Nakae S, Iwakura Y, Suto H, Galli SJ. Phenotypic differences between Th1 and Th17 cellsand negative regulation of Th1 cell differentiation by IL-17. J. Leukocyte Biol. 2007;81:1258–1268. doi: 10.1189/jlb.1006610. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Kai H, Chang F, Shibata K, Tahara-Hanaoka S, Honda S-I, Shibuya A, Shibuya K. A critical role of LFA-1 in the development of Th17 cells and induction of experimental autoimmune encephalomyelitis. Biochem. Biophys. Res. Commun. 2007;353:857–862. doi: 10.1016/j.bbrc.2006.12.104. [DOI] [PubMed] [Google Scholar]
- 47.Shinkai M, Shinkai T, Puri P, Stringer MD. Increased CXCR3 expression associated with CD3-positive lymphocytes in the liver and biliary remnant in biliary atresia. J. Pediatr. Surg. 2006;41:950–954. doi: 10.1016/j.jpedsurg.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 48.Franklin RM, Remus LE. Conjunctival-associated lymphoid tissue: evidence for a role in the secretory immune system. Invest. Ophthalmol. Visual Sci. 1984;25:181–187. [PubMed] [Google Scholar]
- 49.Dua HS, Gomes JA, Jindal VK, Appa SN, Schwarting R, Eagle RC, Jr, Donoso LA, Laibson PR. Mucosa specific lymphocytes in the human conjunctiva, corneoscleral limbus and lacrimal gland. Curr. Eye Res. 1994;13:87–93. doi: 10.3109/02713689409042401. [DOI] [PubMed] [Google Scholar]
- 50.Dorsey ER, Thompson JP, Noves K, Dick AW, Holloway RG, Schwid SR. Quantifying the risks and benefits of natalizumab in relapsing multiple sclerosis. Neurology. 2007;68:1524–1528. doi: 10.1212/01.wnl.0000260699.09720.ad. [DOI] [PubMed] [Google Scholar]
- 51.Sands BE, Kozarek R, Spainhour J, Barish CF, Becker S, Goldberg L, Katz S, Goldblum R, Harrigan R, Hilton D, Hanauer SB. Safety and tolerability of concurrent natalizumab treatment for patients with Crohn’s disease not in remission while receiving infliximab. Inflamm. Bowel Dis. 2007;13:2–11. doi: 10.1002/ibd.20014. [DOI] [PubMed] [Google Scholar]
- 52.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J. Clin. Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dubree NJP, Artis DR, Castanedo G, Marsters J, Sutherlin D, Caris L, Clark K, Keating SM, Beresini MH, Chiu H, et al. Selective α4β7 integrin antagonists and their potential as anti-inflammatory agents. J. Med. Chem. 2002;45:3451–3457. doi: 10.1021/jm020033k. [DOI] [PubMed] [Google Scholar]
- 54.Zhang M, Vacchio MS, Vistica BP, Lesage S, Egwuagu CE, Yu C-R, Gelderman MP, Kennedy MC, Wawrousek EF, Gery I. T cell tolerance to a neo-self antigen expressed by thymic epithelial cells: the soluble form is more effective than the membrane-bound form. J. Immunol. 2003;170:3954–3962. doi: 10.4049/jimmunol.170.8.3954. [DOI] [PubMed] [Google Scholar]