Abstract
PURPOSE
To investigate the role of decay-accelerating factor (DAF), a cell surface complement regulator that recently has been linked to T-cell responses and autoimmunity in the pathogenesis of experimental autoimmune uveitis (EAU).
METHODS
EAU was induced in wild-type (WT) and Daf1−/− mice, and their disease severities, IRBP specific Th1/Th17 responses, and cytokine expression profiles were compared. In a test of the efficacy of treatment with soluble mouse DAF protein, EAU was induced in disease-susceptible B10.RIII mice, and they were treated with 0.5 mg soluble DAF protein or equal volume of PBS IP every other day. Retinal histology and IRBP-specific T-cell responses were compared after 14 days.
RESULTS
Both EAU incidence and histopathology scores were significantly greater in Daf1−/− mice. There was a >10-fold greater mononuclear cell influx into the retina together with severe vasculitic lesions, retinal folding, and photoreceptor cell layer destruction. There were 5- to 7-fold greater Th1 and 3- to 4-fold greater Th17 responses against IRBP in Daf1−/− mice with EAU, and they expressed significantly elevated levels of GM-CSF, IL-2, IL-3, and IFN-γ. WT B10.RIII mice that received soluble DAF protein treatments exhibited decreased IRBP-specific Th1/Th17 responses and were protected from retinal injury compared with the mice that received PBS treatments.
CONCLUSIONS
DAF significantly influences IRBP-specific Th1 and Th17 responses and disease severity in EAU. Systemic upregulation of DAF levels could be used to suppress retinal antigen(s)–specific autoimmunity to treat autoimmune posterior uveitis.
Experimental autoimmune uveitis (EAU) is a rodent model of human autoimmune posterior uveitis that can be blinding and that affects 150,000 Americans annually.1,2 EAU can be induced by immunization with several retinal proteins including S-Ag (retinal arrestin), rhodopsin, recoverin, phosducin, and interphotoreceptor retinoid-binding protein (IRBP).2 Most studies have used uveitogenic peptides of IRBP, a 148-kDa protein located in the interphotoreceptor matrix which transports vitamin A derivatives between photoreceptors and retinal pigment epithelium (RPE).3 For studies of H-2b C57BL/6 background mice, human IRBP1–20 is uveitogenic, whereas for studies of H-2r B10.RIII mice, human IRBP161–180 is uveitogenic.
It is well established that EAU in mice is CD4+ T-cell mediated.4 Until recently, IFN-γ generated by IRBP-reactive Th1 cells was implicated as the major effector response by both direct effects and indirect effects through activating macrophages and monocytes,5 which produce TNF-α and other proinflammatory cytokines.6–8 Recent work has provided evidence that IL-17 plays a more critical role in both EAU and the human disease.9–11 In support of this, blocking IL-17 by specific mAbs dramatically reduces clinical symptoms and pathologic retinal changes in EAU.9 The latest studies12 suggest that both IFN-γ–producing Th1 and IL-17–producing Th17 cells are pathogenic in EAU.
Recent work by us13 and others14 has found that T-cell responses are modulated by decay-accelerating factor (DAF or CD55), a cell-surface C3/C5 convertase inhibitor, originally characterized as a regulator that, in the context of serum complement, protects self cells from C3/C5 activation on their surface.15 Subsequent studies provided evidence that DAF modulates T-cell response by inhibiting local generation of C5a/C3a, which are integrally involved in antigen-presenting cell (APC) cytokine production and activated T-cell survival. 16,17
In work related to T-cell–mediated autoimmunity thereby to EAU, we18 and others14 have shown that mice deficient in Daf1, the murine homologue of human DAF, have more severe experimental autoimmune encephalomyelitis (EAE), a myelin-specific T-cell–mediated central nervous system (CNS) injury model for multiple sclerosis. We found that enhanced CNS injury in Daf1−/− mice with EAE is due to augmented MOG-specific Th1 and Th17 responses, both of which depend on enhanced APC–T-cell C5a/C3a-C5aR/C3aR interactions due to elevated local C5a/C3a production in the absence of DAF.18 In this study, using Daf1−/− mice and IP-administered recombinant soluble DAF protein, we found evidence that DAF, originally characterized as a complement inhibitor, regulates the IRBP-specific Th1/Th17 responses that lead to retinal injury in EAU.
MATERIAL AND METHODS
Mice and Reagents
Daf1−/− mice were developed as described19 and backcrossed with C57BL/6 mice for more than 12 generations. C57BL/6 and B10.RIII mice 8 to 12 weeks old were purchased from Jackson Laboratory (Bar Harbor, ME). Human IRBP peptide1–20 and IRBP peptide161–180 was synthesized by Anaspec Inc. (San Jose, CA). Purified Bordetella pertussis toxin was ordered from List Biological Laboratories (Campbell, CA) and complete Freund’s adjuvant (CFA) from Sigma-Aldrich (St. Louis, MO). Mycobacterium tuberculosis strain H37RA extract was purchased from Difco (Detroit, MI). All studies were performed in an approved Institutional animal protocol in the animal resource center of Case Western Reserve University. Animal management complied with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Induction and Scoring of EAU
For experiments with Daf1−/− mice on the C57BL/6 background, the mice were immunized subcutaneously in both thighs and at the base of the tail with 300 µg IRBP1–20 emulsified in CFA supplemented with 0.5 mg/mL M. tuberculosis strain H37RA extract. Pertussis toxin (1.5 µg) was administered simultaneously IP. The mice were killed on day 21 and the enucleated eyes were fixed in 4% formaldehyde. Disease severity was scored on a scale of 0 to 4, as described by Chan et al.20 using pupillary– optic nerve sections of each eye in a masked fashion. For treatment experiments, EAU susceptible B10.RIII mice were immunized similarly with 25 µg of IRBP161–180, and pertussis toxin was not used in this strain.
Recombinant Soluble Mouse DAF (rDAF) Preparation
Soluble mouse DAF protein was bulk produced by fermentation with the recombinant yeast Pichia pastoris strain, which was previously developed in the laboratory.21 In brief, P. pastoris expressing the mouse DAF CCP 1–4 with a C terminus 6X His tag were cultured in a 6-L automatic fermentor (NBS, Edison, NJ). After methanol induction, recombinant mouse DAF protein was purified on a nickel column (Qiagen, Valencia, CA) and dialyzed against PBS. The purity and bioactivity of the purified mouse DAF protein were checked by Coomassie blue staining and complement-inhibition assays, as described before.21
Treatment of EAU with rDAF
For treatment experiments, 8-week-old B10.RIII mice were immunized with 5 µg of IRBP161–180 peptide in CFA and randomly divided into two groups. In the treatment group, 0.5 mg rDAF protein was given to each mouse IP every other day after immunization, until day 14, and the control group mice were given the same volume of PBS alone. On day 14, both groups of mice were killed for ocular histology and immunologic evaluations.
T-Cell Response Assays
IFN-γ and IL-17 ELISPOT assays were performed as described.13 Ninety-six well ELISPOT plates (Cellular Technology Ltd., Cleveland, OH) were coated in PBS overnight at 4°C with a capture antibody for IFN-γ or IL-17, after which they were blocked with 150 µL of PBS-1% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO) per well and washed three times with PBS. Splenocytes (600,000) were added to wells containing different concentrations of IRBP1–20 (C57BL/6 mice) or IRBP161–180 (B10.RIII mice), and 24 hours later, the resultant spots were developed and counted on a computer-assisted image analyzer (Immunospot; Cellular Technology, Cleveland, OH).
Cytokine Assays
Splenocytes (2 × 106) from mice euthanatized at day 21 were incubated for 48 hours with 10 µg/mL IRBP1–20, and supernatants were applied to a mouse cytokine antibody array (Ray Biotech, Inc., Norcross, GA) that detects most target proteins at picogram levels for semiquantitative cytokine level measurements. The results were quantified by densitometry and normalized against supplied positive and negative controls, according to the manufacturer’s instructions.
Statistical Analysis
All experiments were performed at least twice with similar results. The data were analyzed by independent t-test. P ≤ 0.05 was considered to be significant.
RESULTS
Severity of Retinal Damage in Daf1−/− Mice
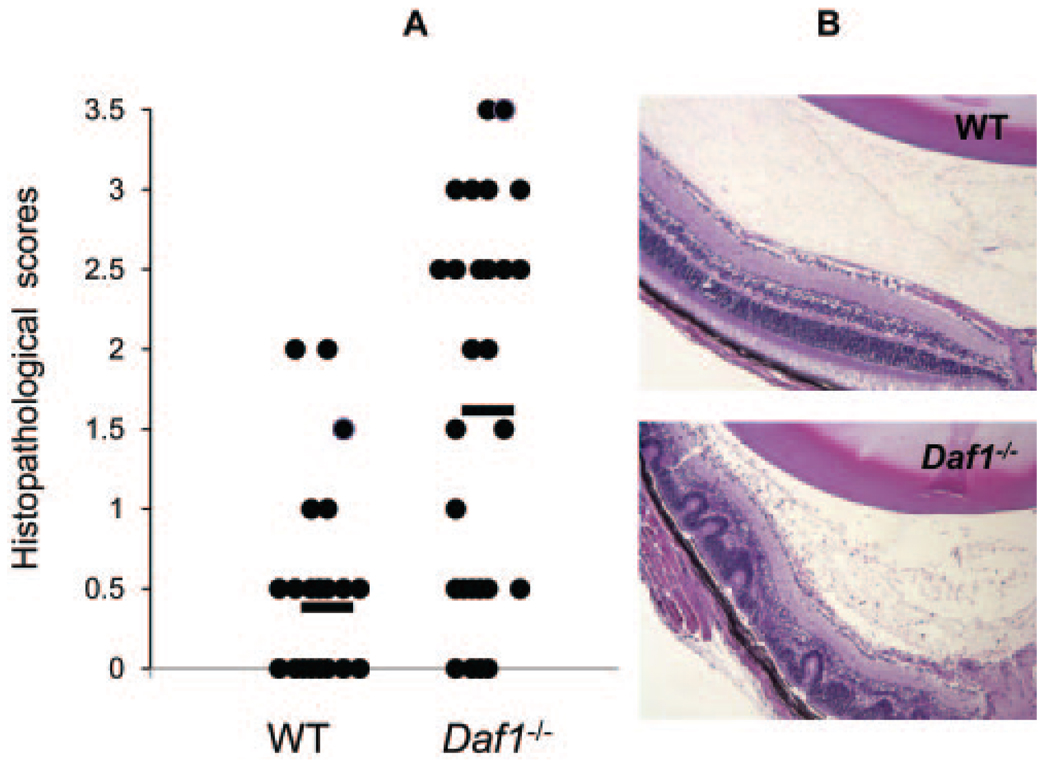
We induced EAU in Daf1−/− and wild-type (WT) mice with IRBP1–20 immunization, together with pertussis toxin and examined eyes enucleated on day 21 as described.2 After H&E staining, we evaluated the severity of EAU in a masked fashion on a scale of 0 to 4 using previously published criteria based on the number, type, and size of lesions. These analyses showed that both EAU incidence (Daf1−/− 87.5% vs. WT 50%) and histopathology scores (Daf1−/− 1.86 ± 1.08 vs. WT 0.73 ± 0.56) were significantly greater in Daf1−/− mice (Table 1). There was a massive mononuclear cell influx into the posterior uvea, together with severe vasculitic lesions, retinal folding, and photoreceptor cell layer destruction in Daf1−/− mice, compared with mild changes in WTs with EAU (Fig. 1).
TABLE 1.
Comparison of EAU Incidence and Severity in WT and Daf1−/− Mice
| Animal Group | Incidence | Mean Histopathology Scores |
|---|---|---|
| WT | 50.0% (8/16) | 0.73 ± 0.56 (0.0–1.0) |
| Daf1−/− | 87.5% (14/16) | 1.86 ± 1.08 (0.0–3.5) |
n = 16.
FIGURE 1.
Eyes enucleated from Daf1−/− mice with EAU exhibited higher histopathological scores and markedly more severe retinal injury than did WT control eyes. (A) Histopathologic scores of WT and Daf1−/− eyes after IRBP1–20 immunization (n = 16 in each group, P < 0.05). (●) the score of one eye; bar: average score. (B) Representative ocular sections from WT and Daf1−/− mice with EAU.
IRBP1–20-Specific T-Cell Responses in Daf1−/− Mice with EAU
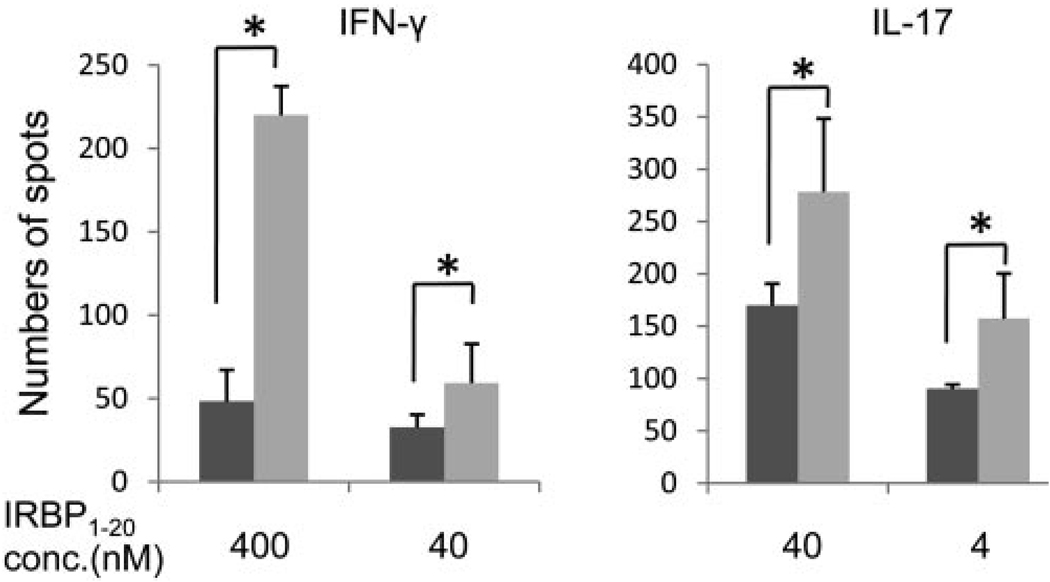
To determine whether the heightened retinal injury in Daf1−/− mice is associated with augmented IRBP-specific T-cell responses, we harvested splenic cells of mice euthanatized on day 21 and quantitated IRBP1–20-specific IFN-γ– and IL-17–producing cells with ELISPOT assays. These assays (Fig. 2) showed that spleens from Daf1−/− mice contained 5- to 7-fold more IFN-γ–producing and 2- to 3-fold more IL-17–producing T cells than did spleens from WT mice.
FIGURE 2.
ELISOPT assays of IFN-γ– and IL-17–producing T cells in 6 × 105 spleen cells on day 21 from WT (dark gray) and Daf1−/− mice (light gray) with EAU, showing Daf1−/− mice exhibited stronger IRBP-specific Th1 and Th17 responses (n = 16, P < 0.01); *P < 0.05.
Cytokine Expression Profile in Splenocytes from Daf1−/− Mice with EAU
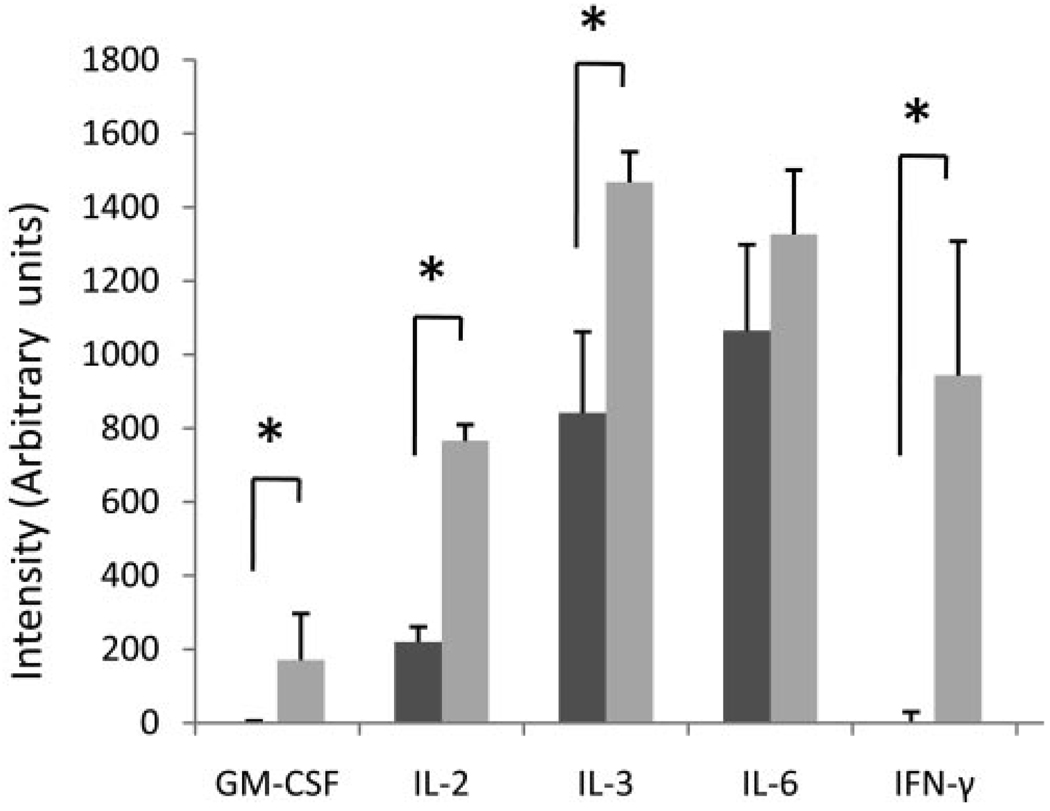
To determine other cytokines differentially expressed by IRBP-responding APCs and T cells in Daf1−/− and WT mice, we incubated spleen cells from diseased Daf1−/− or WT mice together with IRBP1–20 and assayed the supernatants with a cytokine antibody array. This assay provides relative expression level comparisons by measuring densities of respective spots after color development.22 After densitometry analysis, the results (Fig. 3) showed that Daf1−/− mouse splenocytes produced significantly increased levels of GM-CSF, IL-2, IL-3, and IFN-γ, whereas the IL-6 levels were not significantly different.
FIGURE 3.
Cytokine antibody array assessment of culture supernatants from splenocytes of WT and Daf1−/− mice with EAU, showing that after IRBP1–20 restimulation, Daf1−/− splenocytes produced elevated levels of GM-CSF, IL-2, IL-3, and IFN-γ, whereas the IL-6 levels were not significantly different. *P < 0.05.
DAF Protein Protection of WT Mice from Retinal Injury in EAU
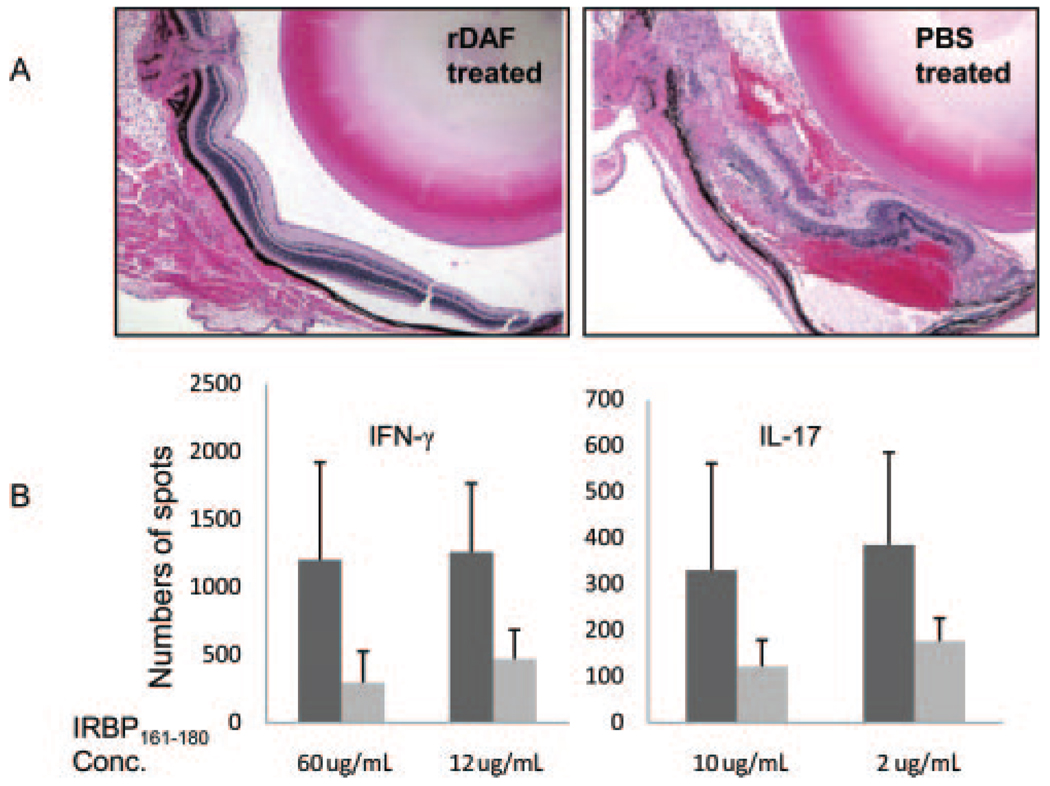
In view of the findings that Daf1−/− mice exhibited more severe retinal injury and elevated IRBP-specific Th1/Th17 responses in EAU, we next tested whether administration of recombinant DAF protein would affect the generation of IRBP-reactive T-cell responses in WT mice and affect EAU disease severity. Because EAU severity is mild in C57BL/6 mice, for these experiments, we used the disease-susceptible B10.RIII mice. After immunizing 10 B10.RIII mice with 5 µg IRBP161–180 peptide in CFA without pertussis toxin, we administered 0.5 mg purified mouse DAF protein IP every other day to five mice and an equal volume of PBS to the other five. On day 14, we killed the mice and compared T-cell responses and ocular histopathology. As shown in Fig. 4A, Compared with splenocytes from PBS-treated control mice, recombinant DAF-treated mice showed fourfold less IRBP-specific IFN-γ– and IL-17–producing cells (Fig. 4A. Consistent with this, in contrast to massive leukocyte infiltration, retinal folding and hemorrhage in PBS-treated control animals, little if any disease was observed in the recombinant DAF-treated mice (Fig. 4B).
FIGURE 4.
Soluble DAF treatment prevented mice from retinal injury in EAU. (A) Representative histology of ocular sections from soluble recombinant mouse DAF protein–treated mice and PBS-treated mice (n = 5 in each group). The eyes were sectioned on day 14 and stained with H&E. (B) ELISPOT assays of Th1 and Th17 responses in recombinant (light gray) DAF protein– and (dark gray) PBS-treated mice. Spleens were collected the same day mice were killed for ocular histology. Spleen cells (6 × 105/well) were assayed for the numbers of IFN-γ (Th1)– and IL-17 (Th17)–producing cells in response to different concentrations of IRBP161–180. *P < 0.05.
DISCUSSION
Using Daf1−/− mice and IP-administered recombinant DAF protein, we found that IRBP-specific T-cell responses and the severity of retinal damage in EAU are greatly influenced by DAF. Histopathologic analysis of mouse eyes showed that both the incidence and severity of the retinal injury were greater in Daf1−/− mice. There was markedly greater leukocyte infiltration within the retina and greater disruption of retinal structure compared to mild changes in WT mice. Consistent with this, ELISPOT assays showed 5- to 7-fold more IRBP1–20 specific IFN-γ– and 2- to 3-fold more IL-17–producing T cells in Daf1−/− mice with EAU. Cytokine array assays showed significantly elevated levels of GM-CSF, IL-2, IL-3, and IFN-γ produced by Daf1−/− splenocytes. In accordance with these results, systemic administration of soluble recombinant DAF protein in the EAU susceptible B10.RIII mice efficiently inhibited the IRBP-reactive Th1/Th17 responses and protected the mice from retinal injury in EAU.
The findings in this study show for the first time that DAF is integrally involved in the pathogenesis of EAU and provide further evidence that DAF modulates T-cell responses in autoimmune diseases. As indicated in the introduction, we18 and others14 have shown that DAF suppresses MOG-specific T-cell responses in EAE, an autoimmune disease model similar to EAU in which MO- specific T cells target the myelin sheath and cause central nervous system (CNS) injury. We found that DAF functions by modulating the activation of C3, fB, fD, and C5, which are locally produced by APCs and T cells, during their cognate interactions.13 Under conditions of reduced restraint on local complement activation in the absence of DAF, more C5a and C3a are generated, and these anaphylatoxins interact with APC and T-cell C5aR/C3aR, both of which induce bidirectional GPCR signal transduction, which confers costimulation and survival signals to T cells.16,17
The elevated production of GM-CSF, IL-2, IL-3, and IFN-γ by Daf1−/− mouse splenocytes reflects the more severe retinal injury in these mice. GM-CSF is a cytokine that activates neutrophils, macrophages, and eosinophils and drives dendritic cell (DC) generation.23 Elevated levels of GM-CSF are associated with heightened disease severity in many autoimmune diseases and their animal models, including collagen-induced arthritis24,25 and EAE.26,27 Moreover, GM-CSF Tg mice which express elevated levels of GM-CSF spontaneously develop retinal injury.28 IL-2 stimulates Th1 and Th17 cell9 expansions that drive the pathologic development of EAU. Elevated levels of IFN-γ in the supernatants were consistent with the ELISPOT assay results, whereas no IL-17 was detected. The immunization protocol (CFA with pertussis toxin), the experimental conditions, or the sensitivity of the array could explain the lack of detection of the suppressive cytokines, such as IL-4, IL-5, IL-10, and TGF-β.
Recent studies29 have shown that EAU incidence and severity in IRBP1–20-immunized C3−/− mice are significantly reduced compared with that of WT mice and that the severity of EAU is ameliorated in transgenic mice expressing soluble complement receptor 1-related protein Y (Crry), a rodent-specific complement inhibitor that has activities similar to DAF.30 Although that study clearly indicated that complement is integrally involved in the pathogenesis of EAU, and cell surface complement regulators could protect mice from retinal injury in EAU, the underlying mechanisms were not explored. Although Daf1−/− mice possess similar levels of Crry on their cell surfaces compared with WT mice,31 DAF deficiency was not compensated by the Crry molecules in different disease models in which the pathologic effects were either caused by conventional terminal complement activation product membrane attack complex (MAC)31 or by autoreactive T cells.18 This report, together with our previous studies,13,16,17,32 indicates that by inhibiting local complement activation between interacting APC-T cells, DAF regulates IRBP-specific Th1 and Th17 responses modulate EAU disease severity.
Previous studies have found that complement and complement regulators are important in other experimental uveitis models.33,34 In rats with anterior chamber zymosan injection, anterior uveitis develops because zymosan activates complement through the alternative pathway and thereby promotes ocular inflammation. Depleting complement with cobra venom factor (CVF) prevented zymosan-initiated anterior uveitis while blocking the function of Crry in specific mAb-induced anterior chamber inflammation.33 In another rat model of anterior uveitis, melanin-associated antigen (MAA) immunization induces T-cell–mediated anterior chamber inflammation. 35 In this model, blocking Crry function with mAbs or its expression with siRNAs resulted in the early onset of disease and the exacerbation of intraocular inflammation. However, MAA-specific T-cell responses were not analyzed in this study.34
Different from rats, many gene knockout and transgenic mice have been developed. Studies in the mouse EAU model not only have been important for understanding the pathogenesis of human autoimmune posterior uveitis but also have been valuable for testing potential therapies.36 We have shown that Daf1−/− mice elicit augmented IRBP-specific Th1/Th17 responses and heightened retinal injury and that systemic administration of soluble recombinant DAF protein inhibits both IRBP-specific Th1/Th17 responses thereby protecting mice from retinal injury. Our results not only support the new finding12 that both IRBP-specific, IFN-γ–producing Th1 cells and IL-17–producing Th17 cells are pathogenic in EAU, but also argue that upregulating DAF levels by pharmaceuticals or by administering soluble recombinant DAF protein could have clinical value in autoimmune posterior uveitis. In fact, one group of drugs known to be able to upregulate DAF expression levels are statins,37 which have been shown to be effective in treating mice with EAU.38
Acknowledgments
The authors thank Edward Medof for helpful discussions, Scott Howell at the Vision Science Research Center (VSRC) imaging core for digital imaging analysis, and Catherine Doller at the histology core for excellent histology services.
Supported by National Institute of Health Grant NS052471 (FL). QL and HB were supported in part by Natural Science Foundation of China Grant 30671988.
Footnotes
Disclosure: F. An, None; Q. Li, None; Z. Tu, None; H. Bu, None; C.-C. Chan, None; R.R. Caspi, None; F. Lin, None
References
- 1.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500. doi: 10.1016/j.ophtha.2003.06.014. discussion, 500. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal RK, Caspi RR. Rodent models of experimental autoimmune uveitis. Methods Mol Med. 2004;102:395–419. doi: 10.1385/1-59259-805-6:395. [DOI] [PubMed] [Google Scholar]
- 3.Pepperberg DR, Okajima TL, Wiggert B, Ripps H, Crouch RK, Chader GJ. Interphotoreceptor retinoid-binding protein (IRBP): molecular biology and physiological role in the visual cycle of rhodopsin. Mol Neurobiol. 1993;7:61–85. doi: 10.1007/BF02780609. [DOI] [PubMed] [Google Scholar]
- 4.Singh VK, Biswas S, Anand R, Agarwal SS. Experimental autoimmune uveitis as animal model for human posterior uveitis. Indian J Med Res. 1998;107:53–67. [PubMed] [Google Scholar]
- 5.Singh VK, Rai G. Cytokines in posterior uveitis: an update. Immunol Res. 2001;23:59–74. doi: 10.1385/IR:23:1:59. [DOI] [PubMed] [Google Scholar]
- 6.de Kozak Y, Naud MC, Bellot J, Faure JP, Hicks D. Differential tumor necrosis factor expression by resident retinal cells from experimental uveitis-susceptible and -resistant rat strains. J Neuroimmunol. 1994;55:1–9. doi: 10.1016/0165-5728(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 7.Caspi RR. Th1 and Th2 responses in pathogenesis and regulation of experimental autoimmune uveoretinitis. Int Rev Immunol. 2002;21:197–208. doi: 10.1080/08830180212063. [DOI] [PubMed] [Google Scholar]
- 8.Caspi RR. Immune mechanisms in uveitis. Springer Semin Immunopathol. 1999;21:113–124. doi: 10.1007/BF00810244. [DOI] [PubMed] [Google Scholar]
- 9.Amadi-Obi A, Yu CR, Liu X, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 10.Peng Y, Han G, Shao H, Wang Y, Kaplan HJ, Sun D. Characterization of IL-17+ interphotoreceptor retinoid-binding protein-specific T cells in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2007;48:4153–4161. doi: 10.1167/iovs.07-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamzaoui K, Hamzaoui A, Guemira F, Bessioud M, Hamza M, Ayed K. Cytokine profile in Behcet's disease patients: relationship with disease activity. Scand J Rheumatol. 2002;31:205–210. doi: 10.1080/030097402320318387. [DOI] [PubMed] [Google Scholar]
- 12.Luger D, Silver PB, Tang J, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heeger PS, Lalli PN, Lin F, et al. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201:1523–1530. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Miwa T, Hilliard B, et al. The complement inhibitory protein DAF (CD55) suppresses T cell immunity in vivo. J Exp Med. 2005;201:567–577. doi: 10.1084/jem.20040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medof ME, Kinoshita T, Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984;160:1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strainic MG, Liu J, Huang D, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112:1759–1766. doi: 10.1182/blood-2008-04-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Lin F, Strainic MG, et al. IFN-gamma and IL-17 production in experimental autoimmune encephalomyelitis depends on local APC-T cell complement production. J Immunol. 2008;180:5882–5889. doi: 10.4049/jimmunol.180.9.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin F, Fukuoka Y, Spicer A, et al. Tissue distribution of products of the mouse decay-accelerating factor (DAF) genes: exploitation of a Daf1 knock-out mouse and site-specific monoclonal antibodies. Immunology. 2001;104:215–225. doi: 10.1046/j.0019-2805.2001.01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan CC, Caspi RR, Ni M, et al. Pathology of experimental autoimmune uveoretinitis in mice. J Autoimmun. 1990;3:247–255. doi: 10.1016/0896-8411(90)90144-h. [DOI] [PubMed] [Google Scholar]
- 21.Lin F, Immormino RM, Shoham M, Medof ME. Bulk production and functional analyses of mouse CD55’s native and deglycosylated active domains. Arch Biochem Biophys. 2001;393:67–72. doi: 10.1006/abbi.2001.2488. [DOI] [PubMed] [Google Scholar]
- 22.Berruyer C, Pouyet L, Millet V, et al. Vanin-1 licenses inflammatory mediator production by gut epithelial cells and controls colitis by antagonizing peroxisome proliferator-activated receptor gamma activity. J Exp Med. 2006;203:2817–2827. doi: 10.1084/jem.20061640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 24.Campbell IK, Rich MJ, Bischof RJ, Dunn AR, Grail D, Hamilton JA. Protection from collagen-induced arthritis in granulocyte-macrophage colony-stimulating factor-deficient mice. J Immunol. 1998;161:3639–3644. [PubMed] [Google Scholar]
- 25.Lawlor KE, Wong PK, Campbell IK, van Rooijen N, Wicks IP. Acute CD4+T lymphocyte-dependent interleukin-1-driven arthritis selectively requires interleukin-2 and interleukin-4, joint macrophages, granulocyte-macrophage colony-stimulating factor, interleukin-6, and leukemia inhibitory factor. Arthritis Rheum. 2005;52:3749–3754. doi: 10.1002/art.21495. [DOI] [PubMed] [Google Scholar]
- 26.McQualter JL, Darwiche R, Ewing C, et al. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med. 2001;194:873–882. doi: 10.1084/jem.194.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ponomarev ED, Shriver LP, Maresz K, Pedras-Vasconcelos J, Verthelyi D, Dittel BN. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:39–48. doi: 10.4049/jimmunol.178.1.39. [DOI] [PubMed] [Google Scholar]
- 28.Lang RA, Metcalf D, Cuthbertson RA, et al. Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell. 1987;51:675–686. doi: 10.1016/0092-8674(87)90136-x. [DOI] [PubMed] [Google Scholar]
- 29.Read RW, Szalai AJ, Vogt SD, McGwin G, Barnum SR. Genetic deficiency of C3 as well as CNS-targeted expression of the complement inhibitor sCrry ameliorates experimental autoimmune uveoretinitis. Exp Eye Res. 2006;82:389–394. doi: 10.1016/j.exer.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Molina H, Wong W, Kinoshita Brenner TC, Fole S, Holers VM. Distinct receptor and regulatory properties of recombinant mouse complement receptor 1 (CR1) and Crry, the two genetic homologues of human CR1. J Exp Med. 1992;175:121–129. doi: 10.1084/jem.175.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin F, Emancipator SN, Salant DJ, Medof ME. Decay-accelerating factor confers protection against complement-mediated podocyte injury in acute nephrotoxic nephritis. Lab Invest. 2002;82:563–569. doi: 10.1038/labinvest.3780451. [DOI] [PubMed] [Google Scholar]
- 32.Lalli PN, Strainic MG, Lin F, Medof ME, Heeger PS. Decay accelerating factor can control T cell differentiation into IFN-gamma-producing effector cells via regulating local C5a-induced IL-12 production. J Immunol. 2007;179:5793–5802. doi: 10.4049/jimmunol.179.9.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sohn JH, Kaplan HJ, Suk HJ, Bora PS, Bora NS. Chronic low level complement activation within the eye is controlled by intraocular complement regulatory proteins. Invest Ophthalmol Vis Sci. 2000;41:3492–3502. [PMC free article] [PubMed] [Google Scholar]
- 34.Jha P, Sohn JH, Xu Q, et al. Suppression of complement regulatory proteins (CRPs) exacerbates experimental autoimmune anterior uveitis (EAAU) J Immunol. 2006;176:7221–7231. doi: 10.4049/jimmunol.176.12.7221. [DOI] [PubMed] [Google Scholar]
- 35.Bora NS, Woon MD, Tandhasetti TM, Cirrito TP, Kaplan HJ. Induction of experimental autoimmune anterior uveitis by a self-antigen: melanin complex without adjuvant. Invest Ophthalmol Vis Sci. 1997;38:2171–2175. [PubMed] [Google Scholar]
- 36.Liao T, Ke Y, Shao WH, et al. Blockade of the interaction of leukotriene b4 with its receptor prevents development of autoimmune uveitis. Invest Ophthalmol Vis Sci. 2006;47:1543–1549. doi: 10.1167/iovs.05-1238. [DOI] [PubMed] [Google Scholar]
- 37.Mason JC, Ahmed Z, Mankoff R. Statin-induced expression of decay-accelerating factor protects vascular endothelium against complement-mediated injury. Circ Res. 2002;91:696–703. doi: 10.1161/01.res.0000038151.57577.19. [DOI] [PubMed] [Google Scholar]
- 38.Kohno H, Sakai T, Saito S, Okano K, Kitahara K. Treatment of experimental autoimmune uveoretinitis with atorvastatin and lovastatin. Exp Eye Res. 2007;84:569–576. doi: 10.1016/j.exer.2006.11.011. [DOI] [PubMed] [Google Scholar]