Obesity has reached epidemic proportions, and complications related to obesity contribute substantially to health care costs and mortality. Since the accumulation of fat is the net result of a prolonged state of imbalance between energy intake and energy expenditure, one would think that an ideal fat mass in obese persons could be achieved relatively simply by either decreasing food intake or increasing energy expenditure, ultimately causing a sustained negative energy balance. Unfortunately, this is not so easy to achieve, because evolutionary pressure has rewarded those individuals and species able to store sufficient energy to survive famines; also, the unrestricted availability of food represents an unnatural condition.1 Currently, most interventions, whether behavioral or pharmacologic, are aimed at the energy-intake side of the equation and result in only moderate, often temporary improvements, with the notable exception of bariatric surgery.
Interventions designed to increase energy expenditure are relatively limited. An increase in physical activity, although effective, is not easy to sustain. The pharmacologic approach has also been disappointing. Supraphysiologic doses of thyroid hormones or adrenergic agonists result in an increase in energy expenditure, but their systemic adverse events preclude their use for the treatment of obesity.
Brown adipose tissue represents a natural target for the modulation of energy expenditure. This tissue is far from being a fat depot. When activated, it requires the uptake of substrate from the circulation, mostly free fatty acids, but also glucose (Fig. 1). The physiologic role of brown adipose tissue in small mammals (and human newborns) is the maintenance of core temperature. In brown adipose tissue, mitochondria release chemical energy in the form of heat by means of the uncoupling of the oxidative phosphorylation, making the process of respiration exceedingly inefficient. This phenomenon is mediated by the uncoupling protein 1 (UCP1), which renders the inner membrane of the mitochondria “leaky” and hence releases energy in the form of heat rather than storing it as ATP.2 UCP1 is, in turn, regulated by triiodothyronine, which is generated within brown adipose tissue by means of 5′ deiodination of the prohormone thyroxine, effectively creating a local, tissue-specific hyperthyroid state in the absence of changes in circulating levels of thyroid hormones.3 β3-adrenergic signaling plays an important role in the modulation of this process, and recent evidence indicates that food intake results in a similar activation,4 suggesting that brown adipose tissue could play an important role in short-term energy homeostasis.
Figure 1. The Activation of Brown Adipose Tissue.
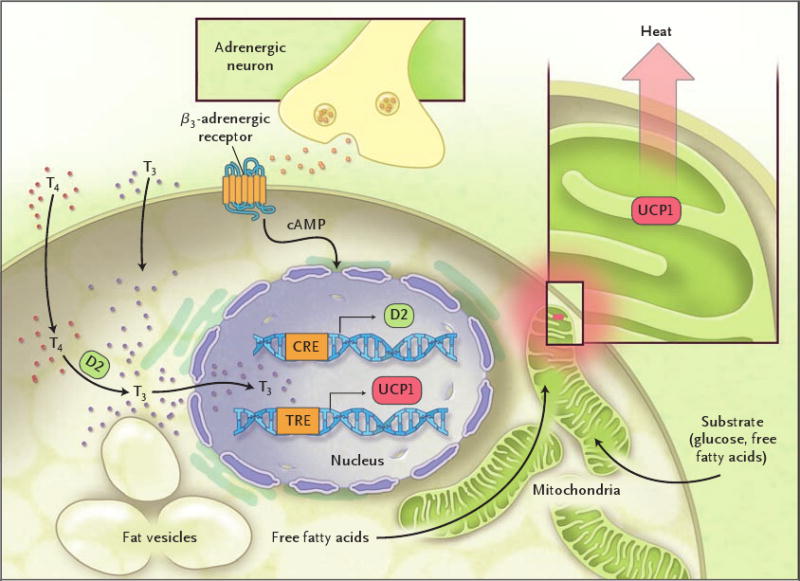
Stimulation of β3-adrenergic receptors leads to the dramatic increase in the intracellular concentration of triiodothyronine (T3) by means of the type 2 5′ deiodinase (D2); T3 in turn stimulates the transcription of uncoupling protein 1 (UCP1), which causes the leakage of protons from the inner membrane of the mitochondria, hence dissipating energy in the form of heat. The abbreviation cAMP denotes cyclic adenosine monophosphate, CRE cAMP response element, T4 thyroxine, and TRE thyroid hormone response element.
To date, the study of the physiology of brown adipose tissue has been limited mostly to rodents, because human intrascapular brown adipose tissue disappears shortly after birth, and small depots of cells resembling brown adipose tissue have been considered vestigial and devoid of a physiologic role. Nonetheless, some clinical observations, such as “nests” of brown adipose tissue within the white adipose tissue of persons exposed to cold for a sustained period,5 or brown adipose tissue in patients with pheochromocytoma6 who, owing to the tumor, have chronic exposure to supraphysiologic levels of catecholamines, have pointed to the fact that brown adipose tissue might be relevant in adult humans. Recently, the discovery of PRDM16 (PRD1-BF1-RIZ1 homologous domain containing 16),7 the genetic master switch of brown-adipose-tissue differentiation, and its regulation by BMP7 (bone morphogenetic protein 7)8 has reinvigorated interest in the study of this tissue in humans.
The presence of multiple depots of brown adipose tissue in the cervical region has been recognized for many years and considered a nuisance from the perspective of positron-emission tomography (PET) performed with the tracer 18F-fluorodeoxyglucose (18F-FDG), a technique that relies on the uptake of radiolabeled glucose by metabolically active (neoplastic) tissues. Indeed, several interventions, most notably warming of the room occupied by the study subject and the use of benzodiazepines and beta-blockers, are commonly used to quench the activity of brown adipose tissue, ultimately reducing the rate of false positive signals in diagnostic scans.9 In this issue of the Journal, articles about three very different studies that took full advantage of the PET technology address the presence and relevance of brown adipose tissue in adult humans.10-12
Virtanen and colleagues,10 using a purely morphologic approach, demonstrated that brown adipose tissue in adult humans can be rapidly activated by exposure to cold temperatures. Biopsy specimens obtained from the supraclavicular region, an area of enhanced uptake of 18F-FDG on PET scans, clearly demonstrated the presence of brown adipose tissue, according to morphologic and molecular markers. Cypess and colleagues11 analyzed a large number of patients who underwent combined 18F-FDG PET and computed tomography (PET–CT) and found that brown adipose tissue was present in a substantial fraction of the study population. The authors found an inverse correlation between the activity of brown adipose tissue and age, body-mass index (BMI), and fasting plasma glucose level; in other words, they found a direct correlation between the activation of brown adipose tissue and metabolic measures that indicate the presence or absence of good health. The probability of the detection of brown adipose tissue was inversely correlated with the environmental temperature. The findings are quite robust, despite the confounders associated with observational studies, and it is very likely that the data underestimate the actual presence of active brown adipose tissue.
Using an experimental approach, van Marken Lichtenbelt and colleagues12 examined the presence, distribution, and activity of brown adipose tissue and its potential role in energy metabolism in a group of volunteers. The study used 18F-FDG PET–CT to assess the cold-stimulated brown-adipose-tissue activity in relation to metabolic measures, including energy expenditure at rest and core and surface temperatures. All the subjects, with the notable exception of the most obese volunteer, displayed functional brown adipose tissue; the activity of the tissue correlated inversely with BMI and the percentage of body fat and correlated directly with energy expenditure during rest. A substantial increase in energy expenditure was observed in all the subjects exposed to mild cold, but the authors found no correlation between cold-induced thermogenesis and brown-adipose-tissue activity.
The common message from these studies is that brown adipose tissue is present and active in adult humans, and its presence and activity are inversely associated with adiposity and indexes of the metabolic syndrome. A major shortcoming of the studies is the lack of direct correlation between brown-adipose-tissue activity and cold-stimulated changes in energy expenditure. This could be secondary to the inability of the PET–CT technique to measure the activity of small nests of brown adipose tissue. Another possible (and not mutually exclusive) explanation could be related to the fundamental physical difference between obese people and lean people: obese persons have a natural coat of tissue with a low coefficient of thermal dispersion. In such persons, superficial vasoconstriction would be an efficient means of maintaining core temperature. The data on skin temperature reported by van Marken Lichtenbelt et al. seem to validate this explanation; when exposed to cold, obese subjects showed a greater differential in skin temperature to core temperature than did lean volunteers.12 If this is true, it would represent yet another obstacle to the success of weight-loss strategies.
Taken together, these studies point to a potential “natural” intervention to stimulate energy expenditure: turn down the heat and burn calories (and reduce the carbon footprint in the process). Obviously, this strategy is an oversimplification, and one should expect compensatory mechanisms aimed at maintaining the energy homeostasis — that is, an increase in energy intake in response to a loss of energy secondary to cold exposure. Nonetheless, these studies, by showing the presence and activity of brown adipose tissue in adult humans, are a powerful proof of concept that this tissue might be used as a target for interventions, pharmacologic and environmental, aimed at modulating energy expenditure.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Bellisari A. Evolutionary origins of obesity. Obes Rev. 2008;9:165–80. doi: 10.1111/j.1467-789X.2007.00392.x. [DOI] [PubMed] [Google Scholar]
- 2.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 3.Silva JE, Larsen PR. Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature. 1983;305:712–3. doi: 10.1038/305712a0. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–9. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 5.Huttunen P, Hirvonen J, Kinnula V. The occurrence of brown adipose tissue in outdoor workers. Eur J Appl Physiol Occup Physiol. 1981;46:339–45. doi: 10.1007/BF00422121. [DOI] [PubMed] [Google Scholar]
- 6.English JT, Patel SK, Flanagan MJ. Association of pheochromocytomas with brown fat tumors. Radiology. 1973;107:279–81. doi: 10.1148/107.2.279. [DOI] [PubMed] [Google Scholar]
- 7.Seale P, Kajimura S, Yang W, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng YH, Kokkotou E, Schulz TJ, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–4. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tatsumi M, Engles JM, Ishimori T, Nicely O, Cohade C, Wahl RL. Intense (18)F-FDG uptake in brown fat can be reduced pharmacologically. J Nucl Med. 2004;45:1189–93. [PubMed] [Google Scholar]
- 10.Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–25. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 11.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–8. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]


