Abstract
Chronic immunodeficiency virus infections are characterized by dysfunctional cellular and humoral antiviral immune responses. As such, immune modulatory therapies that enhance and/or restore the function of virus-specific immunity may protect from disease progression. Here, we investigate the safety and immune restoration potential of the blockade of co-inhibitory receptor programmed death-1 (PD-1) during chronic SIV infection in macaques. We demonstrate that PD-1 blockade using an antibody to PD-1 is well tolerated and results in rapid expansion of virus-specific CD8 T cells with improved functional quality. This enhanced T cell immunity was seen in the blood and also in the gut, a major reservoir of SIV infection. PD-1 blockade also resulted in proliferation of memory B cells and increases in SIV envelope-specific antibody. These improved immune responses were associated with significant reductions in plasma viral load and also prolonged the survival of SIV-infected macaques. Impressively, blockade was effective during the early (wk10) as well as late (∼wk90) phases of chronic infection even under conditions of severe lymphopenia. These results demonstrate enhancement of both cellular and humoral immune responses during a pathogenic immunodeficiency virus infection by blocking a single inhibitory pathway and identify a novel therapeutic approach for HIV/AIDS.
Virus-specific T cells exhibit varying degrees of functional impairment during chronic infections1,2. While these T cells retain some anti-viral functions, they are less polyfunctional compared to antiviral T cells seen in acute infections. This defect in T cell function greatly contributes for the inability of the host to eliminate the persisting pathogen. The exhaustion of virus-specific T cells was first shown during persistent LCMV infection of mice3,4 and was quickly extended to other model systems including human immunodeficiency virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV) infections in humans5-7. The co-inhibitory receptor PD-1 has been shown to be highly expressed by the exhausted virus-specific CD8 T cells8,9. PD-1 is also upregulated on HIV-110-12 and SIV13,14-specific CD8 T cells and in vitro blockade of PD-1 enhances cytokine production and proliferative capacity of these cells. However, the importance of this PD-1 inhibitory pathway in regulating T cell function during immunodeficiency virus infection in vivo is not known. Here, we use a SIV/macaque model to evaluate the effects of in vivo blockade of PD-1 on the safety and restoration of both virus-specific cellular and humoral immunity during chronic immunodeficiency virus infections.
PD-1 blockade was performed using an antibody specific to human PD-1 that blocks the interaction between macaque PD-1 and its ligands (PDLs) in vitro13. Blockade was performed during the early (10 weeks) as well as late (∼90 weeks) phases of chronic SIV infection. Nine macaques (5 during the early phase and 4 during the late phase) received the anti-PD-1 Ab and 5 macaques (3 during the early phase and 2 during the late phase) received an isotype control Ab (Sinagis, anti-RSV-specific)15.
PD-1 blockade during chronic SIV infection resulted in a rapid expansion of SIV-specific CD8 T cells in blood of all macaques (Fig. 1a, 1b). We were able to study the CD8 T cell responses to two immunodominant epitopes, Gag CM916 and Tat SL8/TL817, using MHC I tetrameric complexes in seven of the anti-PD-1 Ab treated and three of the control Ab treated macaques that expressed the Mamu A*01 histocompatability molecule. Consistent with previous reports13,14, the majority (>98%) of Gag-CM9 tetramer-specific CD8 T cells expressed PD-1 prior to blockade (data not shown). Following PD-1 blockade, the Gag-CM9 tetramer-specific CD8 T cells expanded rapidly and peaked by 7-21 days. At the peak response, these levels were about 2.5-11 fold higher than their respective levels on day 0 (p=0.007) and remained elevated until 28-45 days (Fig. 1b). Similar results were observed with blockade during the early as well as late phases of chronic SIV infection. A 3-4 fold increase in the frequency of Gag-specific IFN-γ positive CD8 T cells was also observed by day 14 following blockade in the two Mamu A*01 negative animals (RTd11 and RDb11) demonstrating that PD-1 blockade can enhance the frequency of virus-specific CD8 T cells that are restricted by non-Mamu A*01 alleles (data not shown). As expected, expansion of SIV-specific CD8 T cells was not observed in the control Ab treated macaques (Fig. 1).
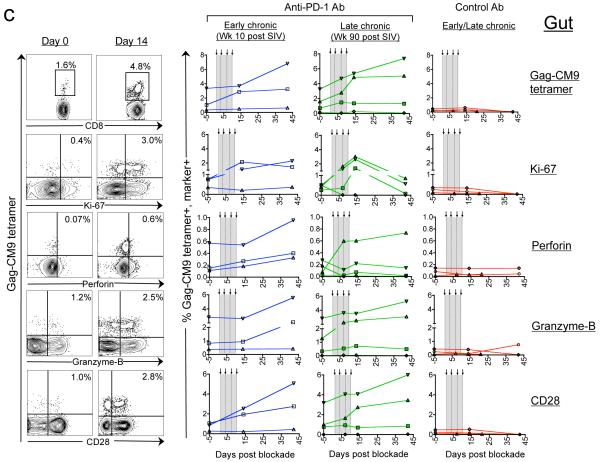
Figure 1. In vivo PD-1 blockade during chronic SIV infection increases the Gag CM9-specific CD8 T cells with improved functional quality in both blood and gut.
a) Representative FACS plots for the macaque RRk10. The magnitude and phenotype of Gag CM9-tetramer positive CD8 T cells in blood (b) and gut (colorectal mucosal tissue) (c). Representative FACS plots are shown on the left and summary for all Mamu A*01 positive animals is shown on the right. Numbers on the FACS plots represent the frequency of tetramer positive cells as a percent of total CD8 T cells. Arrows and vertical dotted lines indicate anti-PD-1 Ab or control Ab treatment.
PD-1 blockade was also associated with significant increase in the frequency of virus-specific CD8 T cells that were undergoing active cell division in vivo with improved functional quality (Fig. 1b). Consistent with the rapid expansion of SIV-specific CD8 T cells, the frequency of Gag-CM9 tetramer-specific CD8 cells that co-expressed Ki-67 (marker for proliferating cells) also increased as early as by day 7 following blockade (p=0.01). Similarly, we observed an increase in the frequencies of Gag-CM9 tetramer-specific CD8 T cells co-expressing perforin (p=0.001) and granzyme B (p=0.03)(cytolytic potential), CD28 (co-stimulation potential; p=0.001), CD127 (proliferative potential; p=0.0003)18 and CCR7 (lymph node homing potential; p=0.001)19. We also observed a transient 1.5-2 fold increase in the frequency of tetramer negative and ki-67 positive CD8 T cells following blockade (data not shown). This could be due to expansion of CD8 T cells specific to other epitopes in Gag as well as other proteins of SIV, and other chronic viral infections in these animals. No significant enhancement was observed for these markers in the three control Ab treated macaques.
Interestingly, no expansion was observed for Tat TL8-specific CD8 T cells following blockade (Supplementary Fig. 1a). This could be due to viral escape from recognition by Tat TL8-specific CD8 T cells as PD-1 blockade is known to result in expansion of T cells only when they simultaneously receive signals through TCR. To test this possibility, we sequenced the viral genomes present in the plasma just prior to the initiation of blockade from all three Mamu A*01 positive macaques that were infected with SIV251 and received the blocking Ab during the early phase of infection. Indeed, we found mutations in the viral genome corresponding to the Tat TL8 epitope region (Supplementary Fig. 1b). All these mutations either have been shown or predicted to reduce the binding of Tat SL8/TL8 peptide to Mamu A*01 MHC molecule and result in escape from recognition by the Tat SL8/TL8-specific CD8 T cells16,17. These results suggest that in vivo blockade of PD-1 may not result in expansion of T cells that are specific to escape mutants of viral epitopes.
PD-1 blockade also resulted in expansion of Gag-CM9-specific CD8 T cells at the colorectal mucosal tissue (gut), a preferential site of SIV/HIV replication20 (Fig. 1c). Expansion was not observed for two of the seven monkeys although expansion was evident for one of them in blood. In contrast to blood, the expansion in gut peaked much later by day 42 and ranged from 2-3 fold compared to their respective day 0 levels (p=0.003). Similar to blood, the Gag-CM9 tetramer-specific cells that co-expressed Ki-67 (p=0.01), perforin (p=0.03), granzyme B (p=0.01), and CD28 (p=0.01) also increased in the gut following blockade.
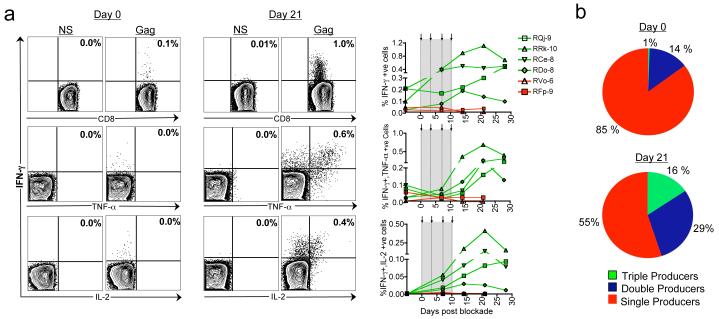
More importantly, PD-1 blockade also enhanced the functional quality of antiviral CD8 T cells and resulted in the generation of polyfunctional cells capable of co-producing cytokines IFN-γ, TNF-α and IL-2 (Fig. 2). On the day of initiation of PD-1 blockade during the late chronic phase of infection, the frequency of Gag-specific IFN-γ positive cells was low and failed to co-express TNF-α and IL-2 (Fig. 2a). However, following the blockade, the frequency of IFN-γ positive cells increased in all four PD-1 Ab treated macaques (p=0.03) and acquired the ability to co-express TNF-α and IL-2. The expansion of IFN-γ positive cells peaked by 14-21 days and the peak levels were 2-10 fold higher than the respective day 0 levels. On day 21, about 16% of the total Gag-specific cells coexpressed all three cytokines, and about 30% coexpressed IFN-γ and TNF-α (Fig. 2b). This is in contrast to <1% of the total Gag-specific cells coexpressing all three cytokines (p=0.01), and about 14% coexpressing IFN-γ and TNF-α on day 0 (p=0.04). Similar results were also observed following blockade during the early chronic phase of infection (data not shown).
Figure 2. In vivo PD-1 blockade during chronic SIV infection increases the polyfunctional virus-specific CD8 T cells.
a) Frequency of Gag-specific cytokine secreting CD8 T cells as a percent of total CD8 T cells. Representative FACS plots are shown on the left and summary for the group is shown on the right. Arrows and vertical dotted lines indicate anti-PD-1 Ab or control Ab treatment. Green lines represent anti-PD-1 Ab treated macaques and red lines represent control Ab treated macaques. b) Cytokine co-expression subsets expressed as a percent of total cytokine positive cells. Mean for the group is shown.
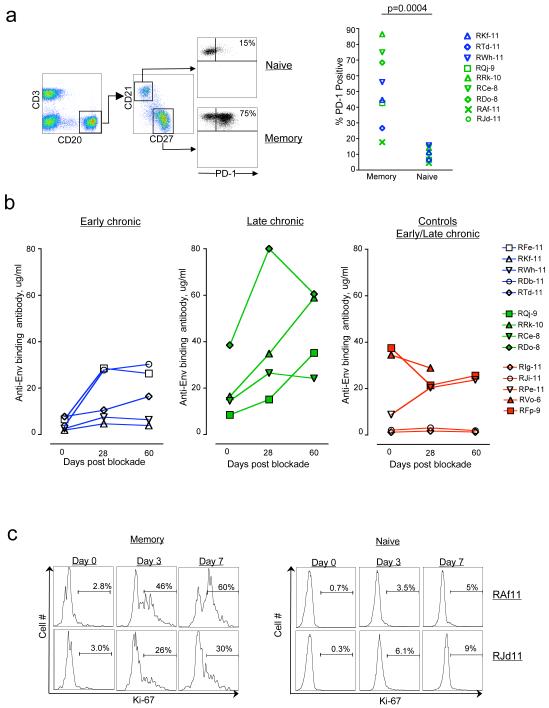
Recent studies have shown that chronic immunodeficiency virus infections are also associated with B cell dysfunction21,22 and very little is known about the role of PD-1 in regulating B cell function/exhaustion. To understand the role of PD-1 in regulating B cell function during chronic immunodeficiency virus infections, we characterized the B cell responses following PD-1 blockade in SIV-infected macaques (Fig. 3). Analysis of PD-1 expression on different B cell subsets prior to PD-1 blockade revealed preferential expression of PD-1 by memory B cells (CD20+CD27+CD21-) compared to naïve B cells (CD20+CD27-CD21+)(Fig. 3a)(p<0.001). In vivo blockade of PD-1 resulted in a 2-8 fold increase in the titer of SIV-specific binding Ab by day 28 following blockade (p<0.001)(Fig. 3b). To understand this further, we studied the proliferation of memory B cells in SIV-infected macaques that were treated simultaneously with anti-PD-1 Ab and anti-retroviral therapy and observed a significant increase in Ki-67+ (proliferating) memory but not naïve B cells as early as day 3 (Fig. 3c). These results demonstrate that PD-1:PDL pathway could play a role in regulating B cell dysfunction during chronic SIV infection. Neutralization assays revealed a 2-fold increase in titers against the easily neutralizable lab adapted SIV251 and no increase in titers against hard to neutralize wild type SIV251 or 239 (data not shown). In 2 of the 9 animals treated with anti-PD-1 Ab, we observed only a minimal (< 2 fold) expansion of SIV-specific Ab following blockade. Interestingly, the frequency of total memory B cells in these two animals was lower (∼40% of total B cells) compared to the remaining 7 animals (60-90% of total B cells) prior to blockade (data not shown) suggesting that the level of SIV-specific memory B cells prior to blockade may determine the level of expansion of SIV-specific Ab following blockade.
Figure 3. In vivo PD-1 blockade during chronic SIV infection enhances SIV-specific humoral immunity.
a) Expression of PD-1 on memory (CD20+CD27+CD21-) and naïve (CD20+CD27-CD21+) B cells in blood following SIV infection and prior to in vivo PD-1 blockade. b) Titers of anti-SIV Env binding Ab in serum following blockade. c) Ki-67 expression (marker for proliferation) on memory and naïve B cells following blockade. Numbers on the FACS plots represent Ki-67 positive cells as a percent of respective total cells. Macaques RAf11 and RJd11 were treated simultaneously with anti-PD-1 Ab and anti-retroviral therapy at 22 weeks post SIV infection.
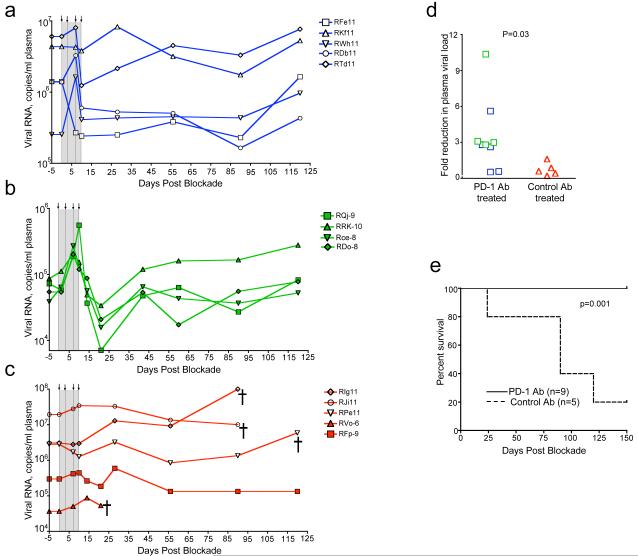
PD-1 blockade resulted in significant reductions in plasma viremia (p=0.03) and also prolonged the survival of SIV-infected macaques (p=0.001)(Fig. 4). In 2 of the 5 macaques treated with anti-PD-1 Ab during the early chronic phase, viral load declined by day 10 and persisted at or below this level until day 90 (Fig. 4a). In one macaque viral load declined transiently and in the remaining two macaques increased transiently and returned to preblockade levels. In contrast to the early chronic phase, all 4 macaques treated with the anti-PD-1 Ab during the late chronic phase exhibited a transient increase in viremia by day 7, but rapidly reduced the virus load by day 21 to levels that were below their respective day 0 levels (Fig. 4b). However, the viral RNA levels returned to pre-blockade levels by day 43. As expected, no significant reductions for the plasma viral loads were observed in any of the five macaques treated with the control Ab (Fig. 4c). By 21-28 days following blockade, the viral RNA levels in the anti-PD-1 Ab treated animals were 2-10 fold lower than their respective day 0 levels (p=0.03) (Fig. 4d). By day 150 after the blockade, 4 of the 5 macaques in the control group were euthanized due to AIDS related symptoms (loss of appetite, diaheria, weight loss etc.), whereas all 9 animals in the anti-PD-1 Ab treated group had survived (p=0.001)(Fig. 4e).
Figure 4. In vivo PD-1 blockade reduces plasma viremia and prolongs survival of SIV-infected macaques.
Plasma viral load in a) macaques treated with anti-PD-1 Ab during the early chronic phase b) macaques treated with anti-PD-1 Ab during the late chronic phase and c) macaques treated with control Ab during the early/late chronic phase of SIV infection. d) Fold reduction in plasma viral load between day 0 and day 28 (early chronic study) or day 0 and day 21 (late chronic study). e) Survival of SIV-infected macaques following PD-1 blockade. =, death of animal.
The observed initial rise in plasma viremia in all of the late phase treated and some of the early phase treated animals could be due to an increase in the frequency of activated CD4 T cells. To determine this, we measured the percent of Ki-67 positive total CD4 T cells as well as the frequency of SIV Gag-specific IFN-γ producing CD4 T cells (preferential targets for virus replication23) following blockade (Supplementary Fig. 2). These analyses revealed a transient increase in the percent of Ki-67 positive CD4 T cells by day 7-14 following blockade (p=0.002) and this increase was higher in animals treated during the late phase than early phase of infection (p=0.015)(Supplementary Fig. 2a). Similarly, an increase in the frequency of Gag-specific CD4 T cells was also observed, but only in animals treated during the late phase of infection (Supplementary Fig. 2b). No significant increases were observed for these activated CD4 T cells in the control Ab treated macaques. These results suggest that the activated CD4 T cells could have contributed for the observed initial rise in plasma viremia following blockade.
It is to be noted that, prior to initiation of PD-1 blockade, the set point viral load in plasma and total CD4 T cells in blood and gut were similar between the anti-PD-1 Ab and control Ab treated groups (Supplementary Fig. 3). However, the frequencies of Gag CM9+ cells and Gag CM9+ cells co-expressing Perforin, Granzyme B or CD28 were not similar between the two treatment groups prior to in vivo blockade (Fig. 1b). This raises the possibility that these differences could have contributed for the expansion of Gag CM9+ cells following PD-1 blockade. To study the influence of the frequency of Gag CM9+ cells prior to blockade on their expansion following blockade, we divided the anti-PD-1 Ab treated group into two subgroups based on the frequency of Gag CM9+ cells prior to initiation of blockade such that one group has similar levels and the other group has higher levels of Gag CM9+ cells compared to control Ab treated group. These subgroups were then analyzed for expansion of CM9+ cells following blockade. Expansion of CM9+ cells was evident in both subgroups of animals following blockade of PD-1, irrespective of whether they were low or high in magnitude prior to blockade (Supplementary Fig.4). Similar results were also observed with subgroup analyses based on the frequency of CM9+ cells co-expressing molecules associated with better T cell function such as perforin, granzyme B, CCR7, CD127 or CD28 (Supplementary Fig.4). However, we observed a trend towards better expansion of CM9+CD28+ cells in animals with higher levels of CM9+CD28+ cells prior to blockade suggesting that CD28 expression may serve as a biomarker for predicting the outcome of in vivo PD-1 blockade.
To evaluate the safety of PD-1 blockade, we performed an extensive analysis of serum proteins, ions, lipids, liver and kidney enzymes, and complete blood count following blockade (Supplementary Tables 1 and 2). These analyses revealed no significant changes for all parameters tested between the anti-PD-1 Ab and control Ab treated macaques (data not shown). Similarly, the levels of anti-nuclear antibodies (ANA) in serum (measure of autoimmunity) also did not change significantly following treatment with anti-PD-1 Ab (Supplementary Fig. 5). In one macaque, the levels of ANA increased about 3 fold by day 10 following blockade, but returned to day 0 levels by day 56. These results demonstrate that anti-PD-1 Ab treatment during chronic SIV infection results in no observable toxicity. This is consistent with a recent study that demonstrated the safety of PD-1 blockade in patients with advanced hematologic malignancies24.
We studied the pharmacokinetics of the partially humanized anti-PD-1 Ab in serum following in vivo blockade. The titer of anti-PD-1 Ab rapidly declined between days 14 and 28 following blockade (Supplementary Fig. 6a) and coincided with monkeys generating antibody response against the mouse Ig variable domains of anti-PD-1 Ab (Supplementary Fig. 6b). These results suggest that the use of completely humanized anti-PD-1 Ab may allow longer periods of treatment that may further enhance the efficacy of in vivo blockade.
In conclusion, our results demonstrate that in vivo blockade of PD-1 during chronic SIV infection is safe and results in rapid expansion and restoration of SIV-specific polyfunctional CD8 T cells and enhanced B cell responses. Impressively, expansion was observed with blockade performed during the early as well as late phases of chronic infection even under conditions of high levels of persisting viremia and AIDS. Expansion was also observed at the colorectal mucosal tissue, a preferential site of SIV/HIV replication20. Importantly, PD-1 blockade resulted in a significant reduction of plasma viral load and also prolonged the survival of SIV-infected macaques. These results are highly significant considering the failure of blockade of a related co-inhibitory molecule CTLA-4 to expand virus-specific CD8 T cells and to reduce plasma viral load in SIV-infected macaques25. The therapeutic benefits of PD-1 blockade may be improved further by using combination therapy with anti-retrovirals and/or therapeutic vaccination.
Methods Summary
Study group
14 Indian rhesus macaques infected with SIV were studied. Eight macaques were used for the early chronic phase and were infected intravenously with 200 TCID50 of SIV251. Six macaques were used for the late chronic phase, 3 were infected with SIV251 intrarectally and 3 were infected with SIV239 intravenously. All macaques, except RDb11, were negative for Mamu B08 and Mamu B17 alleles. RDb11 was positive for Mamu B17 allele. Macaques were housed at the Yerkes National Primate Research Center and were cared for under guidelines established by the Animal Welfare Act and the NIH “Guide for the Care and Use of Laboratory Animals” using protocols approved by the Emory University IACUC.
In vivo antibody treatment
Macaques were infused with either partially humanized mouse anti-human PD-1 Ab (clone EH12-1540)26 or a control Ab (SYNAGIS). The anti-PD-1 Ab has mouse variable heavy chain domain linked to human IgG1 (mutated to reduce FcR and complement binding)27 and mouse variable light chain domain linked to human Kappa. The clone EH12 binds to macaque PD-1 and blocks interactions between PD-1 and its ligands in vitro13. SYNAGIS is a humanized mouse monoclonal antibody (IgG1κ) specific to F protein of respiratory syncytial virus (Medimmune, Gaithersberg, MD). Antibodies were administered intravenously at 3 mg/kg of body weight on days 0, 3, 7 and 10.
Immune responses
PBMCs from blood and lymphocytes from rectal pinch biopsies were isolated as described previously13. Tetramer staining28, Intracellular cytokine production28,29 and measurements of anti-SIV Env binding Ab30 was performed as described previously.
Supplementary Material
Acknowledgements
The authors thank J. D. Altman for provision of Gag CM9 and Tat SL8 tetramers, H. Drake-Perrow for administrative support, D. Watkins and Wisconsin National Primate Research Center Genotyping Service for Mamu typing of animals. The authors also thank the Yerkes Division of Research Resources for excellent pathology support, Emory CFAR virology core for viral load assays and the NIH AIDS Research and Reference Reagent Program for the provision of peptides. This work was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases grants R01 AI057029, R01 AI071852, R01 AI074417 to RRA; the Foundation for the NIH through the Grand Challenges in Global Health initiative P51 RR00165 to RA, GJF and RRA; Yerkes National Primate Research Center base grant, P51 RR00165; Emory CFAR grant P30 AI050409; and R24 RR16038 to David I. Watkins.
Appendix
Additional methods
B cell responses
100μl of blood was surface stained with antibodies to CD3 (Clone SP34-2, BD Biosciences, San Diego, California), CD20 (2H7, e-Biosciences), CD21 (B-ly4, Becton Dickson, San Jose) CD27 (M-T2712 Becton Dickson, San Jose) and PD-1 (clone EH-12) each conjugated to a different fluorochrome. Cells were lysed and fixed with FACS lysing solution, and permeabilized using FACS perm (BD Biosciences) according to manufacturer’s instructions. Cells were then stained for intracellular Ki-67 using an anti-Ki-67 Ab conjuagated to PE (Clone B56, Becton Dickson, San Jose). Following staining, cells were washed and acquired using LSRII (BD Biosciences, San Jose, California), and analyzed using FlowJo software.
Titers of anti-PD-1 Ab and monkey antibody response against anti-PD-1 Ab in serum
To measure the levels of anti-PD-1 antibody, plates were coated with goat anti-mouse Ig (pre absorbed to human Ig, Southern Biotech), blocked and incubated with different dilutions of serum to capture the blocking antibody. Bound antibody was detected using anti-mouse IgG conjugated to HRP (pre absorbed to human Ig, Southern Biotech). Known amounts of blocking antibody captured in the same manner were used to generate a standard curve. To measure the levels of monkey antibody response against the anti-PD-1 antibody, plates were coated with anti-PD-1 Ab antibody (5 μg/ml), blocked and incubated with different dilutions of serum to capture the anti-blocking antibody. Bound antibody was detected using anti-human lambda chain specific Ab conjugated to HRP (Southern Biotech). This detection antibody does not bind to the blocking antibody because only the constant regions of the heavy and light chains were humanized and the constant region of light chain is kappa. The amount of captured monkey Ig was estimated using a standard curve that consisted of known amounts of purified macaque Ig that had been captured using anti-macaque Ig.
Quantitation of SIV copy number
SIV copy number was determined using a quantitative real time PCR as previously described28. All specimens were extracted and amplified in duplicates, with the mean result reported.
Amplification and sequencing of the Tat TL8 epitope
A 350 nucleotide fragment including Tat TL8 epitope was amplified by limiting dilution RT-PCR. Viral RNA was extracted using the QIAamp Viral RNA mini kit (Qiagen, Valencia, CA) from plasma. vRNA was reverse transcribed with the SIVmac239 specific primer Tat-RT3 (5′-TGGGGATAATTTTACACAAGGC-3′) and Superscript III (Invitrogen Corporation, Carlsbad, CA) using the manufacturer’s protocol. The resultant cDNA was diluted and copy number was determined empirically in our nested PCR protocol. Limiting dilution, nested PCR was performed at ∼0.2 copies per reaction using the Expand HiFi PCR kit (Roche Applied Sciences, Indianapolis, IN) with outer primers Tat-F1 (5′- GATGAATGGGTAGTGGAGGTTCTGG -3′) and Tat-R2 (5′- CCCAAGTATCCCTATTCTTGGTTGCAC -3′), and inner primers Tat-F3 (5′- TGATCCTCGCTTGCTAACTG -3′) and Tat-R3 (5′- AGCAAGATGGCGATAAGCAG -3′). The first round reactions were cycled using the following program: 94°C for 1:00, followed by 10 cycles of 94°C for 30 seconds, 55°C for 30 seconds, and 68°C for 1:00, followed by 25 more cycles identical to the first ten but for the addition of 5 seconds to the extension time at every cycle, followed by a final extension at 68°C for 7:00. The second round reactions were cycled using the following program: 94°C for 1:00, followed by 35 cycles of 94°C for 30 seconds, 53°C for 30 seconds, and 68°C for 1:00, followed by a final extension at 68°C for 7:00. After cleanup with ExoSap-IT (USB Corporation, Cleveland, OH), PCR products were sequenced directly using the inner primers on an automated sequencer at The Children’s Hospital of Philadelphia’s Napcore sequencing facility (Philadelphia, PA). Contigs were assembled using Sequencher 4.8 (Gene Codes Corporation, Ann Arbor, MI) amplicons containing nucleotides with double chromatogram peaks were excluded.
Statistical analyses
Linear mixed effects models were employed to determine differences in blood chemistry and complete blood count values between anti-PD-1 Ab and control Ab treated animals. Bonferroni method was used to adjust p values for multiple tests. Paired t-test was used for comparison of immune responses before and after PD-1 blockade. Log-transformed data were used when the data were not normal, but log-normal. Wilcoxon rank-sum test was used to compare the fold reductions in viral loads between the groups. Mantel-Haenszel log rank test was used to compare the survival curves between the groups. Statistical analyses were performed using S-PLUS 8.0. A two sided p<0.05 considered statistically significant.
References
- 1.Wherry EJ, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Klenerman P, Hill A. T cells and viral persistence: lessons from diverse infections. Nat Immunol. 2005;6:873–879. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- 3.Zajac AJ, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. see comments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallimore A, et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Letvin NL, Walker BD. Immunopathogenesis and immunotherapy in AIDS virus infections. Nat Med. 2003;9:861–866. doi: 10.1038/nm0703-861. [DOI] [PubMed] [Google Scholar]
- 6.Pantaleo G, Koup RA. Correlates of immune protection in HIV-1 infection: what we know, what we don’t know, what we should know. Nat Med. 2004;10:806–810. doi: 10.1038/nm0804-806. [DOI] [PubMed] [Google Scholar]
- 7.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 8.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 9.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 10.Petrovas C, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 12.Trautmann L, et al. Upregulation of PD-1 expression on HIV-specific CD8 + T cells leads to reversible immune dysfunction. Nat Med. 2006 doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 13.Velu V, et al. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J Virol. 2007;81:5819–5828. doi: 10.1128/JVI.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrovas C, et al. SIV-specific CD8+T-cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 2007 doi: 10.1182/blood-2007-01-069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malley R, et al. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. J Infect Dis. 1998;178:1555–1561. doi: 10.1086/314523. [DOI] [PubMed] [Google Scholar]
- 16.Allen TM, et al. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. Journal of Immunology. 1998;160:6062–6071. [PubMed] [Google Scholar]
- 17.Allen TM, et al. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature. 2000;407:386–390. doi: 10.1038/35030124. [DOI] [PubMed] [Google Scholar]
- 18.Kaech SM, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 19.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 20.Pierson T, McArthur J, Siliciano RF. Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Annu Rev Immunol. 2000;18:665–708. doi: 10.1146/annurev.immunol.18.1.665. [DOI] [PubMed] [Google Scholar]
- 21.De Milito A. B lymphocyte dysfunctions in HIV infection. Current HIV research. 2004;2:11–21. doi: 10.2174/1570162043485068. [DOI] [PubMed] [Google Scholar]
- 22.Moir S, Fauci AS. Pathogenic mechanisms of B-lymphocyte dysfunction in HIV disease. J Allergy Clin Immunol. 2008;122:12–19. doi: 10.1016/j.jaci.2008.04.034. quiz 20-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Douek DC, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 24.Berger R, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 25.Cecchinato V, et al. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J Immunol. 2008;180:5439–5447. doi: 10.4049/jimmunol.180.8.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorfman DM, Brown JA, Shahsafaei A, Freeman GJ. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2006;30:802–810. doi: 10.1097/01.pas.0000209855.28282.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu D, et al. In vitro characterization of five humanized OKT3 effector function variant antibodies. Cell Immunol. 2000;200:16–26. doi: 10.1006/cimm.2000.1617. [DOI] [PubMed] [Google Scholar]
- 28.Amara RR, et al. Control of a Mucosal Challenge and Prevention of AIDS by a Multiprotein DNA/MVA Vaccine. Science. 2001;292:69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- 29.Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol. 2007;81:8468–8476. doi: 10.1128/JVI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai L, et al. GM-CSF DNA: an adjuvant for higher avidity IgG, rectal IgA, and increased protection against the acute phase of a SHIV-89.6P challenge by a DNA/MVA immunodeficiency virus vaccine. Virology. 2007;369:153–167. doi: 10.1016/j.virol.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.