Abstract
Aims
We investigated the effect of comprehensive periodontal therapy on the levels of multiple systemic inflammatory biomarkers.
Methods
Thirty patients with severe periodontitis received comprehensive periodontal therapy within a 6-week period. Blood samples were obtained at: one week pre- therapy (T1), therapy initiation (T2), treatment completion (T3), and 4 weeks thereafter (T4). We assessed plasma concentrations of 19 biomarkers using multiplex assays, and serum IgG antibodies to periodontal bacteria using checkerboard immunoblotting. At T2 and T4, dental plaque samples were analyzed using checkerboard hybridizations.
Results
At T3, PAI-1, sE-selectin, sVCAM-1, MMP-9, myeloperoxidase, and a composite Summary Inflammatory Score (SIS) were significantly reduced. However, only sE-selectin, sICAM, and serum amyloid P sustained a reduction at T4. Responses were highly variable: analyses of SIS slopes between baseline and T4 showed that approximately 1/3 and 1/4 of the patients experienced marked reduction and pronounced increase in systemic inflammation, respectively, while the remainder were seemingly unchanged. Changes in inflammatory markers correlated poorly with clinical, microbiological and serological markers of periodontitis.
Conclusions
Periodontal therapy resulted in an overall reduction of systemic inflammation, but the responses were inconsistent across subjects and largely not sustainable. The determinants of this substantial heterogeneity need to be explored further.
Keywords: Periodontal therapy, inflammatory mediators, systemic inflammation, atherosclerotic vascular disease
CLINICAL RELEVANCE
Scientific rationale
We investigated the effects of periodontal therapy on multiple inflammatory blood markers simultaneously and their association with clinical, microbiological and serological markers of periodontitis.
Principal findings
Despite a general trend for reduced systemic inflammation immediately after therapy and a significant suppression of specific biomarkers, large differences were noted across patients, with approximately 1/3 experiencing a clear reduction in systemic inflammation, 1/4 an increase, and the remainder no changes.
Practical implications
The heterogeneous responses correlated poorly with clinical and infectious markers of periodontitis. The identification of the subgroup of patients that may benefit systemically from periodontal therapy remains challenging.
INTRODUCTION
Accumulating evidence suggests that periodontal infections are associated with adverse systemic outcomes, including atherosclerotic vascular disease (AVD) and its sequelae (Beck & Offenbacher 2005). This notion is in accordance with the concept of infectious inflammatory diseases as contributors to the risk and progression of AVD (Zebrack & Anderson 2003, Hansson 2005). The common denominator of the multiple biological pathways proposed to underlie this association is the chronic state of systemic inflammation that accompanies severe periodontitis, characterized by elevated acute phase proteins such as CRP (Boucher et al. 1967, Shklair et al. 1968, Ebersole et al. 1997, Loos et al. 2000, Noack et al. 2001, Loos 2005), inflammatory cytokines such as IL-6 (Loos et al. 2000) and coagulation factors such as fibrinogen (Loos 2005). In turn, these mediators contribute to the perturbation of the vascular endothelium, a hallmark of early atherogenesis, leading to cell surface expression of adhesion molecules, thereby facilitating adherence and entry of leukocytes into the vessel wall (Ross 1999). Bacterial products from oral pathogens were also shown to induce endothelial activation (Khlgatian et al. 2002, Roth et al. 2006, Roth et al. 2007).
Recent studies have investigated the potential role of periodontal therapy on systemic inflammation, but the results are somewhat conflicting. Patients treated by non-surgical mechanical periodontal therapy showed a significant increase in plasma TNF-α, CRP and IL-6 levels immediately after the intervention, indicating a systemic acute-phase response, apparently due to a massive bacterial inoculation in conjunction with instrumentation (Ide et al. 2004, D'Aiuto et al. 2005, D'Aiuto et al. 2007). Patients with periodontitis followed for at least 3 months after completion of non-surgical/surgical therapy and adjunctive systemic antibiotics demonstrated no significant changes of serum levels of CRP, IL-6 or TNF-a (Yamazaki et al. 2005). In contrast, a 2-month post-treatment follow-up of subjects enrolled in a randomized controlled trial that compared mechanical periodontal therapy alone versus identical therapy supplemented by local adjunctive antibiotics (D'Aiuto et al. 2005) reported significant reductions in serum CRP and IL-6 in both treatment arms, and a significant reduction in total and LDL cholesterol in the group that received the adjunctive therapy. However, a recent randomized controlled trial by the same group (Tonetti et al. 2007) reported no significant difference in plasma levels of CRP, IL-6, and plasminogen activator inhibitor-1 (PAI-1) levels between treatment and control groups at a 6-month follow-up examination, although the treatment group experienced a reduction in serum sE-selectin and neutrophil counts. In addition, a recent systematic review concluded that there is modest evidence that periodontal therapy results in a reduction of serum CRP levels (Paraskevas et al. 2008).
Little is known about how these responses would be reflected if a wider array of pro- and anti-inflammatory mediators was assessed simultaneously or on the determinants of the apparent heterogeneity. In this study, we examined the effect of comprehensive periodontal therapy, consisting of scaling and root planning, pocket reduction surgery, tooth extractions whenever indicated, but no adjunctive local or systemic antibiotic medications, on multiple inflammatory mediators in the peripheral blood. We further explored the degree to which the systemic inflammatory responses correlate to clinical, microbiological and antibody responses to periodontal therapy.
MATERIAL AND METHODS
Study design overview
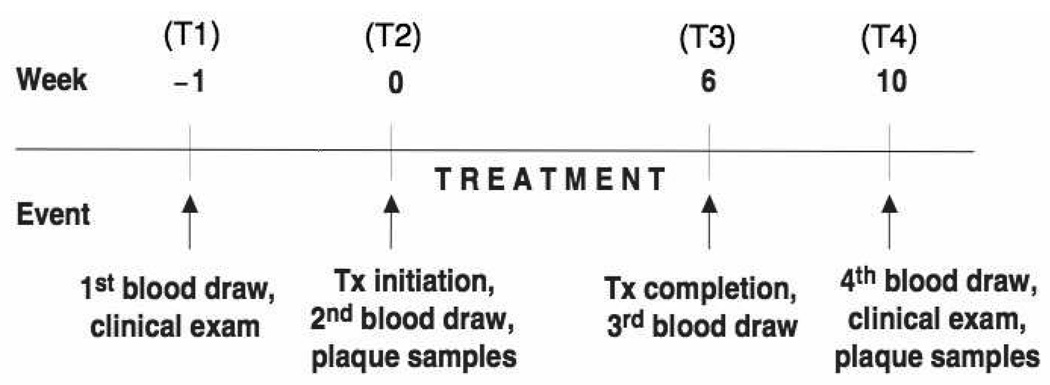
We adopted a prospective, single arm intervention study design that was approved by the Columbia University Medical Center Institutional Review Board. In brief, participants underwent a clinical examination and a first blood sample was obtained 1-week prior to the start of periodontal therapy (T1). A second blood sample was obtained 1 week later on the day of treatment initiation (T2). All periodontal treatment was completed within a 6-week window, at which point a first post-treatment blood sample was obtained (T3). A second post-treatment blood sample (T4) was obtained 4 weeks thereafter, i.e., at 10 weeks after T2, and a final clinical examination was carried out. Figure 1 provides an overview of the study design.
Figure 1.
Study flow chart
Subject sample
Patients were recruited among those seeking treatment at the clinic for post-doctoral Periodontics, Columbia University College of Dental Medicine. To be eligible for enrollment, patients had to (i) be diagnosed with severe periodontitis, with radiographic evidence of bone loss extending to ≥ 30% of the root length at multiple sites, (ii) present with ≥ 2 teeth/quadrant with a pocket depth of ≥ 6mm and concomitant clinical attachment loss of ≥ 3mm; and (iii) have minimum of 20 teeth. Exclusion criteria included: prior periodontal therapy; systemic conditions or genetic disorders that entail the diagnosis of “Periodontitis as manifestation of systemic diseases” (1999) International Workshop for the Classification of Periodontal Disease Conditions); use of systemic antibiotics or regular use of anti-inflammatory drugs for the preceding 6-month period; diabetes mellitus; current use of tobacco products or of nicotine replacement medication; and current pregnancy or lactation. Former smokers were considered eligible if they had quit more than 6 months prior to enrollment. All participants were informed about the scope and the procedures of the study and informed consent was obtained.
Clinical examination
At the time of enrollment (T1) and at T4, all patients underwent a full-mouth periodontal examination at six sites per tooth at all teeth present. A single calibrated examiner who was not involved in the treatment of the patients recorded the following variables: Probing depth (PD), defined as the distance between the gingival margin and the bottom of the probeable pocket, to the nearest whole millimeter.
Location of the gingival margin (GM), i.e., the distance between the cemento-enamel junction (CEJ) and the gingival margin, recorded to the nearest whole millimeter. This measure was given a positive sign in case of a gingival recession and a negative sign when the gingival margin was located coronal to the CEJ. The distance was deemed non-readable whenever the CEJ was obscured by dental restorations or was impossible to identify. The algebraic sum of GM and PD was used to compute the clinical attachment level (CAL).
The presence of dental plaque (PL), without the use of any disclosing agent, and bleeding on probing (BOP) were recorded dichotomously. The latter was deemed positive if it occurred within 15 seconds after the measuring of PD.
All recordings were entered chair-side using the software Epi Info (Centers for Disease Control and Prevention, Atlanta, GA, USA) and were uploaded to a server at the Statistical Analysis Center, Columbia University Medical Center.
Dental plaque samples
Subgingival plaque samples were obtained at T2 and T4, from the same 8 to 10 interproximal surfaces in a randomly selected maxillary quadrant, using sterile curettes as described earlier (Papapanou et al. 2000). The samples were analyzed with respect to 11 bacterial species (Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola, Fusobacterium nucleatum, Prevotella intermedia, Campylobacter rectus, Micomonas micros, Eikenella corrodens, Veillonella parvula, and Actinomyces naeslundii) according to the checkerboard DNA-DNA hybridization method (Haffajee & Socransky 1994) as earlier described (Picolos et al. 2005). Samples from different occasions from the same patient were always processed simultaneously on the same membrane.
Blood sampling and processing
At each of the four time points, approximately 5 ml of blood were collected by venipuncture in untreated Vacutainer™ blood collection tubes (Becton-Dickinson, Franklin Lakes, NJ, USA). Blood samples were always obtained in the morning, before any manipulation of the gingival tissues, and were non-fasting. Serum was obtained by centrifugation at 1300g for 10 min and was aliquoted and stored at −70°C until further analysis. The concentrations of 19 biomarkers were assessed using a multiplex assay (Linco Reseach Inc., St Charles, Mo, USA) for Luminex technology according to the manufacurer’s instructions. The assays used were (i) the Cardiovascular Disease Panel 1, for the assessment of adiponectin, matrix metalloproteinase-9 (MMP-9), myeloperoxidase (MPO), total plasminogen activator inhibitor 1 (PAI-1), soluble E-selectin, soluble intercellular adhesion molecule-1 (sICAM-1), and soluble vascular adhesion molecule 1 (sVCAM-1); (ii) the Cardiovascular Disease Panel 2, for the assessment of high sensitivity C-reactive protein (hsCRP), serum amyloid P (SAP) and serum amyloid A (SAA); and (iii) the high-sensitivity Human Cytokine Panel for the assessment of the following cytokines: IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, and TNF-α.
In brief, serum samples were incubated with a mix of beads coated with antibodies for different serum mediators. After washing, samples were incubated with biotin labeled Detection Antibody Cocktail that was then marked with Streptavidin-Phycoerythrin dye. Readout was performed with a Luminex 100 (Luminex, Austin, TX, USA). Bio-Plex Manager 3.0 (BIO-RAD, Hercules, CA, USA) was used to analyze the readout. Samples from different time points from the same patient were always run on the same plate, and a total of three consecutive runs were used to run all samples from the entire study.
Serum antibodies to periodontal microbiota
Serum IgG antibody levels to the same periodontal microbiota included in the microbial panel was determined using checkerboard immunoblotting (Sakellari et al. 1997), as described previously (Papapanou et al. 2004). Samples from all four occasions from the same patient were always processed simultaneously on the same membrane.
Periodontal therapy
Periodontal therapy was provided by two periodontists (authors JHB and MS). Therapy commenced at T2, and was completed within a 6-week period, i.e., by T3. It included oral hygiene instruction, full-mouth scaling and root planing, periodontal surgery according to each patient’s individual needs, and tooth extractions, when indicated. Following surgery, patients used an adjunctive antimicrobial mouth rinse (chlorhexidine 0.12%), but no local or systemic antibiotics were administered.
Data analysis
In all analyses, SAS version 9.1 (SAS Institute, Cary, NC, USA) was used and the individual patient was the computational unit. Clinical and microbiological variables were averaged within each patient on each occasion.
For the comparison of serum analyte data, the values obtained from T1 and T2 were averaged to create a combined pre-treatment baseline value (BL), to account for biological variation between the two pre-treatment blood draws. We used the Wilcoxon signed-ranked test for paired samples to compare analyte changes between either BL and T3 or BL and T4. In alternate analyses, we also used standard paired t-tests based on both natural log and raw biomarker values. The findings were consistent between the non-parametric and parametric analyses.
To analyze an overall systemic response to treatment, a summary inflammatory score (SIS) was calculated (Papapanou et al. 2007). A standardized within-person z-score was created for each of three time points (BL, T3, T4) by (i) subtracting the mean within person (across time) analyte value from the analyte value at each of the three aforementioned time points, and (ii) dividing it by the within-patient analyte standard deviation (SD). Thus, the calculation of a within-person z-score from analyte i, time j and person k yielded: Zkij = (valuekij−meanki)/(SDki). A total of 57 z-scores (19 analytes×3 time points) were computed for each patient. The standardized z-scores were subsequently used in the calculation of a subject-based SIS at each of three time points, by averaging the 19 analyte z-scores. The use of z-scores, rather than the actual analyte values, in the computation of SIS prevented any one analyte from artificially dominating the SIS as a result of scaling differences. Z-scores corresponding to the anti-inflammatory mediators IL-10 and adiponectin were multiplied by (−1) before their inclusion in the SIS. Thus, a positive SIS reflected a state of elevated systematic inflammation at the time point of interest relative to the mean value across all three time points. Accordingly, a negative SIS value reflected a relative reduced level of systemic inflammation. As a final step, differences between SIS at BL and the SIS at the early (T3) and late (T4) post-treatment time points were used to define the cumulative systemic inflammatory responses at 6 and 10 weeks post-baseline, respectively. Mean values, standard deviations and median values were calculated for each analyte and SIS at BL, T3 and T4.
Linear regression models were used to assess whether changes in clinical parameters, microbial levels or serum antibodies (independent variables) predicted change in any of the systemic biomarkers (dependent variables).
RESULTS
A total of 30 patients (16 females and 14 males) were enrolled. The mean age of the patients was 43.3 years (SD 13.3, range 14–77). With respect to self-reported race, 4 people were Black, 11 White, 12 of mixed race, and 3 declined to report.
Table 1 summarizes the provided therapy and the clinical periodontal characteristics of the patients at T1 and T4. Within the treatment window, patients attended an average of 6.1 treatment visits (SD 1.1, range 4–9), including an average of 3.9 periodontal surgery appointments (SD 0.6, range 2–5). An average of 2.8 teeth per patient were extracted (SD 2.9, range 0–12). The pre-treatment number of sites with PD ≥ 6mm was reduced from an average of 79.7 sites per patient (SD 30.5, range 36–156) to a post-treatment mean of 11.6 sites per patient (SD 8.8, range 0–39). Likewise, the pre-treatment percentage of sites with BOP was reduced from 82.6% (SD 14.7, range 39–100) to 26.5% (SD 9.8, range 7–46). In contrast, the high plaque levels pre-treatment (96.3%, SD 4.4, range 86–100)remained high at the last visit (71.4%, SD 8.8, range 56 – 100).
Table 1.
Periodontal therapy and clinical periodontal variables before (T1) and after treatment (T4).
| Variable | Mean | Standard Deviation | Range |
|---|---|---|---|
| # of treatment visits | 6.1 | 1.1 | 4 – 9 |
| # of surgical sessions | 3.9 | 0.6 | 2 – 5 |
| # of extractions | 2.8 | 2.9 | 0 – 12 |
| Pre-treatment PD (mm) | 4.5 | 0.9 | 3.2 – 6.7 |
| Post-treatment PD (mm) | 2.7 | 0.5 | 2.0 – 3.5 |
| Pre-treatment CAL (mm) | 5.0 | 1.3 | 3.4 – 8.2 |
| Post-treatment CAL (mm) | 4.4 | 1.1 | 2.7 – 7.3 |
| Pre-treatment # of sites with PD ≥ 6 mm | 79.7 | 30.5 | 36 – 156 |
| Post-treatment # of sites with PD ≥ 6 mm | 11.6 | 8.8 | 0 – 39 |
| Pre-treatment % BOP | 82.6 | 14.7 | 39 – 100 |
| Post-treatment % BOP | 26.5 | 9.8 | 7 – 46 |
| Pre-treatment % Plaque | 96.3 | 4.4 | 86–100 |
| Post-treatment % Plaque | 71.4 | 8.8 | 56–100 |
PD = probing depth; CAL = clinical attachment level
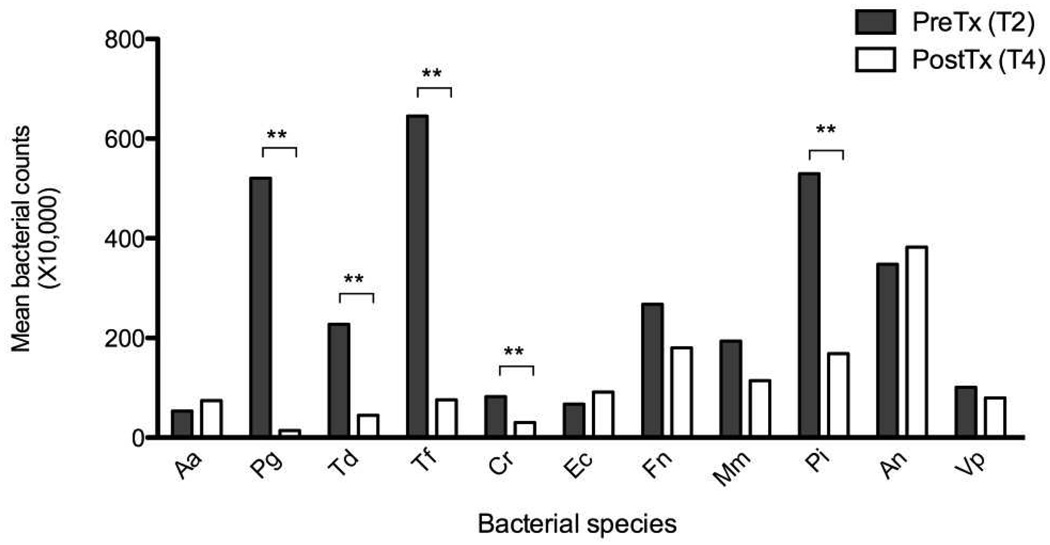
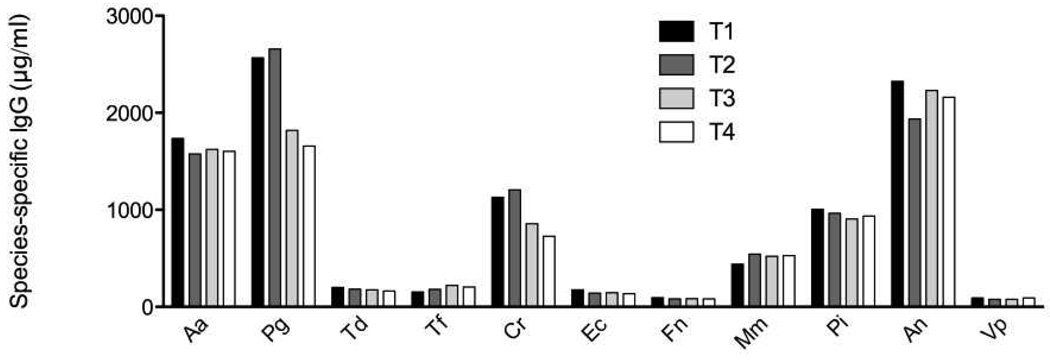
Figure 2 shows average subgingival bacterial levels for individual species at T2 and T4. Therapy resulted in a substantial and statistically significant (p<0.01) reduction in the levels of several periodontal pathogens including P. gingivalis, T. denticola, P. intermedia, T. forsythia and C. rectus. In contrast, no statistically significant differences in serum IgG levels were noted at any time point and for any titer examined (Figure 3).
Figure 2.
Subgingival bacterial levels before initiation and after completion of periodontal therapy. ** denotes p<0.01.
Figure 3.
Serum IgG antibody levels at the four blood sampling occasions (T1 and T2 pre- treatment; T3 and T4 post-treatment). No statistically significant differences were noted.
Table 2 summarizes the levels of the 19 biomarkers as well as the Summary Inflammatory Scores for Baseline (average of T1 and T2), immediately after completion of active therapy (T3) and four weeks thereafter (T4). The number of observations available for comparisons over time is lower than 30, as (i) one patient missed the T3 visit; (ii) all samples from one patient were not possible to process due to excessive clogging caused by high lipid content, and (iii) additional non-readable values were generated for a few sample/analyte combinations. A number of statistically significant changes at T3 compared to baseline, all of them representing a reduction in mediator levels, were noted for the following analytes: sE-selectin, MMP-9, MPO, PAI-1, and sVCAM-1. However, at T4 only the levels of sE-selectin remained statistically significantly lower than baseline values, while two other analytes whose levels were not found to be significantly reduced at T3 (sICAM-1 and SAP) were suppressed at T4. Reflecting the overall inflammatory responses across the 19 mediators, SIS was significantly lower at T3, but did not sustain its statistical significance difference from baseline at T4.
Table 2.
Serum biomarker levels at Baseline (average of T1 and T2), T3 and T4.
| Baseline | T3 | T4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biomarker | n | Mean | SE | Median | Mean | SE | Median | Mean | SE | Median |
| CRP (mg/l) | 28 | 6.7 | (1.5) | 3.2 | 6.9 | (1.6) | 3.3 | 6.0 | (1.7) | 2.3 |
| PAI-1 (ng/ml) | 28 | 45.2 | (4.6) | 43.3 | 40.0** | (4.6) | 32.9 | 45.0 | (4.9) | 34.4 |
| SAA (µg/ml) | 25 | 9.4 | (2.4) | 4.9 | 9.0 | (2.6) | 4.7 | 9.6 | (3.4) | 3.8 |
| SAP (µg/ml) | 25 | 35.6 | (3.0) | 35.4 | 36.2 | (3.1) | 35.4 | 30.2** | (2.3) | 27.0 |
| sE-selectin (ng/ml) | 28 | 38.1 | (7.1) | 30.2 | 29.2** | (3.2) | 26.5 | 29.6** | (3.1) | 24.1 |
| sICAM-1 (ng/ml) | 28 | 213.4 | (15.6) | 209.3 | 204.0 | (17.5) | 201.0 | 199.8* | (15.4) | 185.4 |
| sVCAM-1 (µg/ml) | 28 | 1.1 | (0.1) | 1.1 | 1.0** | (0.0) | 1.0 | 1.1 | (0.1) | 1.1 |
| MPO (ng/ml) | 28 | 90.5 | (12.1) | 69.5 | 45.8** | (6.1) | 34.3 | 103.7 | (17.3) | 54.7 |
| MMP-9 (ng/ml) | 28 | 246.4 | (28.4) | 191.1 | 146.7* | (10.7) | 139.5 | 240.0 | (31.3) | 185.2 |
| TNF-a (pg/ml) | 27 | 4.4 | (0.4) | 4.3 | 4.1 | (0.4) | 4.0 | 5.5 | (1.3) | 4.2 |
| IL-1β (pg/ml) | 27 | 0.9 | (0.2) | 0.3 | 1.1 | (0.3) | 0.4 | 1.1 | (0.3) | 0.4 |
| IL-2 (pg/ml) | 26 | 6.1 | (1.4) | 3.2 | 6.6 | (1.6) | 2.6 | 6.4 | (1.5) | 3.0 |
| IL-4 (pg/ml) | 25 | 104.8 | (27.4) | 41.2 | 85.4 | (26.4) | 34.7 | 108.0 | (30.5) | 33.7 |
| IL-5 (pg/ml) | 27 | 1.7 | (0.4) | 1.1 | 1.6 | (0.4) | 1.0 | 1.5 | (0.4) | 1.1 |
| IL-6 (pg/ml) | 27 | 19.3 | (5.3) | 8.8 | 21.0 | (6.6) | 10.5 | 16.4 | (4.0) | 9.4 |
| IL-7 (pg/ml) | 27 | 10.1 | (1.1) | 10.1 | 11.6 | (1.4) | 9.6 | 9.4 | (1.1) | 8.6 |
| IL-8 (pg/ml) | 27 | 12.0 | (2.1) | 7.2 | 15.6 | (4.4) | 7.5 | 14.1 | (4.9) | 7.4 |
| IL-10 (pg/ml) | 27 | 15.5 | (2.9) | 8.6 | 16.3 | (3.0) | 8.9 | 16.4 | (3.1) | 12.2 |
| Adiponectin (µg/ml) | 28 | 14.7 | (1.9) | 10.6 | 15.3 | (2.0) | 11.8 | 15.4 | (2.3) | 11.8 |
| SIS | 28 | 0.1 | (0.1) | 0.1 | −0.2** | (0.1) | −0.3 | 0.0 | (0.1) | 0.1 |
* Significant changes compared to Baseline are indicated by * (p<0.05) and ** (p<0.01)
n describes the number of patient samples available for comparison between Baseline and T3 or T4.
T1: One week prior to initiation of periodontal therapy
T2: Therapy initiation
T3: Completion of therapy, 6 weeks after T2
T4: 4 weeks after T3
For biomarkers abbreviations, see Materials and Methods.
SIS: Summary Inflammatory Score
Further exploring the variability in serum inflammatory responses, Table 3 describes changes in biomarker levels at T4, expressed as a percent of their average pre-treatment levels (T1 and T2). The median % change in each biomarker (negative sign indicates a decrease), the range of responses, and the % of patients that experienced a ≥25% increase or decrease of their average levels are listed. Depending on the biomarker, between 19% and 96% of the subjects experienced changes within ±25% of their baseline values, but the range of the individual patient responses was substantial. With respect to specific biomarkers, 43% of the patients experienced a ≥25% reduction in CRP and MPO levels, 36% in MMP-9, 32% in SAA and SAP, and 29% in sE-selectin.
Table 3.
Changes in serum biomarkers at T4, expressed as % of the average pre-treatment values (T1 and T2).
| Biomarker | n | Median change | Min change |
Max change |
% of pts with ≥ 25% increase |
% of pts with ≥ 25% decrease |
|---|---|---|---|---|---|---|
| CRP (mg/l) | 28 | −23% | −76% | 714% | 14% | 43% |
| tPAI-1 (ng/ml) | 28 | 0% | −41% | 71% | 18% | 14% |
| SAA (µg/ml) | 25 | −10% | −82% | 619% | 16% | 32% |
| SAP (µg/ml) | 25 | −16% | −47% | 151% | 4% | 32% |
| sE-selectin (ng/ml) | 28 | −16% | −1% | 39% | 7% | 29% |
| sICAM-1 (ng/ml) | 28 | −7% | −43% | 23% | 0% | 4% |
| sVCAM-1 (µg/ml) | 28 | −4% | −28% | 57% | 4% | 7% |
| MPO (ng/ml) | 28 | −17% | −65% | 345% | 32% | 43% |
| MMP-9 (ng/ml) | 28 | −11% | −86% | 999% | 25% | 36% |
| TNF-a (pg/ml) | 27 | 3% | −86% | 848% | 22% | 15% |
| IL-1β (pg/ml) | 27 | 10% | −100% | 355% | 36% | 24% |
| IL-2 (pg/ml) | 26 | 6% | −100% | 243% | 33% | 24% |
| IL-4 (pg/ml) | 25 | −7% | −100% | 439% | 25% | 30% |
| IL-5 (pg/ml) | 27 | 0% | −100% | 667% | 29% | 33% |
| IL-6 (pg/ml) | 27 | −0.5% | −89% | 228% | 44% | 26% |
| IL-7 (pg/ml) | 27 | −10% | −90% | 179% | 37% | 44% |
| IL-8 (pg/ml) | 27 | 9% | −91% | 1291% | 33% | 30% |
| IL-10 (pg/ml) | 27 | −2.1% | −100% | 208% | 23% | 38% |
| Adiponectin (µg/ml) | 28 | 0% | −48% | 76% | 25% | 14% |
T1: One week prior to initiation of periodontal therapy
T2: Therapy initiation
T4: 10 weeks after T2
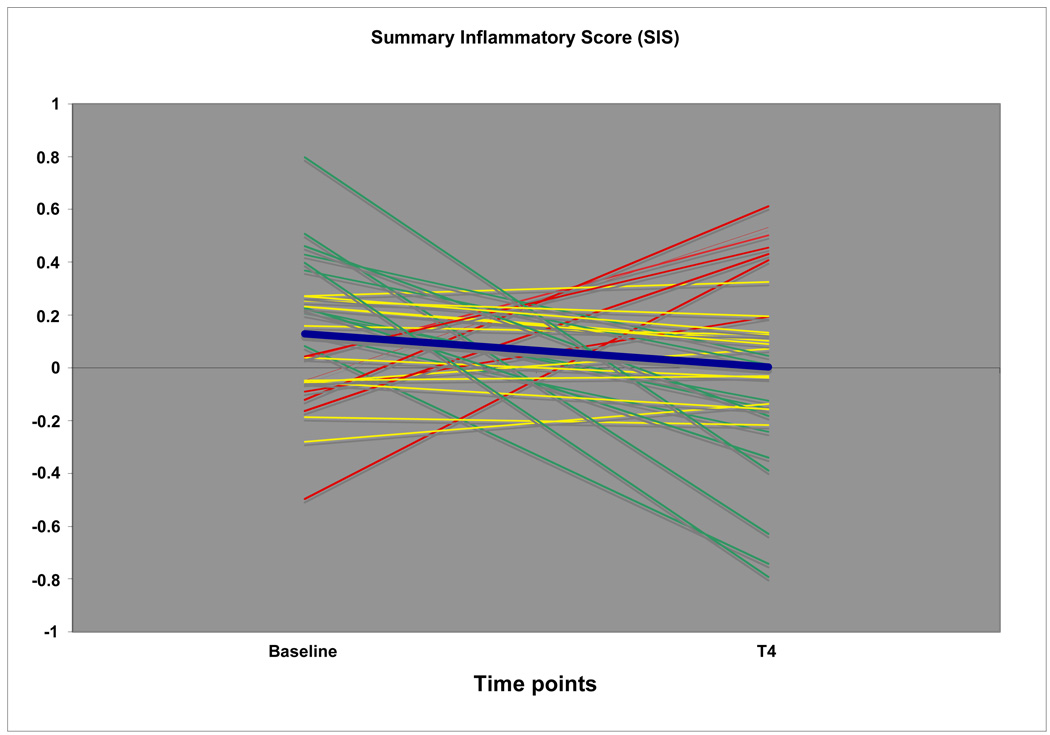
Lastly, to summarize serum biomarker responses after therapy on the individual subject level, we used the standardized Summary Inflammatory Scores (SIS) to explore whether an overall pro- or anti-inflammatory response to therapy was evident among subgroups of subjects. Figure 4 illustrates that 7 patients experienced a marked increase in SIS between baseline and T4, 11 patients a substantial reduction, while the remainder patients were seemingly unchanged.
Figure 4.
Graphic illustration of Summary Inflammatory Scores at Baseline (average of T1 and T2) and T4. Red lines represent patients with markedly increased overall levels of systemic inflammation after therapy, green lines patients with reduced levels of inflammation, and yellow lines patients with seemingly stable levels. The blue line denotes the average SIS response in the entire patient group.
Regression models
In regression analysis, only a few statistically significant correlations between changes in individual mediator levels and clinical, bacterial and serological markers were detected. Clinical improvements in probing depth and BoP were positively associated with reductions in levels of IL-2 (p=0.02) and IL-7 (p=0.05). Changes in subgingival bacterial levels showed an inconsistent association with mediator level changes. P. gingivalis reduction correlated positively to IL-1β (p=0.02), IL-2 (p=0.01) and IL-7 (p=0.003) changes. Similar positive correlations for the same analytes (p=0.04 for all) were noted for T. forsythia. In contrast, A. actinomycetemcomitans reduction related inversely to sICAM-1 (p=0.02) and sVCAM-1 changes (p=0.04), and T. denticola reduction related inversely to sVCAM-1 and positively to IL-7 changes (p=0.02 for both). Changes in IgG antibody levels were not statistically related to any changes in biomarker levels.
DISCUSSION
We used a single arm intervention study design to investigate the effects of comprehensive periodontal therapy on serum markers of systemic inflammation. Our findings suggest that therapy elicits highly heterogeneous systemic inflammatory responses that do not correlate readily with any periodontal clinical, microbiological or serological outcomes.
A number of features of the study design need to be recognized to correctly interpret the present results. Strengths of the study include (i) the simultaneous assessment of multiple markers of systemic inflammation that allowed the computation of an overall, composite inflammatory score (SIS), (ii) the double assessment of the primary outcome variables, i.e., the levels of serum inflammatory mediators, before the initiation of periodontal therapy to partly account for temporal biological variation, and their assessment on two post-treatment time points; (iii) the availability of microbial and serological markers of periodontitis before and after therapy, in addition to clinical variables; and (iv) the standardization of the timing of treatment within the available window of 6 weeks. On the other hand, the study (i) lacks an untreated control group, therefore, the observed differences in serum mediators cannot be unequivocally ascribed to periodontal therapy in their entirety, but may be partly due to a Hawthorn effect or to seasonal variations, (ii) has a limited sample size, and (iii) only provides data on the short-term effects of periodontal therapy, as it covers a 4-week post-therapy time period during which the maturation of the periodontal tissues may still be ongoing. Although our treatment protocol may be considered as relatively unconventional, in as much as all periodontal therapy including periodontal surgery was completed within a relatively short time (6 weeks), it is arguably closer to everyday clinical periodontal practice than previously employed approaches in the study of the systemic effects of periodontal therapy, such as the single appointment full-mouth debridement protocol (Tonetti et al. 2007), or the 2-week full-mouth surgical intervention protocol (Elter et al. 2006). Lastly, although the clinical periodontal conditions improved substantially as a result of therapy in the entire patient cohort, dental plaque rebounded to unacceptably high levels at the last visit (Table 1), and this fact may have influenced the degree of resolution of systemic inflammation achieved between time points T3 and T4. It is also noteworthy that the average CRP level of the study participants prior to treatment was high (Table 2).
The key finding of our study is the considerable inter-patient variability in both the baseline and the post-treatment concentrations for most of the inflammatory markers examined. The same lack of uniformity was reflected when comparisons were based on individual SIS across patients (Fig. 4). Thus, approximately one third of the patients showed a substantial reduction in their aggregate inflammatory scores, one fourth showed a marked increase and the remainder patients were seemingly unchanged. This variability in responses is in accordance with our previously published observations (Lalla et al. 2007, Papapanou et al. 2007), but is also evident in the data from other research groups (D'Aiuto et al. 2004, D'Aiuto et al. 2005, D'Aiuto et al. 2007). Interestingly, regression analyses showed that the changes in inflammatory mediator levels correlated poorly and inconsistently with the change in clinical periodontal status after therapy, the reduction in established or putative periodontal pathogens and with serum IgG antibody levels to periodontal microbiota. Thus, it appears that the broadly defined periodontal characteristics of the patients assessed in this study are clearly not the sole determinants of the systemic inflammatory changes after periodontal therapy. It is tempting to speculate that a pre-existing susceptibility for systemic inflammation, possibly unrelated to the severity of the periodontal infection, may govern the dynamics of these responses. Additional research is required to dissect the determinants of the unexplained variance, and pre-existing atherosclerotic status may be a reasonable candidate property to investigate in this context.
The temporality of the observed effects on specific mediators deserves attention. While statistically significant reductions were observed for sE-selectin, MMP-9, MPO, tPAI-1 and sVCAM-1 immediately after completion of therapy, these levels were not sustainable until the last examination time point and only the levels of sE-selectin, sICAM-1 and SAP were statistically suppressed four weeks after completion of treatment. As mentioned above, the failure of the patients to maintain a proper plaque control between T3 and T4 may partly underlie these observations. Similarly, although approximately half of the patients experienced a ≥ 25% reduction in their CRP levels between baseline and T4 (Table 3), no statistically significant reduction was achieved at either time point. This outcome conflicts with the findings of the most recent meta-analysis available, which concluded that a significant improvement in CRP levels occurs after periodontal therapy (Paraskevas et al. 2008). However, another liver-produced acute phase reactant, SAP, which accelerates accumulation of macrophages and inhibits phagocytosis of amyloid fibers in atherosclerotic lesion (Stewart et al. 2007) was statistically reduced at the last visit, and approximately a third of the patients experienced a ≥ 25% reduction in comparison to their baseline levels.
Interestingly, levels of serum sE-Selectin have been consistently shown to be reduced after periodontal therapy in multiple studies (D'Aiuto et al. 2007, Lalla et al. 2007, Pischon et al. 2007, Tonetti et al. 2007). These findings are of particular interest as E-selectin is expressed exclusively by endothelial cells (Bevilacqua 1993), and the uniformly observed reduction in sE-selectin levels appears to demonstrate a quantifiable effect of periodontal therapy on vascular endothelial activation. A recent randomized clinical trial that demonstrated an increase in endothelium-mediated dilatation 2 and 6 months after completion of periodontal therapy points to the same direction (Tonetti et al. 2007).
Additional markers that were found to be statistically significantly reduced immediately post-therapy have all been reported to play a role in atherogenesis. MPO, a reactive oxidant species produced by neutrophils whose oxidant products are capable of modifying low-density lipoprotein cholesterol, has been shown to be present in human atheromas and instable plaques (Nicholls & Hazen 2005). PAI-1, a highly pro-thrombotic marker of fibrinolysis which has been associated with risk for CVD, was also shown to be elevated in severe periodontitis (Kannel 2005, Bizzarro et al. 2007). VCAM and ICAM, both involved in leukocyte adhesion to vessel walls, have been cross-sectionally associated with CHD, although their prospective association with CHD events is questionable (Malik et al. 2001).
It must be recognized that failure to detect statistically significant differences in the levels of specific inflammatory biomarkers after therapy may be partly attributed to high biological variance which reduced our power to detect treatment effects. Given the association between systemic inflammation and atherogenesis, and the potential of periodontal therapy to promote an anti-atherogenic phenotype, it is essential to further investigate the determinants of these heterogeneous responses in future studies. This may allow the identification of patient subgroups that are most amenable to systemic anti-inflammatory effects after periodontal therapy.
ACKNOWLEDGEMENT
The authors are indebted to Elena Schartz, RDH, Division of Periodontics, Section of Oral and Diagnostic Sciences, Columbia University College of Dental Medicine, for examining the patients.
CONFLICT OF INTEREST & SOURCE OF FUNDING
This study was supported by NIH grant DE015649 (National Institutes of Health, Bethesda, Maryland, USA) and a gift from Colgate-Palmolive, NJ, USA to Dr. Papapanou.
Dr. Demmer was supported by NIH grant K99 DE-018739. Dr. Kebschull was partly supported by a stipend from Neue Gruppe Wissenschaftsstiftung, Wangen/Allgäu, Germany, and the 2008 IADR/Philips Oral Healthcare Young Investigator Research Grant.
Footnotes
The authors declare that they have no conflicts of interest.
REFERENCES
- Consensus report: Periodontitis as a manifestation of systemic diseases. Annals of Periodontology. 1999;4:64. [Google Scholar]
- Beck JD, Offenbacher S. Systemic effects of periodontitis: Epidemiology of periodontal disease and cardiovascular disease. J Periodontol. 2005;76:2089–2100. doi: 10.1902/jop.2005.76.11-S.2089. [DOI] [PubMed] [Google Scholar]
- Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- Bizzarro S, van der Velden U, ten Heggeler JM, Leivadaros E, Hoek FJ, Gerdes VE, Bakker SJ, Gans RO, Ten Cate H, Loos BG. Periodontitis is characterized by elevated PAI-1 activity. J Clin Periodontol. 2007;34:574–580. doi: 10.1111/j.1600-051X.2007.01095.x. [DOI] [PubMed] [Google Scholar]
- Boucher NE, Jr, Hanrahan JJ, Kihara FY. Occurrence of C-reactive protein in oral disease. J Dent Res. 1967;46:624. doi: 10.1177/00220345670460033001. [DOI] [PubMed] [Google Scholar]
- D'Aiuto F, Parkar M, Andreou G, Suvan J, Brett PM, Ready D, Tonetti MS. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004;83:156–160. doi: 10.1177/154405910408300214. [DOI] [PubMed] [Google Scholar]
- D'Aiuto F, Nibali L, Parkar M, Suvan J, Tonetti MS. Short-term effects of intensive periodontal therapy on serum inflammatory markers and cholesterol. J Dent Res. 2005;84:269–273. doi: 10.1177/154405910508400312. [DOI] [PubMed] [Google Scholar]
- D'Aiuto F, Parkar M, Tonetti MS. Acute effects of periodontal therapy on bio-markers of vascular health. J Clin Periodontol. 2007;34:124–129. doi: 10.1111/j.1600-051X.2006.01037.x. [DOI] [PubMed] [Google Scholar]
- Ebersole JL, Machen RL, Steffen MJ, Willmann DE. Systemic acute-phase reactants, C-reactive protein and haptoglobin, in adult periodontitis. Clin Exp Immunol. 1997;107:347–352. doi: 10.1111/j.1365-2249.1997.270-ce1162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elter JR, Hinderliter AL, Offenbacher S, Beck JD, Caughey M, Brodala N, Madianos PN. The effects of periodontal therapy on vascular endothelial function: a pilot trial. Am Heart J. 2006;151:47. doi: 10.1016/j.ahj.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Haffajee AD, Socransky SS. (1994) Microbial etiological agents of destructive periodontal diseases. Periodontol. 2000;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Ide M, Jagdev D, Coward PY, Crook M, Barclay GR, Wilson RF. The short-term effects of treatment of chronic periodontitis on circulating levels of endotoxin, C-reactive protein, tumor necrosis factor-alpha, and interleukin-6. J Periodontol. 2004;75:420–428. doi: 10.1902/jop.2004.75.3.420. [DOI] [PubMed] [Google Scholar]
- Kannel WB. Overview of hemostatic factors involved in atherosclerotic cardiovascular disease. Lipids. 2005;40:1215–1220. doi: 10.1007/s11745-005-1488-8. [DOI] [PubMed] [Google Scholar]
- Khlgatian M, Nassar H, Chou HH, Gibson FC, 3rd, Genco CA. Fimbria-dependent activation of cell adhesion molecule expression in Porphyromonas gingivalis-infected endothelial cells. Infect Immun. 2002;70:257–267. doi: 10.1128/IAI.70.1.257-267.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalla E, Kaplan S, Yang J, Roth GA, Papapanou PN, Greenberg S. Effects of periodontal therapy on serum C-reactive protein, sE-selectin, and tumor necrosis factor-alpha secretion by peripheral blood-derived macrophages in diabetes. A pilot study. J Periodontal Res. 2007;42:274–282. doi: 10.1111/j.1600-0765.2006.00945.x. [DOI] [PubMed] [Google Scholar]
- Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PM, van der Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol. 2000;71:1528–1534. doi: 10.1902/jop.2000.71.10.1528. [DOI] [PubMed] [Google Scholar]
- Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76:2106–2115. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- Malik I, Danesh J, Whincup P, Bhatia V, Papacosta O, Walker M, Lennon L, Thomson A, Haskard D. Soluble adhesion molecules and prediction o coronary heart disease: a prospective study and meta-analysis. Lancet. 2001;358:971–976. doi: 10.1016/S0140-6736(01)06104-9. [DOI] [PubMed] [Google Scholar]
- Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol. 2001;72:1221–1227. doi: 10.1902/jop.2000.72.9.1221. [DOI] [PubMed] [Google Scholar]
- Papapanou PN, Neiderud A-M, Papadimitriou A, Sandros J, Dahlén G. "Checkerboard" assessments of periodontal microbiota and serum antibody responses: A case-control study. J Periodontol. 2000;71:885–897. doi: 10.1902/jop.2000.71.6.885. [DOI] [PubMed] [Google Scholar]
- Papapanou PN, Neiderud AM, Disick E, Lalla E, Miller GC, Dahlen G. Longitudinal stability of serum immunoglobulin G responses to periodontal bacteria. J Clin Periodontol. 2004;31:985–990. doi: 10.1111/j.1600-051X.2004.00599.x. [DOI] [PubMed] [Google Scholar]
- Papapanou PN, Sedaghatfar MH, Demmer RT, Wolf DL, Yang J, Roth GA, Celenti R, Belusko PB, Lalla E, Pavlidis P. Periodontal therapy alters gene expression of peripheral blood monocytes. J Clin Periodontol. 2007;34:736–747. doi: 10.1111/j.1600-051X.2007.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol. 2008;35:277–290. doi: 10.1111/j.1600-051X.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- Picolos DK, Lerche-Sehm J, Abron A, Fine JB, Papapanou PN. Infection patterns in chronic and aggressive periodontitis. J Clin Periodontol. 2005;32:1055–1061. doi: 10.1111/j.1600-051X.2005.00828.x. [DOI] [PubMed] [Google Scholar]
- Pischon N, Hagewald S, Kunze M, Heng N, Christan C, Kleber BM, Muller C, Bernimoulin JP. Influence of periodontal therapy on the regulation of soluble cell adhesion molecule expression in aggressive periodontitis patients. J Periodontol. 2007;78:683–690. doi: 10.1902/jop.2007.060286. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Roth GA, Moser B, Huang SJ, Brandt JS, Huang Y, Papapanou PN, Schmidt AM, Lalla E. Infection with a periodontal pathogen induces procoagulant effects in human aortic endothelial cells. J Thromb Haemost. 2006;4:2256–2261. doi: 10.1111/j.1538-7836.2006.02128.x. [DOI] [PubMed] [Google Scholar]
- Roth GA, Moser B, Roth-Walter F, Giacona MB, Harja E, Papapanou PN, Schmidt AM, Lalla E. Infection with a periodontal pathogen increases mononuclear cell adhesion to human aortic endothelial cells. Atherosclerosis. 2007;190:271–281. doi: 10.1016/j.atherosclerosis.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Sakellari D, Socransky SS, Dibart S, Eftimiadi C, Taubman MA. Estimation of serum antibody to subgingival species using checkerboard immunoblotting. Oral Microbiol Immunol. 1997;12:303–310. doi: 10.1111/j.1399-302x.1997.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Shklair IL, Loving RH, Leberman OF, Rau CF. C-Reactive protein and periodontal disease. J Periodontol. 1968;39:93–95. doi: 10.1902/jop.1968.39.2.93. [DOI] [PubMed] [Google Scholar]
- Stewart CR, Haw A, 3rd, Lopez R, McDonald TO, Callaghan JM, McConville MJ, Moore KJ, Howlett GJ, O'Brien KD. Serum amyloid P colocalizes with apolipoproteins in human atheroma: functional implications. J Lipid Res. 2007;48:2162–2171. doi: 10.1194/jlr.M700098-JLR200. [DOI] [PubMed] [Google Scholar]
- Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Honda T, Oda T, Ueki-Maruyama K, Nakajima T, Yoshie H, Seymour GJ. Effect of periodontal treatment on the C-reactive protein and proinflammatory cytokine levels in Japanese periodontitis patients. J Periodontal Res. 2005;40:53–58. doi: 10.1111/j.1600-0765.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- Zebrack JS, Anderson JL. The role of infection in the pathogenesis of cardiovascular disease. Prog Cardiovasc Nurs. 2003;18:42–49. doi: 10.1111/j.0889-7204.2003.01421.x. [DOI] [PubMed] [Google Scholar]