Abstract
The monoclonal antibody C225 interacts with the ectodomain of the EGF receptor to block ligand binding and initiates receptor endocytosis and intracellular trafficking. The data herein show that C225-dependent EGF receptor trafficking relocalizes the receptor to the endoplasmic reticulum (ER) and nucleus. This mechanism, which also involves interaction of the C225-internalized receptor with the Sec61 translocon within the endoplasmic reticulum (ER), is, in most respect, analogous to the pathway previously described for EGF-induced trafficking to the ER and nucleus (Liao HJ and Carpenter G. Mol Biol Cell 2007; 18: 1064-1072), However, while inhibition of receptor tyrosine kinase activity blocks EGF-induced nuclear localization of the receptor, the same kinase inhibitors stimulate C225-dependent nuclear localization of EGF receptor in the nucleus. In contrast, the kinase inhibitor Lapatinib fails to stimulate nuclear accumulation of the receptor in C225-treated cells and does not provoke receptor dimerization as do inhibitors that recognizing the open conformation of the receptor kinase. This suggests that inhibitor-dependent receptor dimerization may facilitate C225-induced receptor trafficking.
INTRODUCTION
Agents that prevent the activation of the EGF receptor and ErbB-2 receptor tyrosine kinases are prominent in current clinical practice and trials. Among these is the C225 monoclonal antibody (Cetuximab, Erbitux©) that blocks growth factor binding to EGF receptor (1, 2). Crystallographic analysis demonstrates that the antibody binding site overlaps the ligand binding site (3). This reagent is approved for the treatment of colon and head and neck tumors and is in clinical trials for other cancers (4). In many tumor cell lines, C225 provokes growth arrest (5–11), while in a few, cell death is induced (12, 13). Whether these responses are mediated by the antibody’s capacity to interact with the EGF receptor ligand binding site is unclear. The binding of C225 to the ectodomain of EGF receptor does not provoke a significant level of receptor tyrosine phosphorylation, but does bring about receptor internalization by an uncertain route (14, 15). The internalized receptor is not extensively processed to the lysosome, but rather is recycled to the cell surface (16). Whether the bound antibody is also recycled is not known. Also, it is not known whether antibody-induced trafficking of the receptor is related to the antibody’s biologic activity.
EGF provokes nuclear localization of full-length EGF receptor (17) and a novel intracellular trafficking pathway has been identified for this intracellular destination (18). This pathway involves sorting of the internalized cell surface receptor to the endoplasmic reticulum (ER) and its interaction with the Sec61 translocon, which facilitates bidirectional movement of proteins, including transmembrane proteins, between the cytoplasm and the ER. The Sec61 complex is able to retrotranslocate the mature EGF receptor from the ER to the cytosol, as a prerequisite for receptor translocation to the nucleus (18). This pathway is required for EGF to induce cyclin D and therefore constitutes a signal transduction pathway (17).
In this manuscript we present an evaluation of the capacity of C225 to induce intracellular translocation of EGF receptor to the ER, its interaction with the Sec61 trafficking pathway, and nuclear localization.
MATERIALS AND METHODS
Materials
Dulbecco’s Modified Eagle’s Medium (DMEM) containing L-glutamine and high glucose, Ham’s F-12 medium and fetal bovine serum (FBS) were purchased from Life Technologies, Inc. Human breast cancer cell line MDA-MB-468 from ATCC. Recombinant human EGF was obtained from R & D Systems, Inc. DiFi cells, C225 and 528 antibodies were the gifts from Dr. Robert Coffey, Vanderbilt University, Nashville, TN. Mouse monoclonal antibody 455 was from Oncogene. Fab fragments of C225 were generously provided by Dr. Carlos Arteaga, Vanderbilt University, Nashville, TN. EGFR kinase inhibitor AG 1478 was from Calbiochem. Lipofectamine 2000 reagent was from Invitrogen. Antibodies to EGF receptor and Sec61β were from Upstate, Inc. Antibody to HDAC was from Santa Cruz Biotechnology, Inc. The pDsRed2-ER construct (calreticulin~RFP) was from Clontech. The EGFR~mGFP construct was previously described (18). Lapatinib was a generous gift obtained from Drs. William Bronnann and Ashotosh Pal, MD Anderson Cancer Center, Houston, TX.
Cell culture and treatment
MDA-MB-468 cells were cultured in DMEM containing 10% FBS. DiFi cells were maintained in a mixture of DMEM and Ham’s F-12 medium (1:1, v/v) with 10% FBS. Cultures were incubated in a 5% CO2 humidified atmosphere. 40–50% confluent cells were incubated for overnight in DMEM (for MDA-MB-468) or DMEM and F-12 (1:1 v/v) plus 0.5% FBS prior to treatment with EGF (4 nM) or C225 (5nM) for the indicated times.
Preparation of nuclear extracts and SDS lysates
The nuclear fractionation protocol was described previously (17, 18). Briefly, cells in a 10 cm dish were rinsed twice with ice-cold PBS and removed with a rubber cell scraper in 1 ml Buffer A (10 mM HEPES pH 7.5, 10 mM KCl, 2 mM MgCl2, protease inhibitor tablet with EDTA at 1 tablet/10 ml) containing 1% NP-40. Cells were disrupted by 10 passesthrough a 21 gauge needle and the extent of nuclear isolation was monitored microscopically. Nuclei were centrifuged (500 × g, 5 min) and washed once with Buffer A. The resulting supernatant was designated as the non-nuclear fraction. The nuclear pellet was resuspended in 50 μl Buffer A supplemented with 500 mM NaCl and 25% glycerol, and kept on ice for 30 min. Samples were centrifuged (12,000 × g, 5 min), and the supernatant (nuclear extracts) was aliquoted and frozen at -80°C. The pellet (SDS lysate) was solubilized in 1 x SDS-PAGE sample loading buffer.
Co-precipitation and western blotting
Cells were lysed in cold Buffer A containing 1 % NP-40 and incubated for 30 min on ice. After centrifugation (12,000 x g, 5 min), Sec61β antibody and Protein A beads were added to the supernatant and incubated overnight. The precipitate was then washed three times with Buffer A. After SDS-PAGE and transfer to nitrocellulose membranes, the samples were probed with the indicated antibody. For western blots, cell lysates were subjected to SDS-PAGE, transferred to nitrocellulose membranes, and probed with the indicated antibody. Bound antibody was detected by enhanced chemiluminescence (ECL).
Confocal microscopy
MDA-MB-468 cells were co-transfected with pEGFR~mGFP and pDsRed2-ER DNA (Clontech) using Lipofectamine 2000 according to the manufacturer instruction. The cells were subcultured (1:1 split) 24 hrs after transfection and placed into normal culture medium for 24 hrs. Cells were serum starved overnight and incubated with or without C225 (5 nM) for the indicated time. Cells were imaged with a Zeiss LSM510 confocal scanning microscope and a Plan-Neofluar 40 x 1.3 NA oil immersion lens was used for imaging all the samples with a 1μm optical slice. GFP was excited with an argon laser with excitation at 488 nm and RFP was excited at a 543 nm. The emission was detected with filter sets (505–550 band pass for GFP and 560 long pass for RFP). Image analysis was performed using Metamorph software (Universal Imaging Corp). Line intensity scan was used to show co-localization of GFP and RFP.
RESULTS
C225-induced nuclear localization of EGF receptor
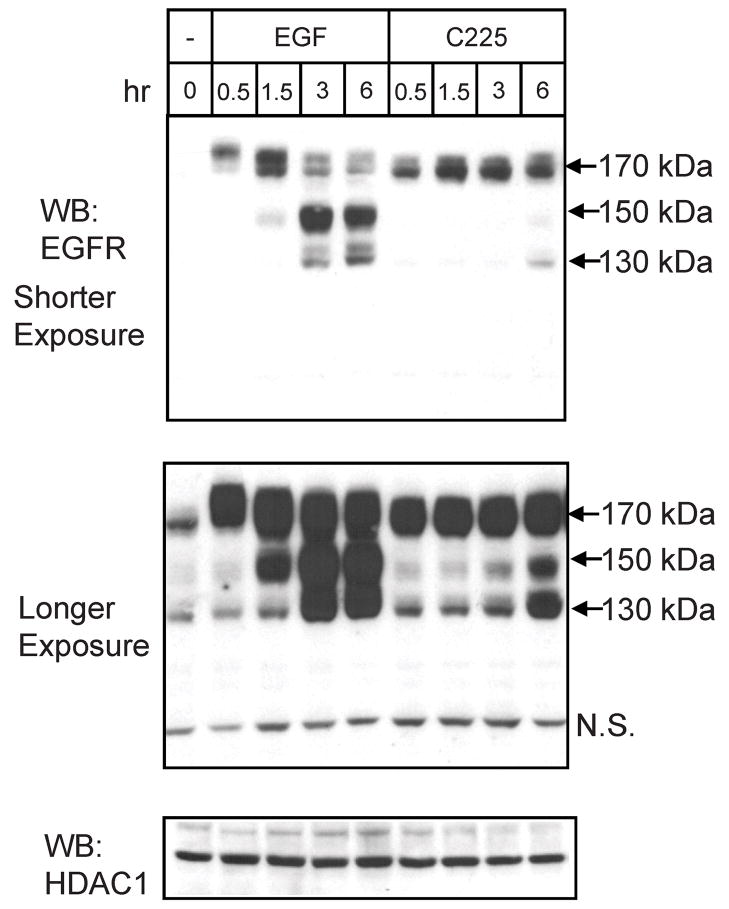
To assess the capacity of C225 to provoke translocation of EGF receptor to the nucleus, the experiment described in Figure 1 was performed. MDA-MB-468 cells were incubated with EGF or C225 for increasing periods of time. Cell fractionation was used to prepare a nuclear fraction and this was extracted with high-salt to release non-membrane and non-chromatin bound molecules (17). EGF induced translocation of receptor to the salt-extracted nuclear fraction with the appearance of intact receptor together with 150 kDa and 130 kDa degradation products, which have previously been reported to represent the loss of ectodomain residues (18). Incubation with C225 induced a similar time course of receptor translocation to the salt-extracted nuclear fraction, but with a substantially decreased level of the lower molecular mass fragments and increased retention of the native 170 kDa receptor species. Upon longer exposure (Figure 1, middle panel) it is clear that the similar receptor fragments are produced following treatment with C225, but at a markedly lower level compared to EGF. Exposure of MDA-MB-468 cells to C225 for three hours does not alter the total level of EGF receptor (Supplementary Figure 1).
Figure 1.
EGF and C225-induced EGFR translocation to the nucleus. MDA-MB-468 cells were incubated with EGF or C225 for the indicated times. High-salt nuclear extracts were blotted with anti-EGFR and reblotted with the nuclear maker HDAC1, as a loading control. Arrows mark the 170 kDa mature EGF receptor and the 150, 130 kDa fragments. N.S. identifies a non-specific band.
To determine whether the capacity of C225 to provoke nuclear localization of the EGF receptor was specific to that antibody, two other antibodies to the EGF receptor plus a Fab fragment of C225 were tested. The results are presented in Figure 2 and indicate that antibodies C225 or 528, but not antibody 445 or the Fab C225, induce substantial levels of nuclear EGF receptor. Interestingly, both C225 and 528 block EGF binding to EGF receptor (2) and it is clear from structural data that C225 directly contacts the ligand binding site (3). Antibody 445, which does recognize the EGF receptor ectodomain, does not block ligand binding (2). The results with C225 Fab indicate that antibody bivalence is necessary for the C225 effect on EGF receptor trafficking.
Figure 2.
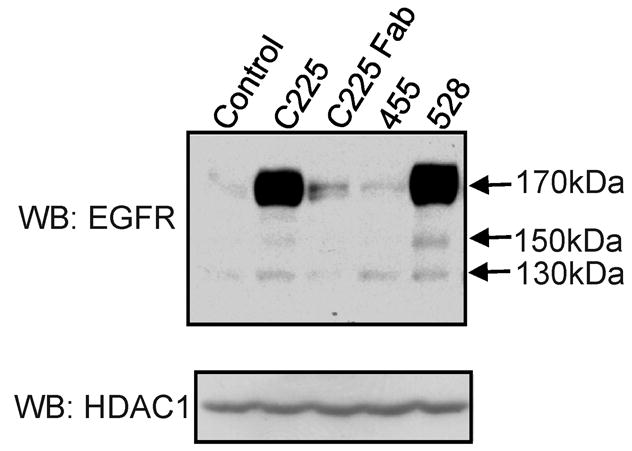
Capacity of different antibodies to induce EGFR translocation to the nucleus. MDA-MB-468 cells were incubated with each at the indicated antibodies (5nM) for 3 hrs. High- salt nuclear extracts were blotted with anti-EGFR and reblotted with the nuclear maker HDAC1, as a loading control. The 455 antibody is a mouse monoclonal antibody to a carbohydrate EGFR epitope. The 528 antibody is a mouse monoclonal antibody to the ecto- domain of the EGFR. Arrows mark the 170 kDa mature EGF receptor and the 150, 130 kDa fragments.
C225-dependent EGF receptor trafficking to the ER
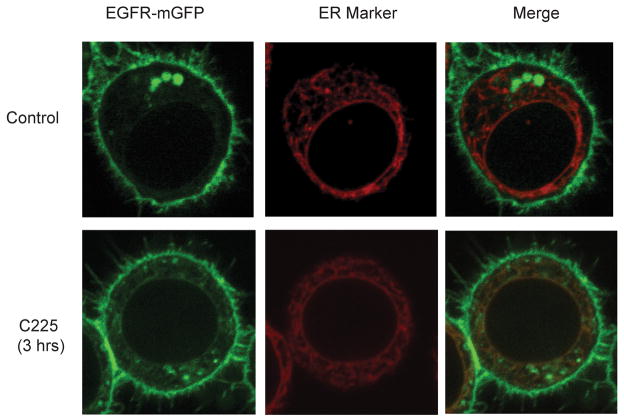
In cells treated with EGF, EGF receptor is slowly trafficked from the cell surface to the ER prior to nuclear localization (18). This trafficking pathway was examined in cells treated with C225. Cells were transfected to co-express the ER marker calreticulin~RFP and EGF receptor ~mGFP. The data in Figure 3 demonstrate the individual signals produced by these two proteins plus the overlap of the signals. In the absence of C225 there is no overlap of the two markers. However, following addition of C225 for 3 hrs there is substantial overlap (brown yellow) of the calreticulin and EGF receptor signals, indicating ER localization of the receptor. These results are very similar to those published for EGF induced trafficking of EGF receptor to the ER (18). In this experiment the confocal plane was chosen to visualize the ER and the presence of nuclear EGF receptor is not obvious in this plane.
Figure 3.
C225-induced translocation of EGFR into ER. MDA-MD-468 cells were stably co-transfected with cDNAs encoding the ER protein calreticulin~RFP and EGFR~mGFP. The quiescent cells were then treated with C225 for 3 hrs. Live cell images were taken by confocal microscopy with a 1 μm optical slice. In this figure, the ER is red, while the plasma membrane, and intracellular markers containing EGFR~mGFP appear green.
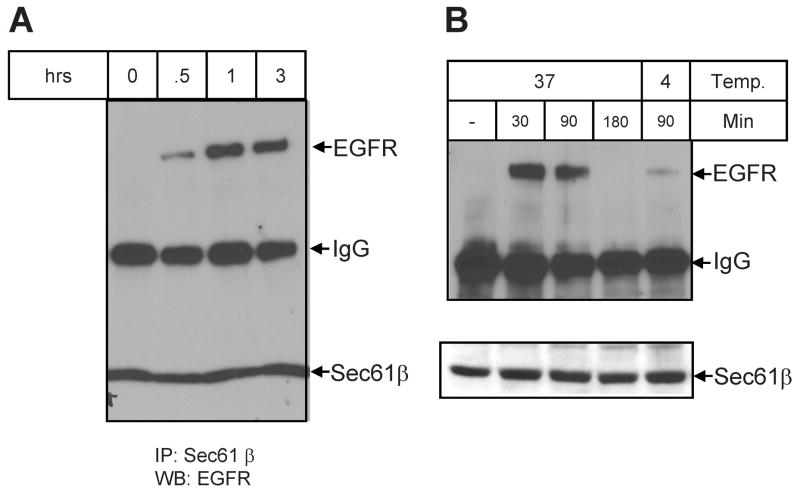
The data in Figure 1 indicate, based on extractability with high salt, that nuclear EGF receptor is not membrane bound, similar to that reported for EGF-induced nuclear localization of the receptor (17, 18). Following EGF treatment, extraction of the transmembrane EGF receptor from the ER lipid bilayer is accomplished by retrotranslocation of the receptor though the Sec61 translocon into the cytosol as a soluble protein (18). In this process, EGF receptor association with the Sec61β subunit can be detected in EGF-treated cells. The results shown in Figure 4A demonstrate that when cells are treated with C225, a time-dependent association of EGF receptor and Sec61β is similarly detected. There is detectable association at 30 min and a maximal level of association at 1 hr after addition of the antibody. This experiment has also been accomplished in DiFi cells along with an additional control (Figure 4B). Incubation of C225 with cells at 4˚ C does not result in significant co-association, indicating that post lysis association is unlikely and that active cell metabolism is required.
Figure 4.
C225-induced association between Sec61β and EGFR. Panel A. MDA-MB-468 cells were incubated with C225 for the indicated times. The non-nuclear fraction was precleared with Protein A beads and then precipitated with Sec61 β antibody and Protein A beads. The precipitates were subjected to SDS-PAGE and then blotted with anti-EGFR and Sec61β antibody. Panel B. DiFi cells were incubated with C225 antibody for the indicated times. The non-nuclear fraction was precleared by Protein A beads and then precipitated with anti-Sec61 β antibody and Protein A beads. The precipitates were subjected to SDS-PAGE and then blotted with anti-EGFR and anti-Sec61β.
Influence of tyrosine kinase inhibition on C225-induced nuclear EGF receptor
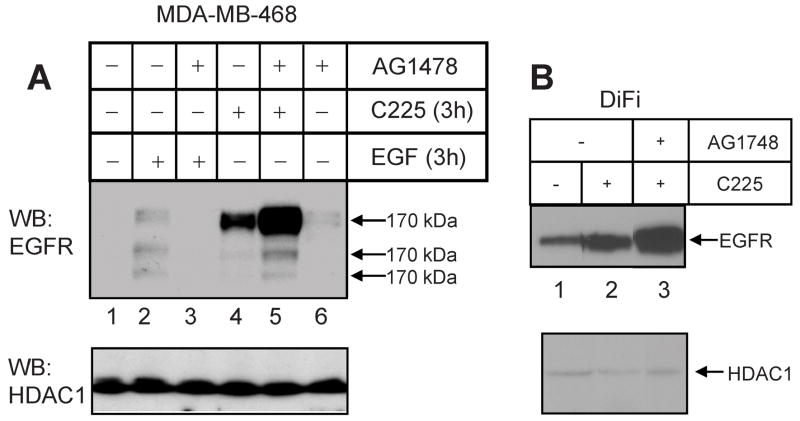
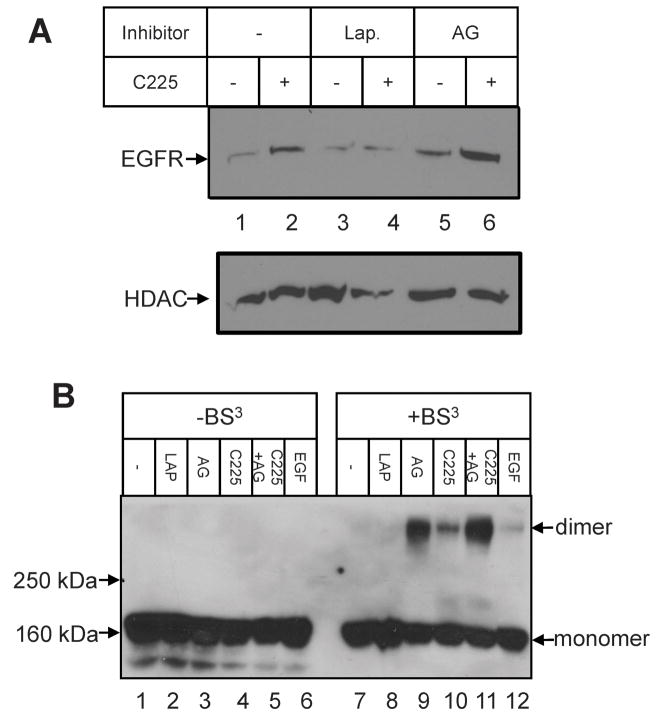
Since EGF receptor tyrosine kinase activity is reported to be necessary for receptor internalization (19) and nuclear localization (17, 18) following the addition of EGF, the influence of kinase inhibitors was tested for C225-dependent nuclear localization of EGF receptor. Surprisingly, the results shown in Figure 5 demonstrate that AG1478 significantly potentiates C225-dependent EGF receptor nuclear localization in both MDA-MB-468 (Panel A) and DiFi (Panel B) cells. In the experiment with MDA-MB-468 cells, EGF receptor nuclear localization following the addition of EGF was also assessed. As previously reported (17, 18), the kinase inhibitor prevented growth factor-induced nuclear translocation of EGF receptor. Also, this experiment allows a direct comparison of the levels of nuclear EGF receptor at the same time point following exposure to EGF or C225. Clearly, C225 provokes a significantly greater level of nuclear receptor at the same period of incubation time. When this experiment was repeated using other EGF receptor kinase inhibitors (Tarceva, Iressa) the results were similar to those obtained with AG1478 (data no shown). Lapatinib, however, did not increase the C225-dependent EGF receptor presence in the nuclear fraction (Figure 6A, lanes 3 and 4).
Figure 5.
Influence of EGFR kinase inhibition on C225-induced EGFR translocation to the nucleus. MDA-MB-468 (Panel A) and DiFi (Panel B) cells were preincubated with or without AG1478 (1μM) for 30 min before incubation with EGF or C225 for 3 hrs. Aliquots of nuclear high-salt extracts were blotted with anti-EGFR. The same blots were reblotted with anti-HDAC1, a nuclear marker. Arrows mark the 170 kDa full-length EGF receptor plus receptor fragments of 150 and 130 kDa.
Figure 6.
Influence of Lapatinib on C225-induced EGFR translocation and dimerization to the nucleus. Panel A. MDA-MB-468 cells were preincubated with or without Lapatinib (1μM) and AG1478 (1μM) for 30 min before incubation with C225 for 3 hrs. Upper Panel. Aliquots of nuclear high-salt extracts were blotted with anti-EGFR. Lower Panel. Reblotted with HDAC1, a nuclear marker. Panel B. The cells were pretreated with or without Lapatinib (1μM) and AG1478 (1μM) for 10 min, then incubated with C225 or EGF for 30 min. The cells were lysed by TGH buffer, then incubated with or without crosslinker BS3 for 30 min at room temperature. Aliquots of lysates were subjected to SDS-PAGE and blotted with anti-EGFR.
AG1478, Iressa and Tarceva are thought to bind to an open conformation of the EGF receptor kinase (20, 21), while Lapatinib is reported to bind to a closed conformation of the kinase (22). Also, it has been shown that AG1478 and related inhibitors provoke dimerization of receptor EGF receptor in cells (23, 24). Therefore, we tested whether Lapatinib could also produce receptor dimerization. The data shown in Figure 6B demonstrate that, while AG1478 (lane 9), C225 (lane 10), or EGF (lane 12) provoked receptor dimerization, Lapatinib does not increase EGF receptor dimerization. These data show that following treated with AG1478 plus C225 (lane 11) the level of receptor dimer was greater than that with C225 alone (lane 10). Under the same conditions, Iressa and Tarceva did provoke EGF receptor dimerization (data not shown).
DISCUSSION
The manner in which EGF induces internalization and intracellular trafficking of the EGF receptor has been described in detail (19). The ligand induces rapid receptor internalization in a manner that requires receptor tyrosine kinase activity for entrance into clathrin-coated pits. When the receptor is overexpressed there is evidence that kinase-independent slow internalization can occur, but the mechanism of internalization is unclear. In the case of C225 antibody-induced internalization, it is known that receptor kinase activity is not necessary and that the internalization process is slow compared to ligand-dependent internalization (16).
Once endocytosis has occurred it is well established that ligand: receptor complexes are trafficked primarily to the lysosome, but also can be recycled to the cell surface (19). Recently, it has been reported that EGF also induces intracellular trafficking of a small fraction of the receptor to the ER (18). In the ER the receptor interacts with the Sec61 translocon and is thereby exported from the ER to the cytosol and subsequently translocates into the nucleus. Nuclear localization sequences have been reported for residues within the intracellular domain (25).
In the case of antibody-dependent intracellular trafficking of the EGF receptor, most of the internalized antibody: receptor complex is recycled to the cell surface (16). Whether any of the antibody-internalized receptor is trafficked to the lysosome is not known directly. However, since C225 and other receptor antibodies provoke a slow down-regulation of the receptor (8, 16, 26, 27), it seems likely that some receptor is degraded in the lysosomes. The data in this manuscript show an additional destination for antibody-dependent internalized EGF receptor. In this trafficking route the receptor is trafficked to the ER, interacts with the Sec61 translocon, and is ultimate found in the nucleus. The most significant difference for trafficking of the receptor through this pathway under the influence ligand or antibody is tyrosine kinase activity requirement for EGF-induced nuclear localization, but not for antibody-induced nuclear localization. This difference in kinase activity requirement is most likely due to the kinase requirement for ligand-dependent entry of receptor into coated pits, while C225-induced cellular entry may occur through a different cell surface portal. Whether C225 translocation of the EGF receptor to the nucleus influences the biologic responses of cells to the antibody is not known.
Other extracellular ligands that are trafficked by their receptors from the cell surface to the ER include certain toxins (28) and the SV40 virus (29). In neither case is there a reported requirement for tyrosine kinase activity and for each of these two different ligands, the Sec61 translocon mediates export from the ER. In the case of toxins export is to the cytoplasm, while for the virus cytoplasmic localization would seem to be a precursor step for nuclear localization. Neither toxin nor virus is internalized though coated pits, but rather are internalized through caveolae. Based on our results with C225-dependent receptor trafficking it would seem that receptor trafficking to the ER and nucleus occurs in the absence of tyrosine kinase activity.
The mechanism by which some tyrosine kinase inhibitors potentiate antibody-induced receptor trafficking to nucleus is not clear. Based on the fact that Lapatinib, which binds to a closed kinase conformation, fails to provoke receptor dimerization or C225-dependent nuclear localization of the receptor, the most likely mechanism might seem to be that inhibitors, such as, AG1478 stabilize an inactive open form of receptor dimer at the cell surface and this may provide for either enhanced antibody association and /or for a more efficiently internalized antibody: receptor complex.
Supplementary Material
Acknowledgments
The authors acknowledge support of NIH grant CA125649 (G.C). Experiments and data analysis were performed in part through the use of the VUMC Cell Imaging Shared Resource (supported by NIH grants CA68485, DK20593, DK58404, HD15052, DK59637 and EY08126)
References
- 1.Fan Z, Masui H, Altas I, et al. Blockade of epidermal growth factor receptor function by bivalent and monovalent fragments of 225 anti-epidermal growth factor receptor monoclonal antibodies. Cancer Res. 1993;53:4322–8. [PubMed] [Google Scholar]
- 2.Gordon G, Tomoyuki K, Claude C, et al. Monoclonal anti-epidermal growth factor receptor antibodies which are inhibitors of epidermal growth factor binging and antagonists of epidermal growth-stimulated tyrosine protein kinase activity. J Boil Chem. 1984;259:7755–60. [PubMed] [Google Scholar]
- 3.Li S, Schmitz KR, Jeffrey PD, et al. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–11. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–74. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 5.Rivera F, Vega-Villegas ME, Lopez-Brea MF. Cetuximab, its clinical use and future perspectives. Anticancer Drugs. 2008;19:99–113. doi: 10.1097/CAD.0b013e3282f23287. [DOI] [PubMed] [Google Scholar]
- 6.Galizia G, Lieto E, De Vita F, et al. Cetuximab, a chimeric human mouse anti-epidermal growth factor receptor monoclonal antibody, in the treatment of human colorectal cancer. Oncogene. 2007;26:3654–60. doi: 10.1038/sj.onc.1210381. [DOI] [PubMed] [Google Scholar]
- 7.Bernier J. Drug Insight: cetuximab in the treatment of recurrent and etastatic squamous cell carcinoma of the head and neck. Nat Clin Pract Oncol. 2008;5:705–13. doi: 10.1038/ncponc1228. [DOI] [PubMed] [Google Scholar]
- 8.Fan Z, Lu Y, Wu X, Mendelsohn J. Antibody-induced epidermal growth factor receptor dimerization mediates inhibition of autocrine proliferation of A431 squamous carcinoma cells. J Biol Chem. 1994;269:27595–602. [PubMed] [Google Scholar]
- 9.Wu X, Rubin M, Fan Z, et al. Involvement of p27KIP1 in G1 arrest mediated by an anti-epidermal growth factor receptor monoclonal antibody. Oncogene. 1996;12:1397–403. [PubMed] [Google Scholar]
- 10.Kiyota A, Shintani S, Mihara M, et al. Anti-epidermal growth factor receptor monoclonal antibody 225 upregulates p27(KIP1) and p15(INK4B) and induces G1 arrest in oral squamous carcinoma cell lines. Oncology. 2002;63:92–8. doi: 10.1159/000065726. [DOI] [PubMed] [Google Scholar]
- 11.Peng D, Fan Z, Lu Y, et al. Anti-epidermal growth factor receptor monoclonal antibody 225 up-regulates p27KIP1 and induces G1 arrest in prostatic cancer cell line DU145. Cancer Res. 1996;56:3666–9. [PubMed] [Google Scholar]
- 12.Liu B, Fan Z. The monoclonal antibody 225 activates caspase-8 and induces apoptosis through a tumor necrosis factor receptor family-independent pathway. Oncogene. 2001;20:3726–34. doi: 10.1038/sj.onc.1204490. [DOI] [PubMed] [Google Scholar]
- 13.Huang SM, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation,apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:1935–40. [PubMed] [Google Scholar]
- 14.Sunada H, Magun BE, Mendelsohn J, et al. Monoclonal antibody against epidermal growth factor receptor is internalized without stimulating receptor phosphorylation. Proc Natl Acad Sci U S A. 1986;83:3825–29. doi: 10.1073/pnas.83.11.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sunada H, Yu P, Peacock JS, et al. Modulation of tyrosine, serine, and threonine phosphorylation and intracellular processing of the epidermal growth factor receptor by antireceptor monoclonal antibody. J Cell Physiol. 1990;142:284–92. doi: 10.1002/jcp.1041420210. [DOI] [PubMed] [Google Scholar]
- 16.Jaramillo ML, Leon Z, Grothe S, et al. Effect of the anti-receptor ligand-blocking 225 monoclonal antibody on EGF receptor endocytosis and sorting. Exp Cell Res. 2006;312:2778–90. doi: 10.1016/j.yexcr.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Lin SY, Makino K, Xia W, et al. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802–8. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 18.Liao HJ, Carpenter G. Role of the Sec61 translocon in EGF receptor trafficking to the nucleus and gene expression. Mol Biol Cell. 2007;18:1064–72. doi: 10.1091/mbc.E06-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2008;314:3093–106. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stamos J, Sliwkowski MX, Eigenbrot C. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J Biol Chem. 2002;277:46265–72. doi: 10.1074/jbc.M207135200. [DOI] [PubMed] [Google Scholar]
- 21.Yun CH, Boggon TJ, Li Y, et al. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell. 2007;11:217–27. doi: 10.1016/j.ccr.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood ER, Truesdale AT, McDonald OB, et al. A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 2004;64:6652–9. doi: 10.1158/0008-5472.CAN-04-1168. [DOI] [PubMed] [Google Scholar]
- 23.Arteaga CL, Ramsey TT, Shawver LK, et al. Unliganded epidermal growth factor receptor dimerization induced by direct interaction of quinazolines with the ATP binding site. J Biol Chem. 1997;272:23247–54. doi: 10.1074/jbc.272.37.23247. [DOI] [PubMed] [Google Scholar]
- 24.Lichtner RB, Menrad A, Sommer A, et al. Signaling-inactive epidermal growth factor receptor/ligand complexes in intact carcinoma cells by quinazoline tyrosine kinase inhibitors. Cancer Res. 2001;61:5790–5. [PubMed] [Google Scholar]
- 25.Hsu SC, Hung MC. Characterization of a novel tripartite nuclear localization sequence in the EGFR family. J Biol Chem. 2007;282:10432–40. doi: 10.1074/jbc.M610014200. [DOI] [PubMed] [Google Scholar]
- 26.Friedman LM, Rinon A, Schechter B, et al. Synergistic down-regulation of receptor tyrosine kinases by combinations of mAbs: implications for cancer immunotherapy. Proc Natl Acad Sci U S A. 2005;102:1915–20. doi: 10.1073/pnas.0409610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez-Torres M, Guix M, Gonzalez A, et al. Epidermal growth factor receptor (EGFR) antibody down-regulates mutant receptors and inhibits tumors expressing EGFR mutations. J Biol Chem. 2006;281:40183–92. doi: 10.1074/jbc.M607958200. [DOI] [PubMed] [Google Scholar]
- 28.Sandvig K, van Deurs B. Transport of protein toxins into cells: pathways used by ricin, cholera toxin and Shiga toxin. FEBS Lett. 2002;529:49–53. doi: 10.1016/s0014-5793(02)03182-4. [DOI] [PubMed] [Google Scholar]
- 29.Marsh M, Helenius A. Virus entry: open sesame. Cell. 2006;124:729–40. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.