Abstract
Thiamine-dependent enzymes are diminished in multiple neurodegenerative diseases. Thiamine deficiency (TD) reduces the activity of thiamine dependent-enzymes [e.g., the α-ketoglutarate dehydrogenase complex (KGDHC)], induces regional selective neurodegeneration and serves as a model of a mild impairment of oxidative metabolism. The current experiments tested whether changes in KGDHC protein subunits (E1k, E2k and E3) or activity or message levels underlie the selective loss of neurons in particular brain regions. Thus, TD-induced changes in these variables in the brain region most vulnerable to TD [the sub-medial thalamic nucleus (SmTN)] were compared to those in a region that is relatively resistant to TD (cortex) at stages of TD when the neuron loss in SmTN is not present, minimal or severe. Impaired motor performance on rotarod was apparent by 8 days of TD (-32%) and was severe by 10 days of TD (-97%). At TD10, the overall KGDHC activity measured by an in situ histochemical staining method declined 52% in SmTN but only 20% in cortex. Reductions in the E2k and E3 mRNA in SmTN occurred as early as TD6 (-28% and -18%, respectively) and were more severe by TD10 (-61% and -66%, respectively). On the other hand, the level of E1k mRNA did not decline in SmTN until TD10 (-48%). In contrast, TD did not alter mRNA levels of the subunits in cortex at late stages. Western blots and immunocytochemistry revealed different aspects of the changes in protein levels. In SmTN, the immunoreactivity of E1k and E3 by Western blotting increased 34% and 40%, respectively, only at TD8. In cortex, the immunoreactivity of the three subunits was not altered. Immunocytochemical staining of brain sections from TD10 mice indicated a reduction in the immunoreactivity of all subunits in SmTN, but not in cortex. These findings demonstrate that the response of the KGDHC activity, mRNA and immunoreactivity of E1k, E2k and E3 to TD is region- and time-dependent. Loss of KGDHC activity in cortex is likely related to post-translational modification rather than a loss of protein, whereas in SmTN transcriptional and post-translational modifications may account for diminished KGDHC activity. Moreover, the earlier detection in TD induced-changes of the transcripts of KGDHC indicates that transcriptional modification of the two subunits (E2k and E3) of KGDHC may be one of the early events in the cascade leading to selective neuronal death.
Keywords: α-ketoglutarate dehydrogenase complex, micropunch, oxidative stress, rotarod, submedial thalamic nucleus, thiamine deficiency
Introduction
Thiamine deficiency (TD) in rodents induces mild oxidative stress, microglial activation, selective neuronal cell death, diminished energy metabolism and behavioral abnormalities (Langlais et al., 1997; Calingasan et al., 1999, 2000; Hakim and Pappius, 1981; Gibson et al., 1982; Freeman et al., 1987) that model important aspects of a number of neurodegenerative diseases including Alzheimer’s disease (AD) (Gibson and Zhang, 2001, 2002a), Parkinson’s disease (PD) (Schwab et al., 1996; Gibson et al., 2003), progressive supranuclear palsy (Park et al., 2001) and Wernicke-Korsakoff’s syndrome (Victor et al., 1989). Diminished activities of thiamine dependent-enzymes such as the α-ketoglutarate dehydrogenase complex (KGDHC) have been consistently observed in brains of TD animal models and in post mortem brains of humans with age-related diseases (Sheu et al., 1998; Gibson et al., 1999; Mastrogiacoma et. al, 1996; Bubber et al., 2004). However, the mechanisms leading to reductions in this metabolically important enzyme and its role in the cascade of events leading to selective neurodegeneration and behavioral abnormalities are unknown. Modifications of the enzyme complex at various levels including post-translational modification may contribute to its reduced activity. Whether the decline is related to altered gene expression has never been tested.
KGDHC consists of multiple copies of three subunits: α-ketoglutarate dehydrogenase (E1k, EC 1.2.4.2), dihydrolipoyl succinyltransferase (E2k, EC 2.3.1.61) and dihydrolipoyl dehydrogenase (E3, EC 1.6.4.3). KGDHC catalyzes an important regulatory reaction of the tricarboxylic acid (TCA) cycle that converts α-ketoglutarate to succinyl-CoA and generates NADH (Reed, 2001). A decline in KGDHC activity would be expected to diminish metabolism and promote neurodegeneration. KGDHC activity can be inhibited by multiple oxidants, and is one of the enzymes that are most sensitive to oxidative stress (Albers et al., 2000; Park et al., 2001). Oxidant-induced reductions in production of NADH by mitochondria are due to effects on KGDHC (Tretter and Adam-Vizi, 2000). Immunochemical studies demonstrate that the three subunits of KGDHC are selectively sensitive to oxidants (Park et al., 1999), and selectively reduced in the diseases in a region dependent manner (Mastrogiacoma et al., 1996a, 1996b). Thus, diminished KGDHC activity is a plausible link between oxidative stress and neurodegeneration.
TD in rodents is a useful and practical in vivo model to monitor and analyze biochemical, cellular and behavioral responses to mild impairment of oxidative metabolism and oxidative stress in a convenient experimental time frame. In TD rodents, endothelial cell changes, neuronal loss and microglial activation first occur in the submedial thalamic nucleus (SmTN) (Calingasan et al., 2000; Ke et al., 2003), a relative small region in which all types of brain cells can be studied in detail. Initial microglial activation (+16%) and neuronal loss (-29%) occur after 8 or 9 days of TD (TD8-9) and are dramatically increased to +400% and -90% by TD 10-11 (Ke et al., 2003). Although the temporal and regional response of various cell types to TD has been characterized in SmTN, the corresponding biochemical changes of KGDHC have not been assessed in the same region. Previous studies that tested the relation of KGDHC to neurodegeneration did not compare changes in the SmTN to non-damaged regions of brain because the prior studies were completed before the sensitivity of the SmTN was identified (Gibson et al., 1984; Butterworth et al., 1986; Sheu et al., 1998).
The role of KGDHC in TD-induced pathology has been studied extensively, but the experiments did not test for changes in gene expression or protein levels of the subunits of KGDHC within SmTN. TD diminishes KGDHC activity in whole brain homogenates (Freeman et al., 1987; Bubber et al., 2004) or dissected brain regions such as cerebral cortex, entire thalamus or inferior colliculus (Butterworth et al., 1986; Sheu et al., 1998). For these brain regions, the changes in in vitro glucose flux reflected selective vulnerability better than KGDHC activity (Gibson et al., 1989). Immunoblotting analysis of whole thalamus and cortex from TD11 and TD13 rats reveals no changes in the immunoreactivity in any of the subunits of KGDHC compared to controls (Sheu et al., 1998). Immunocytochemical staining of a typical coronal section of TD13 and control rats brains with an anti-KGDHC antibody also reveals a similar staining pattern in both unaffected and vulnerable regions (Sheu et al., 1998). In the current experiments, a novel micropunch technique was utilized to precisely sample brain samples from small brain regions such as SmTN, which is one third the size of the Substantia nigra (Karuppagounder et al., 2007) and to quantitatively measure changes of gene expression and immunoreactivity of KGDHC in SmTN and nonvulnerable regions such as cortex. Thus, co-localization of the regional responses with changes in KGDHC could be readily evaluated.
A range of behavioral tests have been used to monitor changes in TD mice including memory, tight rope test performance and open field behavior. Single time point measures suggest that TD-induced deficits in open field behavior correlate to decreases in whole brain KGDHC activity (Freeman et al., 1987). The majority of these studies has been performed in rats, involve labor intensive protocols that are difficult to replicate and the temporal appearance of changes in these measures does not parallel changes in SmTN. Thus, the current experiments tested the motor performance in control and TD mice with a highly standardized rotarod test (Jones and Roberts, 1968; Barlow et al., 1996; Carter et al., 1999). Thus, rotarod performance was measured at multiple times so it could be compared to TD-induced biochemical responses of KGDHC at levels of activity, mRNA and protein in SmTN and cortex regions. Possible correlations between changes of KGDHC, neuron loss and impaired rotarod performance were discussed.
Experimental procedures
Animal treatments
Adult Male C57BL/6 mice (6-8 weeks; 22-26 g) from Harlan Sprague-Dawley (Indianapolis, IN, USA) were used in all experiments. The animals were housed with constant temperature (22 ± 2°C), humidity (50 ± 5 %) and illumination (12 h light/dark cycles). TD was induced as described in previous studies (Ke et al., 2003). Experimental mice (n = 12) received a thiamine-deficient diet (ICN Nutrition Biomedicals, Cleveland, OH) ad libitum and daily intraperitoneal injections of the thiamine pyrophosphokinase inhibitor (Liu et ., 2006), pyrithiamine hydrobromide (Sigma Chemical Co., St. Louis, MO; 5 μg in 0.1 ml saline/10 g body weight). Control mice (n = 12) received a thiamine containing diet (ICN Nutrition Biomedicals, Cleveland, OH) ad libitum and daily intraperitoneal injections of saline (0.1 ml saline/10 g body weight). The Institutional Animal Care and Use Committee of Weill Medical College of Cornell University approved all procedures with the animals.
Rotarod performance
The rotarod apparatus (Columbus Instruments, Columbus, OH) was used to measure the fore- and hind limb motor coordination and balance. Mice were trained for two consecutive days to become acquainted with the rotarod apparatus. Motor performances were assessed in control and TD mice by rotarod from day 1 to 10. Control and TD mice were tested at each speed level ranging from 6, 12, 18, 24 and 30 rpm for a maximum of 120 seconds. The latency to fall off the rotating rod per day per rotation speed level was recorded as a measure of motor function.
In situ KGDHC activity
Following 4, 8 or 10 days of TD induction, mice were administrated a lethal dose of sodium pentobarbital (2 mg/10 g body weight; Abbott Laboratories, North Chicago, IL) intraperitoneally, and were perfused via the ascending aorta with approximately 50 ml of saline to wash away the blood (5 ml/min). The brains were removed immediately and stored at -80°C. The brain block containing the thalamus and cortex (primary and secondary motor cortex) regions was dissected on a Rodent Brain Matrix (ASI Instruments, Warren, MI), and 20 μm serial sections were cut using a cryostat (Hacker-Bright OTF microtome cryostat, Fairfield, NJ). Sections from the Bregma level -0.94 to -1.94 (Franklin and Paxinos, 1997) were collected and used for in situ KGDHC activity stain according to a previously described method with slightly modifications (Park et al., 2000). In brief, sections were incubated with reaction mixture [50 mM Tris-HCl, 1 mM MgCl2, 0.1 mM CaCl2, 50 μM EDTA, 0.2%Triton X-100, 0.3 mM thiamine pyrophosphate (TPP), 5 μg/ml rotenone, 35 mg/ml polyvinyalcohol, 3 mM β-NAD, 0.75 mM Co-A, 3 mM α-ketoglutarate (α-KG), 0.75 mM nitroblue tetrazolium (NBT) and 0.05 mM phenazine methosulfate (PMS)] for 20 or 30 min. A reaction mixture without Co-A and α-KG was used for blank. NBT and PMS were added immediately before the reaction was initiated. The reaction was stopped by removing the sections from the reaction mixture and rinsing the sections twice with distilled water for 10 seconds each time. The sections were air-dried overnight before quantification. Images were acquired (4x magnification) with Nikon eclipse 80i microscope using ACT-1 software. The MetaMorph program (Molecular Devices, Downingtown, PA) was used to draw the region of interest (SmTN or cortex) and to quantify staining density of each region. The density of each region after normalization to the blank was compared between TD and control groups. The percentage change of the regional density following TD represented the change of the in situ KGDHC activity.
Real-time RT PCR
Micropunches were obtained according to the method described previously (Karuppagounder et al., 2007). Micropunches from either cortex (primary and secondary motor cortex) or SmTN from three control or TD mice were pooled together and stored immediately at -80°C. Total RNA was extracted from micropunches using Trizol reagent (Invitrogen, Carlsbad, CA) and concentrated with RNeasy MinElute™ Cleanup kit (Qiagen, Valencia, CA) according to the suppliers’ instructions. This was followed by first strand cDNA synthesis using the First Strand cDNA synthesis kit for RT-PCR (AMV) (Roche Applied Science, Indianapolis, IN) with oligo-p(dT)15 as primer. Real-time PCR of E1k, E2k and E3 was performed using an Applied Biosystems 7500 Real-Time PCR system with pre-designed Taqman® gene expression assays (Applied Biosystems, Foster City, CA). In brief, each amplification mixture (50 μl) contained 22.5 μl of cDNA template, 25 μl of TaqMan® Universal PCR Master Mix, 2.5 μl of a FAM™ dye labeled TaqMan® MGB probe and two PCR primers. Thermal cycler conditions were 95°C for 10 min, and 40 cycles of 95°C for 15 sec and 60°C for 1 min. All samples were normalized for beta-2-microglobulin (b2m) expression in parallel in the same run. A comparative Ct (the threshold cycle of PCR at which amplified product was first detected) method was used to compare the mRNA expression in samples from TD to that of the control.
SDS-PAGE and Western blotting
Total proteins were extracted from the same micropunchs used for total RNA isolation using Trizol reagent according to the supplier’s instructions (Invitorgen, Carlsbad, CA). Protein concentrations were determined by a bicinchoninic colorimetric acid (BCA) assay (Pierce Chemical Company, Rockford, IL). A total of 4 μg of protein was premixed with SDS loading buffer and denatured at 100°C. Denatured samples were then loaded on a 10% Novex Tris-Glycine gel (Invitrogen; Carlsbad, CA). After electrophoresis (100 volts, 4.5 hr), separated proteins were then electrotransferred to a nitrocellulose membrane (Amersham Biosciences; Piscataway, NJ) at 45 volts for 3 hr. Blots were blocked with Tris-buffered saline (TBS)/0.1% Tween-20/3% BSA (for E1k /E2k /E3 detection) or TBS/0.1% Tween-20/5% non-fat milk (for actin detection) at 4°C overnight, and incubated with rabbit E1k, E2k or E3 antibody (1:1,000 dilution for E1k antibody; 1:5,000 for E2k and E3 antibodies) at 4°C overnight [E1k and E2k antibodies were generated in collaboration with Rockland Immunochemicals Inc. (Gilbertsville, PA); E3 was a generous gift from Gordon Lindsay]. Duplicate membranes were incubated with goat actin polyclonal antibody (1:1,000, Santa Cruz Biotechnology Inc.; Santa Cruz, CA) at 4 °C overnight. After washing thoroughly with TBS/0.1% Tween-20 at room temperature, the blots were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (H+L) (1:15,000; Jackson ImmunoResearch Laboratory, Inc.; West Grove, PA) at room temperature for 1 hr for E1k/E2k/E3 detection, or donkey anti-goat IgG-HRP (1:2,000; Santa Cruz Biotechnology Inc.; Santa Cruz, CA) for actin detection. After extensive washing, the bands were visualized by enhanced chemiluminescence (ECL) (Amersham Biosciences; Piscataway, NJ) and analyzed by densitometry.
Immunocytochemistry
At day 10, mice were administered a lethal intraperitoneal dose of pentobarbitone sodium (200 mg/kg; i.p.; Abbott Laboratories, North Chicago, IL) and perfused via the ascending aorta with 50 ml of normal saline, followed by 100 ml of 4% paraformaldehyde (Sigma Chemical Co., St. Louis, MO) in 0.1 M phosphate buffer (pH 7.2) using a pump (Masterflex, Model 7518-00, Cole-Parmer Instrument Company, Barrington, IL) at 5ml/min. The brains were removed and post-fixed in 4% paraformaldehyde overnight, and then transferred to 30% sucrose (Sigma Chemical Co., St. Louis, MO) for at least an additional 24 hours. The brain block that contained the thalamus and cortex (primary and secondary motor cortex) region was dissected on a Rodent Brain Matrix (ASI Instruments; Warren, MI) and sectioned (40 μm) with a sliding microtome (Microm Laborgerate GmbH, Welldorf, Germany). Sections were collected from Bregma level - 0.94 to -1.94 (Franklin and Paxinos, 1997). Briefly, sections were washed with 0.1 M potassium phosphate buffered saline (PBS, pH 7.4) and incubated in 1% H2O2 in PBS for 30 min to quench the endogenous peroxidase. Then sections were treated with 0.1% Triton X-100 (Sigma Chemical Co., St. Louis, MO) for 15 min. Sections were washed with PBS and blocked with 2% bovine serum albumin (BSA) in PBS for 1 hr. Sections were incubated with rabbit anti-E1k, E2k or E3 antibodies (1:500 for E1k and E3; 1:1000 for E2k) in PBS containing 1% BSA (Sigma Chemical Co., St. Louis, MO) overnight at room temperature. After rinsing in PBS, sections were incubated with biotinylated anti-rabbit (Vector Laboratories Inc., Burlingame, CA; 1:200 in PBS containing 0.25% BSA) for one hr. Sections were then incubated in avidin-biotinperoxidase complex for one hr (Vector Laboratories Inc., Burlingame, CA; 1:100 in PBS), rinsed in PBS and developed in 0.05% 3,3′-diaminobenzidine (DAB) and nickel (Vector Laboratories Inc., Burlingame, CA) and 0.003% H2O2 in PBS.
Statistical analysis
All values are expressed as means ± standard error of the mean (SEM). SPSS (SPSS Co., Chicago, IL) was used for statistical analysis. Statistical significance of group differences (P < 0.05) was tested by two-way analysis of variance (ANOVA) followed by Student-Newman-Keuls post hoc test.
Results
Rotarod performance of control and TD mice
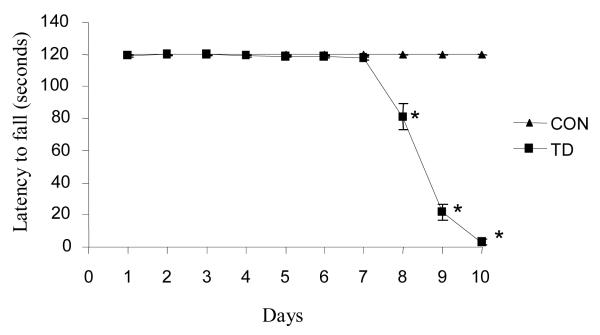
Rotarod performance proved to be a highly reproducible method to assess the motor deficit in mice over the 10 days of TD. Rotarod performance declined 34% on the eighth day of TD and the deficit increased to near 97% at TD10 compared to controls (Fig. 1).
Figure 1.
TD impaired rotarod performance from day 8. Control and TD mice were tested daily from day 1 to 10 on an accelerating rotarod with speed ranged from 6, 12, 18, 24 and 30 rpm for a maximum of 120 seconds. Motor performance was significantly reduced by 8-10 days of TD. The mean ± SEM represents the latency to fall. *p ≤ 0.05 as compared to control group (n = 8) [treatment (df = 1, F = 686.433); time (df = 9, F = 196.810)].
Temporal regional response of in situ KGDHC activity to TD
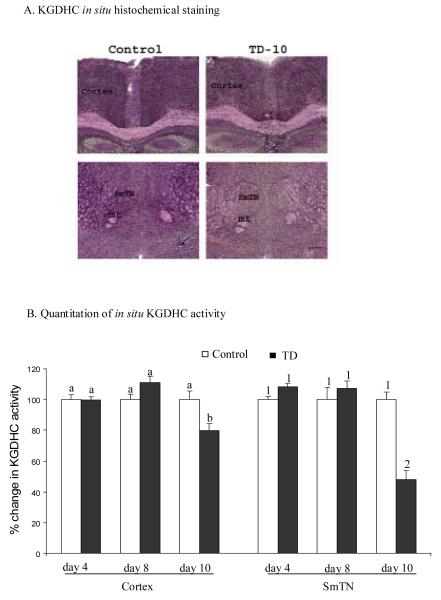
KGDHC activities in cortex and SmTN were assessed on days 4, 8 and 10 of TD by an in situ histochemistry activity stain method. This stain permits activity to be measured with minimal disruption of the cyto-architecture. Both 20 min and 30 min activity staining of brain sections from the same mouse brain were performed to assure linearity of the reaction. The density of the staining at 30 min was nearly doubled compared to that of 20 min (data not shown). Only results at 30 min are presented. A representative in situ KGDHC staining of brain sections from SmTN and cortex of TD10 and control mice are shown in Figure 2A. No differences were apparent before TD10. At TD10 the reduction of KGDHC activity compared to control in SmTN (-52%) was much greater than in cortex (-20%) (Figure 2B).
Figure 2.
In situ KGDHC activity in SmTN and cortex of animals from control and thiamine deficient groups after different periods of treatment. KGDHC activity was measured by an in situ histochemistry activity stain at 4, 8 and 10 days of TD. Values were assessed as density over a blank without Co-A and α-ketoglutarate. The change in the density of TD group compared to control group was presented as the % change in KGDHC activity. A. representative sections showing in situ KGDHC activity staining after a 30 min incubation. Scale bar in lower right corner 200 μm. B. quantitation of in situ KGDHC activity. Regions of interest (cortex and SmTN) are indicated in panel A. Values in panel B represent means ± SEM of % change in KGDHC activity from at least three independent experiments in quadruplicate. Values with different letters or numbers vary significantly from each other (P<0.05) [cortex: treatment (df = 1, F = 0.81); time (df = 2, F = 6.830). SmTN: treatment (df = 1, F = 7.758); time (df = 2, F = 21.022)].
Temporal response of gene expression of KGDHC subunits to thiamine deficiency (TD)
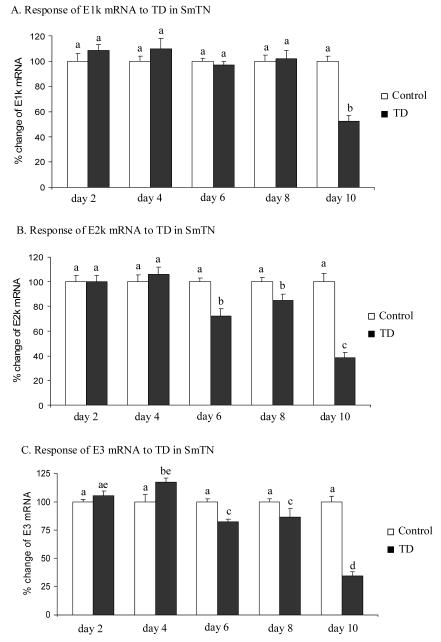
Whether the expression of genes encoding the three subunits of KGDHC responded to TD was investigated in SmTN by quantitative real-time RT-PCR. The reductions in the E2k and E3 mRNA in SmTN were detectable as early as TD6 (-28% and -18%) and were more severe (-61% and -66%) by TD10 (Fig. 3B and 3C). The level of E1k mRNA declined by 48%, and only on TD10 (Fig. 3A).
Figure 3.
Temporal response of gene expression of KGDHC subunits E1k, E2k and E3 to TD in SmTN. Micropunches from SmTN were subjected to quantitative real-time RT-PCR to assess TD-induced changes in the gene expression of E1k (A), E2k (B) and E3 (C). mRNA for β-2-microglobulin (β2m) was measured in the same sample in parallel as an internal control. Values in panels A, B and C are the means ± SEM of percent changes over controls from four independent experiments done in triplicate after normalization to β2m. Values with different letters vary statistically from each other (P<0.05) [E1k: treatment (df = 1, F = 4.986); time (df = 4, F = 7.426). E2k: treatment (df = 1, F = 38.868); time (df = 4, F = 13.822). E3: treatment (df = 1, F = 28.698); time (df = 4, F = 26.565)].
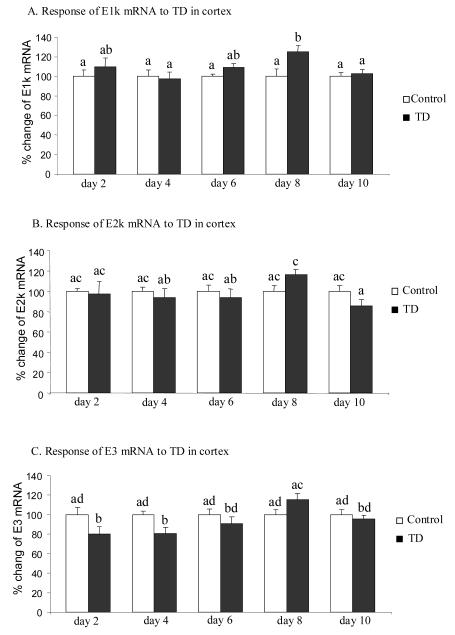
Whether TD altered the mRNA level of the three genes of KGDHC in cortex was also tested. The gene expression of E1k increased 25% but only at TD8 (Fig. 4A). In contrast, the level of E3 mRNA declined 20% at early stage of TD (day 2 and 4) and did not differ from controls at later stages (TD 6, 8 and 10) (Fig. 4C). E2k mRNA levels did not change through the 10 days of TD (Fig. 4B).
Figure 4.
Temporal response of gene expression of KGDHC subunits E1k, E2k and E3 to TD in cortex. Micropunches from cortex were subjected to quantitative real-time RT-PCR to assess TD-induced changes in the gene expression of E1k (A), E2k (B) and E3 (C). mRNA of β-microglobulin (β2m) used as an internal control was measured from the same sample in parallel. Values in panels A, B and C are the means ± SEM of percent changes over controls from four independent experiments done in triplicate after normalization to β2m. Values with different letters vary statistically from each other (P<0.05) [E1k: treatment (df = 1, F = 5.024); time (df = 4, F = 1.491). E2k: treatment (df = 1, F = 0.33); time (df = 4, F = 1.291). E3: treatment (df = 1, F = 28.698); time (df = 4, F = 26.565)].
Response of immunoreactivity of KGDHC to thiamine deficiency (TD)
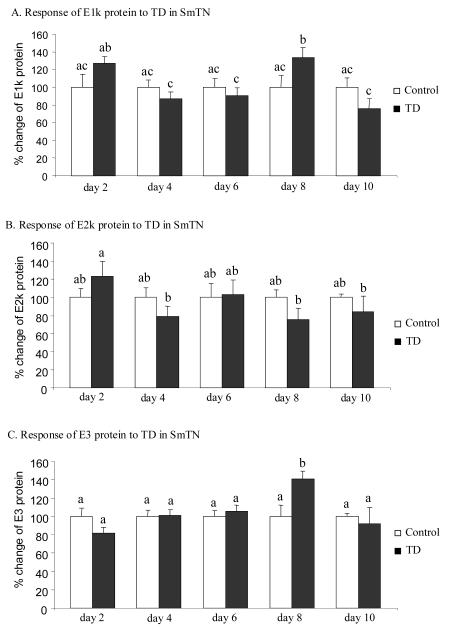
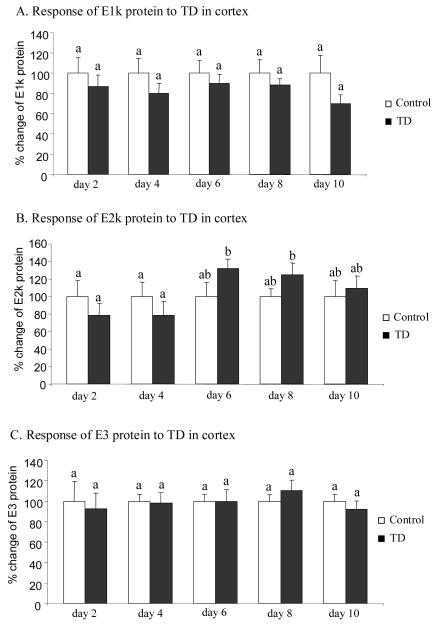
The effect of TD on the immunoreactivity of the three subunits of KGDHC in SmTN and cortex was assessed by Western blotting. Total protein isolated from micropunches obtained from SmTN and cortex of control and TD mice were subjected to SDS-PAGE followed by Western blotting probed with three antibodies against E1k, E2k and E3 subunit. In SmTN, TD increased the immunoreactivity of E1k and E3 subunits by 34% (Fig. 5A) and 41% (Fig. 5C), but only at day 8. The immunoreactivity of E2k was unaltered through 10 days of TD (Fig. 5B). In cortex, TD did not induce any significant change in the immunoreactivity of the three subunits of KGDHC (Fig. 6).
Figure 5.
Temporal response of immunoreactivity of the three subunits of KGDHC to TD in SmTN. Total protein was isolated from micropunches obtained from the SmTN and subjected to SDS-PAGE followed by Western blotting probed with antibodies against E1k (A), E2k (B) or E3 (C). β-actin immunoreactivity was used as an internal control. Values are means ± SEM of relative densities of the subunit from four independent experiments after normalization to β-actin. Values with different letters vary significantly from each other (P<0.05) [E1k: treatment (df = 1, F = 0.186); time (df = 4, F = 3.034). E2k: treatment (df = 1, F = 0.734); time (df = 4, F = 1.233). E3: treatment (df = 1, F = 0.470); time (df = 4, F = 3.055)].
Figure 6.
Temporal response of immunoreactivity of the three subunits of KGDHC to TD in cortex. Total proteins isolated from micropunches from cortex were subjected to SDS-PAGE, Western blotting followed by immunodetection with antibody against E1k (A), E2k (B) or E3 (C). Values are means ± SEM of relative densities of the subunit from four independent experiments after normalization to β-actin. Values with different letters vary significantly from each other (P<0.05) [E1k: treatment (df = 1, F = 4.080); time (df = 4, F = 0.113). E2k: treatment (df = 1, F = 0.007); time (df = 4, F = 1.422). E3: treatment (df = 1, F = 0.036); time (df = 4, F = 0.235)].
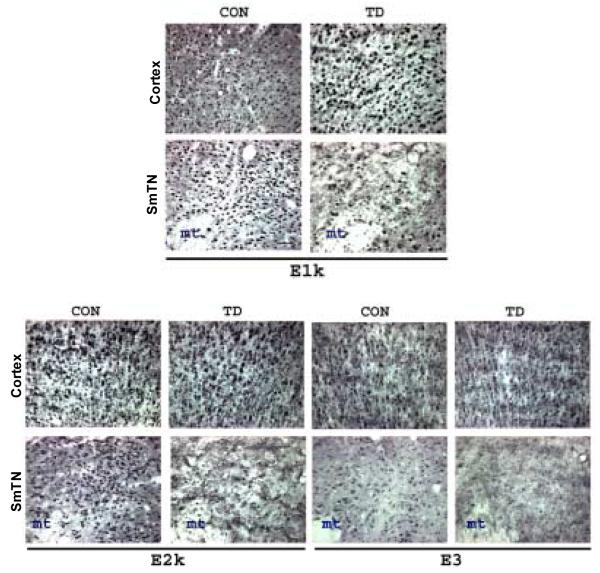
Immunocytochemical staining patterns of the three subunits of KGDHC were also compared between TD10 and control mice. Brain sections from TD10 and control mice were immunostained with antibodies to E1k, E2k and E3. Ten days of TD diminished the immunoreactivity of all subunits in SmTN, but not in cortex (Figure 7).
Figure 7.
Ten days of TD diminished immunoreactivity of KGDHC subunits only in SmTN as determined by immunocytochemistry. Representative photomicrographs from cortex and SmTN stained with antibodies specific against KGDHC subunits E1k, E2k and E3. TD brains show decrease in E1k, E2k and E3-immunoreactivity in SmTN compared to control, while the cortex was spared. Scale bar 50 μm.
Discussion
The cause of the regional selective neuronal cell death in TD has not been fully elucidated. Many mechanisms including diminished energy metabolism, focal lactic acidosis, NMDA receptor-mediated excitotoxicity and oxidative stress have been implicated (Desjardins and Butterworth, 2005; Ke and Gibson, 2004). Involvement of oxidative stress in TD is evident. For example, gene expression and protein level of endothelial nitric oxide synthase (eNOS) increase in regions that are vulnerable to TD at symptomatic stages of TD (Kruse et al., 2004). An early response of endoplasmic reticulum to TD occurs by day 6 of TD (Wang et al., 2007). The up-regulation of markers for endoplasmic reticulum stress precedes eNOS induction and neuropathological lesion. Thus, identification of factors involved in early responses to TD may provide better understanding of mechanisms underlying TD-induced selective neuronal loss. The present study tested the relationship of the selective neurodegeneration to rotarod performance over the 10 days of TD, temporal and regional responses of KGDHC including activity, mRNA and protein levels to TD.
In rats, TD induces behavioral abnormalities in “tight rope” walking, maze learning, avoidance tasks and motor performance (for references see Gibson et al., 1982). Early central muscarinic cholinergic lesion also occurs in TD rats (Barclay et al., 1981). Fewer behavioral studies have been conducted in TD mice. TD does alter open field behavior in mice. For example, a 37% and 39% decline in total distance and numbers of vertical movements occurs by day 7 of TD, respectively (Freeman et al., 1987). Rotarod performance is a reproducible and simple behavioral task. Although this behavioral test has been widely used in other animal models, it has not previously been tested in TD mouse model. In the present study, TD produced a 32% decline in the rotarod performance by day 8 of TD that was exaggerated to 82% and 97% loss by day 9 and 10, respectively. Although initial neuronal loss in SmTN also occurs at day 8 of TD (-29%), neuronal loss at TD9 is similar with that of TD8. TD does not induce severe neuronal loss until day 10 of TD (-90%) (ke et al., 2003). Thus, in addition to a moderate neuronal loss, other factors may contribute to exaggerated deficit in motor performance at TD9.
The temporal measurement of in situ KGDHC activity in SmTN and cortex of TD and control mice allowed us to identify early and regional response of KGDHC activity to TD. Previous studies of TD only assess KGDHC activity on homogenates of either whole brain (Gibson et al., 1984; Bubber et al., 2004) or various brain regions which are pathologically vulnerable and nonvulnerable (Butterworth et al., 1986; Sheu KF et al., 1998) at late stages of TD (≥TD8). The in situ measure of KGDHC activity employed in the present study complements previous measures of activities of thiamine-dependent enzymes in TD mice. The technique reveals morphological variations in activity. In addition, the activity may reflect in situ activity better than homogenized brain because the structure of the tissue is still apparent. A temporal and regional response of the KGDHC activity to TD was tested in both a non-vulnerable area (cortex) and a vulnerable region (SmTN) by the in situ KGDHC staining. Diminished KGDHC activity did not occur until 10 days of TD in either cortex or SmTN (Fig. 2). Moreover, the reduction in the KGDHC activity in SmTN (-52%) was more severe than cortex (-20%). These results are consistent with observations from a previous study that the reduction in the KGDHC activity at late stages is more dramatic in vulnerable areas than in spared regions (Sheu KF et al., 1998). Omission of thiamine from the assay mix may have revealed changes in activity at earlier stages of TD, since thiamine in the assay mix may have replenished the thiamine depleted by TD. In cultured lymphocytes, TD does not affect the KGDHC holo-protein but in the absence of TPP in the assay mix, the activity is much lower in the TD samples than controls (Pekovich et al., 1996). Thus, the actual KGDHC activities in the brains of TD mice are likely to be lower than that indicated from the in situ histochemistry assay in the presence of thiamine.
The temporal and regional response of gene expression to a treatment can be very revealing about cellular mechanisms. Previous studies of TD in cultured lymphoblasts suggest that thiamine has a direct effect on expression of the gene for transketolase (a thiamine dependent enzyme), but not the genes for the KGDHC proteins (Pekovich et al., 1996). Previous experiments on brains have not tested whether TD alters the gene expression of the three subunits of KGDHC. A previous study on thiamine-dependent transketolase reveals no significant changes in the mRNA level from the whole brain in response to TD (Sheu et al., 1996). However, TK mRNA declines up to 25% in TD rats in a regional dependent manner when assessed by an in situ hybridization method (Sheu et al., 1996). Thus, it is important to assess gene expression regionally. The present study tested the temporal and regional response of the mRNA level of KGDHC to TD by combining the micropunch technique (Karuppagounder et al., 2006) with real-time RT-PCR. TD induced an earlier reduction (TD6) in the gene expression of both E2k and E3 in SmTN. The gene expression of the E1k was not down-regulated until TD10. Regional and selective responses of microglia and neurons to TD have been studied extensively (Calingasan et al., 1998, 2000, Ke et al., 2003). Initial microglial activation (+16%) and neuronal loss (-29%) occur only after 8 or 9 days of TD (TD8-9) and only in SmTN, but not in the cortex (Ke et al., 2003). Thus, the down-regulation in gene expression of the two subunits of KGDHC precedes microglial activation and neuronal loss (TD8-9) in the vulnerable region-SmTN.
The pattern of gene expression is very different in the cortex which is not vulnerable to TD than in the SmTN. Only minimal changes occurred in cortex including a 25% increase in E1k mRNA level at TD8 and a 25% reduction in E3 mRNA level at TD2 and TD4. The mechanisms of these changes in the mRNA level of the E1k and E3 in cortex are unknown. The 25% increased E1k mRNA level at only TD8 is likely to be a compensatory effect. A DNA array study shows that the mRNA level of the E1k is increased in brains of patients with moderate AD and no significant change occurs in brains of patients with severe AD compared to control individuals (Blalock et al., 2004). A 40% increase of the E1k protein in an E2k deficient cell line also suggests a possible compensation in response to diminished E2k (Shi et al., 2005). Therefore, the 20% diminished KGDHC activity in cortex at TD10 is not likely due to altered gene expression of KGDHC. Post-translational modifications of the enzyme complex may account for such reduction in the KGDHC activity in cortex.
In cortex, determination of protein levels of the three subunits of KGDHC by Western blotting and immunocytochemical staining revealed no change in spite of the decline in KGDHC activities. These results are consistent with previous observations that TD does not alter immunoreactivities of KGDHC subunits in cortex (Sheu et al., 1998). These findings further support the suggestion that TD-induced reduction of KGDHC activity in cortex is due to post-translational modification of the complex. Since KGDHC consists of multiple copies of three subunits, TD may induce dissociation of subunits through post-translational modifications, which will diminish enzyme activity and leave protein contents of the subunits unaltered. For example, different oxidants selectively alter KGDHC subunits (Park et al., 1999). Assessment of TD-induced oxidative stress has mostly focused on vulnerable areas (Calingasan et al., 1999), but it may occur to a less extent in non-vulnerable regions such as cortex (Langlais et al., 1997). KGDHC is sensitive to a wide range of oxidants (Humphries et al., 1998; Gibson et al., 2002b; Jeitner et al., 2005). Thus, mild oxidative stress induced by TD may play an important role in mediating the loss of KGDHC activity in cortex. Further studies on post-translational modification of KGDHC are required to understand the mechanism for diminished KGDHC activity without loss of proteins in cortex during TD.
In SmTN, the two methods for assessing protein levels revealed different responses. The immunoreactivity of the three subunits of KGDHC determined by Western blotting was unaltered by 10 days of TD. In contrast, immunocytochemical staining of the three subunits of KGDHC showed significant reductions in their immunoreactivities in TD10 mice compared to the control. The decreased immunoreactivity of KGDHC subunits determined by immunocytochemistry parallels the reductions in the mRNA level of the three subunits and the overall KGDHC activity in SmTN at 10 days of TD. Although further experiments are required to understand these differences, it is likely that profound oxidative stress in SmTN at late stages of TD may modify the enzyme complex extensively and alter antigenicity of the subunits significantly. However, the denaturing gels used in these experiments may have masked these modifications, so that it was not possible to observe differences with the Western blots employed for these studies.
In summary, rotarod performance reveals a TD- induced impairment of behavior that corresponds to neuronal loss in SmTN. TD-induced changes in KGDHC enzyme activity, mRNA and immunoreactivity were time and region-dependent. Diminished KGDHC activity in cortex is likely due to TD-induced post-translational modification of the complex. Transcription and post-translational modifications of KGDHC subunits may contribute to diminished KGDHC activity in SmTN. Moreover, down-regulated mRNA levels of the E2k and E3 of KGDHC in SmTN is an early event in the cascade leading to selective neurodegeneration and behavioral abnormalities in TD.
Acknowledgement
This work was supported by NIH grants: AG14600, AG11921 and AG14930.
Abbreviations used
- AD
Alzheimer’s disease
- ANOVA
analysis of variance
- b2m
beta-2-microglobulin
- DAB
3,3′-diaminobenzidine
- ECL
enhanced chemiluminescence
- E1k
α-ketoglutarate dehydrogenase (EC 1.2.4.2)
- E2k
dihydrolipoyl succinyltransferase (EC 2.3.1.61)
- E3
dihydrolipoyl dehydrogenase (EC 1.6.4.3)
- KGDHC
α-ketoglutarate dehydrogenase complex
- NBT
nitroblue tetrazolium
- PD
Parkinson’s disease
- PMS
phenazine methosulfate
- SmTN
submedial thalamic nucleus
- TCA
tricarboxylic acid
- TD
thiamine deficiency
- TK
transketolase
- TPP
thiamine pyrophosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albers DS, Augood SJ, Park LC, Browne SE, Martin DM, Adamson J, Hutton M, Standaert DG, Vonsattel JP, Gibson GE, Beal MF. Frontal lobe dysfunction in progressive supranuclear palsy: evidence for oxidative stress and mitochondrial impairment. J Neurochem. 2000;74:878–81. doi: 10.1046/j.1471-4159.2000.740878.x. [DOI] [PubMed] [Google Scholar]
- Barclay LL, Gibson GE, Blass JP. Impairment of behavior and acetylcholine metabolism in thiamine deficiency. J Pharmacol Exp Ther. 1981;217:537–43. [PubMed] [Google Scholar]
- Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, Wynshaw-Boris A. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–71. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Bubber P, Ke ZJ, Gibson GE. Tricarboxylic acid cycle enzymes following thiamine deficiency. Neurochem. Int. 2004;45:1021–8. doi: 10.1016/j.neuint.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Butterworth RF, Giguere JF, Besnard AM. Activities of thiamine-dependent enzymes in two experimental models of thiamine-deficiency encephalopathy. 2. alpha-Ketoglutarate dehydrogenase. Neurochem. Res. 1986;11:567–77. doi: 10.1007/BF00965326. [DOI] [PubMed] [Google Scholar]
- Calingasan NY, Chun WJ, Park LC, Uchida K, Gibson GE. Oxidative stress is associated with region-specific neuronal death during thiamine deficiency. J. Neuropathol. Exp. Neurol. 1999;58:946–58. doi: 10.1097/00005072-199909000-00005. [DOI] [PubMed] [Google Scholar]
- Calingasan NY, Huang PL, Chun HS, Fabian A, Gibson GE. Vascular factors are critical in selective neuronal loss in an animal model of impaired oxidative metabolism. J. Neuropathol. Exp. Neurol. 2000;59:207–17. doi: 10.1093/jnen/59.3.207. [DOI] [PubMed] [Google Scholar]
- Calingasan NY, Park LC, Calo LL, Trifiletti RR, Gandy SE, Gibson GE. Induction of nitric oxide synthase and microglial responses precede selective cell death induced by chronic impairment of oxidative metabolism. Am. J. Pathol. 1998;153:599–610. doi: 10.1016/S0002-9440(10)65602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Characterization of progressive motor deficits in mice transgenic for the human Huntington’s disease mutation. J. Neurosci. 1999;19:3248–57. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW. Incipient Alzheimer’s disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci U S A. 2004;101:2173–8. doi: 10.1073/pnas.0308512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins P, Butterworth RF. Role of mitochondrial dysfunction and oxidative stress in the pathogenesis of selective neuronal loss in Wernicke’s encephalopathy. Mol. Neurobiol. 2005;31:17–25. doi: 10.1385/MN:31:1-3:017. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 1997. [Google Scholar]
- Freeman GB, Nielsen PE, Gibson GE. Effect of age on behavioral and enzymatic changes during thiamin deficiency. Neurobiol. Aging. 1987;8:429–34. doi: 10.1016/0197-4580(87)90037-6. [DOI] [PubMed] [Google Scholar]
- Gibson G, Barclay L, Blass J. The role of the cholinergic system in thiamin deficiency. In: Sable HZ, Gubler CJ, editors. Thiamin. New York Academy of Science; New York: 1982. pp. 382–403. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Kingsbury AE, Xu H, Lindsay JG, Daniel S, Foster OJ, Lees AJ, Blass JP. Deficits in a tricarboxylic acid cycle enzyme in brains from patients with Parkinson’s disease. Neurochem. Int. 2003;43:129–35. doi: 10.1016/s0197-0186(02)00225-5. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Ksiezak-Reding H, Sheu KF, Mykytyn V, Blass JP. Correlation of enzymatic, metabolic, and behavioral deficits in thiamin deficiency and its reversal. Neurochem. Res. 1984;9:803–14. doi: 10.1007/BF00965667. [DOI] [PubMed] [Google Scholar]
- Gibson G, Nielsen P, Mykytyn V, Carlson K, Blass J. Regionally selective alterations in enzymatic activities and metabolic fluxes during thiamin deficiency. Neurochem. Res. 1989;14:17–24. doi: 10.1007/BF00969752. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Park LC, Zhang H, Sorbi S, Calingasan NY. Oxidative stress and a key metabolic enzyme in Alzheimer brains, cultured cells, and an animal model of chronic oxidative deficits. Ann. N. Y. Acad. Sci. 1999;893:79–94. doi: 10.1111/j.1749-6632.1999.tb07819.x. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Zhang H. Abnormalities in oxidative processes in non-neuronal tissues from patients with Alzheimer’s disease. J. Alzheimers Dis. 2001;3:329–338. doi: 10.3233/jad-2001-3308. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Zhang H. Interactions of oxidative stress with thiamine homeostasis promote neurodegeneration. Neurochem. Int. 2002a;40:493–504. doi: 10.1016/s0197-0186(01)00120-6. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Zhang H, Xu H, Park LC, Jeitner TM. Oxidative stress increases internal calcium stores and reduces a key mitochondrial enzyme. Biochim. Biophys. Acta. 2002b;1586:177–89. doi: 10.1016/s0925-4439(01)00091-6. [DOI] [PubMed] [Google Scholar]
- Hakim AM, Pappius HM. The effect of thiamine deficiency on local cerebral glucose utilization. Ann. Neurol. 1981;9:334–9. doi: 10.1002/ana.410090404. [DOI] [PubMed] [Google Scholar]
- Humphries KM, Szweda LI. Selective inactivation of alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with 4-hydroxy-2-nonenal. Biochemistry. 1998;37:15835–41. doi: 10.1021/bi981512h. [DOI] [PubMed] [Google Scholar]
- Jeitner TM, Xu H, Gibson GE. Inhibition of the alpha-ketoglutarate dehydrogenase complex by the myeloperoxidase products, hypochlorous acid and mono-N-chloramine. J. Neurochem. 2005;92:302–10. doi: 10.1111/j.1471-4159.2004.02868.x. [DOI] [PubMed] [Google Scholar]
- Jones BJ, Roberts DJ. A rotarod suitable for quantitative measurements of motor incoordination in naive mice. Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1968;259:211. doi: 10.1007/BF00537801. [DOI] [PubMed] [Google Scholar]
- Karuppagounder SS, Shi Q, Xu H, Gibson GE. Changes in inflammatory processes associated with selective vulnerability following mild impairment of oxidative metabolism. Neurobiol Dis. 2007 doi: 10.1016/j.nbd.2007.01.011. In press (doi: 10.1016/j.nbd.2007.01.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke ZJ, Gibson GE. Selective response of various brain cell types during neurodegeneration induced by mild impairment of oxidative metabolism. Neurochem. Int. 2004;45:361–9. doi: 10.1016/j.neuint.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Ke ZJ, DeGiorgio LA, Volpe BT, Gibson GE. Reversal of thiamine deficiency-induced neurodegeneration. J. Neuropathol. Exp. Neurol. 2003;62:195–207. doi: 10.1093/jnen/62.2.195. [DOI] [PubMed] [Google Scholar]
- Kruse M, Navarro D, Desjardins P, Butterworth RF. Increased brain endothelial nitric oxide synthase expression in thiamine deficiency: relationship to selective vulnerability. Neurochem Int. 2004;45:49–56. doi: 10.1016/j.neuint.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Langlais PJ, Anderson G, Guo SX, Bondy SC. Increased cerebral free radical production during thiamine deficiency. Metab. Brain Dis. 1997;12:137–43. [PubMed] [Google Scholar]
- Liu JY, Timm DE, Hurley TD. Pyrithiamine as a substrate for thiamine pyrophosphokinase. J Biol Chem. 2006;281:6601–7. doi: 10.1074/jbc.M510951200. [DOI] [PubMed] [Google Scholar]
- Mastrogiacoma F, Lindsay JG, Bettendorff L, Rice J, Kish SJ. Brain protein and alpha-ketoglutarate dehydrogenase complex activity in Alzheimer’s disease. Ann. Neurol. 1996a;39:592–8. doi: 10.1002/ana.410390508. [DOI] [PubMed] [Google Scholar]
- Mastrogiacomo F, LaMarche J, Dozic S, Lindsay G, Bettendorff L, Robitaille Y, Schut L, Kish SJ. Immunoreactive levels of alpha-ketoglutarate dehydrogenase subunits in Friedreich’s ataxia and spinocerebellar ataxia type 1. Neurodegeneration. 1996b;5:27–33. doi: 10.1006/neur.1996.0004. [DOI] [PubMed] [Google Scholar]
- Park LC, Albers DS, Xu H, Lindsay JG, Beal MF, Gibson GE. Mitochondrial impairment in the cerebellum of the patients with progressive supranuclear palsy. J. Neurosci. Res. 2001;66:1028–34. doi: 10.1002/jnr.10062. [DOI] [PubMed] [Google Scholar]
- Park LC, Calingasan NY, Sheu KF, Gibson GE. Quantitative alpha-ketoglutarate dehydrogenase activity staining in brain sections and in cultured cells. Anal. Biochem. 2000;277:86–93. doi: 10.1006/abio.1999.4359. [DOI] [PubMed] [Google Scholar]
- Park LC, Zhang H, Sheu KF, Calingasan NY, Kristal BS, Lindsay JG, Gibson GE. Metabolic impairment induces oxidative stress, compromises inflammatory responses, and inactivates a key mitochondrial enzyme in microglia. J. Neurochem. 1999;72:1948–58. doi: 10.1046/j.1471-4159.1999.0721948.x. [DOI] [PubMed] [Google Scholar]
- Pekovich SR, Martin PR, Singleton CK. Thiamine pyrophosphate-requiring enzymes are altered during pyrithiamine-induced thiamine deficiency in cultured human lymphoblasts. J. Nutr. 1996;126:1791–8. doi: 10.1093/jn/126.7.1791. [DOI] [PubMed] [Google Scholar]
- Reed LJ. A trail of research from lipoic acid to alpha-keto acid dehydrogenase complexes. J. Biol. Chem. 2001;276:38329–36. doi: 10.1074/jbc.R100026200. [DOI] [PubMed] [Google Scholar]
- Schwab C, Steele JC, Akiyama H, McGeer PL. Distinct distribution of apolipoprotein E and beta-amyloid immunoreactivity in the hippocampus of Parkinson dementia complex of Guam. Acta. Neuropathol. (Berl) 1996;92:378–85. doi: 10.1007/s004010050533. [DOI] [PubMed] [Google Scholar]
- Sheu KF, Calingasan NY, Dienel GA, Baker H, Jung EH, Kim KS, Paoletti F, Gibson GE. Regional reductions of transketolase in thiamine-deficient rat brain. J. Neurochem. 1996;67:684–91. doi: 10.1046/j.1471-4159.1996.67020684.x. [DOI] [PubMed] [Google Scholar]
- Sheu KF, Calingasan NY, Lindsay JG, Gibson GE. Immunochemical characterization of the deficiency of the alpha-ketoglutarate dehydrogenase complex in thiamine-deficient rat brain. J. Neurochem. 1998;70:1143–50. doi: 10.1046/j.1471-4159.1998.70031143.x. [DOI] [PubMed] [Google Scholar]
- Shi Q, Chen HL, Xu H, Gibson GE. Reduction in the E2k subunit of the alpha-ketoglutarate dehydrogenase complex has effects independent of complex activity. J Biol Chem. 2005;280:10888–96. doi: 10.1074/jbc.M409064200. [DOI] [PubMed] [Google Scholar]
- Tretter L, Adam-Vizi V. Inhibition of Krebs cycle enzymes by hydrogen peroxide: A key role of α-ketoglutarate dehydrogenase in limiting NADH. J. Neurosci. 2000;20:8972–9. doi: 10.1523/JNEUROSCI.20-24-08972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor M, Adams RD, Collins GH. In: The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders due to Alcoholism and Malnutrition. Davies FA, editor. Philadelphia: 1989. [Google Scholar]
- Wang X, Wang B, Fan Z, Shi X, Ke ZJ, Luo J. Thiamine deficiency induces endoplasmic reticulum stress in neurons. Neuroscience. 2007;144:1045–56. doi: 10.1016/j.neuroscience.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]