Abstract
Previous research has demonstrated stability of cognitive ability and marked heritability during adulthood, but questions remain about the extent to which genetic factors account for this stability. We conducted a 35-year longitudinal assessment of general cognitive ability using the Armed Forces Qualification Test administered to 7,232 male twins in early adulthood and readministered to a subset of 1,237 twins during late middle age. The proportion of variance in cognitive functioning explained by genetic factors in young adulthood was .49 and it was .57 in late middle age. The correlation between the two administrations was .74 with a genetic correlation of 1.0, indicating that the same genetic influences operated at both times. Genetic factors were primarily responsible for stability and nonshared environmental factors for change. The genetic factors influencing cognition may change across other eras, but the same genetic influences are operating from early adulthood to late middle-age.
Spearman described a general factor common to many mental abilities that he called “g” (Neisser et al., 1996), which accounts for at least 50% of the variance across a range of mental tests and is one of the most replicated findings in psychology (Neisser et al., 1996). General cognitive ability correlates with numerous other characteristics, such as academic and occupational success (Neisser et al., 1996). There has been very little longitudinal, genetically informative research that addresses this trait during the period from early adulthood to late-middle adulthood, a period that has been quite understudied in many domains. There are three important questions about general cognitive ability during this period: What are the magnitudes of genetic and environmental influences during this period? Do the same or different genetic and environmental influences operate at different ages? Finally, what are the genetic and environmental influences on change and stability over time? To answer these questions unambiguously, a study must have longitudinal data, a long time interval, the same measure at each assessment, and a genetically informative sample. To our knowledge, no study to date has met all of these criteria. Midlife remains a sorely understudied period, with the bulk of developmental research investigating childhood, adolescence, or old age. Most of the extant genetically informative studies are cross-sectional, and the few longitudinal studies cover relatively short time intervals. In many studies, different measures of g are used at different ages, and each measure will have its own unique method variance.
What are the Magnitudes of Genetic and Environmental Influences During This Period?
Bouchard and McGue (2003) reviewed the behavioral genetics studies of cognitive ability and suggested that the relative strength of genetic and environmental influences on intelligence varies with age. A number of investigators have concluded that the influence of genetic factors increases with age, whereas the influence of shared environmental factors decreases with age, at least until middle age (McCartney, Harris, & Bernieri, 1990; McGue, Bouchard, Iacono, & Lykken, 1993; Plomin & Spinath, 2004). Vogler (2006) suggested that the heritability of cognitive functioning appears to be relatively stable over time with some decline in heritability in older cohorts and the results of several studies support this (Finkel, Pedersen, McGue, & McClearn, 1995; Finkel, Pedersen, Plomin, & McClearn, 1998; McGue & Christensen, 2002; Pedersen, Plomin, Nesselrode, & McClearn, 1992; Plomin, Pedersen, Lichtenstein, & McClearn, 1994; Posthuma, de Geus, & Boomsma, 2001). In a study with assessments at ages 50, 60, 70, and 80 years, Reynolds et al. (2005) found an inverted U-shaped pattern for genetic variance; in other words, genetic variance increased somewhat from age 50 to 60 and then decreased. Among studies of adults, the limited number that utilized a true longitudinal design, the relatively brief time intervals utilized, and the preponderance of subjects over the age of 65 years essentially preclude a definitive answer to the question of whether the magnitude of genetic and environmental influences changes from early adulthood to late middle age.
Do the Same or Different Genetic and Environmental Influences Operate at Different Ages?
The extent to which genetic influences change at different ages is not well understood for most traits, but it has important implications for gene-association studies. If the sample being studied is heterogeneous with regard to age, and different genes are influencing the trait at different ages, this heterogeneity would increase the difficulty of detecting an association. Because an individual's genotype is fixed at conception, it is tempting to think of the influence of genetic factors as a stable, static phenomenon. McClearn (1993) quotes Francis Galton's observation, made in 1876: “it must be borne in mind that the divergence of development, when it occurs, need not be ascribed to the effect of different nurtures, but it is quite possible that it may be due to the appearance of qualities inherited at birth, though dormant” (Galton, 1876, pp. 402). Although the individual's genotype does not change during the lifespan, different genes may be expressed at different developmental periods (Vogler, 2006). As Plomin et al. (1993) pointed out, even if a trait is equally heritable at two ages, it is not necessarily stable with regard to its genetic determinants, and different heritabilities at two ages do not necessarily reflect instability of genetic influences.
Previous longitudinal studies addressing the question of whether different genetic factors were operating at different ages included children only. A number of studies have found evidence for new genetic influences arising over the course of childhood and adolescence (Cardon, Fulker, Defries, & Plomin, 1992; Defries, Plomin, & Labuda, 1987; Eaves, Long, & Heath, 1986; Petrill, Lipton, Hewitt, & Plomin, 2004; Wilson & Matheny, 1983)), whereas other studies have not (Bartels, Rietveld, Van Baal, & Boomsma, 2002; Polderman et al., 2006).
What are the Genetic and Environmental Influences on Change and Stability over Time?
McGue and Christensen (2002), using a cohort-sequential design, examined a sample of twins over age 70 on a battery of cognitive measures and found a heritability of .06 for the linear change over four occasions spanning 6 years. In a longitudinal study of subjects in the second half of life, Plomin et al. (1994) found that genetic factors accounted for nearly 90% of the stability. In a study of latent growth parameters at age 65, Reynolds et al. (2005) found that the heritability of linear change was .01 and that the nonshared environment explained .99 of the variance. The quadratic trend (acceleration of cognitive change over time or “change in the change”) had a heritability of .43 and a contribution from the nonshared environment of .57. DeFries et al. (1987) found that the genetic contribution to the cognitive stability observed between age 4 and adulthood was .28.
We are unaware of any genetically informative studies that span the period from early adulthood/adolescence to late middle age that utilize a true longitudinal design and repeated administration of the same cognitive measure. The Vietnam Era Twin Study of Aging (VETSA; Kremen et al., 2006) provides a unique opportunity to investigate the determinants of cognitive development from adolescence/early adulthood to late middle age, an important but understudied period in developmental aging research.
Method
Subjects
Age 20 Armed Forces Qualification Test (AFQT) scores were obtained from military records for 7,232 men (1,669 identical pairs, 1,303 fraternal pairs, and 1,288 unpaired twins) from the Vietnam Era Twin Registry (VETR). Age 55 AFQT scores were obtained from the 1,237 men who participated in VETSA. VETSA participants were assessed at one of the two testing sites (Boston University and the University of California, San Diego) or, in rare circumstances, elected to have a research assistant travel to them. Informed consent was obtained after the nature and possible consequences of the studies were explained. The study was approved by the Boston University and University of California, San Diego, Institutional Review Boards. Zygosity for VETR members was initially determined by a combination of questionnaire and blood-group type. Eisen, Neuman, Goldberg, Rice, and True (1989) reported that zygosity determined utilizing this protocol was 95% accurate. VETSA participants underwent additional zygosity testing via analysis of 25 microsatellite markers.
VETR members are representative of all twins who served in the military during the Vietnam War on a variety of socio-demographic variables (Eisen, True, Goldberg, Herderson, & Robinette, 1987; Goldberg, True, Eisen, Henderson, & Robinette, 1987). Of the VETSA subjects, 48.5% of those that we contacted agreed to participate. Given that participation entailed a 2- or 3-day trip to Boston or San Diego, we believe that the participation rate is reasonable. Moreover, we have carefully compared participants to nonparticipants.
Studies have shown that differences in socioeconomic status between the military veterans and nonveterans, when observed, are modest in size, contrary to a popular assumption that military men came from lower socioeconomic strata (Boulanger, Kadushin, & Martin, 1981; Cooper, 1977). The demographic characteristics of VETSA participants are very similar to those of 2003 U.S. Census data for men in their 50s in regard to education, median self-income, ethnicity, marital status, and employment. VETSA participants do not differ significantly from the overall subject pool in regard to age at induction, race, marital status, education at induction, branch of military, Vietnam service, combat experience, or lifetime prevalence of the more common types of psychopathology. Although the VETSA sample was drawn from individuals who were members of the military, two-thirds of our participants were not stationed in a war zone.
Measures
AFQT
The AFQT is a 50-min paper-and-pencil test consisting of 100 multiple-choice items administered just before military induction (Bayroff & Anderson, 1963). It is highly correlated with measures of general cognitive ability (Uhlaner & Bolanovich, 1952). For example, McGrevy, Knouse, and Thompson (1974) found a correlation of .84 (after correcting for restriction of range) between the AFQT and Wechsler Adult Intelligence Scale (Wechsler, 1955) scores. Items equally represent the four domains of vocabulary, arithmetic word problems, knowledge and reasoning about tools and mechanical relations, and visual-spatial organization (Uhlaner & Bolanovich, 1952). We administered the same AFQT version given to participants approximately 35 years earlier.
Analysis of Twin Data
AFQT scores were recorded as percentiles based on military norms. For the purposes of genetic model fitting, the raw percentiles were transformed to their normal deviates. We conducted biometrical modeling to quantify genetic and environmental determinants of the traits being studied. In biometric analysis, we assumed that observed variation in the trait is due to a mixture of latent additive genetic (A), shared environmental (C), and nonshared environmental (E) factors. The additive genetic component of variance, usually called “heritability,” is due to genes with influences that combine additively. The common environmental variance includes all environmental influences shared by members of a twin pair that serve to make the twins similar to one another (e.g., the family environment). The unique environment entails nonshared environmental factors that make members of a twin pair different from one another, plus measurement error. Our analyses utilized the simplest and most frequently applied multivariate model, the correlated factors model. In the correlated factors model, in addition to the A, C, and E parameters of the univariate model, we also add correlations between latent variables, rA, rC, and rE. These symbols represent the genetic correlation, the shared environmental correlation and the nonshared environmental correlation, respectively.
Results
The mean age of participants at the baseline AFQT administration was 19.8 years (SD = 1.5 years; range = 16–31 years). Mean age for readministration during VETSA was 55.4 years (SD = 2.48 years; range 51–60 years). The correlation of AFQT at age 20 with AFQT at age 55 was .74 (p < .001). Mean baseline AFQT score (n = 7,232) was 54.6 (SD = 23.5). Mean AFQT score at age 55 (n = 1,237) was 64.1 (SD = 20.9). Change could only be computed for the 1,237 subjects who participated in VETSA. Although the magnitude of the change was small (3 percentile points; SD = 0.16), the mean at age 55 was significantly higher than at age 20 (paired samples t test = −6.26, p < .001). At age 55, the correlation between the AFQT and the Wechsler Abbreviated Scale of Intelligence IQ was .84 (after correcting for restriction of range).
Maximum likelihood estimates of correlations for monozygotic (MZ) and dizygotic (DZ) twins are given in Table 1; these results indicate the principal trends in the data. The cross-pair correlations for MZ twins are all greater than those for DZ twins, suggesting that genetic effects play some role in individual differences. However, cross-twin correlations for DZ twins exceed one half of their respective MZ correlations, which may reflect environmental effects shared by twins, genetic consequences of assortative mating, or both (Eaves, 1982). The cross-twin, cross-age correlations for MZ twins are similar to the cross-twin, within-age correlations, indicating a very high level of long-term stability in the effects of familial (genetic, shared environmental, or both) influences.
TABLE 1. Maximum Likelihood Estimates of Correlations Between Twins' Armed Forces Qualification Test Scores at Age 20 and at Age 55.
| Twin | Twin 1 at age 20 | Twin 2 at age 20 | Twin 1 at age 55 |
|---|---|---|---|
| Monozygotic twins | |||
| Twin 2 at age 20 | .75 | — | — |
| Twin 1 at age 55 | .74 | .70 | — |
| Twin 2 at age 55 | .69 | .75 | .75 |
| Dizygotic twins | |||
| Twin 2 at age 20 | .52 | — | — |
| Twin 1 at age 55 | .70 | .42 | — |
| Twin 2 at age 55 | .45 | .79 | .47 |
Note. Age 20 Armed Forces Qualification Test (AFQT) scores served as baseline. The Age 55 AFQT scores were taken during the Vietnam Era Twin Study of Aging. All correlations were statistically significant (p < .001).
We obtained maximum-likelihood estimates of the contributions of additive genetic effects (A), environmental effects shared by twins (C), and nonshared (individual-specific) environmental effects unique to individual twins (E) by using the structural equation modeling program Mx (Neale, Boker, Xie, & Maes, 2003). Several structural models were fitted to the data, and likelihood-ratio tests were conducted for differences between means and covariance structures (see Table 2). Model 1 allowed each variance, covariance, and mean to take its own value in MZ and DZ twins. Subsequently, reduced models with fewer parameters were tested to determine whether they fit the data as well as the full model (Model 1). We compared Models 2 through 6, presented in Table 2, to Model 1. These tests indicated that there is no mean difference in AFQT scores between twins randomly designated as Twin 1 and Twin 2 or between MZ and DZ twins. These tests also indicated a significant mean difference between AFQT scores at baseline and follow-up and a significant difference in covariances for MZ versus DZ pairs, as would be expected if there were genetic effects. We see that the full bivariate-saturated ACE model (Model 6), which allows means to differ only between baseline and follow-up, gives a fit that is comparable to the most general model (i.e., Model 1) that imposes no constraints. Although Model 6 can be reduced by omitting the genetic (p = .32) and shared environmental (p = .26) effects unique to follow-up, we took the conservative approach of including these parameters to preclude any bias in the estimate of heritability that may occur at follow-up.
TABLE 2. Statistical Comparison of Models 1 Through 6.
| Model | Description | −2LL | k | LR | Δdf | p | AIC |
|---|---|---|---|---|---|---|---|
| Model 1 | Saturated model | 15,097.344 | 28 | — | — | — | — |
| Model 2 | Means equal for Twins 1 and 2 | 15,101.134 | 24 | 3.790 | 4 | .435 | −4.210 |
| Model 3 | Means equal for monozygotic and dizygotic twins | 15,099.980 | 24 | 2.636 | 4 | .620 | −5.364 |
| Model 4 | Means equal for induction and follow-up | 15,171.044 | 24 | 73.700 | 4 | <.001 | 65.700 |
| Model 5 | Variances and covariances equal for monozygotic and dizygotic twins | 15,340.484 | 18 | 243.141 | 10 | <.001 | 223.141 |
| Model 6 | Bivariate ACE model, means differ only between baseline and follow-up | 15,122.444 | 11 | 25.100 | 17 | .092 | −8.900 |
Note. The table presents the following values: −2 times the log likelihood (−2LL), the number of free parameters in the model (k), the results of a likelihood ratio test (LR, distributed as a chi-square statistic), the change in degrees of freedom of the saturated model and submodel (Δdf), the significance of the chi-square test (p), and an index of the balance between goodness of model fit and parsimony (Akaike's information criterion, AIC). The bivariate ACE model includes additive genetic factors (A), shared family environment (C) and nonshared environment (E).
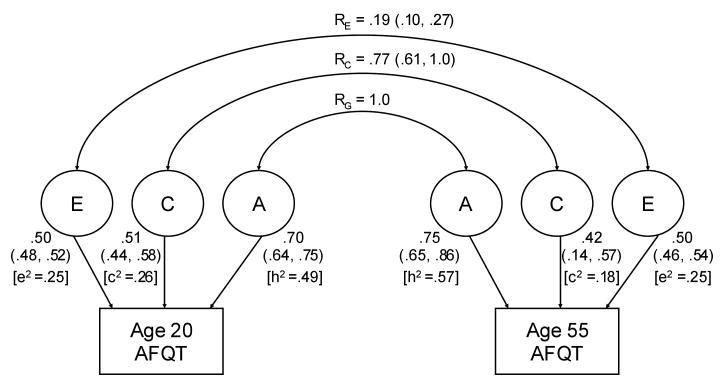
Effects of A, C, and E at baseline and at follow-up derived from the full bivariate model are presented in Figure 1 as both path coefficients and proportions of variance; correlations between the latent factors (e.g., shared environmental influences) are also presented. There was no statistically significant difference between the percentage of variance in AFQT scores explained by genetic effects at age 20 (49%) and the percentage of variance in AFQT scores explained by genetic effects at age 55 (57%). However, before concluding that these two values are the same, it is prudent to consider the statistical power of the comparison. Therefore, we calculated the power of the test for differences in heritability assuming that the heritability during early adulthood is .5 and heritability at follow-up is .6, and the genetic correlation between occasions is assumed to be 1.00. Sample sizes approximated those of the current study; that is, 200 MZ and 170 DZ pairs were measured on both occasions, and a further 1,800 MZ and 1,500 DZ pairs were measured only at induction. The power of the test for heterogeneity of heritability over waves (α = .05) is 43%. This result indicates that, even if the observed values are truly different from one another, with our sample size, we would fail to conclude that the difference is significant 57% of the time.
Fig. 1.
Bivariate correlated factors model for Armed Forces Qualification Test (AFQT) scores at baseline (age 20) and during the Vietnam Era Twin Study of Aging (age 55). A = additive genetic factors; C = shared family environment; E = nonshared environment; rG = genetic correlation; rC = shared environment correlation; rE = nonshared environment correlation. Values adjacent to lines are path coefficients; values in parentheses are the 95% confidence intervals; values in brackets are standardized variance components (h2 = genetic influences; c2 = shared environment; e2 = nonshared environment).
The correlation between genetic influences at baseline and late middle age did not differ significantly from unity. The correlation between shared environmental effects in early adulthood and late middle age was .77. The shared environmental and genetic influences that affected twins' performance as young adults persisted into later life. The phenotypic correlation between AFQT at age 20 and at age 55 was .74; modeling results indicated that 71.3% of this phenotypic correlation is attributable to genetic influences operating at both occasions, 22.4% is due to shared environmental influences on both occasions, and 6.3% due to nonshared environmental influences. Although the average change in AFQT scores from age 20 to age 55 was significant, but rather small, we conducted analyses to determine the extent to which genetic and shared environmental factors contributed to change. These two factors had very modest effects on change during this interval (1.7% and 15.2% for genetic and shared environmental influences, respectively). By far the greatest influence on change in AFQT was the nonshared environment, which accounted for 83.1% of the variance in change.
Discussion
Our findings with regard to the stability of an individual's general cognitive ability over 3.5 decades of adulthood indicate that there is a slight, but significant, increase in the level of performance and there is a substantial coefficient of stability (i.e., correlation from baseline to follow-up). The pattern of correlations within individuals, across twin pairs, and across twins and across times provides for some interesting additional conclusions. If one wanted to predict how a given individual would score on the AFQT when he is 55 years old, one would do approximately equally well by predicting from the individual's own score at age 20 or from his MZ cotwin's score at age 55. MZ cotwins are as similar to one another when measured at the same time as an individual is similar to himself over the 35-year interval. The observed genetic and shared environmental correlations indicate no new genetic or shared environmental influences that manifest themselves over the extended interval of adulthood. This genetic correlation is different from several studies of children, adolescent twins, and adoptees assessed at different ages that suggested that, during some developmental periods, there are changes in the genes that are influencing cognitive ability. However, it is also possible that the use of different measures at different occasions in those studies of younger subjects resulted in the lower genetic correlations. Although we found that the same genes were operating over the 35-year period from age 20 to age 55, it is quite possible that some different genetic influences could be operating 35 years later, when subjects are 90 years old. Indeed it is not known whether the same genes would be operating 10 or even 5 years later. Although the estimates of heritability during early adulthood and late middle age were 49% and 57%, respectively, our data do not provide the statistical power necessary to determine whether heritability increases significantly.
The cross-temporal correlation for nonshared environmental effects, though significant, is only moderate. This result is consistent with the view that nonshared environmental effects are highly age-specific; that is, much of the variation within MZ pairs reflects short-term environmental influences contributing to differences in test performance across time. Our data confirm this view: The average correlation between early adult and follow-up scores (.74) is similar to the correlation between MZ twins measured at the same time (.71). From the genetic and shared environmental correlations, it is possible to infer that the observed phenotypic stability primarily reflects genetic and shared environmental influences; 71.3 % of the correlation between age 20 and age 55 scores is due to genetic factors, 22.4% of the correlation is due to shared environmental factors, and 6.3% of the correlation is due to nonshared environments.
Although there was considerable stability over time, there was still a substantial amount of variability in the entire sample: The greatest increase in AFQT score was 57 points, and the greatest decrease was 55 points. There was a change of at least 10 points (0.5 SD) among 44.6% of the subjects, and a change of at least 20 points (1.0 SD) among 17.6% of the subjects. Changes during this time were overwhelmingly (83.1%) due to aspects of the environment that were not shared by twins. That is, the same type of influences that make identical twins different from each other are the ones that make an individual perform differently at age 55 from how he performed at age 20.
The VETSA sample includes only men, so our findings may not generalize to women. Our likelihood computations assume that data are missing at random or missing completely at random; however, we know that is not the case. Individuals who scored below the 10th percentile did not qualify for induction. VETSA participants also had slightly higher AFQT scores at age 20 than VETR members who did not participate in VETSA. This difference may be due to factors such as people of higher cognitive ability being more inclined to participate in scientific research and being easier to locate. In principle, correction for biases of ascertainment and follow-up are possible as long as we can specify the selection criteria at both stages. However, it has been shown that far more extreme selection typically does not lead to substantial biases in the patterns of twin correlation (Martin & Wilson, 1982).
The findings reported here regarding cognitive ability have some interesting similarities and differences with results we obtained for another widely studied human characteristic: body mass index (BMI). In a sample of twins from the Vietnam Era Twin Registry that overlaps somewhat with our VETSA sample, the average BMI increased dramatically from age 20 to age 48, from a mean of 22.7 (SD = 3.0) to a mean of 27.8 (SD = 4.2; Franz et al., 2007). Genetic factors were the primary source of variation in BMI at age 20 and age 48, but unlike our findings for cognitive ability, the heritability of BMI decreased significantly from age 20 to age 48 (heritability 76% and 59%, respectively). Shared environmental influences accounted for a negligible (7%, n.s.) amount of variance in BMI at each time point. The phenotypic correlation for BMI across the 28-year interval was .52. The genetic correlation of the overlap between age 20 and age 48 BMI was estimated at .60, indicating that some, but not all, of the genetic influence on BMI is accounted for by the same genes over time. Some of the genes that influence BMI during middle age were not operating in early adulthood. This result is quite different from that for general cognitive ability.
Our results support the remarkable conclusion that virtually all of the genetic influences on cognitive performance expressed in young adulthood are still manifest 35 years later. We found little support for the possibility that “new” genes exert their influence over the course of adult development. A practical implication of this finding is that studies seeking to identify specific alleles that influence general cognitive ability, such as genome-wide association studies, may safely combine subjects from age 20 to 60 years. However, it is quite possible that, in older age, genetic and environmental influences that were not operating earlier may begin to do so. The relative influence of the nonshared environment remained stable over time, but there was only very modest overlap of the features of the nonshared environment influencing cognition in early adulthood versus late middle age. These findings provide long-term longitudinal evidence that challenges suggestions that decades of exposure to environmental influences over the course of the lifespan might attenuate the influence of genetic factors.
Acknowledgments
The Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Dr. Jordan Grafman provided AFQT materials. This VETSA project is supported by National Institutes of Health/National Institute on Aging Grants R01 AG018384, R01 AG018386, R01 AG022381, and R01 AG022982. We gratefully acknowledge the cooperation and participation of the members of the VET Registry.
References
- Bartels M, Rietveld MJH, Van Baal GCM, Boomsma DI. Genetic and environmental influences on the development of intelligence. Behavior Genetics. 2002;32:237–249. doi: 10.1023/a:1019772628912. [DOI] [PubMed] [Google Scholar]
- Bayroff AG, Anderson AA. Development of Armed Forces Qualification Tests 7 and 8. Arlington, VA: U.S. Army Research Institute; 1963. Technical Research Report 1122. [Google Scholar]
- Boulanger G, Kadushin C, Martin J. Legacies of Vietnam: Vol IV Long-term stress reactions. Washington, DC: U.S. Government Printing Office; 1981. [Google Scholar]
- Bouchard TJ, McGue M. Genetic and environmental influences on human psychological differences. Journal of Neurobiology. 2003;54:4–45. doi: 10.1002/neu.10160. [DOI] [PubMed] [Google Scholar]
- Cooper RVL. Military manpower and the all-volunteer force. Santa Monica, CA: RAND; 1977. [Google Scholar]
- Cardon LR, Fulker DW, Defries JC, Plomin R. Continuity and change in general cognitive-ability from 1 to 7 years of age. Developmental Psychology. 1992;28:64–73. [Google Scholar]
- Defries JC, Plomin R, Labuda MC. Genetic stability of cognitive-development from childhood to adulthood. Developmental Psychology. 1987;23:4–12. [Google Scholar]
- Eaves LJ. The utility of twins. In: Anderson VE, Hauser WA, Penry JK, Sing CF, editors. Genetic basis of epilepsies. New York: Raven; 1982. pp. 249–276. [Google Scholar]
- Eaves LJ, Long J, Heath AC. A theory of developmental-change in quantitative phenotypes applied to cognitive-development. Behavior Genetics. 1986;16:143–162. doi: 10.1007/BF01065484. [DOI] [PubMed] [Google Scholar]
- Eisen S, True W, Goldberg J, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: Method of construction. Acta Geneticae Medicae et Gemellologicae. 1987;36:61–66. doi: 10.1017/s0001566000004591. [DOI] [PubMed] [Google Scholar]
- Eisen S, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam Era Twin Registry: An approach using questionnaires. Clinical Genetics. 1989;35:423–432. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Finkel D, Pedersen NL, McGue M, McClearn GE. Heritability of cognitive-abilities in adult twins: Comparison of Minnesota and Swedish data. Behavior Genetics. 1995;25:421–431. doi: 10.1007/BF02253371. [DOI] [PubMed] [Google Scholar]
- Finkel D, Pedersen NL, Plomin R, McClearn GE. Longitudinal and cross-sectional twin data on cognitive abilities in adulthood: The Swedish adoption/twin study of aging. Developmental Psychology. 1998;34:1400–1413. doi: 10.1037//0012-1649.34.6.1400. [DOI] [PubMed] [Google Scholar]
- Franz CE, Grant MD, Jacobson KC, Kremen WS, Eisen SA, Xian H, et al. Response to genetics of body mass stability and risk for chronic disease: A 28-year longitudinal study. Twin Research and Human Genetics. 2007;10:893. doi: 10.1375/twin.10.4.537. [DOI] [PubMed] [Google Scholar]
- Galton F. The history of twins as a criterion of the relative powers of nature and nurture. The Journal of the Royal Anthropological Institute. 1876;5:391–406. [Google Scholar]
- Goldberg J, True W, Eisen S, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: Ascertainment bias. Acta Geneticae Medicae et Gemellologicae. 1987;36:67–78. doi: 10.1017/s0001566000004608. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Thompson-Brenner H, Leung YM, Grant MD, Franz CE, Eisen SA, et al. Genes, environment, and time: The Vietnam Era Twin Study of Aging (VETSA) Twin Research and Human Genetics. 2006;9:1009–1022. doi: 10.1375/183242706779462750. [DOI] [PubMed] [Google Scholar]
- Martin NG, Wilson SR. Bias in the estimation of heritability from truncated samples of twins. Behavior Genetics. 1982;12:467–472. doi: 10.1007/BF01065638. [DOI] [PubMed] [Google Scholar]
- McCartney K, Harris MJ, Bernieri F. Growing up and growing apart: A developmental meta-analysis of twin studies. Psychological Bulletin. 1990;107:226–237. doi: 10.1037/0033-2909.107.2.226. [DOI] [PubMed] [Google Scholar]
- McClearn GE. Behavior genetics: The last century and the next. In: Plomin R, McClearn GE, editors. Nature, nurture, and psychology. Washington, DC: American Psychological Association; 1993. pp. 27–51. [Google Scholar]
- McGrevy DF, Knouse SB, Thompson RA. Relationships among an individual intelligence test and two air force screening and selection tests. San Antonio, TX: Personnel Research Division, Air Force Human Resources Laboratory, Brooks Air Force Base; 1974. Technical Report AFHRL-TR-74-25. [Google Scholar]
- McGue M, Bouchard TJ, Jr, Iacono WG, Lykken DT. Behavior genetics of cognitive abilities: A life-span perspective. In: Plomin R, McClearn GE, editors. Nature, nurture, and psychology. Washington, DC: American Psychological Association; 1993. pp. 59–76. [Google Scholar]
- McGue M, Christensen K. The heritability of level and rate-of-change in cognitive functioning in Danish twins aged 70 years and older. Experimental Aging Research. 2002;28:435–451. doi: 10.1080/03610730290080416. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. 6th. Richmond: Virginia Commonwealth University, Department of Psychiatry; 2003. [Google Scholar]
- Neisser U, Boodoo G, Bouchard TJ, Boykin AW, Brody N, Ceci SJ, et al. Intelligence: Knowns and unknowns. American Psychology. 1996;51:77–101. [Google Scholar]
- Pedersen NL, Plomin R, Nesselrode JR, McClearn GE. A quantitative genetic analysis of cognitive abilities during the second half of the life span. Psychological Science. 1992;3:346–353. [Google Scholar]
- Petrill SA, Lipton PA, Hewitt JK, Plomin R. Genetic and environmental contributions to general cognitive ability through the first 16 years of life. Developmental Psychology. 2004;40:805–812. doi: 10.1037/0012-1649.40.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, Emde RN, Braungart JM, Gampos J, Corley R, Fulker DW, et al. Genetic change and continuity from fourteen to twenty months: The MacArthur Longitudinal Twin Study. Child Development. 1993;64:1354–1376. [PubMed] [Google Scholar]
- Plomin R, Pedersen NL, Lichtenstein P, McClearn GE. Variability and stability in cognitive abilities are largely genetic later in life. Behavior Genetics. 1994;24:207–215. doi: 10.1007/BF01067188. [DOI] [PubMed] [Google Scholar]
- Plomin R, Spinath FM. Intelligence: Genetics, genes, and genomics. Journal of Personality and Social Psychology. 2004;86:112–129. doi: 10.1037/0022-3514.86.1.112. [DOI] [PubMed] [Google Scholar]
- Polderman TJ, Gosso MF, Posthuma D, Van Beijsterveldt TC, Heutink P, Verhulst FC, et al. A longitudinal twin study on IQ, executive functioning, and attention problems during childhood and early adolescence. Acta Neurologica Belgica. 2006;106:191–207. [PubMed] [Google Scholar]
- Posthuma D, de Geus EJ, Boomsma DI. Perceptual speed and IQ are associated through common genetic factors. Behavior Genetics. 2001;31:593–602. doi: 10.1023/a:1013349512683. [DOI] [PubMed] [Google Scholar]
- Reynolds CA, Finkel D, McArdle JJ, Gatz M, Berg S, Pedersen NL. Quantitative genetic analysis of latent growth curve models of cognitive abilities in adulthood. Developmental Psychology. 2005;41:3–16. doi: 10.1037/0012-1649.41.1.3. [DOI] [PubMed] [Google Scholar]
- Uhlaner JE, Bolanovich DJ. Development of the Armed Forces Qualification Test and predecessor Army screening tests, 1946–1950. Washington, DC: Personnel Research Section, Department of the Army; 1952. [Google Scholar]
- Vogler GP. Behavior genetics and aging. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. Amsterdam: Academic Press; 2006. pp. 41–55. [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale. New York: Psychological Corporation; 1955. [Google Scholar]
- Wilson RS, Matheny AP. Assessment of temperament in infant twins. Developmental Psychology. 1983;19:172–183. [Google Scholar]