Abstract
Abnormalities in oxidative metabolism and reductions of thiamine-dependent enzymes accompany many age-related neurodegenerative diseases. Thiamine deficiency (TD) produces a cascade of events including mild impairment of oxidative metabolism, activation of microglia, astrocytes and endothelial cells that leads to neuronal loss in select brain regions. The earliest changes occur in a small, well-defined brain region, the submedial thalamic nucleus (SmTN). In the present study, a micropunch technique was used to evaluate quantitatively the selective regional changes in mRNA and protein levels. To test whether this method can distinguish between changes in vulnerable and non-vulnerable regions, markers for neuronal loss (NeuN) and endothelial cells (eNOS) and inflammation (IL-1β, IL-6 and TNF-α) in SmTN and cortex of control and TD mice were assessed. TD significantly reduced NeuN and increased CD11b, GFAP and ICAM-1 immunoreactivity in SmTN as revealed by immunocytochemistry. When assessed on samples obtained by the micropunch method, NeuN protein declined (-49%), while increased mRNA levels were observed for eNOS (3.7 fold), IL-1β (43 fold), IL-6 (44 fold) and TNF-α (64 fold) in SmTN with TD. The only TD-induced change that occurred in cortex with TD was an increase in TNF-α (22 fold) mRNA levels. Immunocytochemical analysis revealed that IL-1β, IL-6 and TNF-α protein levels increased in TD brains and colocalized with glial markers. The consistency of these quantitative results with immunocytochemical measurements validates the micropunch technique. The results demonstrate that TD induces quantitative, distinct inflammatory responses and oxidative stress in vulnerable and non-vulnerable regions that may underlie selective vulnerability.
Keywords: Oxidative stress, Submedius thalamus, Neurodegeneration, Inflammation
Introduction
Thiamine deficiency (TD) models the selective neurodegeneration that accompanies mild impairment of oxidative metabolism (Gibson et al., 1999). Reductions in thiamine-dependent enzymes have been implicated in many neurological disorders including Alzheimer’s disease (Gibson et al., 1988; Mastrogiacomo et al., 1993), Parkinson’s disease (Mizuno et al., 1994), progressive supranuclear palsy (Albers et al., 2000), Huntington’s disease (Klivenyi et al., 2004), Friedreich’s ataxia and spinocerebellar ataxia type 1 (Mastrogiacomo et al., 1996) and Wernicke-Korsakoff syndrome (Blass and Gibson, 1977; Butterworth et al., 1993). Disturbances in thiamine metabolism selectively damage certain brain structures. In humans, TD causes bilateral lesions in the midline, medial and intralaminar nuclei of the thalamus, mammillary bodies, periaqueductal grey, cerebellar vermis and brainstem periventricular regions (Trovik et al., 1982; Victor et al, 1989).
TD causes a time-dependent, selective neuronal death in specific brain regions, while other cell types are either activated or unaffected (Ke and Gibson, 2004). Experimental TD in rodents produces bilateral lesions in thalamus and mammillary bodies, including medial dorsal thalamus, anterior medial thalamus, pulvinar thalamus, sparing the other brain regions (Langlais et al., 1996; Ke and Gibson, 2004; Todd and Butterworth, 1998; Troncoso et al., 1981; Watanabe and Kanabe, 1978;). The earliest neuronal death occurs in a small, well-defined brain region, the submedial thalamic nucleus (SmTN) (Calingasan et al., 2000; Ke et al., 2003; Zhang et al., 1995). The submedial thalamus is also called thalamus submedius or gelatinous nuclei (Franklin and Paxinos, 1997; Paxinos and Watson, 1986).
Inflammation occurs in many neurodegenerative diseases and possesses unique pathology and symptoms. The pathophysiology of TD-induced damage is unknown, but evidence strongly suggests that oxidative stress and inflammation lead to neuronal loss. Regionally selective neuronal death, activation of other cell types such as astrocytes, microglia and endothelial cells, vascular changes, inflammatory responses and oxidative stress in TD recapitulates many neurodegenerative diseases (Ke and Gibson, 2004). Our previous studies and those of others show that TD elevates markers of oxidative stress, nitric oxide synthase (NOS), intracellular adhesion molecule-1 (ICAM-1) in early stages and induces 4-hydroxy-2-nonenal (HNE) and hemeoxygenase-1 (HO-1) in late stages (Calingasan et al., 1999; Ke and Gibson, 2004; Kruse et al., 2004). TD increases the inflammatory molecules CD40 and CD40L. Furthermore, genetic deletion of molecules involved in either oxidative stress (ICAM-1 and eNOS) or inflammation (CD40 and CD40L) delayed TD-induced neuronal death (Ke et al., 2005 a, b). Glial cells are critical components of immunological insults to neurons. Astrocytes and microglia can become activated by a variety of factors, and produce pro-inflammatory cytokines such as tumor necrosis factor- α (TNF-α) and interleukin-1β, free radicals such as nitric oxide (NO°) and superoxide (Sankarapandi et al., 1998) that can lead to the subsequent neuronal death.
Previous studies, using immunohistochemical analysis or in situ hybridization reveal a selective loss of neurons and activation of other cell types during TD. However, immunohistochemical studies do not allow quantitative studies of small regions, such as SmTN. Although in situ hybridization is the best semi-quantitative method, it is subject to many errors. Thus, evaluation of the molecular and cellular events that accompany TD requires development of a reliable method for sampling in desired regions. In the present study, a micropunch technique was developed to study the selective regional changes in response to TD. Although punch techniques have been used for other small regions such as substantia nigra (Palkovits, M., 1973; Palkovits and Brownstein, M.J., 1988; Jacobowitz and Kallarakal, 2004; Saravanan et al., 2005), the SmTN is only about 30% the size of these regions. Protein and mRNA levels of markers for neuronal loss and pro-inflammatory processes following TD were assessed in the small vulnerable nucleus (SmTN) and non-vulnerable region (cortex).
Materials and Methods
Animals
Adult Male C57BL/6 mice (6-8 weeks; 22-26 g) from Harlan (Indianapolis, IN, USA) were used in all experiments. The animals were housed with constant temperature (22 ± 2°C), humidity (50 ± 5 %) and illumination (12 h light/dark cycles). The Institutional Animal Care and Use Committee of Weill Medical College of Cornell University approved all procedures with the animals.
Drug treatment
Thiamine deficiency was induced in C57BL/6 male mice (n=12 for each group as described in our previous studies (Ke et al., 2003; 2004). Experimental mice received a thiamine deficient diet (ICN Nutrition Biomedicals, Cleveland, OH) ad libitum, and daily intraperitoneal injections of the thiamine pyrophosphate inhibitor (Liu et al., 2006), pyrithiamine hydrobromide (Sigma Chemical Co.; St. Louis, MO; 5 μg in 0.1 ml saline/10 g body weight) for 10 days. Control animals received a thiamine containing diet (ICN Nutrition Biomedicals; Cleveland, OH) ad libitum and daily intraperitoneal injections of saline (0.1ml/10 g). One of the earliest indicators of TD is loss of appetite. Thus, controlling for this loss of food intake is the goal of pair feeding. We used pair fed controls in our early studies of thiamine deficiency (Barclay et al., 1981; Gibson et al., 1983). We did not find any differences between ad libitum controls and pair fed controls even for metabolic studies which one would surmise would be more sensitive than the measures here. Even theoretically, pair feeding is far from a perfect control. An animal that is starved (i.e. pair fed) likely has a very different response than one whose intake is restricted voluntarily. Indeed, we find that dietary restriction protects against TD (Calingasan and Gibson, 2000).
Tissue preparation and Immunohistochemistry
Following treatment, the mice were administered with a lethal intraperitoneal dose of pentobarbitone sodium (200 mg/kg; i.p.; Abbott Laboratories, North Chicago, IL) and perfused via the ascending aorta with 50 ml of normal saline, followed by 100 ml of 4% paraformaldehyde (Sigma Chemical Co., St. Louis, MO) in 0.1 M phosphate buffer (pH 7.2) using a pump (Masterflex, Model 7518-00, Cole-Parmer Instrument Company, Barrington, IL) at 5ml/min. The brains were removed and post-fixed in 4% paraformaldehyde overnight, and then transferred to 30% sucrose (Sigma Chemical Co., St. Louis, MO) for at least an additional 24 hours. The brain block that contained the thalamus and cortex region was dissected on a Rodent Brain Matrix (ASI Instruments; Warren, MI) and sectioned (40 μm) with a sliding microtome (Microm Laborgerate GmbH, Welldorf, Germany). Sections were collected from Bregma level -0.94 to -1.94 (Franklin and Paxinos, 1997).
The staining protocol employed a modified ABC immunohistochemistry procedure (Calingasan et al., 2000; Ke et al., 2003). Briefly, sections were washed with 0.1 M potassium phosphate buffered saline (PBS, pH 7.4) and incubated in 1% H2O2 for 30 min to quench the endogenous peroxidase. Then sections were treated with 0.1% Triton X-100 (Sigma Chemical Co., St. Louis, MO) for 15 min. Sections were washed with PBS and blocked with 2% bovine serum albumin (BSA) in PBS for 1 hr. Sections were incubated with mouse monoclonal anti-NeuN (Chemicon, Temecula, CA; 1:1000), rat anti- CD11b (Chemicon, Temecula, CA; 1:500), rabbit polyclonal anti-GFAP (DAKO, Carpinteria, CA; 1:1000), hamster anti- ICAM-1 (PharMingen, San Diego, CA; 1:500) or mouse monoclonal anti-eNOS (BD Transduction Laboratories, San Jose, CA; 1:1000) in PBS containing 1% BSA (Sigma Chemical Co., St. Louis, MO) overnight at room temperature. After rinsing in PBS, sections were incubated with biotinylated anti-mouse, anti-rat, anti-rabbit and anti-hamster IgG, (Vector Laboratories Inc., Burlingame, CA; 1:200 in PBS containing 0.25% BSA) for one hr. Sections were then incubated in avidin-biotin-peroxidase complex for one hr (Vector Laboratories Inc., Burlingame, CA; 1:100 in PBS), rinsed in PBS and developed in 0.05% 3,3′-diaminobenzidine (DAB) (Vector Laboratories Inc., Burlingame, CA) and 0.003% H2O2 in PBS.
Double immunofluorescence was performed to demonstrate the proinflammatory cytokines localization with glial markers. Briefly, Sections were incubated with anti-IL-1β, anti-IL-6, anti-TNF-α (R&D systems, Minneapolis, MN) or rat anti- CD11b (Chemicon, Temecula, CA) or rabbit polyclonal anti-GFAP (DAKO, Carpinteria, CA) antibodies in PBS containing 1% BSA (Sigma Chemical Co., St. Louis, MO) overnight at room temperature. After rinsing in PBS, sections were incubated with Alexa 488-donkey anti-goat IgG (Molecular Probes, Eugene, OR), Rhodamine Red conjugated anti- rat IgG and anti-rabbit IgG (Jackson ImmunoResearch Laboratories Inc, West Grove, PA) in PBS containing 0.25% BSA for one hour.
Tissue preparation and Micropunch
Mice were injected with a lethal dose of pentobarbitone sodium (200 mg/kg; i.p.; Abbott Laboratories, North Chicago, IL) and transcardially perfused with 50 ml of normal saline, using a pump (Masterflex, Model 7518-00, Cole-Parmer Instrument Company, Barrington, IL) at 5ml/min. Brains were rapidly removed from the skull and placed on clean glass slides without disturbing the orientation. Then the brains were kept over a cold plate at -25 to -30°C for 30 min inside the Cryostat (Hacker-Bright OTF Microtome Cryostat, Fairfield, NJ). Brains were mildly warmed by placing the forefinger underneath the glass slide for few seconds and held gently with a curved forceps and placed on its dorsal surface inside the stainless steel mouse brain matrix (ASI Instruments, Warren, MI) with coronal orientation to make sure that the brain is oriented correctly and evenly. The mouse brain matrix was maintained below -10°C through out the slicing procedure by placing it inside the cryostat. Samples of brain were obtained following the atlas of Franklin and Paxinos (1997). Serial sections of brains were cut at 1mm thickness, using a stainless steel razor blade and mounted onto clean glass slides and kept over the cold plates (-10 to -30°C) inside the cryostat. Tissues were micropunched from submedius thalamus and cortex region. The SmTN nucleus is located between bregma level -0.94 and -1.94. The predetermined anatomical landmark for submedial thalamus is the appearance of hippocampus. Sections were placed over the Lovins Micro-Slide Field (Electron Microscopy Sciences, Hatfield, PA) finder to identify the location of the SmTN. The SmTN is situated about 0.5 mm away from the midline, 4 mm dorsoventrally from the cortex and 3 mm from the hippocampus. Cortex punches were taken from primary and secondary motor cortex area (See Fig. 2b) at Bregma level 0.98 to -0.02. Bilateral punches were taken using Harris Uni-Core™, Size 0.75 mm micropunch (Electron Microscopy Sciences, Hatfield, PA). Samples were punched carefully by holding the punch firmly; the tip was positioned at a right angle above the SmTN or cortex and pushed downward into the brain section, until the micropunch made contact with the slide. Punches were lifted away slowly from the section along with the brain samples retrieved in the cutting tip. The sample was expelled by positioning the tip over precooled microfuge tubes and these samples were stored at -80°C. The complete process was carried out inside the cryostat. The temperature was maintained at -10 to -30°C to minimize sample degradation.
Fig. 2. Micropunch technique allows sampling of SmTN.
The left and right panels show the SmTN and cortex, respectively. Fig. a and b show the location of the SmTN and cortex in the atlas. The arrow in Fig. c and d points to the NeuN immunoreactivity in control SmTN and cortex respectively. The arrow in Fig. e and f reveals the loss of NeuN immunoreactivity in TD SmTN but not in cortex. Fig. g and h show the section after it has been micropunched using the Harris Uni-Core™, Size 0.75mm. Scale bars: c-h, 1 mm.
Western blotting
The micropunched brain samples from either cortex or SmTN from three control or three TD-10 mice were pooled together and treated as one sample. In general, two samples that reflected a pooled sample of three (i.e. six control and treated mice) were analyzed. Total RNA and protein were isolated from same micropunches using Trizol reagent according to the suppliers’ instructions (Invitrogen, Carlsbad, CA). Protein concentrations of the lysates were determined by a bicinchoninic colorimetric acid (BCA) assay (Pierce Chemical Company, Rockford, IL). A total of 10 μg of protein was loaded on a 10% SDS-PAGE gel. Separated proteins were then electro-transferred to a nitrocellulose membrane (Amersham Biosciences) using 45 volts for about 3 h. Membrane blots were blocked with 50% Odyssey blocking buffer, 50% TBS overnight at 4°C, and incubated with primary antibody NeuN (1:1000). After washing thoroughly with TBS, 0.1% Tween-20 at room temperature, the blots were incubated at room temperature for one hour with Odyssey Goat anti-Mouse IRDye 800 antibody (1:10,000; LICOR Biosciences, Lincoln, NB) and analyzed by odyssey imaging system.
Real-time RT-PCR
Total RNA was extracted from micropunched brain samples pooled from three mice brains using Trizol reagent (Invitrogen, Carlsbad, CA) and concentrated with RNeasy MinElute™ Cleanup kit (Qiagen, Valencia, CA). This was followed by first strand cDNA synthesis using the First Strand cDNA synthesis kit for RT-PCR (AMV) (Roche Applied Science, Indianapolis, IN) with oligo-p(dT)15 as primer. Real-time PCR of eNOS, IL1β, IL6 or TNF alpha was performed using an Applied Biosystems 7500 Real-Time PCR system with pre-designed Taqman® gene expression assays (Applied Biosystems, Foster City, CA). In brief, each amplification mixture (50 μl) contained 22.5 μl of cDNA template, 25 μl of TaqMan® Universal PCR Master Mix, 2.5 μl of a FAM™ dye labeled TaqMan® MGB probe and two PCR primers. Thermal cycler conditions were 95°C for 10 min, and 40 cycles of 95°C for 15 sec and 60°C for 1 min. All samples were normalized for beta-2-microglobulin (b2m) expression in parallel in the same run. A comparative Ct (the threshold cycle of PCR at which amplified product was first detected) method was used to compare the mRNA expression in samples from TD to that of the control.
Statistical Analysis
All the values were expressed as mean ± SEM. SPSS (SPSS Co., Chicago, IL) software was used to perform statistical analysis. P < 0.05 was considered significant. Statistical significance of group differences was tested by one-way analysis of variance (ANOVA) followed Student-Newman-Keuls post hoc test.
Results
TD-induced changes in multiple cell types
To determine TD-induced changes in neurons, microglia, astrocytes and endothelial cells, sections from control and TD-10 brains from bregma level -0.94 to -1.94 were processed with NeuN, CD11b, GFAP or ICAM-1 antibodies. TD caused a significant loss of NeuN immunoreactivity in the SmTN region. Control brains did not show any loss of neurons in SmTN region (Fig.1a, b and Fig. 2c, e). CD11b immunoreactivity in SmTN region of control brains revealed small cell bodies which reflect resting microglia. The large cell bodies with branches illustrate significant microglial activation with TD (Fig. 1c, d). A robust astrocytic response was also evident. At TD-10, increased numbers of GFAP-immunoreactive astrocytes were found in the SmTN region relative to control brains (Fig. 1e, f). Similar to our previous studies (Calingasan et al., 2000) in control brain there is no ICAM-1 immunoreactivity in the SmTN, while TD brains showed intense staining of capillaries, indicating constitutive expression of ICAM-1 in endothelial cells (Fig. 1g, h).
Fig. 1. TD alters the response of multiple cell types.
Adult male C57BL/6 mice were treated with saline or pyrithiamine hydrobromide (0.5 mg/kg; i.p.) once daily for 10 days. Animals were sacrificed on the 10th day and the brains were fixed and processed for NeuN, CD11b, GFAP or ICAM-1 immunostaining. Left and right panels show the control and TD, respectively. NeuN (a, b), CD11b (c, d), GFAP (e, f), ICAM-1 (g, h). Insets show the magnified images of the multiple cell types (n=5). Scale bars: a-h, 200 μm; Insets; a-h, 10 μm.
Micropunch technique allows sampling of very small submedial thalamic nuclei
This micropunch technique allows precise examination of pathologically altered regions. Once the punch has been removed, any component can be analyzed to reflect the in vivo condition. In these studies we focused on mRNA and protein levels. SmTN which is a part of medial thalamus lies medial to mammillothalamic tract. In fig. 2a and b, the right and left panels show the location of the SmTN and the cortical area respectively in the atlas. In control brain, NeuN staining showed neurons were intact in SmTN and cortex (Fig. 2c and d). TD induced neuronal loss in the vulnerable region SmTN (Fig. 2e), while neurons were spared in the non-vulnerable cortex (Fig. 2f). Figures 2g and h show the sections after the SmTN and cortex has been micropunched. The 0.75 mm micropunch includes the whole area of SmTN and some adjacent nuclei as shown precisely in the Fig.2g.
TD decreased NeuN protein levels in micropunches from SmTN samples
NeuN (Neuron-specific nuclear protein) is expressed in the nucleus of most neurons. As shown in Figs.1a, b and 2c, e, NeuN immunohistochemical reactivity declines in the SmTN, but not cortex of TD mice (Fig. 2 d, f). The punched brain samples from the SmTN and cortex region were analyzed by Western blotting to determine quantitatively, the reduction in NeuN protein levels (Fig. 3a). Western blot analysis of NeuN antibody revealed two strong bands at 48 and 46 kDa. Analysis of the band intensity from TD-10 SmTN showed a significant (34%) reduction in 48kDa, while 46 kDa showed only 23% compared to control SmTN (Fig. 3b). On the other hand, punches from cortex did not show any significant changes in NeuN protein levels between control and TD-10 animals (Fig. 3b). Qualitatively similar results were observed with two more control and two more TD samples when analysis was done by ECL.
Fig. 3. TD decreased NeuN protein levels.
Western blots were performed with the protein homogenates prepared from the SmTN and cortex micropunches of three control and TD brains probed with NeuN antibody and visualized by LI-COR IR dyes (A). Panel (B) shows the densitometric analysis of band intensity for NeuN (48 and 46 kDa) were quantified using Odyssey software. Data are represented as mean ± SEM in each group. * p <0.05 compared with control group (n=2). Although the statistics were done with n’s of two, a total of six animals in each group were used. Qualitatively similar results were observed with two more control and two more TD samples when analysis was done by ECL.
Selective increases in mRNA levels of endothelial NOS following TD
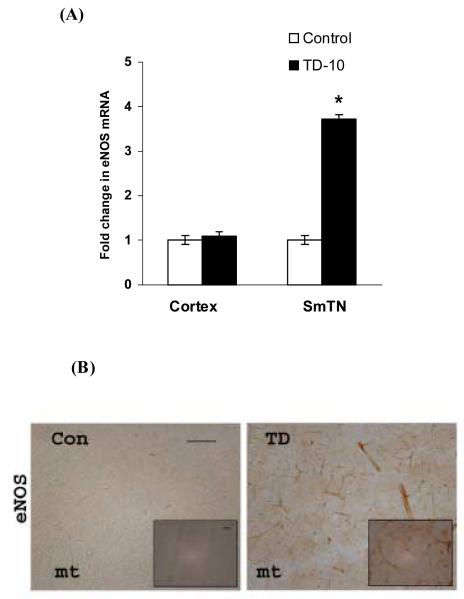
The mRNA expression level of eNOS was analyzed in the vulnerable SmTN and non-vulnerable cortex region. In TD-10 animals, eNOS mRNA increased 3.7 fold compared to control in the SmTN, whereas there was no change in cortex compared to the control (Fig. 4). Further, the eNOS expression was examined by immunohistochemical staining. TD-10 brains showed increased eNOS immunoreactivity in SmTN compared with the control (Fig. 4b).
Fig. 4. TD increased mRNA and protein levels of endothelial NOS.
Quantitative RT-PCR of eNOS mRNA was performed in total RNA extracts from SmTN and cortex punches of three control or TD brains. The expression levels of mRNA were normalized relative to the levels of GAPDH mRNA and fold differences were calculated relative to the control group. Values are mean ± SEM in each group. * p <0.05 compared with control group (n=3) (A). Values are mean ± SEM in each group. * p <0.05 compared with control group (n=3). Although the statistics were done with n’s of three, the results reflect a total of nine animals in each group. Panel (B) shows the representative control and TD brains sections stained with an eNOS antibody. TD-10 brains showed increased eNOS immunoreactivity (n=4). Scale bars: 100 μm; Insets 20 μm.
Selective increase in mRNA levels of pro-inflammatory cytokines in SmTN following TD
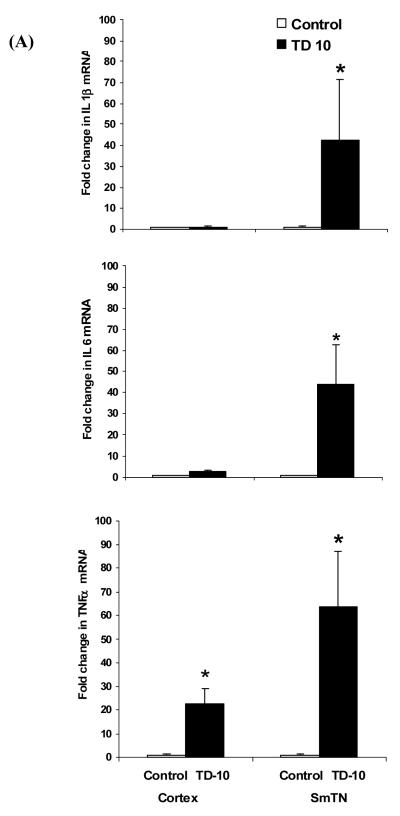
Activated microglia and astrocytes release pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α subsequent to an injury. The mRNA levels of inflammatory markers were analyzed in the vulnerable SmTN and non-vulnerable cortex region using quantitative real-time PCR. TD significantly increased mRNA levels of IL-1β, IL-6 and TNF-α in SmTN, by 42.5, 43.7 and 63.6 fold, respectively, compared to control SmTN region (Fig. 5 A). Interestingly, IL-1β and IL-6 expression levels were not altered in TD cortex region compared to control cortex. In contrast, TD increased TNF-α expression levels in TD cortex, compared to control (Fig. 5 A).
Fig. 5. TD increased mRNA and protein levels of pro-inflammatory markers.
Quantitative RT-PCR of pro-inflammatory markers IL-1β, IL-6 and TNF-α mRNA was performed on total RNA extracts from SmTN and cortex punches of three control or TD 10 brains. The expression levels of mRNA were normalized relative to the levels of GAPDH mRNA and fold differences were calculated relative to the control group. Values are mean ± SEM in each group. * p <0.05 compared with control group (n=3). Although the statistics were done with n’s of three, the results reflect a total of nine animals in each group. Panel (B) shows the representative TD-10 brains sections stained with IL-1β, IL-6 and TNF-α in SmTN region and colocalization with glial markers. IL-1β and TNF-α (green) double immunofluoresence with CD11b (red) showed that IL-1β and TNF-α colocalized in microglia (yellow). Reactive astrocytes labeled with GFAP (red) showed IL-6 (green) colocalized with GFAP (yellow) (n=4). Scale bars: 100 μm.
The effect of TD on the proinflammatory markers IL-1β, IL-6 and TNF-α protein levels were tested by immunocytochemistry. Control brains did not show any immunoreactivity for the proinflammatory markers (data not shown). TD induced IL-1β proteins weakly, while moderate increase were observed for TNF-α and colocalized with CD11b (Fig. 5B). Immunofluorescence analysis showed a moderate staining for IL-6 immunoreactivity in TD brains. IL-6 staining occurred mainly in reactive astrocytes as demonstrated by colocalization with astrocyte marker GFAP (Fig. 5B).
Discussion
This study is the first to assess TD-induced selective regional changes in the SmTN and cortex using the micropunch technique to quantitatively analyze the neuronal loss and pro-inflammatory markers. This approach allows the quantitative analysis of multiple mRNA and protein levels in the same sample. By comparison, only one or two molecules can be analyzed in immunohistochemistry or in situ hybridization methods. The ability to make multiple measures in the same sample facilitates comparison between vulnerable and non-vulnerable regions in testing for mechanisms of neurodegeneration in the regions. One disadvantage of the micropunch technique is the loss of the ability to identify individual cell types that reflect the complexity of the brain. This can be partially overcome by assessing cell specific messages or proteins (eg. GFAP). Additional limitations are that it is time consuming and requires a high degree of manual dexterity. The micropunch technique has been widely used to study many brain regions in various neurodegenerative disorders. For example, a similar approach has been used to study the dopaminergic neuron cell bodies in substantia nigra and their terminal in the nucleus caudate putamen that is involved in Parkinson’s disease. SmTN is about 30% the size of substantia nigra. The diameter of the SmTN is around 0.70 mm. Thus, the 0.75 mm micropunch included the whole area and some parts of adjacent nuclei as shown precisely in the fig. 2g. Although the sample size is small, this can be partially overcome by pooling multiple samples. This negates the opportunity to utilize the variations between individual mice to relate the variables such as weight, rotorod performance or memory. The requirement to keep the brain sections cold (-10 to -30°C) during cutting also minimizes degradation of proteins and nucleic acids.
The neuronal specific nuclear protein (NeuN) is increasingly being used as a specific marker to differentiate neurons from other cell types and has proven particularly useful for quantification of the number of neurons. NeuN is expressed in nucleus and cell body of most neurons in rodents and humans. Exceptions include mitral, purkinje and photoreceptor cells (Mullen et al., 1992). Our previous studies show that TD causes a time-dependent, selective neuronal death in specific brain regions, while other cell types are either activated or unaffected (Ke and Gibson, 2004). The present studies tested whether NeuN by Western blotting could be used to estimate the loss of neurons in SmTN. This is important because the overall goal is to determine the mechanism for neuronal loss in SmTN during TD. Immunocytochemistry revealed that the number of NeuN positive cells decreased by 90% in the SmTN (present study, Ke et al., 2003). Western blot analysis suggested that TD reduced the 48 kDa band of NeuN by 34% (p<0.05) and the 46 kDa band of NeuN by 23% compared to the controls. The larger change in the 48 kDa band than the 46 kDa band suggests that the immunocytochemistry reflects the 48 kDa band more than the other. A lack of concordance of changes in NeuN immunoreactivity by Western blot and immunocytochemistry has also been reported for cerebral ischemia and soman poisoning in mice (Unal-Cevik et al., 2004; Collombet et al., 2006). Thus, it seems that NeuN by Western is a weak marker of neuronal loss. Nevertheless, reduction in the NeuN protein level in the SmTN of TD mice validates the use of the micropunch technique to examine neuronal loss in this small region.
Microglia are key components of the inflammatory responses in brain. Microglial activation occurs early in the injury process and activated microglia exhibit morphological and immunological responses to insults by releasing the various cytokines, chemokines (Ransohoff et al., 1996 and de Bock et al., 1996) and cytotoxic products, such as reactive oxygen and nitrogen species (Colton and Gilbert 1987). Microglia are considered the most potent endogenous antigen-presenting cells (APC) in the CNS, and are critical for the induction of inflammatory responses. Activated microglia express surface molecules necessary for antigen presentation including CD40 (Ke et al., 2005a). IL-1 and TNF-α are two major pro-inflammatory cytokines with pleiotropic and largely overlapping functions, produced by microglia and blood-derived macrophages during CNS inflammation. Overexpression of IL-1 has been reported in AD, Down syndrome, traumatic brain injury and epilepsy (Griffin et al., 1989 and Sheng et al., 1994). Brain TNF-α levels are typically increased in various CNS disorders including ischemia (Liu et al., 1994), multiple sclerosis (Rieckmann et al., 1995) and trauma (Goodman et al., 1990). TD induced proinflammatory markers IL-1β, IL-6 and TNF-α mRNA and protein levels in SmTN have not been reported earlier.
Microglial activation also occurs in the early and late stages of TD in both rat and mouse models of TD (Calingasan et al., 1998, Todd and Butterworth, 1999 and Ke and Gibson, 2004). The current results show microglial activation is observed only in SmTN as revealed by large cell bodies with branches by both morphological and immunological (ie. CD11b immunoreactivity) criteria. IL-1β and TNF-α mRNA levels in TD were determined in the vulnerable SmTN and the non-vulnerable cortex. TD significantly increased IL-1β and TNF-α mRNA levels in SmTN, compared to control, which is consistent with the elevation in CD11b immunoreactivity in the SmTN (Fig. 1d). TD also increased TNF-α mRNA levels in cortex (Fig. 5A), while CD11b immunoreactivity was not observed in the cortex. This indicates TD induced changes in cortex are unique and unlike in SmTN. Further, the induction of proinflammatory cytokine protein expression levels were confirmed by immunohistochemistry. IL-1β stained weakly, while TNF-α stained moderately in TD SmTN (Fig. 5B). Double immunofluorescence analysis revealed the colocalization of TNF-α with CD11b positive cells (Fig. 5B).
Astrocytes serve as a structure support matrix for neurons, are vital in the formation of the structural integrity of the blood brain barrier (BBB) (Janzer and Raff, 1987) and are an important component of the inflammatory response. When activated, astrocytes proliferate, change morphologically, increase GFAP and S100B levels, and secrete a variety of cytokines and inflammatory molecules. As a source of immunologically relevant cytokines and chemokines, astrocytes play a pivotal role in the type and extent of CNS immune and inflammatory responses. CD40L-positive astrocytes increase during the progressive TD-induced neuronal death (Ke et al., 2005a). IL-6 is a pleiotropic cytokine with effects on the immune and nervous system. IL-6 is mainly produced by activated astrocytes, exerts multiple effects, both beneficial and destructive on brain cells (Van Wagoner and Benveniste, 1999). IL-6 is elevated in animal model of PD (Grunblatt et al., 2000). IL-6 was determined in the vulnerable SmTN and non-vulnerable cortex to assess its role in TD. Following TD, IL-6 expression increased significantly in SmTN, but not in cortex (Fig. 5A). Further, Double immunofluorescence analysis revealed IL-6 is colocalized with GFAP indicating that IL-6 is expressed in reactive astrocytes (Fig. 5B). The increase in pro-inflammatory cytokine expression levels in SmTN likely reflects role of inflammatory processes in the pathogenesis of TD.
Endothelial cells are critical components of the BBB, which regulates the movement of cells and molecules between the brain and systemic circulation. This transmigration is mediated by intracellular adhesion molecule-1 (ICAM-1). ICAM is a glycoprotein expressed on endothelial cells that facilitates leukocyte adhesion to endothelium and is a ligand for the CD11a/CD18 (LFA-1) and CD11b/CD18 (Mac-1) integrins that are expressed in leukocytes. Pro-inflammatory cytokines such as IL-1 and TNF-α increase ICAM-1 expression (Dustin et al., 1986). ICAM-1 expression on endothelial cells increases in the early stages of TD (Calingasan et al., 2000 and Ke and Gibson 2004) and genetic knockouts of ICAM expression are protective (Calingasan et al., 2000). Endothelial NOS (eNOS) also increases in TD (Calingasan et al., 2000; Kruse et al., 2004) and genetic knockouts of eNOS are protective (Calingasan et al., 2000). Recently, Kruse et al., has shown that mRNA and protein levels of eNOS isoforms increased selectively in the medial thalamus region following TD (Kruse et al., 2004). Similarly, in the current study a significant increase in eNOS mRNA and protein levels was observed in SmTN compared to cortex following TD. Together, these results support the suggestion that vascular endothelium is a major site of nitric oxide (NO•) production in TD, and that eNOS-derived NO• could account for the selective damage in medial thalamus.
Several mechanisms for TD-induced selective degeneration of SmTN neurons are proposed. The present study is the first to report TD-induced proinflammatory cytokine levels in the region most vulnerable region to TD, the SmTN. This supports our previous experiments indicating an increase in the inflammatory markers CD40, CD40L, ICAM-1 and hemeoxygenase. These results indicate a role for the inflammatory processes in TD pathogenesis. This micropunch technique allowed us to obtain the samples from the small SmTN and to study quantitatively the changes in the mRNA levels of the pro-inflammatory molecules IL-1β, IL-6 and TNF-α. They also indicate that TD does induce changes in the cortex that are very different than in the SmTN. These findings provide strong evidence for a substantial role of glial activation and those inflammatory mediators in the TD-induced selective neuronal loss.
Acknowledgements
This work was supported by NIH grants: AG14600, AG11921, and AG14930
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Albers DS, Augood SJ, Park LC, Browne SE, Martin DM, Adamson J, Hutton M, Standaert DG, Vonsattel JP, Gibson GE, Beal MF. Frontal lobe dysfunction in progressive supranuclear palsy: evidence for oxidative stress and mitochondrial impairment. J. Neurochem. 2000;74:878–881. doi: 10.1046/j.1471-4159.2000.740878.x. [DOI] [PubMed] [Google Scholar]
- Barclay LL, Gibson GE, Blass JP. Impairment of behavior and acetylcholine metabolism in thiamine deficiency. J. Pharm. Exp. Ther. 1981;217:537–543. [PubMed] [Google Scholar]
- Blass JP, Gibson GE. Abnormality of a thiamine-requiring enzyme in patients with Wernicke-Korsakoff syndrome. N. Engl. J. Med. 1977;297:1367–1370. doi: 10.1056/NEJM197712222972503. [DOI] [PubMed] [Google Scholar]
- Butterworth RF, Kril JJ, Harper CG. Thiamine-dependent enzyme changes in the brains of alcoholics: relationship to the Wernicke-Korsakoff syndrome. Alcohol Clin. Exp. Res. 1993;17:1084–1088. doi: 10.1111/j.1530-0277.1993.tb05668.x. [DOI] [PubMed] [Google Scholar]
- Calingasan NY, Gibson GE. Dietary restriction attenuates the neuronal loss, induction of heme oxygenase-1 and blood brain barrier breakdown induced by impaired oxidative metabolism. Brain Res. 2000;885:62–69. doi: 10.1016/s0006-8993(00)02933-4. [DOI] [PubMed] [Google Scholar]
- Calingasan NY, Park LC, Calo LL, Trifiletti RR, Gandy SE, Gibson GE. Induction of nitric oxide synthase and microglial responses precede selective cell death induced by chronic impairment of oxidative metabolism. Am J Pathol. 1998;153:599–610. doi: 10.1016/S0002-9440(10)65602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calingasan NY, Chun WJ, Park LC, Uchida K, Gibson GE. Oxidative stress is associated with region-specific neuronal death during thiamine deficiency. J Neuropathol. Exp. Neurol. 1999;58:946–958. doi: 10.1097/00005072-199909000-00005. [DOI] [PubMed] [Google Scholar]
- Calingasan NY, Huang PL, Chun HS, Fabian A, Gibson GE. Vascular factors are critical in selective neuronal loss in an animal model of impaired oxidative metabolism. J Neuropathol. Exp. Neurol. 2000;59:207–217. doi: 10.1093/jnen/59.3.207. [DOI] [PubMed] [Google Scholar]
- Collombet JM, Masqueliez C, Four E, Burckhart MF, Bernabe D, Baubichon D, Lallement G. Early reduction of NeuN antigenicity induced by soman poisoning in mice can be used to predict delayed neuronal degeneration in the hippocampus. Neurosci Lett. 2006;398:337–342. doi: 10.1016/j.neulet.2006.01.029. [DOI] [PubMed] [Google Scholar]
- Colton CA, Gilbert DL. Production of superoxide anions by a CNS macrophage, the microglia. FEBS Lett. 1987;223:284–288. doi: 10.1016/0014-5793(87)80305-8. [DOI] [PubMed] [Google Scholar]
- de Bock F, Dornand J, Rondouin G. Release of TNF alpha in the rat hippocampus following epileptic seizures and excitotoxic neuronal damage. Neuroreport. 1996;26:1125–1129. doi: 10.1097/00001756-199604260-00004. [DOI] [PubMed] [Google Scholar]
- Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J. Immunol. 1986;137:245–254. [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 1997. [Google Scholar]
- Gibson GE, Sheu KF, Blass JP, Baker A, Carlson KC, Harding B, Perrino P. Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer’s disease. Arch. Neurol. 1988;45:836–840. doi: 10.1001/archneur.1988.00520320022009. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Park LC, Zhang H, Sorbi S, Calingasan NY. Oxidative stress and a key metabolic enzyme in Alzheimer brains, cultured cells, and an animal model of chronic oxidative deficits. Ann. N.Y. Acad. Sci. 1999;893:79–94. doi: 10.1111/j.1749-6632.1999.tb07819.x. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Pelmas C, Blass JP. A central cholinergic deficit in rats with dietary thiamin deficiency. Neurochem. Pathol. 1983;1:125–135. [Google Scholar]
- Goodman JC, Robertson CS, Grossman RG, Narayan RK. Elevation of tumor necrosis factor in head injury. J. Neuroimmunol. 1990;30:213–217. doi: 10.1016/0165-5728(90)90105-v. [DOI] [PubMed] [Google Scholar]
- Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc. Natl. Acad. Sci. U S A. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunblatt E, Mandel S, Youdim MB. MPTP and 6-hydroxydopamine-induced neurodegeneration as models for Parkinson’s disease: neuroprotective strategies. J. Neurol. 2000;247(Suppl 2):II95–102. doi: 10.1007/pl00022909. [DOI] [PubMed] [Google Scholar]
- Jacobowitz DM, Kallarakal AT. Flotillin-1 in the substantia nigra of the Parkinson brain and a predominant localization in catecholaminergic nerves in the rat brain. Neurotox Res. 2004;6:245–257. doi: 10.1007/BF03033435. [DOI] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Ke ZJ, DeGiorgio LA, Volpe BT, Gibson GE. Reversal of thiamine deficiency-induced neurodegeneration. J. Neuropathol. Exp. Neurol. 2003;62:195–207. doi: 10.1093/jnen/62.2.195. [DOI] [PubMed] [Google Scholar]
- Ke ZJ, Gibson GE. Selective response of various brain cell types during neurodegeneration induced by mild impairment of oxidative metabolism. Neurochem. Int. 2004;45:361–369. doi: 10.1016/j.neuint.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Ke ZJ, Calingasan NY, Karuppagounder SS, DeGiorgio LA, Volpe BT, Gibson GE. CD40L deletion delays neuronal death in a model of neurodegeneration due to mild impairment of oxidative metabolism. J Neuroimmunol. 2005a;164:85–92. doi: 10.1016/j.jneuroim.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Ke ZJ, Calingasan NY, DeGiorgio LA, Volpe BT, Gibson GE. CD40-CD40L interactions promote neuronal death in a model of neurodegeneration due to mild impairment of oxidative metabolism. Neurochem. Int. 2005b;47:204–215. doi: 10.1016/j.neuint.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Klivenyi P, Starkov AA, Calingasan NY, Gardian G, Browne SE, Yang L, Bubber P, Gibson GE, Patel MS, Beal MF. Mice deficient in dihydrolipoamide dehydrogenase show increased vulnerability to MPTP, malonate and 3-nitropropionic acid neurotoxicity. J. Neurochem. 2004;88:1352–1360. doi: 10.1046/j.1471-4159.2003.02263.x. [DOI] [PubMed] [Google Scholar]
- Kruse M, Navarro D, Desjardins P, Butterworth RF. Increased brain endothelial nitric oxide synthase expression in thiamine deficiency: relationship to selective vulnerability. Neurochem. Int. 2004;45:49–56. doi: 10.1016/j.neuint.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Langlais PJ, Zhang SX, Savage LM. Neuropathology of thiamine deficiency: an update on the comparative analysis of human disorders and experimental models. Metab Brain Dis. 1996;11:19–37. doi: 10.1007/BF02080929. [DOI] [PubMed] [Google Scholar]
- Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, Feuerstein GZ. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994;25:1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- Liu JY, Timm DE, Hurley TD. Pyrithiamine as a substrate for thiamine pyrophosphokinase. J. Biol. Chem. 2006;281:6601–6607. doi: 10.1074/jbc.M510951200. [DOI] [PubMed] [Google Scholar]
- Mastrogiacomo F, Bergeron C, Kish SJ. Brain alpha-ketoglutarate dehydrogenase complex activity in Alzheimer’s disease. J. Neurochem. 1993;61:2007–2014. doi: 10.1111/j.1471-4159.1993.tb07436.x. [DOI] [PubMed] [Google Scholar]
- Mastrogiacomo F, LaMarche J, Dozic S, Lindsay G, Bettendorff L, Robitaille Y, Schut L, Kish SJ. Immunoreactive levels of alpha-ketoglutarate dehydrogenase subunits in Friedreich’s ataxia and spinocerebellar ataxia type 1. Neurodegeneration. 1996;5:27–33. doi: 10.1006/neur.1996.0004. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Matuda S, Yoshino H, Mori H, Hattori N, Ikebe S. An immunohistochemical study on alpha-ketoglutarate dehydrogenase complex in Parkinson’s disease. Ann. Neurol. 1994;35:204–210. doi: 10.1002/ana.410350212. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd Edition Academic Press; New York: 1986. [Google Scholar]
- Palkovits M. Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Res. 1973;59:449–450. doi: 10.1016/0006-8993(73)90290-4. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Brownstein MJ. Maps and Guide to Microdissection of the Rat Brain. Elsevier; New York: 1988. [Google Scholar]
- Ransohoff RM, Glabinski A, Tani M. Chemokines in immune-mediated inflammation of the central nervous system. Cytokine Growth Factor Rev. 1996;7:35–46. doi: 10.1016/1359-6101(96)00003-2. [DOI] [PubMed] [Google Scholar]
- Rieckmann P, Albrecht M, Ehrenreich H, Weber T, Michel U. Semi-quantitative analysis of cytokine gene expression in blood and cerebrospinal fluid cells by reverse transcriptase polymerase chain reaction. Res. Exp. Med. (Berl) 1995;195:17–29. doi: 10.1007/BF02576770. [DOI] [PubMed] [Google Scholar]
- Sankarapandi S, Zweier JL, Mukherjee G, Quinn MT, Huso DL. Measurement and characterization of superoxide generation in microglial cells:evidence for an NADPH oxidase-dependent pathway. Arch. Biochem. Biophys. 1998;353:312–321. doi: 10.1006/abbi.1998.0658. [DOI] [PubMed] [Google Scholar]
- Saravanan KS, Sindhu KM, Mohanakumar KP. Acute intranigral infusion of rotenone in rats causes progressive biochemical lesions in the striatum similar to Parkinson’s disease. Brain Res. 2005;1049:147–155. doi: 10.1016/j.brainres.2005.04.051. [DOI] [PubMed] [Google Scholar]
- Sheng JG, Boop FA, Mrak RE, Griffin WS. Increased neuronal beta-amyloid precursor protein expression in human temporal lobe epilepsy: association with interleukin-1 alpha immunoreactivity. J. Neurochem. 1994;63:1872–1879. doi: 10.1046/j.1471-4159.1994.63051872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd KG, Butterworth RF. Evaluation of the role of NMDA-mediated excitotoxicity in the selective neuronal loss in experimental Wernicke encephalopathy. Exp. Neurol. 1998;149:130–138. doi: 10.1006/exnr.1997.6677. [DOI] [PubMed] [Google Scholar]
- Todd KG, Butterworth RF. Early microglial response in experimental thiamine deficiency: an immunohistochemical analysis. Glia. 1999;25:190–198. doi: 10.1002/(sici)1098-1136(19990115)25:2<190::aid-glia9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Torvik A, Lindboe CF, Rogde S. Brain lesions in alcoholics. A neuropathological study with clinical correlations. J. Neurol. Sci. 1982;56:233–248. doi: 10.1016/0022-510x(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Troncoso JC, Johnston MV, Hess KM, Griffin JW, Price DL. Model of Wernicke’s encephalopathy. Arch. Neurol. 1981;38:350–354. doi: 10.1001/archneur.1981.00510060052007. [DOI] [PubMed] [Google Scholar]
- Unal-Cevik I, Kilinc M, Gursoy-Ozdemir Y, Gurer G, Dalkara T. Loss of NeuN immunoreactivity after cerebral ischemia does not indicate neuronal cell loss: a cautionary note. Brain Res. 2004;1015:169–174. doi: 10.1016/j.brainres.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Van Wagoner NJ, Benveniste EN. Interleukin-6 expression and regulation in astrocytes. J. Neuroimmunol. 1999;100:124–39. doi: 10.1016/s0165-5728(99)00187-3. [DOI] [PubMed] [Google Scholar]
- Victor M, Davis RD, Collins GH. The Wernicke-Korsakoff syndrome and related neurologic disorders due to alcoholism and malnutrition. 2nd edn F. A. Davis Co.; Philadelphia: 1989. [Google Scholar]
- Watanabe I, Kanabe S. Early edematous lesion of pyrithiamine induced acute thiamine deficient encephalopathy in the mouse. J. Neuropathol. Exp. Neurol. 1978;37:401–413. doi: 10.1097/00005072-197807000-00004. [DOI] [PubMed] [Google Scholar]
- Zhang SX, Weilersbacher GS, Henderson SW, Corso T, Olney JW, Langlais PJ. Excitotoxic cytopathology, progression, and reversibility of thiamine deficiency-induced diencephalic lesions. J. Neuropathol. Exp. Neurol. 1995;54:255–267. doi: 10.1097/00005072-199503000-00012. [DOI] [PubMed] [Google Scholar]