Abstract
Background
Chromium picolinate (CrPic) has been shown to attenuate weight gain, but the mechanism underlying this effect is unknown.
Methods
We assessed the effect of CrPic in modulating food intake in healthy, overweight, adult women who reported craving carbohydrates (Study 1) and performed confirmatory studies in Sprague-Dawley rats (Study 2). Study 1 utilized a double-blind placebo-controlled design and randomly assigned 42 overweight adult women with carbohydrate cravings to receive 1,000 μg of chromium as CrPic or placebo for 8 weeks. Food intake at breakfast, lunch, and dinner was directly measured at baseline, week 1, and week 8. For Study 2, Sprague-Dawley rats were fasted for 24 h and subsequently injected intraperitoneally with 0, 1, 10, or 50 μg/kg CrPic. Subsequently, rats were implanted with an indwelling third ventricular cannula. Following recovery, 0, 0.4, 4, or 40 ng of CrPic was injected directly into the brain via the intracerebroventricular cannula, and spontaneous 24-h food intake was measured.
Results
Study 1 demonstrated that CrPic, as compared to placebo, reduced food intake (P < 0.0001), hunger levels (P < 0.05), and fat cravings (P < 0.0001) and tended to decrease body weight (P = 0.08). In study 2, intraperitoneal administration resulted in a subtle decrease in food intake at only the highest dose (P = 0.03). However, when administered centrally, CrPic dose-dependently decreased food intake (P < 0.05).
Conclusions
These data suggest CrPic has a role in food intake regulation, which may be mediated by a direct effect on the brain.
Introduction
Despite widespread efforts to combat the increasing obesity epidemic, which parallels the increase in prevalence of type 2 diabetes, limited progress has been made.1,2 Compliance with most weight loss programs is notoriously poor, particularly over the long-term.3 Thus, there is a need for effective and safe alternative options.
Dietary supplements may offer an alternative or adjunctive treatment option for many overweight individuals desiring to lose weight. Sales of dietary supplements increased dramatically following the passage of the Dietary Supplement Health and Education Act.4 There are currently over 29,000 nutritional supplements available to consumers, and Americans spend over 12 billion dollars per year on these products.5,6 For the vast majority of products, however, there is a paucity of data supporting their use in humans for weight loss purposes.
Unlike many commercial weight loss products, there is some support for the beneficial effects of chromium picolinate (CrPic) on body weight and body composition. To form this popular nutritional supplement, the element and naturally occurring mineral chromium is combined with picolinic acid, which assists in efficient chromium absorption. A recent meta-analysis of 10 randomized, double-blind, placebo-controlled trials found CrPic had a modest but significant differential effect on body weight (1 kg) as compared to placebo.7 Recently, in a double-blind, placebo-controlled study in subjects with type 2 diabetes, CrPic did not promote weight loss but was reported to significantly attenuate body weight gain and enhance insulin sensitivity as compared to the placebo group.8 Although the mechanism underlying these effects is unknown, Cr-Pic has been suggested to impact neurotransmitters involved in the regulation of eating behavior, mood, and food cravings.9–11
Attenuation of weight gain would suggest an effect on energy balance to either reduce food intake or increase energy expenditure. However, the effect that CrPic supplementation has on food intake in humans has not been addressed, despite its popularity among consumers desiring weight loss or improved body composition.7 The primary objective of this study was to evaluate the effect of CrPic on food intake in healthy, overweight, adult women who reported craving carbohydrates. Secondary objectives were to evaluate the effect of CrPic on food cravings, satiety, and body weight. This sample was selected because previous reports have concluded that CrPic may reduce food cravings.10 Based on the positive clinical outcome, animal studies were conducted to evaluate potential mechanisms.
Subjects and Methods
Study 1
Participants
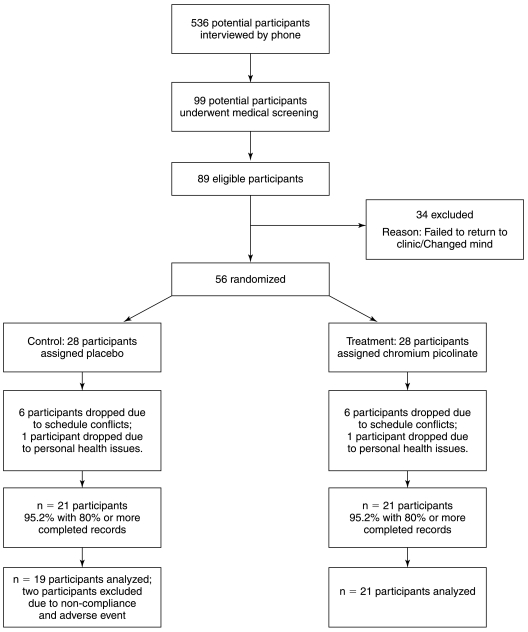
The study was approved by the Institutional Review Board of the Pennington Biomedical Research Center (PBRC), Baton Rouge, LA. Forty-two overweight, nonsmoking, healthy adult women who reported intense cravings for carbohydrates at least 2 days per week completed this 8-week randomized, double-blind, placebo-controlled study. Figure 1 summarizes the recruitment, enrollment, and collection of data for study participants.
FIG. 1.
Illustration of the number of participants from recruitment to the end of the study.
Screening Procedures
Potential participants attended a screening visit, during which psychological questionnaires and blood samples were taken to identify physical or psychological contraindications to participation in the study. Participants were required to (1) be a healthy female without any chronic disease, (2) be a carbohydrate craver, determined by self-reported carbohydrate cravings on 2 or more days of the week, (3) be >18 years of age and <50 years of age, (4) have a body mass index (BMI) between 25 and 39.9 kg/m2, and (5) be a nonsmoker. Participants were also excluded if they had a diagnosable eating disorder or were taking any medications or dietary supplements (including CrPic) that could influence appetite, hunger, or satiety.
Study Design and Procedure
Participants were randomly assigned to receive either 1,000 μg of chromium as CrPic or placebo (dicalcium phosphate) daily (chromium picolinate was supplied as Chromax by Nutrition 21). The safety of this dose is well established.12,13 Eligible participants were scheduled to complete food test days during the luteal phase of their menstrual cycle on three separate occasions (baseline, week 1, and week 8). On each food test day, participants were instructed to consume a standard breakfast of cereal and orange juice, an ad libitum lunch of sandwiches, chips, and cookies, and a self-selected buffet-type dinner meal (i.e., macronutrient self-selection paradigm).14 Participants were given product following their baseline and week 1 food test days, as well as at their week 4 clinic visit. Compliance was monitored through pill counts, as well through a 24-h urine content analysis.
Eating behavior measures
Food intake
Food intake was directly measured in the PBRC's Eating Laboratory and measured using Mettler (Columbus, OH) Toledo ISO 9001 scales.
Visual Analogue Scales (VAS)
VAS were used to assess subjective ratings of hunger, satiety, fullness, and carbohydrate cravings before and after each meal, as well as at 3 and 4 h post-lunch.15
Food Craving Inventory (FCI)
The FCI16 was used to provide a reliable and valid assessment of cravings for four different types of foods: carbohydrates/starches, fast-food fats, high-fat foods, and sweets.
Eating Inventory
The Eating Inventory17 has established reliability and validity.18 It consists of three subscales: Dietary Restraint, Disinhibition, and Perceived Hunger.
Physiological measures
Weight
Metabolic weights, the weight taken from patients in hospital gowns during a fasting state and following voiding in the morning, were taken at each visit.
Dual energy X-ray absorptiometry (DEXA)
DEXA scans were performed using a Hologics (Bedford, MA) QDR 4500A whole-body scanner. The scans were analyzed with the latest software QDR for Windows version 11.1.
Glucose
Glucose was measured on the Beckman Coulter (Fullerton, CA) Synchron CX7 using a glucose oxidase electrode.
Insulin
Insulin was measured on the Diagnostic Products Corp. (Los Angeles, CA) 2000 using an immunoassay with chemiluminescent detection.
Study 2
Animals
All animal procedures were performed in accordance with National Institutes of Health guidelines for the care and use of animals and were approved by the Animal Care and Use Committee of the PBRC. Male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) were housed singly and maintained on a 12:12-h light–dark cycle with ad libitum access to standard rat chow and water unless otherwise noted. To assess the effects of peripheral CrPic injection on food intake, rats (weighing 224 ± 2.2 g) were fasted for 24 h. Two hours prior to the end of the fast, CrPic was injected intraperitoneally at doses of 0, 1, 10, and 50 μg/kg (eight animals per group), and food intake over the subsequent 24 h was recorded. CrPic was dissolved in 0.9% (wt/vol) NaCl such that the injected volume was similar in all treatment groups and could be delivered in a dose of 2.5 mL/kg, which was the vehicle volume.
To assess direct effects of CrPic on the brain, rats were subsequently implanted with an indwelling cannula directed to the third cerebroventricle.19 Rats were anesthetized and placed into a stereotaxic device, and a 22-gauge stainless steel cannula was implanted at coordinates −2.2 from bregma and −7.5 from dura. This cannula was then anchored with dental acrylic, the incision was sutured, and a 28-gauge obdurator placed into the cannula. Animals were treated with analgesics and allowed to recover at least 1 week before further study. Following surgical recovery, rats were again fasted for 24 h and injected intracerebroventricularly with 0, 0.4, 4, and 40 ng of CrPic (seven or eight animals per group), and food intake over the subsequent 24 h was recorded.
Statistical analysis
Change from baseline was defined as the value at time t minus the value observed at baseline for all response measures. A repeated-measures analysis of covariance was used to test if food intake varied as a function of the two different conditions (active or placebo) with change from baseline in kcal intake being the dependent variable. All other variables were analyzed using a similar analysis of covariance design with change from baseline being the dependent variable and baseline levels of these variables being covariates. Post hoc analyses of means were conducted. All analyses were carried out using SAS (Cary, NC) version 9.12 software package.
Results
Study 1
Participant recruitment and descriptive characteristics of the study sample. Participants were relatively young and, with the exception of being overweight, healthy adult women. Participant recruitment is outlined in Figure 1, and the descriptive characteristics of the sample are summarized in Table 1. Participants in the placebo group had significantly higher BMIs than participants in the CrPic group. Participants in the two groups did not differ on any other demographic characteristic.
Table 1.
Descriptive Characteristics of the Study Sample at Baseline
| |
Mean (SD) |
||
|---|---|---|---|
| Entire sample (n = 40) | CrPic (n = 21) | Placebo (n = 19) | |
| Race (white/African American) | 26/14 | 13/8 | 13/6 |
| Age (years) | 33.2 (9.9) | 32.0 (10.2) | 34.5 (9.7) |
| Body weight (kg) | 83.7 (12.2) | 83.2 (12.8) | 84.4 (11.7) |
| Body mass index (kg/m2) | 31.3 (4.5) | 30.7 (4.2)a | 31.9 (4.7) |
| Body fat (%) | 40.2 (5.0) | 39.7 (1.1) | 40.7 (1.2) |
| Blood pressure (mm Hg) | |||
| Diastolic | 74.0 (10.2) | 74.1 (9.7) | 73.7 (10.9) |
| Systolic | 113.5 (11.3) | 115.1 (12.7) | 111.8 (9.6) |
| Waist circumference (cm) | 92.1 (11.7) | 91.3 (12.1) | 92.9 (11.5) |
| Glucose (mg/dL) | 87.9 (6.8) | 87.1 (1.4) | 88.7 (1.7) |
| Insulin (μIU/mL) | 13.7 (7.3) | 14.3 (1.8) | 13.0 (1.4) |
| HOMA-Sb | 53.6 (4.5) | 55.6 (7.2) | 51.5 (5.5) |
| Food intake | |||
| Total kCal | 1,648 (415) | 1,496 (437) | 1,814 (322) |
| Fat (kCal) | 666 (202) | 612 (217) | 726 (169) |
| Carbohydrate (kCal) | 744 (196) | 673 (184) | 823 (182) |
| Protein (kCal) | 235 (69) | 209 (71) | 264 (54) |
| Fat (kCal) (%) | 40.1 (5.3) | 40.3 (5.1) | 39.9 (5.7) |
| Carbohydrate (kCal) (%) | 45.4 (6.2) | 45.5 (6.1) | 45.3 (6.5) |
| Protein (kCal) (%) | 14.4 (3.0) | 14.4 (3.4) | 14.7 (2.6) |
| Eating behavior measures | |||
| Eating Inventory | |||
| Restraint | 8.7 (5.4) | 7.4 (4.9) | 10.0 (5.7) |
| Disinhibition | 8.0 (3.1) | 7.2 (2.9) | 8.8 (3.2) |
| Hunger | 7.1 (3.2) | 6.7 (2.7) | 7.5 (3.7) |
| FCI | |||
| Sweets | 3.1 (0.7) | 3.2 (0.7) | 3.0 (0.8) |
| High fats | 2.3 (0.8) | 2.5 (0.8) | 2.1 (0.7) |
| Carbs/starches | 2.8 (0.8) | 2.9 (0.8) | 2.7 (0.7) |
| Fast food fats | 3.3 (0.6) | 3.4 (0.7) | 3.2 (0.5) |
| General | 2.8 (0.5) | 3.0 (0.5) | 2.7 (0.5) |
| VAS ratings | |||
| Hunger | |||
| Before breakfast | 67.3 (25.0) | 64.4 (25.3) | 70.5 (24.9) |
| After breakfast | 14.2 (22.2) | 16.7 (27.0) | 11.4 (15.7) |
| Before lunch | 69.3 (24.1) | 67.5 (24.5) | 71.4 (24.1) |
| After lunch | 4.2 (8.2) | 5.5 (7.8) | 2.8 (8.7) |
| 3 h after lunch | 38.2 (21.4) | 40.0 (20.5) | 36.2 (22.7) |
| 4 h after lunch | 53.5 (26.3) | 55.4 (28.5) | 51.5 (24.4) |
| Before dinner | 62.9 (23.3) | 59.4 (27.5) | 66.6 (17.7) |
| After dinner | 6.3 (12.5) | 9.2 (15.2) | 3.1 (7.9) |
| Fullness | |||
| Before breakfast | 23.9 (23.2) | 24.0 (19.3) | 23.7 (27.4) |
| After breakfast | 72.8 (25.9) | 73.1 (28.5) | 72.5 (23.4) |
| Before lunch | 22.7 (17.8) | 26.5 (20.1) | 18.4 (14.3) |
| After lunch | 88.0 (12.6) | 87.0 (15.0) | 89.2 (9.4) |
| 3 h after lunch | 47.1 (20.1) | 50.6 (21.2) | 43.2 (18.6) |
| 4 h after lunch | 37.8 (25.0) | 34.4 (27.6) | 41.4 (22.1) |
| Before dinner | 32.0 (24.9) | 31.4 (28.6) | 32.7 (20.8) |
| After dinner | 87.9 (15.8) | 85.6 (18.8) | 90.6 (11.6) |
Participants were randomly assigned to receive either 1,000 μg of CrPic or placebo (dicalcium phosphate) daily for an 8-week period.
P < 0.05.
HOMA-S = glucose (mmol/L) × insulin (μU/mL)/22.5.
Compliance and Adverse Events
Pill counts suggested good compliance for both the CrPic (>94%) and placebo (>93%) groups. Additionally, the 24-h urine content analysis indicated chromium levels were significantly higher in the CrPic group as compared to the placebo (mean = 12.8 vs. 0.19 ng/mL; F = 2274; P < 0.0001).
One participant in the placebo group dropped out of the study because of an adverse emotional reaction reportedly due to the study medication. No other adverse events were reported. Of the 42 participants who completed this study, two participants in the placebo group were excluded from final analyses because of (1) noncompliance to protocol and (2) abnormally high scores on all study measures. Thus, analyses were conducted on a study sample of 40 participants (21 CrPic and 19 placebo).
Food Intake
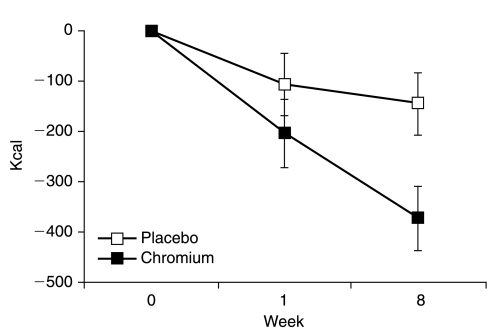
As presented in Figure 2, food intake was significantly decreased versus baseline with both treatments (P < 0.05), but this decrease was significantly more pronounced with CrPic treatments at week 8 (−25% vs. −8%; P < 0.05). Participants receiving CrPic decreased their caloric consumption at their dinner meal to a greater extent than participants receiving placebo (P < 0.05; for chromium, Δ = −252 kcal, P < 0.0001; for placebo, Δ = −113 kcal, P < 0.01) and also significantly decreased their caloric intake during their lunch meal (Δ = −112 kcal, P < 0.05), but this change was not significantly different between the two treatments (for placebo, Δ = −44 kcal, P = 0.35). Macronutrient composition was not found to differ over time or between groups.
FIG. 2.
Change in food intake over the entire day for participants assigned to receive CrPic (n = 21) versus placebo (n = 19).
Hunger and Satiety
There were no differences by condition in hunger ratings on VAS throughout the baseline test meal day (day 0). At week 8, however, participants receiving CrPic had lower average hunger ratings than participants in the placebo condition at 4.5 h after lunch (i.e., before dinner; between group difference = 17.1, P < 0.02) and also tended to have lower average hunger ratings at 4 h after lunch (between group difference = 12.8, P = 0.08). These differences appear to be due to participants in the placebo group reporting increased hunger at 4 and 4.5 h after lunch, as compared to baseline (mean increase for placebo = 12.5 [P < 0.05] and 6.4 [P = 0.25], respectively). There was also a trend for participants receiving chromium to have higher average hunger ratings than participants receiving placebo immediately after lunch (between group difference = 13.8, P = 0.06). Fullness ratings did not vary over time or condition. On the Eating Inventory, participants receiving CrPic reported significantly decreased hunger levels from week 0 to week 8 (Δ = −1.6; P < 0.01); no change was found for participants receiving placebo. There were no differences by condition in change in hunger levels over time. Participants receiving placebo also had significantly decreased levels of disinhibition over time (Δ = −1.1; P < 0.05), and there was a trend for participants receiving CrPic to also decrease their levels of disinhibition (Δ = −0.84; P = 0.08).
Food Cravings
Participants in both conditions had significantly lower scores on the FCI over time (Δ = 0.3 for placebo, P <.05; Δ = 0.5 for CrPic, P < 0.01). Participants receiving CrPic had lower scores on all four FCI subscales: Carbohydrates/Starches (Δ = −0.5), Fast-Food Fats (Δ = −0.5), High-Fat Foods (Δ = −0.4), and Sweets (Δ = −0.6) (all Ps < 0.001). Participants receiving placebo had lower scores on all subscales as well: Carbohydrates/Starches (Δ = −0.3), Fast-Food Fats (Δ = −0.4), High-Fat Foods (Δ = −0.2), and Sweets (Δ = −0.5) (Ps < 0.05). Participants receiving CrPic decreased their cravings on the High-Fat Foods subscale (e.g., bacon) to a greater extent than participants assigned to placebo treatment (between group difference = −0.2, P < 0.05).
Body Weight
Participants receiving CrPic decreased body weight from baseline to week 8 (Δ = −0.5 kg). In contrast, participants receiving placebo increased body weight during this same time period (Δ = 0.5 kg). There was a trend for change in body weight to differ by group at week 8 (between group difference = 1 kg, P < 0.08).
Glucose and Insulin
Fasting blood glucose increased a small amount in both conditions (for CrPic, Δ = 3.5 mg/dL, P < 0.05; for placebo, Δ = 2.7 mg/dL, P = 0.08) but did not differ between groups during the study. Fasting insulin and homeostatic model assessment of insulin sensitivity (HOMA-S) levels did not change significantly in either group.
Study 2
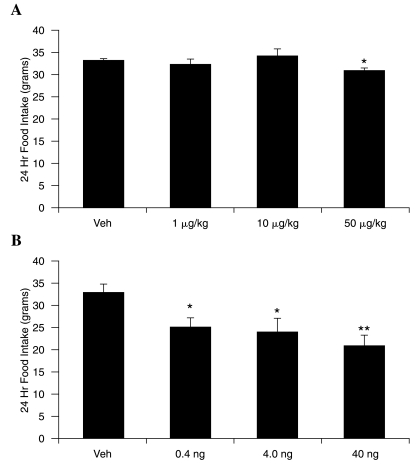
To assess whether CrPic also suppresses food intake in an animal model, chow-fed male Sprague-Dawley rats were treated with increasing doses of CrPic (1, 10, and 50 μg/kg). Intraperitoneal injection of CrPic to 24-h fasted rats (n = 8) resulted in a small but statistically significant decrease in 24-h food intake at the highest dose administered (50 μg/kg; P = 0.03) but had no effect on food intake at lower doses (Fig. 3A).
FIG. 3.
Effect of intraperitoneal and intra-cerebroventricular CrPic injection on 24-h food intake in Sprague-Dawley rats. (A) 24-h fasted rats were injected intraperitoneally with increasing doses of CrPic, and 24-h food intake was assessed. (B) Rats implanted with an indwelling intracerebroventricular cannula were injected intracerebroventricularly with lower doses of CrPic, and 24-h food intake was assessed. *P < 0.05, **P < 0.01 versus vehicle (Veh).
To determine whether the observed changes in food intake could be mediated by a direct action on the brain, much lower doses of CrPic (0.4, 4, and 40 ng) were administered directly into the third cerebroventricle of fasted rats. In this acute model (Fig. 3B), intracerebroventricularly administered CrPic suppressed food intake at all doses (P < 0.05) compared to vehicle (2 μL of phosphate-buffered saline), with the highest dose (40 ng) producing a 50% decrease in food intake over the subsequent 24 h (P < 0.01). These data indicate that CrPic may function by a central mechanism.
Discussion
The primary finding of this series of studies was that CrPic reduced food intake in both humans and Sprague-Dawley rats. CrPic decreased food intake in healthy, overweight adult women who reported craving carbohydrates. To our knowledge, this is the first study to examine whether CrPic affects food intake in humans. The reduction in food intake did not appear to be related to illness or other adverse effects of CrPic; the only adverse event related to the intervention was reported by a participant assigned to placebo treatment.
Despite significantly reducing their calorie intake, participants receiving CrPic did not report increased hunger levels. In contrast, participants receiving placebo reported increased hunger levels at 4 and 4.5 h after lunch, even though they did not reduce their food intake to as great an extent as participants receiving CrPic. This finding suggests that CrPic may impact physiological satiety signals and potentially sustain satiety levels during periods of caloric restriction.
As a follow-up to the human study, we tested whether CrPic had similar effects on 24-h food intake in male Sprague-Dawley rats. In this series of studies, CrPic decreased 24-h food intake when administered both peripherally and centrally. The effect on food intake, however, was much more dramatic when CrPic was administered centrally versus peripherally, and a dose-dependent effect was observed only when CrPic was administered centrally. Few studies have directly tested the effect of CrPic on eating behavior in animal models. While some studies have found no effect,20 at least one study found higher doses of CrPic decrease food intake.21 Our observation that direct brain administration of CrPic was sufficient to suppress food intake suggests that CrPic may be acting on central mechanisms controlling food intake. Studies in peripheral tissues demonstrate that CrPic may enhance insulin sensitivity,8 and a large body of work demonstrates that brain insulin signaling is critical for the appropriate regulation of food intake and body weight.22,23 Therefore, it is conceivable that CrPic may be acting on insulin-sensitive signaling systems in the brain, although additional work is required to clearly define the mechanism underlying CrPic-dependent changes in food intake.
A secondary goal of Study 1 was to evaluate the effects of CrPic on body weight. Participants receiving CrPic lost a small amount of weight (0.5 kg), while participants given placebo treatment gained a small amount of weight (0.5 kg) during this same time period, although the difference was not statistically significant. Our findings are consistent with previous reports7 and suggest that CrPic may attenuate weight gain or induce small weight losses over time in a population that may be predisposed to weight gain. Our data suggest the mechanism through which CrPic may reduce body weight is by decreasing food intake. However, since we did not measure physical activity levels or energy expenditure, it is unknown if CrPic also influences energy expenditure.
Participants receiving CrPic reported decreased cravings for carbohydrates, fast foods, high-fat foods, and sweets over time. A similar pattern of results was found for participants given placebo treatment. However, participants receiving CrPic decreased their cravings for high fat foods to a greater extent than participants receiving placebo. This finding is novel and is significant since cravings for high fat foods may lead to weight gain.24 Our finding that CrPic decreased cravings for carbohydrates and sweets is consistent with previous reports.25 CrPic did not decrease cravings for carbohydrates and sweets, however, to a greater extent than placebo; thus, it is unclear whether CrPic has a specific effect upon any particular food craving (e.g., fats vs. carbohydrates/sweets).
One limitation of the human study was that the sample only included relatively young women who reported craving carbohydrates. To be generalized to other populations, these findings need to be replicated. Another limitation is that eating behavior was measured in a laboratory rather than the participant's natural environment. Eating behavior in the laboratory, however, is consistent with eating behavior in the natural environment26 and has been found to be stable over time.27 Moreover, participants receiving CrPic lost a small amount of weight (0.5 kg) over this 8-week study, which suggests they may have also reduced their food intake outside of the laboratory. However, participants receiving CrPic lost a smaller amount of weight than would be expected based on the difference in food intake (i.e., 210 kcal) between the two groups during their final (week 8) food test day. This suggests that participants receiving CrPic may not have maintained a consistent reduction in energy intake throughout the entire 8-week period. It is also worth noting that participants receiving placebo gained a small amount of weight (0.5 kg) during this study, suggesting CrPic may attenuate body weight gain.
A potential limitation of our animal study was the use of a single intraperitoneal application. Although this protocol suggested CrPic's effects were centrally mediated, the single intraperitoneal dose may not have been sufficient to detect peripheral effects. It is possible that if CrPic was applied more chronically, peripheral effects may have been detected. Another potential limitation of our animal study was that we only measured changes in food intake and did not assess other potential metabolic changes that may have occurred following CrPic administration. Future studies should explore whether CrPic produces any metabolic changes, as well as the potential mechanism through which CrPic may be acting to affect food intake.
In summary, this is the first trial testing the effects of CrPic on food intake in both humans and animals. In Study 1, human participants receiving CrPic reduced food intake, hunger levels, and fat cravings, as compared to participants receiving placebo. Our human data were supported by animal studies demonstrating that CrPic suppressed food intake, particularly following central administration. These studies indicate that exogenous administration of CrPic suppresses food intake, particularly when administered in large doses. If future studies confirm these results, then CrPic may be a useful alternative or adjunctive treatment for individuals desiring to reduce their food intake.
Acknowledgments
The authors would like to express their appreciation to the participants and research associates who made it possible to complete this research project. This research was supported by the Health and Performance Enhancement Division of the Pennington Biomedical Research Center.
References
- 1.Key TJ. Allen NE. Spencer EA. Travis RC. The effect of diet on risk of cancer. Lancet. 2002;360:861–868. doi: 10.1016/S0140-6736(02)09958-0. [DOI] [PubMed] [Google Scholar]
- 2.Kenchaiah S. Evans JC. Levy D. Wilson PW. Benjamin EJ. Larson MG. Kannel WB. Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 3.Mann T. Tomiyama AJ. Westling E. Lew A. Samuels B. Chatman J. Medicare's search for effective obesity treatments: diets are not the answer. Am Psychol. 2007;62:220–33. doi: 10.1037/0003-066X.62.3.220. [DOI] [PubMed] [Google Scholar]
- 4.Dietary Supplement Health Education Act of 1994. US PUB Law 103–417. Oct 25, 1994.
- 5.Gibson JE. Taylor DA. Can claims, misleading information, and manufacturing issues regarding dietary supplements be improved in the United States? J Pharmacol Exp Ther. 2005;314:939–944. doi: 10.1124/jpet.105.085712. [DOI] [PubMed] [Google Scholar]
- 6.Neuhouser ML. Dietary supplement use by American women: challenges in assessing patterns of use, motives and costs. J Nutr. 2003;133(Suppl):1992S–1996S. doi: 10.1093/jn/133.6.1992S. [DOI] [PubMed] [Google Scholar]
- 7.Pittler MH. Stevinson C. Ernst E. Chromium picolinate for reducing body weight: meta-analysis of randomized trials. Int J Obes Relat Metab Disord. 2003;27:522–529. doi: 10.1038/sj.ijo.0802262. [DOI] [PubMed] [Google Scholar]
- 8.Martin J. Wang ZQ. Zhang XH. Wachtel D. Volaufova J. Matthews DE. Cefalu WT. Chromium picolinate supplementation attenuates body weight gain and increases insulin sensitivity in subjects with type 2 diabetes. Diabetes Care. 2006;29:1826–1832. doi: 10.2337/dc06-0254. [DOI] [PubMed] [Google Scholar]
- 9.Attenburrow MJ. Odontiadis J. Murray BJ. Cowen PJ. Franklin M. Chromium treatment decreases the sensitivity of 5-HT2A receptors. Psychopharmacology (Berl) 2002;159:432–436. doi: 10.1007/s00213-001-0960-7. [DOI] [PubMed] [Google Scholar]
- 10.Docherty JP. Sack DA. Roffman M. Finch M. Komorowski JR. A double-blind, placebo-controlled, exploratory trial of chromium picolinate in atypical depression: effect on carbohydrate craving. J Psychiatr Pract. 2005;11:302–314. doi: 10.1097/00131746-200509000-00004. [DOI] [PubMed] [Google Scholar]
- 11.McLeod MN. Golden RN. Chromium treatment of depression. Int J Neuropsychopharmacol. 2000;3:311–314. doi: 10.1017/S146114570000208X. [DOI] [PubMed] [Google Scholar]
- 12.Anderson RA. Bryden NA. Polansky MM. Chi J. Feng J. Elevated intakes of supplemental chromium improves glucose and insulin variables in individuals with type 2 diabetes. Diabetes. 1997;46:1786–1791. doi: 10.2337/diab.46.11.1786. [DOI] [PubMed] [Google Scholar]
- 13.Cefalu W. Bell-Farrow AD. Stegner J. Wang ZQ. King T. Terry JG. Effect of chromium picolinate on insulin sensitivity in vivo. J Trace Elem Exp Med. 1999;12:71–83. [Google Scholar]
- 14.Geiselman PJ. Anderson AM. Dowdy ML. West DB. Redmann SM. Smith SR. Reliability and validity of a macronutrient self-selection paradigm and a food preference questionnaire. Physiol Behav. 1998;63:919–928. doi: 10.1016/s0031-9384(97)00542-8. [DOI] [PubMed] [Google Scholar]
- 15.Flint A. Raben A. Blundell JE. Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 16.White MA. Whisenhunt BL. Williamson DA. Greenway FL. Netemeyer RG. Development and validation of the food-craving inventory. Obes Res. 2002;10:107–114. doi: 10.1038/oby.2002.17. [DOI] [PubMed] [Google Scholar]
- 17.Stunkard AJ. Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 18.Schlundt DG. Johnson WG. Eating Disorders: Assessment, Treatment. Boston: Allyn & Bacon; 1990. [DOI] [PubMed] [Google Scholar]
- 19.Morrison CD. White CL. Wang ZQ. Lee SY. Lawrence DS. Cefalu WT. Zhang ZY. Gettys TW. Increased hypothalamic protein tyrosine phosphate 1B contributes to leptin resistance with age. Endocrinology. 2007;148:1433–1440. doi: 10.1210/en.2006-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernao A. Meseguer I. Aguilar MV. Para MC. Munoz MJ. Effect of different doses of chromium picolinate on protein metabolism in infant rats. J Trace Elem Med Biol. 2004;18:33–39. doi: 10.1016/j.jtemb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Page TG. Southern LL. Ward TL. Thompson DL., Jr Effect of chromium picolinate on growth and serum and carcass traits of growing-finishing pigs. J Anim Sci. 1993;71:656–662. doi: 10.2527/1993.713656x. [DOI] [PubMed] [Google Scholar]
- 22.Bruning JC. Gautam D. Burks DJ. Gillette J. Schubert M. Orban PC. Klein R. Krone W. Muller-Wieland D. Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 23.Porte D., Jr Baskin DG. Schwartz MW. Insulin signaling in the central nervous system: a critical role in metabolic homeostasis, C. elegans to humans. Diabetes. 2005;54:1264–1276. doi: 10.2337/diabetes.54.5.1264. [DOI] [PubMed] [Google Scholar]
- 24.White MA. Whisenhunt BL. Williamson DA. Greenway FL. Netemeyer RG. Development and validation of the food craving inventory. Obes Res. 2002;10:107–114. doi: 10.1038/oby.2002.17. [DOI] [PubMed] [Google Scholar]
- 25.Wurtman RJ. Wurtman JJ. Brain serotonin, carbohydrate-craving, obesity and depression. Obes Res. 1995;4(3 Suppl):477S–480S. doi: 10.1002/j.1550-8528.1995.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 26.Kissileff HR. Thornton J. Becker E. A quadratic equation adequately describes the cumulative food intake curve in man. Appetite. 1982;3:255–272. doi: 10.1016/s0195-6663(82)80022-6. [DOI] [PubMed] [Google Scholar]
- 27.Martin CK. Williamson DA. Geiselman PJ. Walden H. Smeets M. Morales S. Redmann S., Jr Consistency of food intake over four eating sessions in the laboratory. Eat Behav. 2005;6:365–372. doi: 10.1016/j.eatbeh.2005.03.002. [DOI] [PubMed] [Google Scholar]