Abstract
The objective of this study was to identify mechanisms through which valproic acid (VPA) causes weight gain. Healthy participants (N = 52) were randomized to VPA or placebo in a double-blind study. Energy intake (EI) was measured in the laboratory at lunch and dinner, and physical activity (PA) was measured with accelerometry. Glucose levels and hormones [Peptide YY3–36, glucagon-like peptide-1 (GLP-1), leptin, ghrelin, insulin] that regulate EI were measured. Assessments occurred at baseline and week 3. Change from baseline was evaluated with mixed models (α = 0.05). Weight significantly increased in the VPA group (+0.49 kg), but not the placebo group. The VPA group increased fast food fats cravings and decreased glucose levels compared with placebo. Change in weight, EI and PA did not differ by group. Within group analyses indicated that the VPA group increased PA, hunger, binge eating, depression and GLP-1. VPA-associated weight gain is not likely due to changes in PA or the gut hormones studied. Although EI did not increase when measured after 3 weeks of treatment, VPA decreased glucose levels and increased motivation to eat; hence, EI might have increased in the short-term. Research testing VPA on short-term (1 week) EI, metabolism, and substrate partitioning is warranted.
Keywords: divalproex sodium, energy balance, energy expenditure, energy intake, food intake, physical activity, valproic acid, VPA, weight gain
Introduction
Valproic acid (VPA) is an anticonvulsant medication used to treat epilepsy (Brodie and French, 2000), mania associated with bipolar disorder (Bowden, et al., 1994; Pope, et al., 1991), and as a prophylaxis for migraine headache (Mathew, et al., 1995). One side effect of VPA that negatively influences its appeal is weight gain. Weight gain has been found to occur in 57% of adults (Dinesen, et al., 1984) and 58% of older children and teenagers (Wirrell, 2003) treated with VPA. The average amount of weight gained during VPA treatment is approximately 6 kg (Biton, et al., 2001; Chengappa, et al., 2002) and women gain more weight compared with men (El-Khatib, et al., 2007).
The mechanism by which VPA increases body weight is not understood. The balance between energy intake and expenditure influences body weight; therefore, VPA may alter energy intake, physical activity (PA)/energy expenditure or both. Alterations in energy intake or expenditure can result from, or be associated with, changes in biological mechanisms, including hormone levels. In vitro studies suggest that VPA initiates pancreatic insulin secretion that might increase appetite and energy storage and result in weight gain (Luef, et al., 2003). Indeed, VPA treatment has been found to increase post-prandial insulin and proinsulin levels (Luef, et al., 2002). Similarly, adults treated with VPA who develop obesity have been found to have higher levels of serum insulin and leptin compared with those who do not gain weight (Verrotti, et al., 1999), and VPA-treated children have increased insulin levels and decreased glucose levels (Demir and Aysun, 2000). Pylvanen, et al. (2002) found that VPA-treated participants had higher serum insulin levels, but not leptin levels, compared with controls who had similar body mass index. They concluded that hyperinsulinemia and possibly insulin resistance contributed to weight gain, but leptin had no independent role in VPA-related weight gain.
Hormones released in the gut might be affected by VPA and alter energy balance. Candidate hormones include ghrelin, glucagon-like peptide-1 (GLP-1) and peptide YY3–36 (PYY3–36). Ghrelin increases food intake (Bray, 2003), and GLP-1 and PYY3–36 decrease food intake (Batterham, et al., 2003; Small and Bloom, 2005; Stanley, et al., 2004). One study found that ghrelin was lower in patients who gained weight compared with those who did not gain weight during VPA treatment (Greco, et al., 2005). To our knowledge, the effect of VPA treatment on GLP-1 and PYY3–36 has not been studied.
One study tested the effects of VPA on energy intake and expenditure, and this study failed to find changes in either energy intake or expenditure after 4 weeks of VPA administration. Nevertheless, the study had a small sample size (n = 8), was uncontrolled, and relied on methods to measure energy intake (food records and recall) that may have introduced bias and were unlikely to detect changes in such a small sample (Breum, et al., 1992).
The purpose of the present study was to test the effects of VPA on: (i) body weight, (ii) food and beverage intake, (iii) free-living PA levels, (iv) the endocrine response to a meal and (v) eating attitudes and behaviours. To our knowledge, this randomized, placebo-controlled trial was the first to test the effects of VPA on food and beverage intake measured in the laboratory, objectively measured PA levels, and hormones that regulate energy balance. It was hypothesized that VPA treatment would result in: (i) increased food intake, (ii) reduced levels of PA and increased sedentary behaviour, (iii) increased hunger, food cravings and disinhibition or the tendency to overeat, and (iv) diminished physiological satiety signals (i.e. increased ghrelin and decreased GLP-1 and PYY).
Materials and methods
Research design and procedure
Participants completed a screening visit to determine eligibility. Participants provided written informed consent and the study was approved by the Institutional Review Board of the Pennington Biomedical Research Center.
During this double-blind randomized controlled trial, participants were followed for 3 weeks while taking either VPA or placebo, and they completed a follow-up visit 4 weeks after treatment was discontinued. The outcome variables were measured at baseline before random assignment to VPA or placebo. After 3 weeks of treatment, the outcome variables were measured again and treatment was discontinued. Participants returned 4 weeks later for a follow-up visit.
The specific days on which procedures were conducted follow. Physical activity was measured with accelerometry for 2 days at baseline (days −2 and −1, which were the 2 days prior to the baseline food intake tests) and 2 days at week 3 (days 19 and 20). Participants completed test meals on days 0 and 21. Following the assessments on day 0, participants were randomly assigned to receive placebo or VPA. On days 3, 7 and 14, participants reported to the clinic for a blood draw, dose management, and assessment of adverse events (AEs) and medication adherence. Participants returned to the clinic on day 49 for follow-up.
Participants
Healthy adult volunteers were recruited through media advertisements for this randomized placebo-controlled trial. The inclusion criteria were: (1) age, 18–54 years and (2) BMI (kg/m2), 20–30, inclusive. The exclusion criteria included: (1) urea cycle disorders, (2) impaired liver function, (3) pancreatitis, (4) dietary restraint score >14 or disinhibition score >12 on the Eating Inventory (Stunkard and Messick, 1988) to exclude people with irregular eating patterns that contribute to weight fluctuations, (5) weight gain or loss >2.3 kg (five pounds) in the 6 months prior to the study and (6) use of drugs that are contraindicated for VPA treatment, including anticonvulsants, barbiturates, tranquilizers, antidepressants and blood thinners. For females, additional exclusion criteria were: (1) pregnant or unwilling to use an effective form of contraception, (2) irregular menstrual cycles, (3) use of any oral contraceptive other than monophasic oral contraceptives, (4) history of partial hysterectomy, (5) nursing, and (6) history of polycystic ovarian syndrome. Exclusion No. 3 for females provided experimental control of hormone levels that affect food intake. All females were tested during the luteal phase of the menstrual cycle and monophasic oral contraceptive use results in a hormone profile similar to the luteal phase. Participants received monetary compensation for completion of the study.
The study statistician (HH) randomly assigned participants to receive either placebo or VPA in a 1:1 ratio (sequence was concealed) from February to August 2006. Randomization was stratified in an attempt to include equal numbers of men and women in the VPA and placebo groups. Body mass was also stratified according to low (20 ≤ BMI < 25) and high (25 ≤ BMI ≤ 30) BMI to help ensure mean BMI values were similar between groups in the event that VPA differentially affected people by body mass. Efforts were made to recruit 26 people in both the low and high BMI groups.
Materials and procedures
Valproic acid
Weight gain appears to be less severe with the extended release (ER) formulation compared with the delayed release (DR) formulation of VPA (Smith, et al., 2004), and higher serum levels are associated with greater weight gain (Bowden and Singh, 2005). In the present study, the DR formulation of VPA was administered to participants twice a day. The starting dose of VPA was 10 mg/kg/day, with a dose escalation of 5 mg/kg/day at day 4 and a maximum dose of 1500 mg/day. Dose was rounded to the nearest 250 mg (the size of the tablet). On day 4, participants in the placebo group had their regimen increased by two pills to reduce the possibility that participants could determine their group assignment. Pill taking adherence was quantified with pill counts on days 3, 7, 14, and 21. Additionally, plasma VPA concentrations were quantified as a measure of adherence and to determine if the therapeutic range of VPA (50–100 μg/ml) was achieved, which would increase generalizability of the study findings. Plasma VPA concentrations were measured at screening and days 3, 7, 14, 21 and 49 (on day 49, participants had not taken VPA or placebo for 28 days). Plasma VPA levels and blood test results were reviewed by the study physician and VPA dose was adjusted if laboratory results were abnormal or if serum levels exceeded 120 μg/ml.
Test meals
Food intake was measured in the Pennington Biomedical Research Center's Ingestive Behavior Laboratory. Participants completed food intake tests individually at lunch and dinner before (day 0) and after (day 21) treatment. Participants were asked not to eat food or drink calorie-containing beverages for 12 h prior to each test lunch, and between lunch and dinner (4 h separated lunch and dinner). The energy and macronutrient content of the foods served during the ad libitum lunch and dinner are outlined in Table 1. Sugar-sweetened and diet sodas and teas were provided at dinner to determine if VPA treatment increased consumption of calorie-containing beverages. Total food intake (kcal) and intake of protein, fat and carbohydrate was calculated from weighing food provision and plate waste. Participants remained at the center for 1 h after the start of their lunch to complete a blood draw. Participants were then free to leave the center and were given explicit instructions not to consume food or any beverages other than water prior to returning for dinner 3 h later. Measures of eating behaviour in the laboratory have been found to be reliable over time and appropriate for serial evaluation (Martin, et al., 2005).
Table 1.
Description (energy and macronutrient content) of the foods served during the food intake tests at lunch and dinner
| Serving size (g) | Energy (kcal) | Fat (g) | Protein (g) | Carb. (g) | |
|---|---|---|---|---|---|
| Luncha | |||||
| Ham sandwiches (3 hoagies) | 708.0 | 1161 | 36.8 | 81.4 | 123.2 |
| Roast beef sandwiches (3 hoagies) | 708.0 | 1253 | 36.1 | 105.5 | 120.4 |
| Turkey sandwiches (3 hoagies) | 708.0 | 1112 | 28.3 | 89.2 | 120.4 |
| Ruffles® Reduced Fat Potato Chips | 122.8 | 608 | 26.0 | 8.7 | 82.4 |
| Famous Amos® Chocolate Chip Cookies | 224.0 | 1158 | 54.0 | 15.5 | 154.6 |
| Water | 692.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Totals | 1746.8 | 2878–3019 | 108.3–116.8 | 105.6–129.7 | 357.4–360.2 |
| Dinnerb | |||||
| Spaghetti noodles with Prego | 300.0 g noodles, 265.0 g sauce |
722 | 12.4 | 19.1 | 131.1 |
| Spaghetti noodles with Alfredo | 300.0 g noodles, 265.0 g sauce |
922 | 45.5 | 19.0 | 97.7 |
| Arezzo® meatballs (10 meatballs) | 480.0 | 483 | 42.0 | 16.6 | 8.4 |
| Pepperidge Farm® Texas Toast with Cheese (4 slices) | 192.0 | 720 | 44.0 | 16.0 | 68.0 |
| Dole® Tropical Fruit Salad (1 container) | 732.0 | 480 | 0.0 | 0.0 | 126.0 |
| Pepperidge® Farm Iced Brownie (4 brownies) | 296.0 | 1200 | 48.4 | 14.4 | 189.2 |
| Sweetened tea | 692.0 | 210 | 0.0 | 0.0 | 54.0 |
| Unsweetened tea | 692.0 | 45 | 0.0 | 0.0 | 3.0 |
| Dr Pepper | 692.0 | 300 | 0.0 | 0.0 | 80.0 |
| Diet Dr Pepper | 692.0 | 0 | 0.0 | 0.0 | 0.0 |
| Coca-Cola | 692.0 | 280 | 0.0 | 0.0 | 39.0 |
| Diet Coca-Cola | 692.0 | 0 | 0.0 | 0.0 | 0.0 |
| Sprite | 692.0 | 300 | 0.0 | 0.0 | 74.0 |
| Diet Sprite | 692.0 | 8 | 0.0 | 0.0 | 2.0 |
| Water | 692.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Total | 6290.0 | 5062–5090 | 192.3 | 85.1 | 716.4–757.4 |
Participants selected and were served one of the three sandwich choices for lunch.
Participants received sweetened and unsweetened tea and they selected and were served the diet and regular version of only one of the soda flavours for dinner.
Physical activity
Each participant was fitted with an MTI (Manufacturing Technology Inc., Fort Walton Beach, Florida, USA) activity monitor that was worn on the hip 24-h per day for two consecutive weekdays at baseline (days −2 and −1) and days 19 and 20. The accelerometer collected data in 1-min epochs. The accelerometers were used to measure the minutes of sedentary behaviour, light PA, moderate PA and hard/very hard PA.1. The MTI accelerometers used in this study have been found to reliably estimate energy expenditure with a predictable deviation compared with the gold standard measurement, doubly labeled water (McCrory, et al., submitted for publication).
Eating attitudes and behaviours
The following questionnaires were used to measure changes in eating attitudes and behaviours during treatment. The questionnaires were completed at baseline and days 21 and 49.
The Eating Inventory (EI) has adequate reliability and validity and measures dietary restraint (the intent to restrict food intake), disinhibition (the tendency to overeat) and perceived hunger (Stunkard and Messick, 1985, 1988). The EI evaluated change in these parameters during treatment and it was used to exclude potential participants who had elevated scores on the dietary restraint (>14) and disinhibition (>12) scales, due to the relation of these scales with eating and body weight (Lawson, et al., 1995; Smith, et al., 1998).
The Multifactorial Assessment of Eating Disorders Symptoms (MAEDS) (Anderson, et al., 1999) assesses six symptoms related to eating disorders: binge eating, purgative behaviour, avoidance of forbidden foods, restrictive eating, fear of fatness and depression. The MAEDS was normed on a large sample of participants, allowing raw scores to be converted to standardized t-scores.
The Food Craving Inventory (FCI) is a valid measure for general cravings, represented by the total score, and cravings for: sweets, high fats, carbohydrates/starches and fast food fats (White, et al., 2002).
The Body Shape Questionnaire (BSQ) is a reliable and valid measure of body size and shape concerns (Cooper, et al., 1987).
Clinical chemistry
A blood sample, collected during the screening process, was utilized to exclude participants for whom VPA administration is contraindicated (impaired liver function). A urine sample was collected from females at screening to confirm that they were not pregnant. The study physician closely monitored liver function, platelet levels, amylase, and plasma concentrations of VPA. Therefore, the study physician was not blind to group assignment, but it is noted that data collection was conducted exclusively by the blinded study staff and the study physician was not involved.
Lipids [total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides], glucose, insulin, creatinine, albumin and platelets were measured on days 0 and 21. All of these parameters except insulin were also measured on day 49. Insulin was measured during a fasting state. These endpoints were processed on a Beckman Coulter DXC600 (Beckman Coulter, Fullerton, California, USA), with the exception of insulin which was processed on a Siemens Immulite 2000 (Siemens Healthcare Diagnostics Inc., Deerfield, Illinois, USA), and platelet count, which was processed on an Hmx (Beckman Coulter, Fullerton, California, USA). Only specimens for platelet counts contained ethylenediaminetetraacetic acid (EDTA) additive. All specimens were processed on a centrifuge at 3500 rpm (g = 2851) for 15 min at room temperature, except the specimens for platelet counts which were not processed on the centrifuge. All specimens were comprised of serum and stored at −80 °C, with the exception of the specimen for platelet counts, which was whole blood.
Satiety hormones
On days 0 and 21, a blood sample was collected half an hour before and 1 h after the start of lunch to test endocrine responses to test meals. The timing of these samples is based on the finding that PYY3–36 and GLP-1 peak 1 h after a meal (Adam & Westerterp-Plantenga, 2005; le Roux, et al., 2005). All specimens except leptin included the additive EDTA. All specimens were processed with a centrifuge at 3500 rpm (g = 2851) for 15 min at room temperature and were stored at −80 °C. All specimens were processed using radioimmunoas-says with a Linco Packard Riastar gamma counter (Perkin Elmer, Shelton, Connecticut, USA), except GLP-1, which was processed on a Bio Rad Microplate (Bio-Rad Laboratories, Hercules, CA, USA) reader using ELISA.
Clinic visits
Height, body weight and waist circumference were measured at screening, and body weight and waist circumference also were measured on days 21 and 49. Anthropo-metrics were collected in the morning after an overnight fast, following voiding. These measures were collected in the Center's Outpatient Clinic by two certified personnel following the Standard Operating Procedures for collection of these data. Height was measured using a wall-mounted stadiometer and weight was measured with a scale that measures to within 0.1 kg. The stadiometer and scale are calibrated weekly. Height was measured without shoes and weight was measured with the participant wearing only a hospital gown that was also weighed and subtracted from the weight of the participant. Height and weight were measured twice and a third measurement was collected if the two height measures differed by more than 0.5 cm or weight differed by more than 0.5 kg (the mean of the two measurements was recorded, or the mean of the two closest measurements if three measurements were needed). When measuring waist circumference, the volunteer stood in a straight upright position with feet together and hands by their side. The waist was identified (the narrowest part of the torso) and measured with undergarments pulled below the waist.
Participants were queried about AEs on days 3, 7, 14 and 21. On day 49, participants were offered services to help them lose weight that they might have gained during the study (no participants accepted this offer).
Data analytic plan
Data analysis
The primary outcome variables included change in body weight, food intake, PA, eating attitudes and endocrine response to a test meal. Difference scores (e.g. weight on day 21 minus weight on day 0) were calculated to quantify change over time. Mixed models were used to test if: (1) change over time differed by group (between group comparisons) and (2) each group's scores changed significantly over time (these within group analyses were planned a priori). Baseline values were entered as covariates and two-tailed tests were utilized. The hypotheses and statistical tests for the primary outcome variables were developed a priori and α was set at 0.05. For the remaining variables, α was set at 0.01. During development of the study design, a power analysis indicated that with 26 participants per group we could detect a difference of 385 kcal in food intake change between groups. The variance estimate for this power analysis was calculated from test meals conducted in our laboratory that were very similar to those used in the present study (the standard deviation from the pooled variance was 488 kcal). The power analysis assumed a two-tailed test and power equal to 0.80. A difference between groups of 385 kcal for change in food intake is a representative effect size for laboratory-based studies that examine the effect of compounds on food intake (e.g. Rolls, et al., 1998).
Results and discussion
Participant recruitment and descriptive characteristics of the study sample
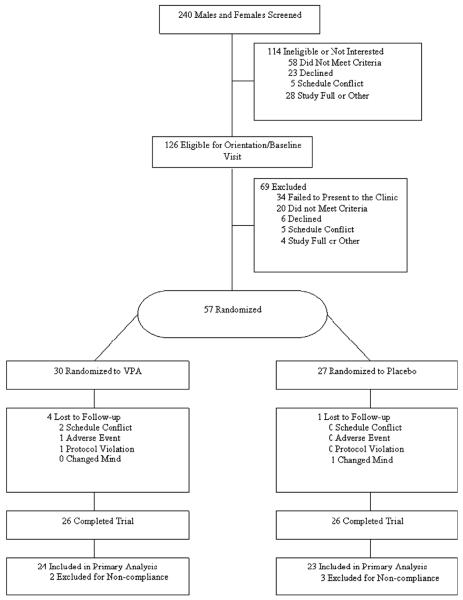
Participant recruitment is outlined in Figure 1 and the descriptive characteristics of the sample are summarized in Table 2. The VPA group had significantly higher baselines levels of hunger compared with the placebo group. Twenty-eight (54%) and 24 (46%) participants were recruited in the high and low BMI categories respectively.
Figure 1.
Participant recruitment.
Table 2.
Baseline characteristics (mean ± SD) of study participants
| Total sample (N = 52) M ± SD |
Placebo group M ± SD |
VPA group M ± SD |
P | |
|---|---|---|---|---|
| Age | 30.3 ± 9.5 | 32. ± 10.2 | 28.2 ± 8.6 | 0.11 |
| Height (cm) | 169.4 ± 9.7 | 169.5 ± 10.7 | 169.3 ± 8.7 | 0.94 |
| Body weight (kg) | 72.8 ± 13.5 | 73.8 ± 4.2 | 71.9 ± 12.9 | 0.60 |
| Waist circumference (cm) | 82.9 ± 10.0 | 83.7 ± 10.6 | 82.1 ± 9.4 | 0.57 |
| BMI (kg/m2) | 25.2 ± 3.3 | 25.5 ± 3.1 | 24.9 ± 3.4 | 0.54 |
| Kcal intake (baseline) | 1796 ± 539 | 1818 ± 517 | 1774 ± 569 | 0.77 |
| Accelerometer (min) | ||||
| Sedentary Beh. | 1034 ± 95 | 1024 ± 95 | 1044 ± 96 | 0.48 |
| Light PA | 377 ± 93 | 391 ± 91 | 362 ± 95 | 0.29 |
| Moderate PA | 25 ± 19 | 20 ± 17 | 29 ± 21 | 0.10 |
| Eating Inventory | ||||
| Dietary restraint | 6.4 ± 3.9 | 6.5 ± 3.7 | 6.3 ± 4.1 | 0.92 |
| Disinhibition | 3.8 ± 2.3 | 3.3 ± 2.0 | 4.2 ± 2.5 | 0.15 |
| Perceived hunger | 4.1 ± 2.7 | 3.2 ± 2.5 | 4.9 ± 2.7 | 0.02 |
| Food Craving Inventory | ||||
| Total score | 2.17 ± 0.67 | 2.10 ± 0.67 | 2.24 ± 0.68 | 0.49 |
| Carbohydrates/starches | 2.12 ± 0.78 | 2.01 ± 0.79 | 2.22 ± 0.78 | 0.34 |
| Fast food fats | 2.35 ± 0.75 | 2.31 ± 0.78 | 2.38 ± 0.72 | 0.71 |
| High fats | 2.00 ± 0.76 | 2.00 ± 0.73 | 1.99 ± 0.81 | 0.95 |
| Sweets | 2.19 ± 0.85 | 2.06 ± 0.77 | 2.33 ± 0.92 | 0.26 |
| MAEDS | ||||
| Binge eating | 43.1 ± 6.7 | 42.2 ± 6.2 | 44.0 ± 7.1 | 0.33 |
| Avoid. of forbidden foods | 43.5 ± 8.6 | 43.9 ± 7.7 | 43.1 ± 9.6 | 0.74 |
| Depression | 39.2 ± 5.9 | 39.4 ± 4.9 | 39.1 ± 6.9 | 0.85 |
| Fear of fatness | 39.7 ± 7.9 | 41.2 ± 8.4 | 38.3 ± 7.3 | 0.21 |
| Purgative behaviour | 46.1 ± 3.8 | 46.2 ± 4.1 | 46.0 ± 3.4 | 0.85 |
| Restrained eating | 43.0 ± 6.1 | 43.0 ± 6.1 | 42.9 ± 6.1 | 0.96 |
| Body Shape Questionnaire | 53.8 ± 16.0 | 53.4 ± 14.5 | 54.2 ± 17.8 | 0.86 |
|
| ||||
| n (%) | n (%) | n (%) | P (X2) | |
| Sex | ||||
| Female | 29 (56) | 15 (58) | 14 (54) | 0.78 |
| Male | 23 (44) | 11 (42) | 12 (46) | |
| Race | ||||
| Caucasian | 28 (54) | 14 (54) | 14 (54) | 0.59 |
| African-American | 23 (44) | 12 (46) | 11 (42) | |
| Hispanic | 1 (2) | 0 (0) | 1 (4) | |
The column on the far right contains P-values from the comparisons between the VPA and placebo group.
Adherence and adverse events
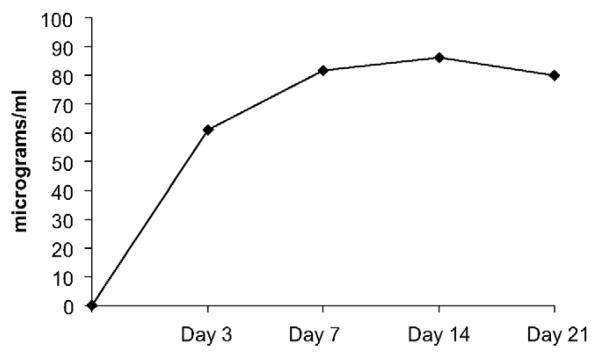
Plasma VPA concentrations for the VPA group are illustrated in Figure 2, demonstrating that the therapeutic range of 50–100 μg/ml was achieved. Pill counts suggested good adherence for the VPA (>95%) and placebo (>93%) group. In the VPA group, a total of 36 AEs were reported (17 gastrointestinal, seven neurological, five respiratory, five genitourinary and two dermatologic). In the placebo group, a total of 18 AEs were reported (five neurological, four gastrointestinal, four respiratory, two musculoskeletal, two oral and one genitourinary).
Figure 2.
Plasma valproic acid concentrations for the VPA group (μg/ml).
Body weight and food intake
The results of the following analyses are summarized in Table 3.
Table 3.
Least squares mean values for difference scores from baseline to day 21
| Difference score (day 21 minus baseline) |
Between group effect |
||||
|---|---|---|---|---|---|
| Placebo | VPA | df | F-value | P-value | |
| Primary outcome variables (α = 0.05) | |||||
| Body weight (kg) | −0.08 | 0.49* | 1,49 | 3.57 | 0.06 |
| Kcal intake (Total) | −90 | −51 | 1,44 | 0.20 | 0.66 |
| Kcal from fat | −28 | −19 | 1,44 | 0.07 | 0.79 |
| Kcal from carbohydrate | −42 | −36 | 1,44 | 0.02 | 0.90 |
| Kcal from protein | −16 | 0 | 1,44 | 1.45 | 0.24 |
| Beverage intake (Kcal) | 9 | 10 | 1,44 | 0.00 | −0.98 |
| Beverage intake (g) | 28 | −71 | 1,44 | 1.76 | 0.19 |
| Hormones & peptides | |||||
| Ghrelin | 17.51 | 23.45 | 1,47 | 0.03 | 0.87 |
| GLP-1 | 0.07 | 0.83* | 1,47 | 1.85 | 0.18 |
| Leptin | −0.15 | −0.49 | 1,47 | 0.70 | 0.41 |
| PYY | −2.47 | 6.12 | 1,47 | 2.41 | 0.13 |
| Eating Inventory | |||||
| Dietary restraint | −0.02 | 0.10 | 1,49 | 0.02 | 0.88 |
| Disinhibition | 0.41 | 0.74 | 1,49 | 0.36 | 0.55 |
| Perceived hunger | 0.54 | 1.07* | 1,49 | 0.75 | 0.39 |
| Food craving Inventory | |||||
| Total score | −0.05 | 0.13 | 1,49 | 2.28 | 0.14 |
| Carbohydrates/starches | −0.02 | 0.06 | 1,49 | 0.35 | 0.56 |
| Fast food fats | −0.11 | 0.24* | 1,49 | 5.41 | 0.02 |
| High fats | −0.04 | 0.13 | 1,49 | 1.60 | 0.21 |
| Sweets | −0.07 | 0.19 | 1,49 | 2.83 | 0.10 |
| MAEDS | |||||
| Binge eating | 0.11 | 2.78* | 1,48 | 1.83 | 0.18 |
| Avoidance of forbidden foods | −0.80 | 0.46 | 1,48 | 0.70 | 0.41 |
| Depression | 0.97 | 2.09* | 1,48 | 0.86 | 0.36 |
| Fear of fatness | 1.05 | 1.99 | 1,48 | 0.40 | 0.53 |
| Purgative behaviour | 0.74 | 1.45 | 1,48 | 0.32 | 0.58 |
| Restrained eating | 1.85 | 0.19 | 1,48 | 1.48 | 0.23 |
| Body Shape Questionnaire | 2.55 | 1.49 | 1, 49 | 0.09 | 0.77 |
| Secondary outcome variables (α = 0.01) | |||||
| Waist circumference (cm) | −0.04 | 0.35 | 1,49 | 0.36 | 0.55 |
| Blood pressure (mm Hg) | |||||
| Systolic | −0.42 | −3.17 | 1,49 | 1.75 | 0.19 |
| Diastolic | −2.54 | −3.94** | 1,49 | 0.53 | 0.47 |
| Clinical chemistry | |||||
| Albumin (g/dl) | 0.01 | −0.17*** | 1,49 | 8.22 | 0.006 |
| Total cholesterol (mg/dl) | 3.2 | −15.9*** | 1,49 | 17.88 | 0.0001 |
| LDL (mg/dl) | 2.44 | −10.78*** | 1,49 | 12.48 | 0.0009 |
| HDL (mg/dl) | −0.98 | −4.34*** | 1,49 | 5.07 | 0.03 |
| Platelets | 8.05 | −19.63** | 1,49 | 7.83 | 0.007 |
| Triglycerides (mg/dl) | 7.05 | −0.94 | 1,49 | 0.52 | 0.48 |
| Creatinine (mg/dl) | 0.02 | 0.02 | 1,49 | 0.07 | 0.80 |
| Insulin (μU/ml) | 1.30 | −0.53 | 1,48 | 6.23 | 0.02 |
| Glucose (mg/dl) | −0.16 | −5.80*** | 1,49 | 13.13 | 0.0007 |
Asterisks indicate that the difference score, which represents change over time within each group, differed significantly from zero
P < 0.05,
P < 0.01,
P < 0.001.
The P-value for the between group comparison is provided in the far right-hand column. This P-value represents the test to determine if change over time differed by group. Significant P-values and difference scores are noted in bold text. Hormone change scores represent the change in the response of hormones to a meal from baseline to day 21. Therefore, positive numbers represent an increased level of the hormone in response to a meal.
As illustrated in the second and third columns of Table 3, within group analyses indicated that the placebo group lost 0.08 kg (P = 0.70) and the VPA group experienced a significant increase in body weight (0.49 kg, P = 0.03) during this 21-day trial. The between group test indicated that body weight change did not differ significantly by group, although the P-value approached significance (P = 0.06, see the last column on the right of Table 3).
As outlined in Figure 1, five participants' data were eliminated from the primary (food intake) analyses for failure to comply with the protocol. Specifically, these participants' eating behaviour negated the ability to accurately estimate food intake and macronutrient consumption. For example, some participants broke apart the sandwiches and ate only certain ingredients from the sandwiches.
Change in food intake from baseline to day 21 did not differ significantly between the groups (Table 3). Within group analyses showed that neither the VPA nor placebo group experienced a significant change in food intake (P > 0.15). Similarly, change in fat, carbohydrate and protein intake did not differ between or within groups.
Change in energy intake from beverages from baseline to day 21 did not differ between or within groups (Table 3). Additionally, change in the number of grams of beverages consumed from baseline to day 21 did not differ between or within groups.
Physical activity
Accelerometers
Two participants' data were not available for analysis due to device malfunction. The VPA and placebo groups did not differ significantly on change in minutes per day of sedentary behaviour or light or moderate PA (p-values >0.44). As illustrated in Figure 3, the VPA group experienced a significant decrease in minutes of sedentary behaviour from baseline to day 21 (P = 0.047, LS Mean = −35.2 min) and a significant increase in minutes of light PA (P = 0.01, 43 min) based on within group comparisons. The placebo group had no changes on these measures (P > 0.12). Data for Hard/Very Hard PA are not shown, because participants engaged in <1 min of this activity per day.
Figure 3.
Change (day 21 minus baseline) in minutes/day of activity, measured by accelerometry, for the placebo and VPA group. Change in activity did not differ by group. *indicate a significant (P < 0.05) change in activity from baseline to day 21 within each group.
Change in endocrine response to a test meal
Changes in endocrine response to a meal were evaluated from baseline to day 21. The groups did not experience significantly different changes for ghrelin, GLP-1, leptin or PYY (Table 3). Within group analyses indicated that neither group experienced a significant change from baseline for ghrelin, leptin or PYY (p-values >0.09). The VPA group experienced a significant increase in GLP-1 (P = 0.04).
Eating attitudes and behaviours
Eating Inventory
There were no differences by group on change scores for any of the subscales of the Eating Inventory (Table 3). The VPA group experienced a significant (P = 0.01) increase in hunger. The VPA group had higher hunger scores at baseline, although the statistical model included baseline hunger values as a covariate and used change scores as the endpoint, providing considerable control for this baseline difference.
Food Craving Inventory
The VPA group had a significant increase in cravings for fast food fats compared with placebo (Table 3). Change in food craving scores did not differ between or within groups for the total score or the carbohydrates/starches, high fats and sweets subscales.
MAEDS
Change in MAEDS subscales did not differ by group. The VPA group had significantly increased scores on the binge eating (P = 0.049) and depression (P = 0.02) subscales of the MAEDS.
Body Shape Questionnaire
Change in concern for body size and appearance from baseline to day 21 did not differ between or within groups.
Waist circumference and blood pressure
Change in waist circumference and blood pressure (systolic and diastolic) from day 0 to 21 did not differ between the groups. Within group analyses indicated that the VPA group had a significant decrease in diastolic (P = 0.006) blood pressure from day 0 to 21.
Clinical chemistry
Change in creatinine and triglycerides from baseline to day 21 did not differ significantly between or within groups. The VPA group had significantly decreased albumin, total cholesterol, LDL, glucose and platelets compared with placebo (all P-values <0.01).
Change in endpoints from baseline to day 49
Change in body weight (P = 0.83), waist circumference (P = 0.65) and systolic and diastolic blood pressure (P > 0.21) from baseline to day 49 did not differ significantly by group. Neither group experienced a significant change in body weight from baseline to day 49 based on within group analyses (P > 0.86).
The groups did not differ on changes in the following self-report measures from baseline to day 49: Eating Inventory (P > 0.12), FCI (P > 0.61), MAEDS (P > 0.24) and BSQ (P = 0.44). Based on within group analyses, the VPA group had a significant increase in hunger from baseline to day 49 (LS Mean = 1.67, P = 0.004), and the placebo group had a significant increase on the fear of fatness subscale of the MAEDS (LS Mean = 2.45, P = 0.02).
Discussion
The results of this randomized controlled trial indicated that VPA treatment was associated with a significant increase in body weight among healthy participants treated with VPA for 3 weeks, although body weight change failed to reach statistical significance between groups (P = 0.06). This finding supports previous literature (Dinesen, et al., 1984; El-Khatib, et al., 2007; Wirrell, 2003) and the amount of weight gained during 3 weeks of VPA treatment in this study (+0.49 kg) is similar to the amount of expected weight gain based on other studies (Biton, et al., 2001), assuming that weight gain occurred steadily during treatment.
The trial reported herein indicates that VPA-induced weight gain is not likely the result of changes in PA or levels of leptin, ghrelin, GLP-1 or PYY. This is the first study to investigate the effects of VPA on food intake measured in the laboratory and although energy intake did not change from baseline to day 21, the VPA group experienced significantly increased cravings for fast food fats compared with placebo. Based on within group analyses, the VPA group experienced significantly increased hunger and binge eating, which are associated with increased energy intake. These findings suggest that the VPA group might have increased energy intake in the short-term and volitionally reduced energy intake by the 21st day of the protocol. Increased depression levels in the VPA group suggest increased dysphoria during the trial. The study design would have been improved by including energy intake measurements earlier in the protocol (day 7) and measuring energy intake over a longer duration to detect subtle changes in intake. For example, doubly labeled water could be used to provide an accurate estimate of energy intake over a 2-week period (Livingstone & Black, 2003; Schoeller, 1990).
In contrast to the hypothesis, the VPA group decreased sedentary behaviour and increased activity levels, although changes in activity levels did not differ significantly by group. These findings indicate that changes in activity levels are not likely responsible for VPA-associated weight gain, i.e. VPA treatment does not induce sedentary behaviour and subsequent weight gain. Although, it is possible that the VPA group volitionally altered their PA levels, activity levels have been found to increase without volition during positive energy balance and this increase helps prevent fat gain (Levine, et al., 1999). It is possible that VPA contributes to a positive energy balance and the body attempts to limit weight gain by increasing activity levels and, subsequently, energy expenditure.
This is the first study to examine the effect of VPA on PYY and GLP-1. Change in these hormones did not differ significantly between the VPA and placebo groups. The VPA group experienced an increase in GLP-1, which suggests that the VPA participants experienced increased physiological satiety signals after treatment. This finding is counterintuitive; if VPA increases energy intake, it is expected that satiety signals would be attenuated. The results of this study indicate that the satiety hormones are not responsible for VPA-associated weight gain. This conclusion builds upon the findings of Pylvanen et al. (2002), who concluded that leptin has no independent role in VPA-associated weight gain. Theoretically, it is reasonable to assume that the satiety hormones might not protect an individual from a positive energy balance that is secondary to VPA treatment. For instance, leptin is most potent when an organism is underweight and in a negative energy balance, but leptin signals are not effective when an organism is obese and in a positive energy balance (Berthoud, 2007; Hofbauer, 2002). Additionally, VPA might have changed the microstructure of eating (e.g. changed eating rate), which subsequently affected levels of satiety hormones. Gastric emptying and GLP-1 levels are associated (Miholic, et al., 2007) and gastric distension activates GLP-1 neurons in rats (Vrang, et al., 2003). In the current study, the VPA group non-significantly increased their rate of eating from baseline to day 21 and the placebo group had a slight decrease in eating rate (data not shown). Therefore, it is possible that changes in eating rate or eating microstructure affected GLP-1 levels. Lastly, VPA treatment might render the body unable to detect satiety signals associated with satiety hormones, rendering them ineffective.
Compared with the placebo group, the VPA group experienced a significant reduction in glucose levels from baseline to day 21. Insulin levels, however, did not change significantly in the VPA group, and GLP-1 infusion decreases glucose in healthy participants (Gutniak, et al., 1992) and type 2 diabetic patients (Willms, et al., 1996). Therefore, it is likely that the decreased glucose levels observed in the VPA group were the result of increased GLP-1. These results do not support previous findings suggesting that hyperinsulinemia contributes to VPA-associated weight gain (Pylvanen, et al., 2002), although the conclusions from the present study are qualified by the short duration of the study and the relatively small amount of weight gain observed.
Twenty-eight days after the last dose of VPA or placebo, almost all of the changes noted during the study no longer differed significantly from baseline values (only the VPA group's hunger score remained significantly elevated compared with baseline). Body weight returned to baseline for the VPA group and was only 0.06 kg on average above baseline body weight. Hence, people who gain weight during VPA treatment will likely lose the weight when treatment is discontinued. Change from baseline to day 49 did not differ between groups on any endpoint.
The present study has many strengths, including: (i) the inclusion of lean and overweight healthy individuals, (ii) random assignment in a double-blind, placebo-controlled trial, (iii) objective measurement of energy intake, including beverage intake, (iv) objective measurement of activity, (v) measurement of eating attitudes and behaviours and (vi) measurement of hormones that regulate energy balance. Additionally, the results of this study suggest that research is warranted on the efficacy of the following interventions to prevent weight gain or promote weight loss among participants taking VPA: (1) cognitive-behavioural interventions to reduce disinhibited eating, hunger, food cravings and binge eating, (2) cognitive interventions to increase dietary restraint, (3) structured meal plans that may include portion-controlled foods and (4) drugs used to treat obesity.
The study also has limitations. First, energy intake and activity levels were measured for 2-day intervals at baseline and week 3; therefore, short-term changes in these parameters could not have been detected. Based on work in our laboratory, some compounds affect food intake acutely, i.e. within 1 or 2 weeks, but food intake frequently returns to baseline after 4–8 weeks of administration of the compound. Second, participants in this study did not have a clinical diagnosis (e.g. epilepsy), although the observed weight gain in this sample was consistent with weight gain observed in clinical samples, and the inclusion of participants without a clinical diagnosis is a strength to the study, as the confounding effects of concomitant or previous medication use on the outcome variables were eliminated. Third, statistical power for some analyses was limited. Fourth, substrate partitioning and energy expenditure were not measured.
In conclusion, VPA-associated weight gain does not appear to be the result of changes in activity levels or hormones. Increased food cravings, hunger and binge eating suggest that energy intake might increase, at least acutely, after VPA treatment. Research is warranted that measures energy intake: (1) in the short-term (1–2 weeks after VPA treatment is initiated), (2) with very sensitive methods and (3) over a number of days to detect relatively subtle changes in energy intake. Additionally, research that measures changes in resting metabolic rate and substrate partitioning in response to VPA treatment would provide important information about the effects of VPA on metabolic processes that affect energy balance.
Acknowledgements
ClinicalTrails.gov (www.clinicaltrials.gov) Identifier: NCT00287053. Results from this study were presented at the 2007 Annual Meeting of the American Psychiatric Association, San Diego, California (May 2007). This research was supported by a grant from Abbott Laboratories. The lead author has received funds from Abbott Laboratories to attend a professional conference.
Footnotes
The remaining authors have no financial conflicts of interest to declare.
The Intelligent Device for Energy Expenditure and Activity (IDEEA™; MiniSun LLC, Fresno, California, USA) was used to measure the energy expended during sedentary and ambulatory behaviours. The IDEEA data are not reported because data collection was hindered by technical difficulties (11 and 21 participants in the VPA and placebo groups, respectively, had complete IDEEA data at baseline and follow-up).
References
- Adam TC, Westerterp-Plantenga MS. Glucagon-like peptide-1 release and satiety after a nutrient challenge in normal-weight and obese subjects. Br J Nutr. 2005;93:845–851. doi: 10.1079/bjn20041335. [DOI] [PubMed] [Google Scholar]
- Anderson DA, Williamson DA, Duchmann EG, Gleaves DH, Barbin JM. Development and validation of a multifactorial treatment outcome measure for eating disorders. Assessment. 1999;6:7–20. doi: 10.1177/107319119900600102. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, et al. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Interactions between the “cognitive” and “metabolic” brain in the control of food intake. Physiol Behav. 2007;91:486–498. doi: 10.1016/j.physbeh.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Biton V, Mirza W, Montouris G, Vuong A, Hammer AE, Barrett PS. Weight change associated with valproate and lamotrigine monotherapy in patients with epilepsy. Neurology. 2001;56:172–177. doi: 10.1212/wnl.56.2.172. [DOI] [PubMed] [Google Scholar]
- Bowden CL, Brugger AM, Swann AC, Calabrese JR, Janicak PG, Petty F, et al. Efficacy of divalproex vs lithium and placebo in the treatment of mania. The Depakote Mania Study Group. JAMA. 1994;271:918–924. [PubMed] [Google Scholar]
- Bowden CL, Singh V. Valproate in bipolar disorder: 2000 onwards. Acta Psychiatr Scand Suppl. 2005;426:13–20. doi: 10.1111/j.1600-0447.2005.00522.x. [DOI] [PubMed] [Google Scholar]
- Bray GA. An Atlas of Obesity and Weight Control. Parthenon Publishing Group; New York: 2003. [Google Scholar]
- Breum L, Astrup A, Gram L, Andersen T, Stokholm KH, Christensen NJ, et al. Metabolic changes during treatment with valproate in humans: implication for untoward weight gain. Metabolism. 1992;41:666–670. doi: 10.1016/0026-0495(92)90061-e. [DOI] [PubMed] [Google Scholar]
- Brodie MJ, French JA. Management of epilepsy in adolescents and adults. Lancet. 2000;356:323–329. doi: 10.1016/S0140-6736(00)02515-0. [DOI] [PubMed] [Google Scholar]
- Chengappa KN, Chalasani L, Brar JS, Parepally H, Houck P, Levine J. Changes in body weight and body mass index among psychiatric patients receiving lithium, valproate, or topiramate: an open-label, nonrandomized chart review. Clin Ther. 2002;24:1576–1584. doi: 10.1016/s0149-2918(02)80061-3. [DOI] [PubMed] [Google Scholar]
- Cooper PJ, Taylor MJ, Cooper Z, Fairburn CG. The development and validation of the Body Shape Questionnaire. Int J Eat Disord. 1987;6:485–494. [Google Scholar]
- Demir E, Aysun S. Weight gain associated with valproate in childhood. Pediatr Neurol. 2000;22:361–364. doi: 10.1016/s0887-8994(00)00133-8. [DOI] [PubMed] [Google Scholar]
- Dinesen H, Gram L, Andersen T, Dam M. Weight gain during treatment with valproate. Acta Neurol Scand. 1984;70:65–69. doi: 10.1111/j.1600-0404.1984.tb00804.x. [DOI] [PubMed] [Google Scholar]
- El-Khatib F, Rauchenzauner M, Lechleitner M, Hoppichler F, Naser A, Waldmann M, et al. Valproate, weight gain and carbohydrate craving: a gender study. Seizure. 2007;16:226–232. doi: 10.1016/j.seizure.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Greco R, Latini G, Chiarelli F, Iannetti P, Verrotti A. Leptin, ghrelin, and adiponectin in epileptic patients treated with valproic acid. Neurology. 2005;65:1808–1809. doi: 10.1212/01.wnl.0000187074.27586.d1. [DOI] [PubMed] [Google Scholar]
- Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S. Anti-diabetogenic effect of glucagon-like peptide-1 (7–36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992;326:1316–1322. doi: 10.1056/NEJM199205143262003. [DOI] [PubMed] [Google Scholar]
- Hofbauer KG. Molecular pathways to obesity. Int J Obes Relat Metab Disord. 2002;26(Suppl 2):S18–S27. doi: 10.1038/sj.ijo.0802124. [DOI] [PubMed] [Google Scholar]
- Lawson OJ, Williamson DA, Champagne CM, DeLany JP, Brooks ER, Howat PM, et al. The association of body weight, dietary intake, and energy expenditure with dietary restraint and disinhibition. Obes Res. 1995;3:153–161. doi: 10.1002/j.1550-8528.1995.tb00131.x. [DOI] [PubMed] [Google Scholar]
- le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ, et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2005;147:1–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283:212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- Livingstone MB, Black AE. Markers of the validity of reported energy intake. J Nutr. 2003;133(Suppl 3):895S–920S. doi: 10.1093/jn/133.3.895S. [DOI] [PubMed] [Google Scholar]
- Luef G, Abraham I, Hoppichler F, Trinka E, Unterberger I, Bauer G, et al. Increase in postprandial serum insulin levels in epileptic patients with valproic acid therapy. Metabolism. 2002;51:1274–1278. doi: 10.1053/meta.2002.34708. [DOI] [PubMed] [Google Scholar]
- Luef GJ, Lechleitner M, Bauer G, Trinka E, Hengster P. Valproic acid modulates islet cell insulin secretion: a possible mechanism of weight gain in epilepsy patients. Epilepsy Res. 2003;55:53–58. doi: 10.1016/s0920-1211(03)00091-3. [DOI] [PubMed] [Google Scholar]
- Martin CK, Williamson DA, Geiselman PJ, Walden H, Smeets M, Morales S, et al. Consistency of food intake over four eating sessions in the laboratory. Eat Behav. 2005;6:365–372. doi: 10.1016/j.eatbeh.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Mathew NT, Saper JR, Silberstein SD, Rankin L, Markley HG, Solomon S, et al. Migraine prophylaxis with divalproex. Arch Neurol. 1995;52:281–286. doi: 10.1001/archneur.1995.00540270077022. [DOI] [PubMed] [Google Scholar]
- Miholic J, Hoffmann M, Holst JJ, Lenglinger J, Mittlbock M, Bergmann H, et al. Gastric emptying of glucose solution and associated plasma concentrations of GLP-1, GIP, and PYY before and after fundoplication. Surg Endosc. 2007;21:309–314. doi: 10.1007/s00464-005-0804-3. [DOI] [PubMed] [Google Scholar]
- Pope HG, McElroy SL, Keck PE, Hudson JI. Valproate in the treatment of acute mania. A placebo-controlled study. Arch Gen Psychiatry. 1991;48:62–68. doi: 10.1001/archpsyc.1991.01810250064008. [DOI] [PubMed] [Google Scholar]
- Pylvanen V, Knip M, Pakarinen A, Kotila M, Turkka J, Isojarvi JI. Serum insulin and leptin levels in valproate-associated obesity. Epilepsia. 2002;43:514–517. doi: 10.1046/j.1528-1157.2002.31501.x. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Shide DJ, Thorwart ML, Ulbrecht JS. Sibutramine reduces food intake in non-dieting women with obesity. Obes Res. 1998;6:1–11. doi: 10.1002/j.1550-8528.1998.tb00308.x. [DOI] [PubMed] [Google Scholar]
- Schoeller DA. How accurate is self-reported dietary energy intake? Nutr Rev. 1990;48:373–379. doi: 10.1111/j.1753-4887.1990.tb02882.x. [DOI] [PubMed] [Google Scholar]
- Small CJ, Bloom SR. The therapeutic potential of gut hormone peptide YY3–36 in the treatment of obesity. Expert Opin Investig Drugs. 2005;14:647–653. doi: 10.1517/13543784.14.5.647. [DOI] [PubMed] [Google Scholar]
- Smith CF, Geiselman PJ, Williamson DA, Champagne CM, Bray GA, Ryan DH. Association of dietary restraint and disinhibition with eating behavior, body mass, and hunger. Eat Weight Disord. 1998;3:7–15. doi: 10.1007/BF03354907. [DOI] [PubMed] [Google Scholar]
- Smith MC, Centorrino F, Welge JA, Collins MA. Clinical comparison of extended-release divalproex versus delayed-release divalproex: pooled data analyses from nine trials. Epilepsy Behav. 2004;5:746–751. doi: 10.1016/j.yebeh.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Stanley S, Wynne K, Bloom S. Gastrointestinal satiety signals III. Glucagon-like peptide 1, oxyntomodulin, peptide YY, and pancreatic polypeptide. Am J Physiol Gastrointest Liver Physiol. 2004;286:G693–G697. doi: 10.1152/ajpgi.00536.2003. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Messick S. Eating Inventory Manual (The Psychological Corporation) Harcourt Brace & Company; San Antonio, TX: 1988. [Google Scholar]
- Verrotti A, Basciani F, Morresi S, de Martino M, Morgese G, Chiarelli F. Serum leptin changes in epileptic patients who gain weight after therapy with valproic acid. Neurology. 1999;53:230–232. doi: 10.1212/wnl.53.1.230. [DOI] [PubMed] [Google Scholar]
- Vrang N, Phifer CB, Corkern MM, Berthoud HR. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol. 2003;285:R470–R478. doi: 10.1152/ajpregu.00732.2002. [DOI] [PubMed] [Google Scholar]
- White MA, Whisenhunt BL, Williamson DA, Greenway FL, Netemeyer RG. Development and validation of the food-craving inventory. Obes Res. 2002;10:107–114. doi: 10.1038/oby.2002.17. [DOI] [PubMed] [Google Scholar]
- Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Nauck MA. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7–36) amide in type 2 (noninsulin-dependent) diabetic patients. J Clin Endocrinol Metab. 1996;81:327–332. doi: 10.1210/jcem.81.1.8550773. [DOI] [PubMed] [Google Scholar]
- Wirrell EC. Valproic acid-associated weight gain in older children and teens with epilepsy. Pediatr Neurol. 2003;28:126–129. doi: 10.1016/s0887-8994(02)00505-2. [DOI] [PubMed] [Google Scholar]