Summary
We have isolated two Drosophila cDNA clones that rescue Saccharomyces cerevisiae deficient in CLN functions. One of these clones is the Drosophila homolog of the cdc2 gene. The second encodes a distant and new member of the cyclin family of proteins, cyclin C. It is highly homologous (72% identity) to a human clone isolated in a similar screen. Yeast cells rescued by a plasmid constitutively expressing this Drosophila cyclin C are unusually small, consistent with an unregulated high level of G1 cyclin function. Sequence comparisons identified regions conserved among the more distantly related cyclins. Based on these conserved elements, we identified homology between cyclins and the ras oncogene.
Introduction
Universality of cell cycle control has been directly demonstrated by cross-species complementation. The cell cycle arrest of cdc2 mutations in the fission yeast Schizosaccharomyces pombe can be rescued by DNA from a variety of different species. In each case, the rescuing sequences are homologous to the S. pombe cdc2 gene and encode a 34 kd kinase. Indeed, all eukaryotes appear to encode a similar cell cycle regulator, the p34cdc2 kinase (see Nurse, 1990 for review). In both S. pombe and Saccharomyces cerevisiae, this p34cdc2 kinase is required at two points in the cell cycle, the transition from G2 to M and the transition from G1 to S (Nurse and Bissett, 1981; Piggott et al., 1982; Reed and Wittenberg, 1990;Surana et al., 1991;Ghiara et al., 1991). The success of cross-species complementation shows that the p34cdc2 from other species can fulfill the requirement for p34cdc2 at both of these control points. This suggests that the dual function of the p34cdc2 kinase has been preserved among eukaryotes.
The p34cdc2 kinase does not act alone. Association with a cyclin protein is required for p34cdc2 activation. Mitotic activation of p34cdc2 is controlled both by the levels of G2 cyclins, which oscillate in each cell cycle as a result of abrupt degradation during mitosis, and by phosphorylation and dephosphorylation of p34cdc2. Like p34cdc2, the G2 cyclins and the regulators of the phosphorylation status are conserved from yeast to humans (for reviews, see Pines and Hunter, 1990; Lewin, 1990; Nurse, 1990).
In metazoans, there are two types of G2 cyclins, cyclin A and cyclin B. These share common sequences that typify cyclins and function similarly in many assays. Nonetheless, because specific signature sequences distinguish the A-type and B-type genes (Minshull et al., 1990) and because these genes have been independently conserved, it seems likely that there are distinctions in their functions. Indeed, the phenotype of a Drosophila cyclin A mutation suggests that cyclin A plays a unique and essential role in the G2 to M transition (Lehner and O’Farrell, 1990b). In S. cerevisiae, no cyclin A–like homolog has been found, but there are at least four cyclin B–like genes, which are largely redundant (Surana et al., 1991; Ghiara et al., 1991; S. Reed, personal communication).
G1 regulators of p34cdc2 have been identified in S. cerevisiae (for review, see Reed, 1991). These regulators, the G1 cyclins, are weakly homologous to the G2 cyclins, and like the G2 cyclins, they interact with p34cdc2 (the product of the CDC28 gene in S. cerevisiae). The G1 cyclins, though presently only characterized in S. cerevisiae, are a family of related proteins. To arrest cells in G1, all three known G1 cyclin genes, CLN1, CLN2, and CLN3, must be mutant. Thus, the CLN genes appear to be functionally redundant, but if regulated differently, they might each mediate different aspects of growth control.
Why is there a diversity of cyclins? According to one model, the distinction among various cyclins determines the properties and thus the function of the p34cdc2 complex. For example, p34cdc2, when complexed to G1 cyclins, would have an S phase–promoting activity (SPF) and, when complexed with G2 cyclins, a mitosis-promoting activity (MPF). Alternatively, a second model does not require that cyclins play this key role as specifiers of p34cdc2 action and suggests that the various cyclins are different only in that they are regulated differently. For example, G1 cyclins may specifically induce S phase and G2 cyclins may specifically induce mitosis, because active G1 cyclins only accumulate in G1, while active G2 cyclins only accumulate in G2. This second model requires that something else, presently unknown, distinguishes the response of G1 and G2 cells to the activated p34cdc2 kinase.
If G1 cyclins are functionally distinct and universally used in regulating the G1 to S transition, the yeast cln mutations might provide a means of identifying these important regulators in other species. Perhaps like other cell cycle genes, G1 cyclin sequences will functionally complement across species boundaries. We have used cross-species complementation to isolate Drosophila genes that substitute for the S. cerevisiae CLN genes. One rescuing clone encodes the Drosophila cdc2(Dmcdc2) gene. While expression of this gene can rescue the requirement for CLN1, CLN2, and CLN3, cells rescued by Dmcdc2 seem to retain a severe delay in progression through the G1 to S transition and grow as very large cells. In contrast, a second rescuing clone yields tiny cells, which is consistent with high levels of Cln function. This clone encodes a protein whose sequence is weakly homologous to the cyclin family and highly homologous (72% identity) to a human gene that has G1 cyclin activity in a similar complementation test (Lew et al., 1991, this issue). Furthermore, characterization of the sequence relationships among more distant cyclin homologs led us to recognize sequence homologies with the ras proteins and to propose that cyclin and ras proteins are structurally and functionally related.
Results
Drosophila Clones That Complement a Yeast CLN Deficiency
We constructed a yeast strain with a conditional cln− phenotype useful for cross-species complementation screening (see Figure 1). In the background of cln1− and cln3− mutations, the wild-type chromosomal CLN2 gene was replaced by a CLN2 gene under the control of the galactose-regulated GAL1 promoter (see Experimental Procedures). The resulting strain, YPL1, grew on galactose-containing medium (GAL1-promoter active). When transferred to glucose media (GAL1-promoter repressed), cells adopted an unbudded shmoo-shaped morphology typical of G1-arrested cells in many yeast strains. The plating efficiency on glucose media was about 10−4 of the efficiency on galactose media. It was suggested to us that the background growth might be due to nonallelic gene conversion of the cln1::TRP1 mutant by the partially homologous CLN2 sequences (R. Deshaies). Such conversion events would eliminate the TRP1 insert. Consistent with this, the background growth was trp−, and selection for TRP+ on glucose plates largely eliminated background growth (<10−6). Growth on glucose was efficiently rescued by transformation with CLN2+ sequences expressed from the ADH1 promoter. Thus, the glucose arrest represents a Cln-deficient state.
Figure 1. Scheme for the CLN Complementation Screen.
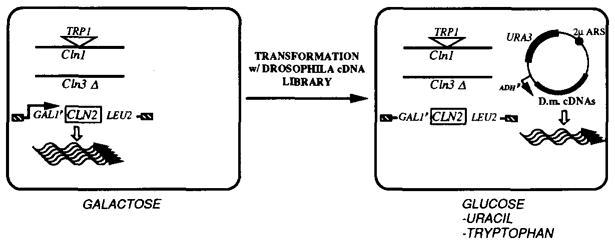
A Drosophila cDNA library in a URA3-marked plasmid was used to transform a strain harboring a deletion of CLN3, a TRP1-disruption of CLN1, and a chromosomal replacement of CLN2 by a GAL1p-driven CLN2 gene. This strain has a triple cln− phenotype when shifted from galactose to glucose media. Cells that contain an introduced plasmid harboring a Drosophila gene providing CLN function can be selected on glucose minimal plates minus uracil. The selection for TRP+ cells was added in order to eliminate the cln+ background, owing to gene conversion between CLN1 and CLN2 sequences.
To screen for complementing Drosophila sequences, we constructed a library of cDNA clones in the S. cerevisiae expression vector pDB20 (Becker et al., 1991). This high copy plasmid gives high levels of expression from the ADH1 promoter, and transformants can be selected by the URA3 marker. Using total RNA from the Drosophila Schneider cell line, a library of more than 106 independent clones was obtained, with more than 95% of the clones containing cDNA inserts. YPL1 was transformed with a pooled plasmid preparation, and 4 × 106 transformants were screened by plating directly on glucose media lacking uracil and tryptophan (Figure 1). Colony formation was slow, perhaps because of the multiple selections imposed on the cells. Approximately 200 clones were retrieved over a period of 4 weeks. Of these, 15% were plasmid-independent revertants (i.e., when replated in the absence of selection for URA+, they lost the plasmid and grew on glucose plates containing uracil and the antimetabolite fluoroorotic acid). Partial sequence analysis of five rescuing plasmids showed that they contained cDNA corresponding to the Drosophila homolog of the S. pombe cdc2 gene (Dmcdc2), which had previously been cloned (Lehner and O’Farrell, 1990a; Jimenez et al., 1990) (see below). A polymerase chain reaction (PCR) test was used to screen the remaining clones for Dmcdc2 sequences. Only 1 clone had a different identity.
Cyclin C, a Cyclin-Related CLN Complementing Gene
The ability of the unique non-Dmcdc2 plasmid, CL24, to rescue the Cln-deficient strain was confirmed by retransformation of YPL1. The CL24 insert was subcloned in a Bluescript vector and sequenced. The 1086 bp sequence showed a single open reading frame of 267 amino acids (Figure 2). Comparison with the size of the RNA transcript detected on Northern blots (see below) suggests that about 150 bp of noncoding sequences are not represented in the cDNA. The absence of a substantial poly(A) sequence in the 3′ sequence of the cDNA suggests that priming of cDNA synthesis by oligo(dT) may have initiated at an internal A-rich sequence. While this implies loss of 3′ sequences, some 5′ sequences might also be missing.
Figure 2. Nucleotide Sequence and Predicted Amino Acid Sequence of a Cyclin C cDNA.

The nucleotide sequence (1086 bp) has an open reading frame encoding a 267 amino acid protein. The putative initiating ATG is underlined along with flanking bases matching the consensus sequences for translational start sites in Drosophila (Cavener, 1987).
Computer searches of data bases with the CL24 aminoacid sequence detected homology with cyclins. This homology was explored in detail by manual comparisons. The many well conserved G2 cyclin sequences served as a guide to align more distantly related cyclin sequences. Alignment of 15 G2 cyclin sequences identified conserved positions, permissible gaps, and allowable substitutions at modestly conserved positions (Figure 3). The CL24 sequence, the CLN sequences, and a distant cyclin homolog (prad1; Motakura et al., 1991) were then aligned by inspection, emphasizing conserved residues and optimizing the matches to alternative residues at other positions. Three regions gave unambiguous and uninterrupted alignments for all of the divergent cyclin sequences (Figure 3). Homologies that are interrupted by frequent gaps or that did not extend to all of the divergent cyclin sequences are considered tenuous and are not discussed here.
Figure 3. Cyclin C Sequence Alignment with Conserved Cyclin Sequences.

The bars in the upper panel are scaled representations of five divergent cyclins. The location of the three regions identified as having high conservation are indicated as shaded segments. The scale is indicated on the right. For each of the conserved regions, a consensus of G2 cyclin sequences is shown. This was derived as follows: 15 G2 cyclin sequences were compared (clam cyclins A [Swenson et al., 1986] and B [Westendorf et al., 1989], human cyclins A [Wang et al., 1990] and B [Pines and Hunter, 1989], Drosophila cyclins A [Lehner and O’Farrell, 1989] and B [Lehner and O’Farrell, 1990], frog cyclins A [Minshull et al., 1990], B1 [Minshull et al., 1989], and B2 [Minshull et al., 1989], sea urchin cyclin [Pines and Hunt, 1987], S. pombe cdc13 [Booher and Beach, 1988], and S. cerevisiae Clb1, Clb2, Clb3, and Clb4 [Ghiara et al., 1991]). At each position, the most commonly occurring amino acid was selected for the consensus. In the few cases of a numerical tie, we selected the residue that was the most widely distributed among the more evolutionarily diverged cyclins. Below the consensus, all of the different residues that occur at a position are listed as alternatives. The cyclin C sequence and the other diverged cyclin sequences were aligned with the G2 consensus sequence. The number to the left of each aligned sequence is the position of the first residue of the segment. As a reference and an example of a G2 cyclin, the clam cyclin A sequence is included in this alignment. Residues matching the G2 consensus are boxed and shaded. Residues considered homologous to the residues of the consensus are boxed, according to the following groupings: A, L, V, I, M; D, E; K, R; N, Q; F, Y; S, T. The scores on the right of each alignment were calculated as follows. The 15 G2 sequences were used as a reference. The number of times each amino acid occurred at a given position in this reference set was determined. For an aligned sequence, each position was given a score corresponding to the number of times the corresponding amino acid occurred in the reference set. The total scores for an alignment were then normalized by dividing by the maximum score (number of positions scored × number of sequences in the reference set). For the ras homology within region III, homology scores were similarly calculated based only on the 21 amino acid sequence indicated for human H-ras.
Pairwise sequence comparisons were made within the stringently aligned regions (Table 1). Two divergent B-type cyclins, human and S. pombe, are 52% identical, while the CL24 sequence has 23% identity with the human cyclin B sequence. The CL24 sequence did not show especially high homology to any of the cyclin subtypes, A, B, or Cln. In particular, CL24 and Cln sequences are both more similar to G2 cyclins than to each other (Table 1). Thus, CL24 represents a distant homolog of both the G2 cyclins and the CLN genes.
Table 1.
Percentage Sequence Identity between Different Members of the Cyclin Family
| Clam cycA | Human cycB | cdc13 | DmcycC | cln1 | Cln3 | prad1 | |
|---|---|---|---|---|---|---|---|
| Clam cycA | 100 | 49 | 55 | 26 | 29 | 19 | 40 |
| Human cycB | 49 | 100 | 52 | 23 | 22 | 23 | 29 |
| cdc13 | 55 | 52 | 100 | 25 | 25 | 35 | 38 |
| DmcycC | 26 | 23 | 25 | 100 | 14 | 16 | 29 |
| Cln1 | 29 | 22 | 25 | 14 | 100 | 25 | 18 |
| Cln3 | 19 | 23 | 35 | 16 | 25 | 100 | 21 |
| prad1 | 40 | 29 | 38 | 29 | 18 | 21 | 100 |
Sequence identity in domains I, II, and III (see Figure 3) was determined after pairwise alignment (clam cyclin A, Swenson et al., 1986; human cyclin B, Pines and Hunter, 1989; S. pombe cdc13, Booher and Beach, 1988; Drosophila cyclin C; S. cerevisiae Cln1 and Cln3, Hadwiger et al., 1989; and human prad1, Motakura et al., 1991).
The above alignments define cyclin consensus sequences, as has been done for other multigene families, such as those encoding homeodomain proteins or zinc finger–containing proteins. Once such precise consensus sequences are defined, computer searches can identify even very distant homologs. Three largely independent cyclin consensus sequences identified the ras oncogene as a homolog (Figure 3 and Experimental Procedures). The match across a 21 amino acid ras region is excellent (it would occur randomly with a probability of about 10−9 for a single comparison) and extends to the diverged cyclin sequences (Cln1, Cln2, Cln3, prad1, and Drosophila cyclin C).
Homology between Human and Drosophila Cyclin C
Using a selection similar to ours, D. Lew and colleagues (see Lew et al., 1991, this issue) isolated human clones that rescued G1 cyclin–deficient S. cerevisiae. In addition to G2 cyclin genes, they isolated three rescuing sequences that have weak cyclin homology. Comparison of the CL24 sequence with these sequences uncovered homology far more extensive than that found in comparisons with any previously known cyclin. The CL24 sequence is 72% identical, with only a single gap of one residue, to one of their human sequences (Figure 4). The human sequence is somewhat longer, extending 20 residues beyond the C-terminus and 17 residues beyond the N-terminus of the Drosophila sequence. Given that CL24 is homologous to cyclins and does not belong to existing classes of cyclins, we will refer to this sequence as Drosophila cyclin C (see Lew et al., 1991, this issue).
Figure 4. Amino Acid Sequence Comparison of Drosophila and Human Cyclin C.
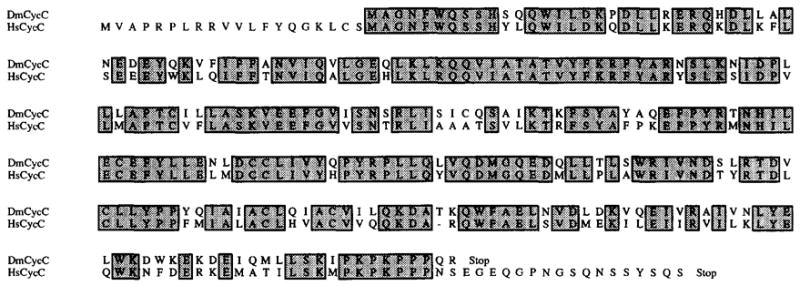
Total Drosophila and human sequences are aligned. Shaded boxes correspond to identical residues. The dash indicates a 1 amino acid gap introduced into the human sequence.
The Drosophila Cyclin C Gene
Cyclin C hybridization probes were prepared and used for Southern blots of Drosophila genomic DNA and for in situ hybridization to polytene chromosomes (Figure 5). The results show that Drosophila cyclin C is encoded by a single copy gene (DmcycC) at 88E on the polytene map.
Figure 5. Characterization of the DmcycC Gene.
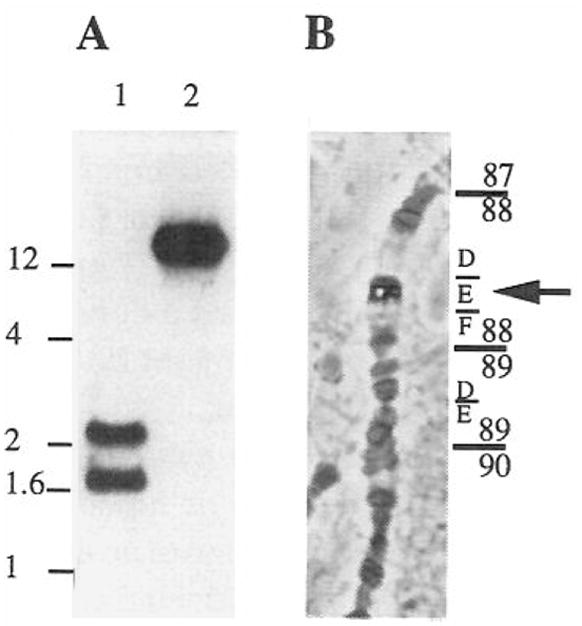
(A) Total genomic DNA from Drosophila was digested with EcoRI (lane 1) and Pstl (lane 2) and probed on Southern blots with a DmcycC probe. The scale on the left is in kilobases.
(B) A DmcycC probe was used to localize the gene on polytene chromosomes from salivary glands of third instar larvae. The cyclin C cDNA clone was labeled with digoxigenin and hybridized to salivary gland squashes. The position of the phosphatase signal following immunological detection was identified in several squashes. It consistently mapped to 88E.
Northern blots of RNA from staged embryos revealed a single low abundance 1.25 kb transcript (Figure 6). The presence of this RNA in newly laid eggs indicates a maternal contribution, and its persistence suggests either that this maternal RNA is stable for about 5 hr or that the RNA levels are maintained by balanced degradation and zygotic synthesis. Importantly, there is no correlation of RNA levels and cell proliferation.
Figure 6. Cyclin mRNA Levels during Embryonic Cell Cycle Progression.

Total RNA samples (corresponding to: lane 1, rapid cleavage stage embryos; lane 2, embryos in interphase of cycle 14; lane 3, embryos engaged in divisions 15 and 16; lane 4, older embryos, whose cell divisions are restricted to the nervous system) were probed with the cyclin C cDNA clone on a Northern blot. The lower panel shows a control hybridization of the same filter with an actin 5C probe. Note that the second lane is shifted down owing to loading of an unusually large volume of sample and that cyclin C and actin bands are affected similarly. Given the specific activities of the cyclin C and actin probes and the exposure times of the two hybridizations, we estimate that the intensity of the cyclin C signal is about 200 times less than that of actin. Signals were detected and analyzed by a Molecular Dynamics Phospholmager, which produces a relatively grainy image. The estimated size of the cyclin C mRNA is indicated in kilobases. Embryos were collected and aged at 25°C for the times indicated.
In situ hybridization of embryos with a digoxigenin-labeled DmcycC cDNA probe gave a faint, dispersed signal. This signal was detected prior to onset of significant transcription in the embryo. Like many maternal transcripts, this RNA is concentrated in the islands of yolk-free cytoplasm associated with nuclei, and it relocates to the cortical region of the syncytial egg upon nuclear migration to the surface. The signal persists through germ band extension, and no evidence of localized accumulation is seen (data not shown). This pattern is most simply consistent with a maternal origin of the embryonic DmcycC RNA with little or no zygotic expression. We have yet to study expression at later stages.
Drosophila p34cdc2 Has CLN-lndependent Function in Yeast
The rescue of cln deficiency by the Dmcdc2 gene was unanticipated but might be explained if the Drosophila p34cdc2 functioned with residual basal level of Cln expression in YPL1 grown on glucose. To test whether leaky expression of GALp–CLN2 is required for Dmcdc2 rescue, we removed the galactose-regulated CLN2 gene using a plasmid shuffle. A triple deletion strain (cln1−, cln2−, cln3−, leu−) bearing a GALp–CLN2, URA3 plasmid was transformed with an ADHp–Dmcdc2 plasmid marked with LEU2+. The LEU+ transformants were picked and streaked on plates that selected against URA3 (plates containing fluoroorotic acid) and for LEU2+ and Dmcdc2 (glucose media lacking leucine). After 4 days of growth, colonies appeared. Thus, when overproduced in S. cerevisiae, Dmcdc2 can bypass the requirement for the known CLN genes. However, these cells grew poorly (see below), and the presence of the GAL1p–CLN2, URA3 plasmid improved growth even when glucose repressed. Thus, leaky expression of GAL1p–CLN2 appears to be significant, though not essential for growth of the Dmcdc2 transformants.
The Dmcdc2 gene might bypass the need for CLN functions either by virtue of properties distinct from the endogenous cdc2 homolog, the CDC28 gene, or by virtue of over-expression. We tested whether high copy overexpression of CDC28 bypasses the CLN requirement. The YPL1 strain was transformed with pRD46a, a multicopy plasmid carrying the CDC28 gene with its own promoter. For comparison we also transformed this strain with ADHp-Dmcdc2-and ADHp-CLN2-expressing plasmids. On glucose plates (minus tryptophan and uracil), CLN2-rescued colonies appeared in 2 days and Dmcdc2-rescued colonies in 3 days, but no growth of CDC28-transformed cells was detected within 5 days. Thus, overproduction of CDC28 does not appear to result in bypass of the CLN requirement.
Growth of Cyclin C- and Dmcdc2-Rescued Cells
Cross (1988) reported that alterations of cln activity change cell size but not the doubling time. Apparently, increased G1 cyclin activity shortens the G1 phase, but some other part of the cell cycle compensates, so that total doubling time is unchanged (Cross, 1988; Hadwiger et al., 1989). Accordingly, the level of G1 cyclin activity should be reflected in the size at which the rescued cells initiate S phase rather than in the growth rate.
We compared the cell size of CLN-deficient cells rescued by CLN2, DmcycC, or Dmcdc2. The DmcycC-rescued cells are smaller than the CLN2-rescued cells, whether assessed by microscopy (Figure 7) or by fluorescence-activated cell sorting analysis (data not shown). This difference suggests that DmcycC encodes high or unregulated G1 cyclin activity. In contrast, the Dmcdc2-rescued cells are larger than the CLN2-rescued cells. This suggests that the basis of the rescue by Dmcdc2 is different from that of DmcycC.
Figure 7. Photomicrographs of cln− Rescued Mutants.
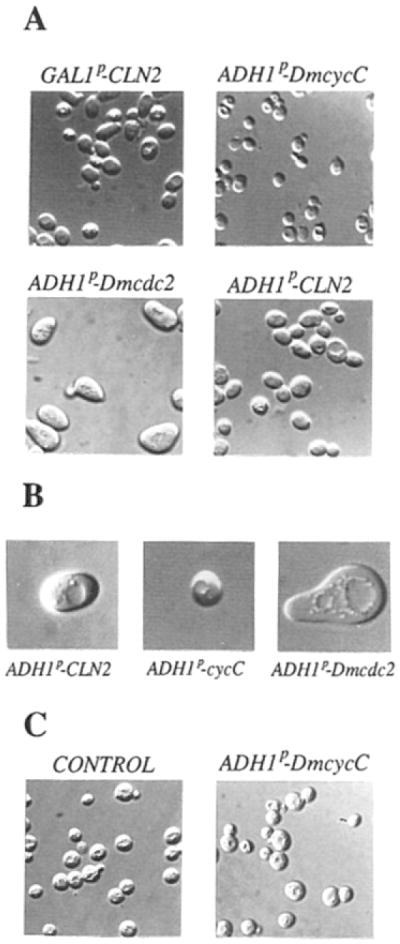
(A) Haploid YPL1 strain grown either in galactose (GAL1p–CLN2) or in glucose after transformation with plasmids containing ADH1 promoter–driven DmcycC, Dmcdc2, or CLN2 (respectively, ADH1p–DmcycC, ADH1p–Dmcdc2, and ADH1p–CLN2).
(B) Enlargements of representative YPL1 transformants with ADH1p–CLN2, ADHIp–DmcycC, and ADH1p–Dmcdc2.
(C) CLN+(bar1-1) cells either transformed with ADH1p–DmcycC or non-transformed (CONTROL), grown in glucose selective media minus uracil, or supplemented with uracil in the case of the nontransformed control.
We expected that DmcycC would drive premature division in CLN+ strains as well as in the CLN-deficient strain. However, when we transformed a CLN+ strain we found that the DmcycC cells were not reduced in size. Thus, the wild-type CLN functions or possibly some other aspect of the strain background blocks DmcycC promotion of the small cell phenotype.
The viability of cells rescued by the Drosophila genes was less than that of the same cells rescued by ADHp–CLN2. The cell number in actively growing cultures was determined by direct counts, and a constant number of cells from each culture were plated. If normalized to the number of colonies obtained from YPL1 grown on galactose media, the plating efficiency was 85% for CLN2-rescued YPL1, 50% for DmcycC-rescued YPL1, 50% for Dmcdc2-rescued YPL1, and 15% for Dmcdc2-rescued cells that lack a GALp–CLN2 gene. Thus, the rescued strains have reduced viability, and a constant attrition of the growing population of cells might be responsible for the slow rate of colony formation.
α factor arrest of the cell cycle appears to be mediated by inhibition of CLN expression or function and can be bypassed by constitutive production of an active CLN product (Cross, 1988; Nash et al., 1988; Hadwiger et al., 1989; Chang and Herskowitz, 1990). Thus, we expected constitutive expression of DmcycC or Dmcdc2 to bypass the α factor block. However, a CLN+ α factor–sensitive strain (CLN+ bar1-1) remained α factor sensitive when transformed with ADHp–Dmcdc2 or ADHp–DmcycC. This sensitivity was evident as an inhibition of budding assessed 4 hr after α factor addition and as growth inhibition on a plate assay (data not shown).
Expression of Drosophila G2 Cyclins in Yeast
Since other investigators have found that expression of G2 cyclins in S. cerevisiae can rescue Cln deficiencies (S. Reed, personal communication), we inserted the known Drosophila cyclin A and cyclin B genes in the pDB20 vector under the control of ADHp and tested their ability to rescue YPL1. No colonies were detected on glucose plates, where growth would require that the plasmids contribute Cln function. More surprisingly, on the galactose plates, which allowed expression of GALp–CLN2, no URA+ colonies were obtained from DmcycB-transformed cells, and DmcycA-transformed cells gave only 50 transformants per mg of DNA. The few DmcycA transformants grew very slowly (detected after 5 days of growth). Since a control transformation gave 1000 transformants per mg after 2 days of growth, it appears that expression of the Drosophila G2 cyclins compromises cell growth or survival. This negative influence of G2 cyclin expression might have prevented isolation of these sequences even if they are capable of exhibiting G1 cyclin function.
Discussion
We have identified a Drosophila gene encoding a protein that has high G1 cyclin activity in yeast. This gene product, cyclin C, is highly homologous (72% sequence identity) to a human gene product that has similar activity (see Lew et al., 1991, this issue). While this gene is an excellent candidate for a regulator of entry into S phase in metazoan cells, such a role has yet to be documented by studies of function in the cognate species. Nevertheless, whatever its normal function in Drosophila might be, the isolation of cyclin C has given us new insights into the family of cyclin proteins. Furthermore, the function of cyclin C in yeast reveals some interesting features of G1 cyclin function.
The Family of Cyclin Homologs
The cell cycle p34cdc2 kinase is thought to require association with a cyclin for activity. What of the other members of the family of cdc2-related kinases (Garrett and Broach, 1989; Courchesne et al., 1989; Lehner and O’Farrell, 1990a; Elion et al., 1990; Paris et al., 1991; Johnson and Smith, 1991)? We propose that kinase activation by cyclin–p34cdc2 association is a more widely used regulatory paradigm and that there is a family of cyclin-like molecules that function to activate cdc2-like kinases.
Overexpression or transfer of a gene to a different species might erode specificity and allow a cyclin-like molecule to activate kinases other than its normal target. How ever, independent isolation of the DmcycC gene (E. Lahue and T. Orr-Weaver, personal communication) and isolation of the homologous human cyclin C gene (Lew et al., 1991, this issue) argue that the screen for G1 cyclin activity in yeast is relatively selective. This selectivity could represent a specificity for genes with a G1 cyclin function. Alternatively, it could represent a broader specificity for genes capable of activating the S. cerevisiae p34cdc2 encoded by the CDC28 gene. Or perhaps, the selectivity is for a still more fundamental ability to interact with and activate cdc2-like kinases.
How extensive might the family of cyclin homologs be? A search for sequences including key amino acid residues found in all cyclin subtypes identified the ras oncogene as a homolog. In a 21 amino acid region, ras and G2 cyclins share the same level of homology as the more distantly related cyclins (Figure 3). Further comparisons of cyclin and ras sequences have identified a second region of extensive ras–cyclin sequence homology (O’Farrell and Léopold, unpublished data). The pattern of sequence homology and alignment with the ras crystal structure suggest that ras and cyclins share a common core structure as well as conserved surface features. The conservation of exposed residues leads us to suggest that these proteins may have common targets of interaction.
The Cyclin C Gene
The distant relationship of cyclin C to the other members of the cyclin family does not appear to be the result of rapid evolutionary divergence of this cyclin. Cyclin C conservation from Drosophila to human (72% identity) is much higher than conservation of other cyclins (e.g., 31% identity between Drosophila and human cyclin A). This suggests that cyclin C has important functions that severely constrain evolutionary divergence. Indeed, genes showing such levels of sequence conservation between species as distant as human and Drosophila tend to be well conserved among all eukaryotes (Wilson et al., 1977).
Despite an expectation that DmcycC should be particularly closely related to the CLN genes because it was selected by its ability to complement defects in these genes, a variety of sequence comparisons failed to detect any particular affinity between these sequences. Indeed, cyclin C appears to be a diverged cyclin homolog and does not appear to belong to any of the cyclin subfamilies.
Based on cyclin homology and independent evolutionary conservation, the name cyclin C seems appropriate. However, it should be noted that there is as yet no indication of whether cyclin C protein levels show the periodic accumulation that originally drew attention to the cyclins and inspired the name. In this context, it should be noted that cyclin C lacks the sequences that are thought to target other cyclins for rapid degradation, the degradation box of the G2 cyclins (Glotzer et al., 1991) and the PEST sequences of the Cln proteins (Rogers et al., 1986).
Cyclin C and Regulation of Cln Function in Yeast
Dominant alleles of CLN1 and CLN3 genes and high level constitutive expression of CLN1 are known to give a small cell phenotype (Cross, 1988; Nash et al., 1988; Hadwiger et al., 1989). On this basis it has been suggested that limitation of CLN function is involved in normal cell size control (Reed, 1991). DmcycC-transformed Cln-deficient yeast are very small, as would be expected for constitutive expression of G1 cyclin activity. In contrast, rescue by CLN2 did not produce a small cell phenotype. This difference leads us to suggest that a component of normal size control still operates in the CLN2-transformed cells but not in the DmcycC-transformed cells. Since both of these introduced genes are expressed from a constitutive ADH promoter, it appears that control at a posttranscriptional level affects Cln2 activity.
We expected constitutive expression of cyclin C to drive premature START in CLN+ cells as well as in cln− cells. However, the size of CLN+ cells was little influenced by introduction of the ADHp–DmcycC gene. Thus, one or more of the CLN genes suppresses the G1 cyclin activity of cyclin C. Such suppression can be accounted for by a competition model in which a yeast Cln forms a complex to the exclusion of cyclin C. This preferential interaction could restore size control, if the Cln complex is regulated while the cyclin C complex is not. Control of a cyclin–p34cdc2 complex has a precedent in the control of cyclin B–p34cdc2 in G2 by phosphorylation and dephosphorylation.
The mating pheromone α factor arrests the yeast cell cycle prior to START. Acting through a complex signal transduction pathway, α factor appears to inhibit Cln function (Cross, 1988; Nash et al., 1988; Hadwiger et al., 1989). We consequently expected that constitutive expression of cyclin C would bypass the α factor block by providing G1 function. However, CLN+ cells transformed with an ADHp–DmcycC plasmid remained α factor sensitive. This suggests either that the yeast mechanisms can inhibit the Drosophila protein, that α factor inhibits a different step, or that the inhibited CLN gene products can exclude cyclin C from participating in the formation of functional complexes.
We do not understand how the Drosophila p34cdc2 kinase rescues the Cln-deficient strain. However, the large size of the Dmcdc2-rescued cells suggests that the nature of the rescuing activity is very different from that of DmcycC. One possibility is that the Drosophila p34cdc2 kinase has a basal level of kinase activity independent of association with a cyclin subunit. Alternatively, the Drosophila protein might be activated by a yeast cyclin–like protein other than Cln1, Cln2, or Cln3.
Cyclin C in Drosophila
The temporal and spatial patterns of expression of a gene can give clues to its function. In our analysis of DmcycC RNA levels in the early embryo, we found no evidence for cell cycle oscillations. However, cell cycle progression during the stages that we have examined may be controlled by other factors (Edgar and O’Farrell, 1990). Alternatively, cyclin C activity, rather than the level of gene expression, might be cell cycle regulated. In any case, the best way to define the function of a gene is by phenotypic analysis of mutations. Among metazoans, the isolation of mutations and phenotypic analysis is, arguably, most advanced in Drosophila. The mapping of DmcycC to polytene chromosome band 88E provides an opening to begin such studies.
Experimental Procedures
cDNA Library Construction
Total RNA isolated from Schneider cells was used to synthesize cDNA, using RNAase H− Moloney murine leukemia virus reverse transcriptase (Superscript RT; Life Technology/BRL) as described by the manufacturers, except for the quantity of total RNA template, which was increased by a factor of 50 relative to conditions used for poly(A)+-selected RNA.
The cloning strategy used was the “inverted BstXI cloning” described by Aruffo and Seed (1987) using the plasmid vector pDB20, as described by Becker et al. (1991).
The ligated cDNAs were transformed into Escherichia coli DH5α F’IQ (BRL), and 106 independent transformants were obtained. Colonies at a density of 5 × 104 per 100 mm petri dish were scraped from the surface of the plates, and plasmid DNA was recovered by alkaline lysis and CsCI gradient. This DNA was used directly to complement S. cerevisiae mutants.
Yeast Strains and Media
Yh131 was derived from BF265-15D (Reed et al., 1985) and includes ura3Δns cln1::TRP1 cln3Δ (kindly provided by H. Richardson) (Richardson et al., 1989). YPL1 was constructed by replacing the chromosomal wild-type CLN2 sequence by a GALp–CLN2 insert in Yh131. To this end, Yh131 was transformed with a Sphl–Xhol fragment of plasmid pREP2 (see below), and chromosomal replacement was selected as LEU+ prototrophy. FC139 (a HMLa HMRa ura3-52 met1 lys−a bar1-1) was kindly provided by F. Chang (Chang and Herskowitz, 1990) and used in the α factor sensitivity experiments.
In the case of the cDNA library, yeast transformations were performed by the spheroplast method of Burgers and Percival (1987). Other transformations were performed by the lithium acetate procedure (Ito et al., 1983). Cell cycle arrest by α factor was performed as described in Chang and Herskowitz (1990). YEP-galactose or YEPD (complete) media, SD (minimal) medium, and supplements were used as described by Guthrie and Fink (1991).
Recombinant DNA Manipulation
Plasmid pREP2 was designed to promote chromosome replacement of the CLN2 gene by a galactose-inducible CLN2 copy. For this purpose, a 5.2 kb EcoRI–Sall fragment from plasmid YCpG2[CLN2] (Richardson et al., 1989) containing GAL1p-CLN2, LEU2 sequences was obtained by partial digestion and cloned into pBluescript. A 530 bp Xhol fragment corresponding to the 5′ region of the CLN2 gene was PCR amplified and cloned into a Xhol site in the pBluescript polylinker, 3′ to the LEU2 gene. A 570 bp Sspl–Xhol fragment from plasmid pSC2-Yep15 (kind gift of H. Richardson) containing 3′ flanking sequences from the CLN2 coding region was inserted into the Smal–Xhol site of pBluescript, next to the GAL1p sequences and in the same orientation as the 5′ flanking sequences. Plasmid pREP2 was then cut with Sphl and Xbal to give a 6.3 kb fragment with free ends within the 3′ and 5′ flanking sequences of the CLN2 gene, and this was used for chromosomal replacement by the method of Rothstein (1983).
For recovery from yeast to E. coli, plasmid DNA was isolated from yeast cultures using the glass bead–phenol method (Hoffman and Winston, 1987), treated with Geneclean, and introduced into E. coli by electroporation. Bacterial colonies were tested directly by the PCR for the presence of cdc2 sequences. Reactions were carried out following conditions described by the manufacturer (Perkin-Elmer Cetus).
In situ hybridization to polytene chromosomes, Southern blots, and Northern blots were performed as described in Lehner and O’Farrell (1989).
For DNA sequencing, a cyclin C cDNA insert was subcloned from the library vector pDB20 into pBluescript SK after NotI digestion. The resulting pSKcycC and pSKcycCR plasmids, containing the insert in both orientations, were submitted to ExoIII–S1 digestion (Henikoff, 1987), and a series of unidirectional deletions were sequenced using a T7 sequencing kit (Pharmacia-LKB biotechnology).
Photomicroscopy of Yeast Cells
Unfixed yeast cells were mounted in growth medium and photographed by using a Nikon Optiphot photomicroscope with differential interference contrast (nomarski) optics. A 63 × objective was used.
Computer Data Base Searches
Most comparisons of a complete protein sequence with the data base sequences test an astronomical number of alignments and inevitably identify weak homologies. Comparisons to short sequences without gaps test many fewer (at least 106 less) alignments, and the significance of homologies are increased by a comparable degree. Consequently, we searched the data base (PIR release 26.0) with sequence “masks” that include key cyclin residues and a specified spacing. These masks constitute cyclin signature sequences. To construct the masks, we first identified regions of sequence that are highly con served among G2 cyclins. We further required that alignment of these regions be uninterrupted and that the conservation extend beyond the G2 cyclins to include the distantly related cyclin sequences (see Figure 3). Within each of the conserved regions we defined a series of sequence “masks” to use in searches of the data base. The mask sequences were derived from the most conserved positions and included as alternatives at each position all the amino acids that appear in a reference set of 15 G2 cyclin sequences (see Figure 3). In the initial searches, the mask sequences were sufficiently degenerate that nearly 100 homologs were identified in the bank. For example, a mask to region III [(Y, C)xxxx(I,V,L) (L,R,K,I)xxExx(I,V,L,M)(L,F,T)] identified 71 sequences that included the 11 cyclin sequences in the data bank and 18 ras sequences. A second mask [(D,E)xx(Y,C)xxxx(I,V,L) (L,R,K,I)xxExx(I,V,L,M)], which overlaps the first mask at five out of the six specified positions, identified 74 sequences that included 11 cyclin sequences, 19 ras sequences, and 2 other sequences that had also been identified in the first search. Alignment of the human H-ras sequence to region III of cyclins shows that, over a 21 amino acid region, it has a level of homology comparable to the more diverged cyclins (Figure 3). In contrast, the 2 other sequences detected in both data base searches did not show a significant level of homology at positions other than those specified by the mask.
A third search of the data base used a consensus sequence that represented the most conserved positions in region III and that did not include any of the alternative residues occurring at the specified positions (DxxYxxxxxxxxExxxLxxL). The search yielded the 11 cyclin sequences and three other proteins. When a single amino acid of this cyclin consensus sequence was changed from aspartic acid to the homologous residue glutamic acid, this consensus became virtually specific for ras sequences, identifying 19 ras sequences and 8 nonras sequences in the protein data bank. The only sequences identified in both these and the previous searches were the cyclin and ras sequences. This search with the consensus sequence is largely independent, since only two of the five positions of this consensus sequence are in common with the mask sequence that we used initially (above).
Acknowledgments
We thank Daniel Lew and Steve Reed for very helpful discussions and for communicating results prior to publication. We are grateful to Helena Richardson, Fred Chang, and Ray Deshaies for providing various strains, plasmids, and extremely valuable advice. We thank Shelagh Campbell, Jim Jaynes, Danesh Moazed, Tony Shermoen, and Madhu Wahi for their comments on the manuscript, and Judy Piccini for her assistance in its preparation. This work was supported by NIH grant PO1 HL43821 (P. H. O.). P. L. acknowledges the support of a Fogarty International Center Fellowship and is on leave from the Institut National de la Santé et de la Recherche Médicale (France).
Footnotes
GenBank Accession Number
The accession number for the sequence reported in this article is M74906.
References
- Aruffo A, Seed B. Molecular cloning of a CD28 cDNA by a high efficiency COS cell expression system. Proc Natl Acad Sci USA. 1987;84:8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DM, Fikes JD, Guarente L. A cDNA encoding a human CCAAT-binding protein cloned by functional complementation in yeast. Proc Natl Acad Sci USA. 1991;88:1968–1972. doi: 10.1073/pnas.88.5.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booher R, Beach D. Involvement of cdc13+ in mitotic control in Schizosaccharomyces pombe; possible interaction of the gene product with microtubules. EMBO J. 1988;6:3441–3447. doi: 10.1002/j.1460-2075.1988.tb03075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgers PM, Percival KJ. Transformation of yeast spheroplasts without cell fusion. Anal Biochem. 1987;163:391–397. doi: 10.1016/0003-2697(87)90240-5. [DOI] [PubMed] [Google Scholar]
- Cavener DR. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucl Acids Res. 1987;15:1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, Herskowitz I. Identification of a gene necessary for cell cycle arrest by a negative growth factor of yeast: FAR1 is an inhibitor of a G1 cyclin, CLN2. Cell. 1990;63:999–1011. doi: 10.1016/0092-8674(90)90503-7. [DOI] [PubMed] [Google Scholar]
- Courchesne WE, Kunisawa R, Thorner J. A putative protein kinase overcomes pheromone-induced arrest of cell cycling in S. cerevisiae. Cell. 1989;58:1107–1119. doi: 10.1016/0092-8674(89)90509-6. [DOI] [PubMed] [Google Scholar]
- Cross F. DAF1, a mutant gene affecting size control, pheromone arrest and cell cycle kinetics of S. cerevisiae. Mol Cell Biol. 1988;8:4675–4684. doi: 10.1128/mcb.8.11.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar BA, O’Farrell PH. The three postblastoderm cell cycles of Drosophila embryogenesis are regulated in G2 by string. Cell. 1990;62:469–480. doi: 10.1016/0092-8674(90)90012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion EA, Grisafi PL, Fink GR. FUS3 encodes a cdc2+/CDC28-related kinase required for the transition from mitosis into conjugation. Cell. 1990;60:649–664. doi: 10.1016/0092-8674(90)90668-5. [DOI] [PubMed] [Google Scholar]
- Garrett S, Broach J. Loss of Ras activity in Saccharomyces cerevisiae is suppressed by disruptions of a new kinase gene, YAKI, whose product may act downstream of the cAMP-dependent protein kinase. Genes Dev. 1989;3:1336–1348. doi: 10.1101/gad.3.9.1336. [DOI] [PubMed] [Google Scholar]
- Ghiara JB, Richardson HE, Sugimoto K, Henze M, Lew DJ, Wittenberg C, Reed SI. A cyclin B homolog in S. cerevisiae: chronic activation of the Cdc28 protein kinase by cyclin prevents exit from mitosis. Cell. 1991;65:163–174. doi: 10.1016/0092-8674(91)90417-w. [DOI] [PubMed] [Google Scholar]
- Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Guthrie C, Fink GR. Guide to Yeast Genetics and Molecular Biology: Methods in Enzymology. San Diego: Academic Press; 1991. [PubMed] [Google Scholar]
- Hadwiger JA, Wittenberg C, de Barros Lopez MA, Richardson HE, Reed SI. A family of cyclin homologs that control G1 phase in yeast. Proc Natl Acad Sci USA. 1989;86:6255–6259. doi: 10.1073/pnas.86.16.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Meth Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Hoffman CS, Winston F. A ten minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of E. coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez J, Alphey L, Nurse P, Glover DM. Complementation of fission yeast cdc2ts and cdc25ts mutants identifies two cell cycle genes from Drosophila: a cdc2 homologue and string. EMBO J. 1990;9:3565–3571. doi: 10.1002/j.1460-2075.1990.tb07567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KW, Smith KA. Molecular cloning of a novel human cdc2/CDC28 protein kinase. J Biol Chem. 1991;266:3402–3407. [PubMed] [Google Scholar]
- Lehner C, O’Farrell PH. Expression and function of Drosophila cyclin A during embryonic cell cycle progression. Cell. 1989;56:957–968. doi: 10.1016/0092-8674(89)90629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C, O’Farrell PH. Drosophila cdc2 homologs: a functional homolog is coexpressed with a cognate variant. EMBO J. 1990a;9:3573–3581. doi: 10.1002/j.1460-2075.1990.tb07568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C, O’Farrell PH. The roles of Drosophila cyclins A and B in mitotic control. Cell. 1990b;61:535–547. doi: 10.1016/0092-8674(90)90535-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin B. Driving the cell cycle: M phase kinase, its partners, and substrates. Cell. 1990;61:743–752. doi: 10.1016/0092-8674(90)90181-d. [DOI] [PubMed] [Google Scholar]
- Minshull J, Blow JJ, Hunt T. Translation of cyclin mRNA is necessary for extracts of activated Xenopus eggs to enter mitosis. Cell. 1989;56:947–956. doi: 10.1016/0092-8674(89)90628-4. [DOI] [PubMed] [Google Scholar]
- Minshull J, Golsteyn R, Hill CS, Hunt T. The A- and B-type cyclin associated cdc2 kinases in Xenopus turn on and off at different times in the cell cycle. EMBO J. 1990;9:2865–2875. doi: 10.1002/j.1460-2075.1990.tb07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motakura T, Bloom T, Kim HG, Juppner H, Ruderman JV, Kronenberg HM, Arnold A. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991;350:512–518. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- Nash R, Tokiwa G, Anand S, Erickson K, Futcher AB. The WHI1+ gene of S. cerevisiae tethers cell division to cell size and is a cyclin homolog. EMBO J. 1988;7:4335–4346. doi: 10.1002/j.1460-2075.1988.tb03332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Nurse P, Bisset Y. Gene required in G1 for commitment to the cell cycle and in G2 for control of mitosis in fission yeast. Nature. 1981;292:558–560. doi: 10.1038/292558a0. [DOI] [PubMed] [Google Scholar]
- Paris J, Le Guellec R, Couturier A, Le Guellec K, Omilli F, Camonis J, MacNeil S, Philippe M. Cloning by differential screening of a Xenopus cDNA coding for a protein highly homologous to cdc2. Proc Natl Acad Sci USA. 1991;88:1039–1043. doi: 10.1073/pnas.88.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot JR, Rai R, Carter BLA. A bifunctional gene product involved in two phases of the yeast cell cycle. Nature. 1982;298:391–393. doi: 10.1038/298391a0. [DOI] [PubMed] [Google Scholar]
- Pines J, Hunt T. Molecular cloning and characterisation of the mRNA for cyclin from sea urchin eggs. EMBO J. 1987;6:2987–2995. doi: 10.1002/j.1460-2075.1987.tb02604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J, Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989;58:833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Pines J, Hunter T. p34cdc2: the S and M kinase? New Biol. 1990;2:389–401. [PubMed] [Google Scholar]
- Reed SI. G1 specific cyclins: in search of an S-phase promoting factor. Trends Genet. 1991;7:95–99. doi: 10.1016/0168-9525(91)90279-Y. [DOI] [PubMed] [Google Scholar]
- Reed SI, Wittenberg C. A mitotic role for the Cdc28 protein kinase of S. cerevisiae. Proc Natl Acad Sci USA. 1990;87:5697–5701. doi: 10.1073/pnas.87.15.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SI, Hadwiger JA, Lorincz AT. Protein kinase activity associated with the product of the yeast cell division cycle CDC28. Proc Natl Acad Sci USA. 1985;82:4055–4059. doi: 10.1073/pnas.82.12.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HE, Wittenberg C, Cross F, Reed SI. An essential G1 function for cyclin-like proteins in yeast. Cell. 1989;59:1127–1133. doi: 10.1016/0092-8674(89)90768-x. [DOI] [PubMed] [Google Scholar]
- Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986;234:364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Rothstein RJ. One-step disruption in yeast. Meth Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Surana U, Robitsch H, Price C, Schuster T, Fitch I, Futcher AB, Nasmyth K. The role of CDC28 and cyclins during mitosis in the budding yeast S. cerevisiae. Cell. 1991;65:145–161. doi: 10.1016/0092-8674(91)90416-v. [DOI] [PubMed] [Google Scholar]
- Swenson KI, Farrell KM, Ruderman JV. The clam embryo protein cyclin A induces entry into M phase and the resumption of meiosis in Xenopus oocytes. Cell. 1986;47:861–870. doi: 10.1016/0092-8674(86)90801-9. [DOI] [PubMed] [Google Scholar]
- Wang J, Chenivesse X, Henglein B, Bréchot C. Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature. 1990;342:942–945. doi: 10.1038/343555a0. [DOI] [PubMed] [Google Scholar]
- Westendorf JM, Swenson KI, Ruderman JV. The role of cyclin B in meiosis. J Cell Biol. 1989;108:1431–1444. doi: 10.1083/jcb.108.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AC, Carlson SS, White TJ. Biochemical evolution. Annu Rev Biochem. 1977;46:573–639. doi: 10.1146/annurev.bi.46.070177.003041. [DOI] [PubMed] [Google Scholar]


