Abstract
Purpose
To investigate the anatomic basis of the optic disc margin in the normal monkey eye by co-localizing optic disc photographs to three-dimensional histomorphometric reconstructions of the same optic nerve head.
Methods
Optic disc photographs from 28 normal monkey eyes were overlaid onto three-dimensional central retinal vessel reconstructions generated as part of post mortem optic nerve histomorphometric reconstructions for each eye. Within each reconstruction, Bruch’s Membrane Opening (BMO) was delineated. Alignment was achieved by matching clinical vessel outline to the vessel reconstruction using parallel viewing software. An experienced observer viewed stereophotographs and marked the disc margin onto clinical photographs using custom software. The alignment of the delineated disc margin to the histomorphometrically-defined BMO was qualitatively assessed within each image.
Results
In twenty eyes, BMO aligned well to the disc margin delineation. In four eyes, alignment improved following repeated co-localization. Careful review of the histomorphometric reconstructions identified that in most cases Bruch’s Membrane extended beyond the termination of the Border Tissue of Elschnig, most substantially in the superior and nasal sectors. Misalignments could be explained either by inaccurate BMO marking or where Bruch’s Membrane terminated external to the inferior edge of the Border Tissue, in which case this latter structure aligned to the disc margin.
Conclusions
BMO was a clinically detectable entity and represented the disc margin in the majority of eyes in this study. The three-dimensional architecture of the Border Tissue combined with the presence of an overhang of Bruch’s Membrane makes an important contribution to disc margin anatomy.
An appreciation of the optic disc margin is centrally important in the examination of all glaucoma patients as it defines the clinically visible boundary of neural tissue within the optic nerve head (ONH). With the advent of semi-automated ONH imaging devices, the ability to clearly identify and delineate the optic disc margin has assumed increasing importance. The disc margin serves both as a basis for cross-sectional neural tissue quantification and as a structural anchor for a reference plane for longitudinal change detection.1
Understanding the anatomic basis of the clinical disc margin remains a significant challenge for all clinicians.2 Although the optic disc is usually examined in a stereoscopic fashion - either by slit lamp biomicroscopy, or by stereophotograph examination - it remains difficult to appreciate the location of the disc margin within the ONH’s complex three-dimensional (3-D) architecture. The traditional view is that the clinical disc margin is defined by the scleral ring of Elschnig which is the anterior-most extension of the Border Tissue of Elschnig.3 The Border Tissue of Elschnig refers to densely compacted connective tissues that rise up from the sclera to join Bruch’s Membrane and thereby enclose the choroid.4-7 We have previously proposed, however, that in monkey eyes, the clinically visible disc margin is the innermost opening in Bruch’s Membrane (Bruch’s Membrane Opening - BMO) which in this species often extends beyond the Border Tissue.8
In the present study we explore the histologic basis of the monkey optic disc margin by co-localizing post mortem 3-D ONH reconstructions to their clinical (in vivo) optic disc photographs in twenty-eight normal monkey eyes. Our findings suggest that the 3-D architecture of the Border Tissue of Elschnig combined with the presence of a pigmented and/or unpigmented overhang of Bruch’s Membrane importantly underlie clinical disc margin anatomy in the normal monkey eye. These findings in monkeys are important because they suggest a preliminary algorithm for clinically assessing the more complicated disc margin anatomy of the human eye.
MATERIALS AND METHODS
Animals
All animals were treated in accordance with the ARVO statement for the use of animals in ophthalmic and vision research. All eyes were histomorphometrically reconstructed post mortem as part of other ongoing research studies. Twenty-eight normal eyes of twenty-one rhesus macaques and one cynomolgus monkey were reconstructed.
Stereophotography
All animals included in the study had regular optic disc stereophotographs acquired as part of imaging protocols developed for other ongoing research studies. Photography was performed while the animal was under pentobarbital anesthesia. IOP in the imaged eye was set at 10 mmHg via a manometer connected to a 27 gauge cannula inserted into the temporal anterior chamber. Pupils were dilated with 1 drop each of 1% tropicamide, 2.5% phenylephrine hydrochloride, and 2% cyclopentolate hydrochloride. Stereophotographs were acquired through a rigid plano contact lens placed on the corneal surface, after at least 30 minutes of IOP stabilization. Stereophotographic pairs in fifteen eyes were acquired using a Topcon TRC-WT Retinal Camera (Topcon, Paramus, NJ). Latterly, this system was replaced with a Nidek 3-DX fundus camera system (Nidek, Fremont, CA) which was used to acquire stereophotographs in the remaining thirteen eyes. Images were captured onto 35mm slide film which was developed and processed into color slides.
For the purposes of the current study, a stereophotograph pair was selected from the day on which the animal was killed. If stereophotographs were not acquired on this date then images acquired on the closest date were selected. Images had to be of sufficient quality to allow co-localization to the histomorphometric 3-D vessel reconstruction, meaning that at least one photograph in the pair should have had clearly discernible central retinal vessels. A good stereo-effect and a clear focus at the disc margin were desirable secondary considerations but were not grounds for exclusion of a stereophotograph pair. Selected stereophotograph slides were digitized at a resolution of 4800 dpi using a color-calibrated Microtek ArtixScan M1 slide scanner (Microtek Lab, Inc., Fontana, CA).
Perfusion Fixation at an IOP of 10 mmHg
The study eyes were cannulated using a 27-gauge needle with the animals under pentobarbital anesthesia, and the IOP was set to 10 mmHg using an adjustable saline reservoir. After a minimum of 30 minutes, the animal was killed with perfusion fixation via the descending aorta with 1 L of 4% buffered hypertonic paraformaldehyde solution followed by 6 L of 5% buffered hypertonic glutaraldehyde solution. IOP was maintained at 10 mmHg for 1 hour, after which each eye was enucleated (including a 0.5 - 1.5 cm section of the optic nerve), all extraorbital tissues were removed, and the anterior chamber was removed 2 to 3 mm posterior to the limbus. The posterior scleral shell with intact optic nerve head, choroid and retina was placed in 5% glutaraldehyde solution for storage.
3-D Histomorphometric Reconstruction and Bruch’s Membrane Opening Delineation
Our initial technique for 3-D optic nerve head reconstruction has been described in detail within a previous report.9 For this study, three of the 28 eyes were reconstructed using this technique and twenty-five eyes were serial sectioned using a second-generation device which increased axial and transverse image resolution to 1.5 × 1.5 × 1.5 μm voxels,1, 10 compared to 2.5 × 2.5 × 3.0 μm using the older device.
Our technique for 3-D delineation of Bruch’s Membrane Opening within the histomorphometric reconstructions has been described in detail elsewhere 8, 11, 12 Briefly, the 3-D ONH reconstruction was loaded into memory on a remote Linux server using a suite of custom software based on the Visualization Toolkit (VTK, Clifton Park, NY). Viewing serial digital transverse and/or radial sagittal sections of the volume, the delineator assigned the approximate center of the neural canal to be the center of rotation for 40, radial sagittal slices of the 3-D reconstruction which were generated at 4.5° intervals.
Within each radial section, the delineator performed a full delineation of the seven landmark surfaces and six pairs of neural canal landmark points (one on each side of the canal) which we have described previously.8 For the purposes of this study, however, only the Bruch’s Membrane Opening (BMO) marks were relevant. Delineations were performed by one of three technicians as part of other ongoing studies. The delineator marked the termination of Bruch’s Membrane on either side of the neural canal as the BMO. Delineation of each BMO point was 3-D in that each radial section pixel was linked to its location within a serial transverse (en face) section through the exact same location (simultaneously displayed on a second monitor). Once the BMO points had been delineated for all 40 sections (80 points in total), the 3-D Cartesian coordinates for each point were saved, allowing a 3-D BMO point cloud to be generated.
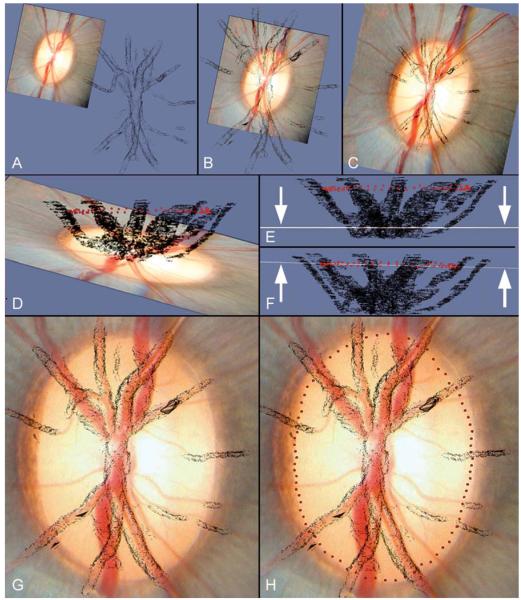
Overlay and Alignment of Histomorphometric Reconstructions onto Clinical Photographs (Figure 1)
Figure 1. Method for co-localizing the clinical disc photograph to the three-dimensional vessel reconstruction using parallel viewing software.
- The clinical photograph is viewed prior to co-localization at a distance from the vessel reconstruction
- X- and Y- axis shifts allow an approximate co-localization in the horizontal plane
- The magnification of the clinical image has been increased to match the dimensions of the vessel reconstruction
- The clinical photograph and vessel reconstruction are viewed in the coronal plane, with the BMO point cloud visible (red glyphs). This maneuver is performed to facilitate Z-axis adjustment. In this panel, the clinical photograph can be seen to be tilted in a different orientation to the long axis of the BMO point cloud
- The Z-axis tilt has been adjusted so that the clinical image (seen in coronal profile, highlighted by arrows) is now orientated in the same plane as the long axis of the BMO point cloud
- The clinical image has been moved vertically in the Z-axis so that the coronal image profile (highlighted by arrows) coincides with the BMO point cloud. Once this adjustment has been completed, the BMO points are switched off.
- The clinical image is now viewed in the en-face orientation following z-axis adjustment. A rotation about the image centroid has been performed to accurately align the clinical vessel outline to the vessel reconstruction
- The final location of the BMO points in relation to the clinical disc photograph is displayed
In order to establish the clinical orientation of each 3-D histomorphometric reconstruction, a high resolution 3-D reconstruction of the central retinal vessels and BMO points was generated for each eye. The 3-D vessel reconstruction was then overlaid onto a digitized clinical photograph (the best focused photo from the stereophotograph pair) using Paraview (Kitware, Inc., Clifton Park, New York) parallel viewing software. All co-localizations were performed by a single operator (NGS). Paraview enabled the operator to move the clinical photograph in 3-D space in terms of x-axis, y-axis and z-axis shifts as well as allowing rotation about the z-axis, z-axis tilt and magnification change.
The 3-D co-localization protocol is depicted in Figure 1. Z-axis adjustments were made with the photograph viewed on its side, along the coronal plane, with the BMO point cloud visualized; this allowed the clinical photograph to be tilted in the same orientation as the BMO point cloud. The BMO points were excluded from view for x- and y-axis manipulations, as well as z-axis rotation and magnification changes so that the final co-localization was based on the alignment of the central retinal vessels rather than by the alignment of the BMO points to the clinically visible disc margin.
Systematic Review of Stereophotographs and Delineation of the Clinical Disc Margin
Once the operator had achieved a satisfactory co-localization of the clinical vessels to the 3-D vessel reconstruction, screen captures were saved of the clinical photographs with and without the co-localized BMO points. The two photographs were then overlaid using Adobe Photoshop CS3 (Adobe Systems, Inc., San Jose, CA) and saved as a two-layer image file.
An experienced clinical observer (CFB) viewed each eye’s digitized stereophotograph pair on a computer monitor using a Screen-Vu stereoscope (PS Mfg., Portland, OR). Where the disc margin structures were not clearly discernible, the observer also had access to the original stereophotograph slides which could be viewed either using a hand-held stereo viewer (Asahi-Pentax Co., Englewood, CO) if the stereophotographs were acquired using the Nidek system or using a light box and mounted stereo viewer (Luminos Photo Corp, Yonkers, NY) if the stereophotographs were acquired using the Topcon system.
For each eye, the observer marked out the disc margin on the clinical photograph using a custom Java-script application. The clinical photograph used for this purpose was the co-localized image layer with the BMO excluded from view. The observer could make two categories of marks - blue marks for where the observer was ‘certain’ of the disc margin and green marks for where the observer was ‘uncertain’ and made a ‘forced’ choice.
For this study the operator marked the innermost clinically visible disc margin structure which was usually the internal edge of an unpigmented, whitish halo or crescent. Where this unpigmented structure was not visible, the termination of the variably mottled disc margin pigment was delineated. In some eyes, the observer could stereoscopically discern two different levels of pigment, an outer superficial layer and an inner deeper layer, with a portion or ‘lip’ of unpigmented tissue variably present at the termination of either pigmented tissue. In these circumstances two rows of marks were made - an outer row of single marks to demarcate the internal edge of the superficial tissue (either pigmented or unpigmented) and an inner row of double marks (a green mark touching a blue mark) to demarcate the termination of the deeper tissue (either pigmented or unpigmented).
Once the observer completed the demarcation of the clinically visible disc margin, the coordinates of the marked points were saved and the points transferred onto the image layer in which the BMO points were also shown. An image of the clinical photograph incorporating the co-localized BMO points and the clinical demarcations was then saved for each eye.
Systematic Review of Histomorphometric Reconstructions
Prior to assessing the alignment of the BMO to the clinical disc margin, the 3-D histomorphometric volumes of all twenty-eight eyes were reviewed by two observers (CFB and NGS). For each eye, the observers documented the quality of the connective tissue staining, the degree of pigment in the reconstruction and the presence or absence of artifacts such as choroidal or retinal detachments. Choroidal and retinal detachments were assumed to be post mortem artifacts as they were not present in optic disc images/disc photographs acquired on the day of sacrifice. The perfusion fixation process is assumed to provide sufficient hydrostatic pressure to cause expansion of the choroidal space in a majority of eyes. Sections at 0°, 45°, 90° and 135° were loaded, enabling the histology in the superior-inferior, superotemporal-inferonasal, temporal-nasal and inferotemporal-superonasal regions to be inspected. In order to establish the histologic underpinnings of the clinical disc margin, the observers carefully reviewed the configuration of Bruch’s Membrane, Bruch’s Membrane Opening and the Border Tissue of Elschnig in each sector of the disc. The observers noted how far Bruch’s Membrane extended beyond the termination of the Border Tissue, the presence or absence of an unpigmented portion of Bruch’s Membrane, the configuration of the Border Tissue in relation to where it met Bruch’s Membrane and whether or not the histomorphometric BMO delineation was felt to be accurate.
Comparison of the Histomorphometrically-Defined BMO with the Clinical Disc Margin
Once delineation of the clinical photos was completed, the images incorporating the histomorphometric BMO marks and disc margin marks were qualitatively reviewed by two observers (CFB and NGS). This was performed to assess how well the co-localized histomorphometric BMO marks aligned with the clinical disc margin delineations. An eye was classified as being well aligned if there was less than a glyph’s diameter separation between adjacent BMO and clinical disc margin glyphs in all disc sectors, with disc sectors being defined as per the systematic histomorphometric review. In eyes with misalignment between the histomorphometric BMO and disc margin marks by the above definition, the following investigations were performed to identify causes for the observed discrepancies. First, the co-localization process was repeated, leading to a ‘second pass’ review of the co-localized BMO and clinical marks. In eyes where the alignment between BMO and clinical marks could not be improved by repeated co-localization, a second, systematic review of the relevant sterephotographs and 3-D histomorphometric reconstructions was carried out so as to identify the salient clinical and/or histologic features that might explain the misalignment.
RESULTS
Systematic Review of 3-D Histomorphometric Reconstructions
Bruch’s Membrane Extension, Pigment and Opening
In most eyes, an extension of Bruch’s Membrane with mottled pigment on its surface, which we defined as ‘pigmented’ Bruch’s Membrane, was usually present beyond the histomorphometric termination of the Border Tissue of Elschnig and choroid. A further extension of Bruch’s Membrane without surface pigment (‘unpigmented’ Bruch’s Membrane) could be detected internal to pigmented Bruch’s Membrane in most high resolution 3-D histomorphometric reconstructions. Figure 2 illustrates the distinction between unpigmented and pigmented Bruch’s Membrane in a histomorphometric section. An extension of Bruch’s Membrane was visible in at least one disc region of every eye examined in this study. A substantial extension of Bruch’s Membrane was observed most frequently in the superior and nasal sectors (both 43% of eyes), followed by the inferonasal (39%), superonasal (36%), temporal (29%), inferotemporal (25%) and superotemporal (21%) sectors. Unpigmented Bruch’s Membrane was usually visualized despite the presence of a darker tissues or poor tissue staining. However, its detection in the three low resolution volumes was less consistent as this resolution was perhaps insufficient to allow reconstruction of unpigmented Bruch’s Membrane.
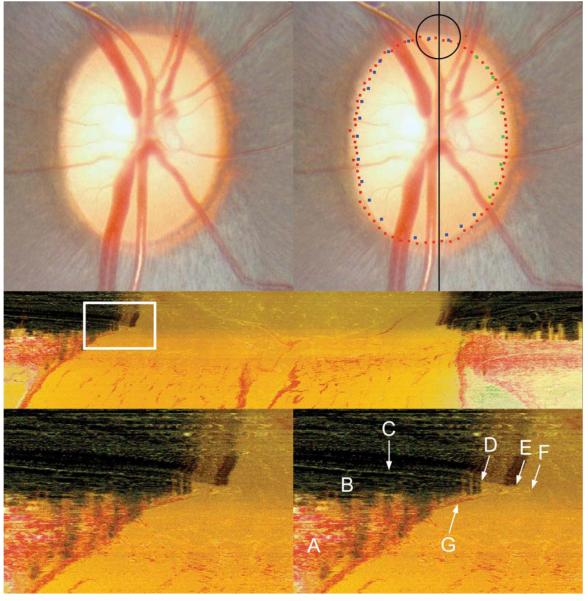
Figure 2. The identification of Bruch’s Membrane and Bruch’s Membrane Opening in a histomorphometric section.
Top left panel: The clinical disc photograph (OD) prior to disc margin delineation
Top right panel: The clinical disc photograph displaying both the co-localized histomorphometric BMO points (red glyphs) and the clinical disc margin points (blue and green glyphs). The black line is the approximate location of the vertical histomorphometric section shown in the middle panel. The area circled in black is the approximate location of the histomorphometric region (white box in the middle panel) magnified in the two bottom panels.
Middle panel: Central vertical histomorphometric section shown as a black line in the top right panel. Superior is left and inferior is right. The area within the white box is magnified in the bottom panels. Note that because the tissues are sectioned from the vitreous (top) to the orbital optic nerve (bottom) a dark shadow is present until the serial sectioning plane passes through the dense pigment of the retinal pigment epithelium, choroid and Bruch’s Membrane
Bottom left panel: Magnified view of the highlighted white box in the middle panel which demonstrates the superior disc margin anatomy
- A = Sclera
- B = Choroid
- C = Bruch’s Membrane
- D = Commencement of pigmented Bruch’s Membrane which in this section appears to co-localize to the termination of the choroid (B)
- E = Termination of pigmented Bruch’s Membrane and the commencement of unpigmented Bruch’s Membrane. Note the presence of pigment ‘shadows’ of variable density cast vertically along the course of the pigmented Bruch’s Membrane which are absent in the regions where Bruch’s Membrane is unpigmented
- F = Termination of unpigmented Bruch’s Membrane, which would be delineated as Bruch’s Membrane Opening in this section
- G = Border Tissue of Elschnig. In this eye, Bruch’s Membrane fuses with the superior edge of the Border Tissue and extends slightly beyond its termination
Accurate delineation of BMO was made difficult where pigment was present on the lamina or scleral surface, as the ‘shadow’ cast by this pigment could obscure the termination of Bruch’s Membrane (Figure 3). Rarely, the Border Tissue met the termination of Bruch’s Membrane and no extension was present. Artifactual choroidal or retinal detachments were present in three eyes (Figure 3), but these did not involve the Bruch’s Membrane/Border Tissue junction and so did not affect the configuration of the disc margin anatomy.
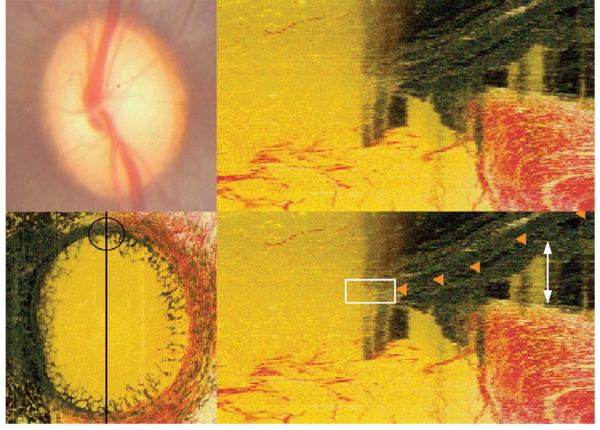
Figure 3. Pigment on the lamina surface causing obfuscation of Bruch’s Membrane Opening.
Top left panel: Clinical disc photograph (OS)
Bottom left panel: En face view of the histomorphometric reconstruction of the same eye. Black line shows the orientation of a histomorphometric section image, a portion of which (the superior disc margin) is magnified in the right panels. The black circle highlights the region viewed in the right panels; note the presence of pigment on the lamina surface
Top right panel: Histomorphometric view of the superior part of the neural canal
Bottom right panel: Bruch’s Membrane delineated (orange glyphs). The white rectangle highlights an area where the view of Bruch’s Membrane is obscured by a dark shadow cast from the lamina pigment below. In this circumstance, accurate delineation of Bruch’s Membrane Opening can be difficult. The white arrowheads highlight the extent of an artifactual choroidal detachment, most likely caused by the perfusion fixation process
Border Tissue of Elschnig Configuration
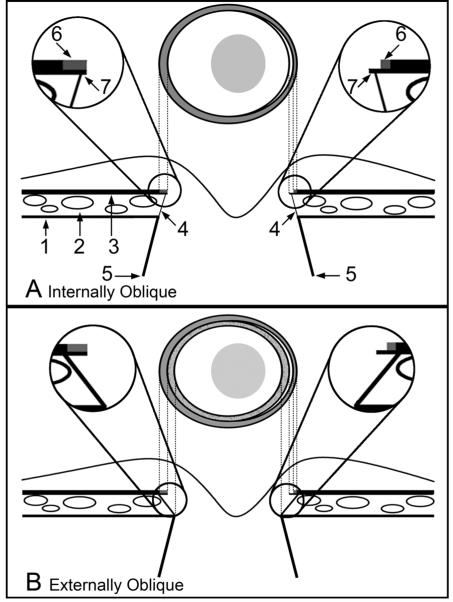
Four principal Border Tissue configurations were recognized. In the most common form, the superior edge of the Border Tissue extended internal to the Border Tissue/scleral junction. We defined this configuration to be ‘internally oblique’ (Figures 4A and 5A). Less commonly, the superior edge of the Border Tissue was external to the Border Tissue/scleral junction. We defined this configuration to be ‘externally oblique’ (Figures 4B and 5B). The Border Tissue also manifested vertical (no obliqueness) and horizontal (an extreme form of internal obliqueness) configurations (Figure 4C and 4D, respectively). These configurations could vary regionally within an individual eye. In rare instances the Border Tissue was regionally not discernible. As noted above, regardless of Border Tissue configuration, a pigmented or unpigmented extension of Bruch’s Membrane beyond its termination was commonly present.
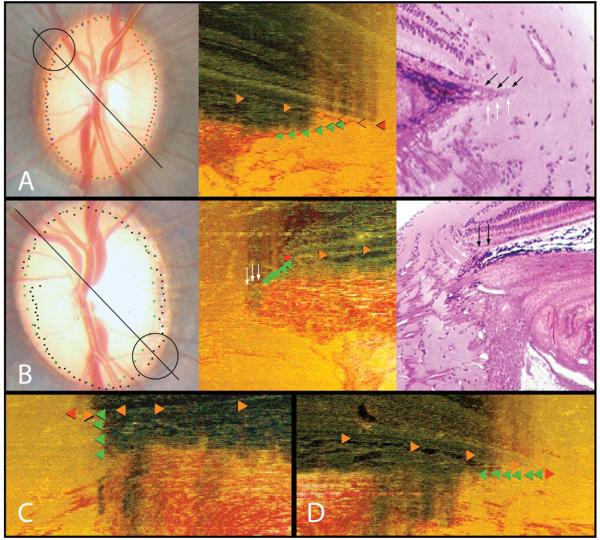
Figure 4. Two Principal Border Tissue Configurations with Variations.
PANEL A. Internally oblique
Left panel: Disc photograph (OS) showing the co-localized BMO (red glyphs) and disc margin delineations (blue and green glyphs). The black line shows the approximate orientation of the histomorphometric section from which the circular region is magnified in the middle panel
Middle panel: Histomorphometric disc margin region. Bruch’s Membrane (orange glyphs), Bruch’s Membrane Opening (red glyph) and the Border Tissue (green glyphs) are each delineated. The inferior edge of the Border Tissue communicates with sclera (the Border Tissue/scleral junction) and the superior edge extends into the neural canal fusing with Bruch’s Membrane (the Border Tissue termination), which extends beyond this point and includes an unpigmented portion
Right panel: Representative histologic section taken from a different, normal monkey eye (perfusion fixed at IOP 10 mmHg; mid-horizontal sagittal section, hematoxylin and eosin stain) demonstrating an internally oblique configuration. White arrows highlight Border Tissue, black arrows highlight an extension of unpigmented Bruch’s Membrane. In this case there is no clear extension of Bruch’s Membrane beyond the termination of the Border Tissues
PANEL B. Externally oblique
Left panel: Disc photograph (OD), demarcated as in panel A. Note that within and around the circled region the clinical disc margin has been marked internal to the histomorphometric BMO points
Middle panel: Histomorphometric section showing the Border Tissue configuration, demarcated as in panel A. Note that the inferior edge of the Border Tissue is internal to its termination at Bruch’s Membrane. Bruch’s Membrane does not extend beyond the Border Tissue’s termination. In this instance, dense pigment within the sclera immediately adjacent to the neural canal casts a shadow upwards that probably explains the lack of a highly reflective “scleral lip” (white arrows) within this region of the clinical photograph
Right panel: representative histologic section taken from a different, normal monkey eye (perfusion fixed at IOP 10 mmHg; mid-horizontal sagittal section, hematoxylin and eosin stain) demonstrating the externally oblique configuration. White arrows highlight the Border Tissue, black arrows highlight Bruch’s Membrane (pigmented)
PANEL C. Vertical configuration
The Border Tissue extends vertically from the sclera to meet Bruch’s Membrane. Bruch’s Membrane extends beyond this point with both pigmented (outer) and unpigmented (inner) portions
PANEL D. Horizontal configuration
The Border Tissue extends horizontally to meet Bruch’s Membrane Opening. This configuration is an extreme form of an internally oblique Border Tissue configuration
Figure 5. Two principal Border Tissue configurations, their relationship to a pigmented or unpigmented extension of Bruch’s Membrane and the resultant clinical disc margin anatomy.
-
Internally ObliqueThe diagram shows the clinical optic disc appearance (above) and a cross section of the optic nerve head (below). Labeling is as follows:
- 1 = Sclera
- 2 = Choriocapillaris
- 3 = Retinal pigment epithelium with Bruch’s Membrane
- 4 = Border Tissue
- 5 = Neural canal boundary
- 6 = Pigment on the surface of Bruch’s Membrane
- 7 = Bruch’s Membrane
Magnified Inset Right - a region of unpigmented Bruch’s Membrane is shown; this corresponds to a white crescent internal to the pigment halo at the disc margin (which corresponds to a portion of pigmented Bruch’s Membrane) -
Externally ObliqueLabeling is as per the schematic in panel AMagnified Inset Left - Bruch’s Membrane is pigmented to its end and does not extend beyond the termination of the Border Tissue. This Bruch’s Membrane extension corresponds to an external crescent of pigment at the disc margin that is internal to the termination of the retinal pigment epithelium. The portion of the Border Tissue that is internal to the end of Bruch’s Membrane (BMO) may be clinically recognizable as an inner reflective (if there is no pigment on the Border Tissue surface) or a pigmented crescent (if there is pigment on the Border Tissue surface) that is posterior to the plane of the retinal pigment epithelium. An inner pigmented halo (lighter grey and stippled) is shown on both sides of the disc diagramMagnified Inset Right-unpigmented Bruch’s Membrane extends internally to the Border Tissue termination, corresponding to a reflective crescent internal to the pigment crescent. Again, pigmented Border Tissue (lighter grey and stippled) extends internal to the reflective crescent. In both the left and right insets the Border Tissue/scleral junction is depicted without a true scleral lip which when present and visible, appears internal and deep to the other structures
Comparison of Clinical Disc Margin and BMO Alignment
Good Alignment
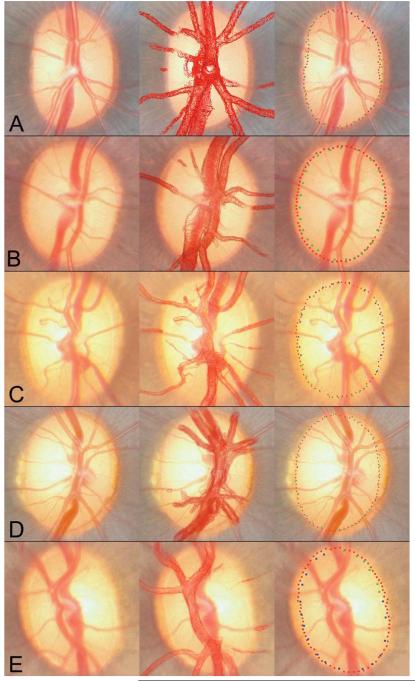
Twenty eyes demonstrated good alignment between histomorphometric BMO marks and clinical disc margin marks (examples shown in Figure 6) within all sectors of the disc. Of these twenty eyes, sixteen had the disc margin marked where an innermost unpigmented region was present at the disc margin (Figure 6C). In these eyes, the termination of unpigmented Bruch’s Membrane corresponded to the delineated disc margin and the clinically visible mottled pigment external to it corresponded to pigmented Bruch’s Membrane.
Figure 6. Five examples (A-E) of good alignment between BMO points and the clinical disc margin, following ‘first pass’ co-localization.
Clinical images are shown in the left panels. The co-localizations of the vessel reconstructions to the clinical images are shown in the middle panels. The co-localized BMO points (red glyphs) and the clinical disc margin delineations (blue and green glyphs) are shown in the right panels
Of the four remaining eyes with good agreement between the clinical markings and histomorphometric BMO, two did not have a clinically visible ‘unpigmented’ disc margin structure, so the termination of mottled pigment was marked instead, which coincided with the histomorphometric BMO. In two eyes, the clinical observer felt that there was pigment at two different planes, with an internal unpigmented ‘stripe’ present at the internal border of the superficial outer pigment. In these eyes, the inner edge of both the unpigmented stripe and the termination of the ‘deeper’ pigment were marked. Following co-localization, however, the BMO was found to be aligned with the innermost clinical markings. Systematic review of the relevant histomorphometric sections confirmed that Bruch’s Membrane was the innermost disc margin structure, and not the Border Tissue, within these areas of the disc. There was, however, no histomorphometric correlate which could explain the pale stripe seen within the pigment, nor the clinically perceived different levels of pigment. It is possible that the pale stripe could represent an area of vitreous attachment to the optic disc, but this could not be confirmed in the absence of access to an in vivo examination of these eyes.
Sources of Misalignment
In eight eyes there were substantial regions in which the clinical disc margin and histomorphometric BMO points were poorly matched. Alignment improved considerably in four of these eyes following repeat co-localization.
Four eyes did not achieve a good match between clinical marks and BMO marks, despite satisfactory co-localization at the second attempt. In each of these four eyes, however, the discrepancy between marks was confined to two or fewer sectors. In the first of these eyes, the clinical disc margin marks were internal to the histomorphometric BMO marks in the superonasal and nasal sectors. Careful review of the histomorphometric sections within the poorly matched regions revealed that a substantial portion of unpigmented Bruch’s Membrane was present, extending internally into the neural canal, beyond where the BMO had been originally delineated. Thus, in this eye, the clinical disc margin and histomorphometric BMO marks would have been more closely matched if the full extension of unpigmented Bruch’s Membrane had been histomorphometrically delineated.
In the second eye, the clinical disc margin marks were again internal to the histomorphometric BMO marks in the temporal sector only. Histomorphometric review confirmed that an extension of unpigmented Bruch’s Membrane was not histomorphometrically detectable in this region of the disc. When a review of the clinical photographs again confirmed the presence of a reflective inner halo within this region, we assumed our histomorphometric technique had failed to reconstruct unpigmented Bruch’s Membrane within this region. As this is one of the low resolution (2.5 × 2.5 × 3.0 micron voxel) histomorphometric reconstructions, this finding may confirm a relative inability to resolve unpigmented Bruch’s Membrane.
In the third eye, the clinical disc margin marks were external to the histomorphometric BMO marks in the nasal and superonasal sectors. In this eye, histomorphometric review revealed that, similar to the first eye, a narrow extension of unpigmented Bruch’s Membrane had been missed in the original delineation. However, in this instance, correcting the histomorphometric BMO points would have left them even more internal to the clinical disc margin points, enhancing rather than resolving the discrepancy. It should be noted, however, that the fundus photograph used for this co-localization (taken from the only available stereophotograph pair for this eye) was of poor quality, making accurate co-localization difficult.
In the remaining eye, there was a marked misalignment in the temporal and inferotemporal sectors, with the clinical disc margin marks coinciding with an internal pigment border and the histomorphometric BMO marks coinciding with a more external pale stripe (defined by crescents of mottled pigment on either side). Histomorphometric review confirmed an externally oblique Border Tissue configuration within this region accompanied by no extension of Bruch’s Membrane beyond the Border Tissue’s termination (Figure 4B). Here, because the Border Tissue was externally oblique to its junction with the sclera, and because there was no extension of Bruch’s Membrane beyond its termination, pigment on the surface of the Border Tissue was clinically visible beyond the termination of Bruch’s Membrane but in a plane that was clearly deeper to it. In this instance, the disc margin was the Border Tissue/scleral junction and the lack of a true unpigmented ‘scleral lip’ on subsequent repeat stereophotograph examination could be explained as the result of a shadow cast by dense pigment present on the surface of the Border Tissue and within the scleral canal wall tissues, themselves (Figure 4B).
DISCUSSION
In the present study we co-localized post mortem 3-D ONH reconstructions to the clinical (in vivo) optic disc photographs of twenty-eight normal monkey eyes. The principal findings of this report are as follows. First, in the majority of eyes, the histomorphometrically delineated BMO aligned with the clinically defined innermost disc margin structure. Thus the innermost termination of Bruch’s membrane (BMO) is a clinically discernible structure in the normal monkey eye. Second, two core components of optic disc margin architecture can be histomorphometrically identified and their multiple combinations invoked to explain clinical disc margin anatomy in the majority of normal monkey eyes. The first component is Border Tissue of Elschnig obliqueness and pigmentation relative to the sclera. The second component is Bruch’s Membrane extension and pigmentation beyond the Border Tissue termination.
In most eyes, a narrow halo (if present for 360°) or crescent (if present in a region less than 360°) of unpigmented Bruch’s Membrane was visible as a pale discrete structure internal to, concentric with, and in the same plane as the termination of pigment at the disc margin. In regions where unpigmented Bruch’s Membrane was not visible, histomorphometrically delineated BMO usually coincided with the clinical termination of the pigmented tissues at the disc margin.
The disc margin pigmented tissues themselves assumed two principal regions, an inner halo or crescent of mottled pigment followed by an outer, dense and dark grey pigment which is traditionally thought to correspond to the termination of the viable retinal pigment epithelium (and underlying choroid).6 It is important to again emphasize that in the overwhelming majority of eyes, the pale halo, mottled pigment and dense pigment border were all in the same plane by stereoscopic examination and co-localized (respectively) to histomorphometrically delineated unpigmented Bruch’s Membrane, pigmented Bruch’s Membrane and the shadow associated with the termination of the choroid (and presumed overlying retinal pigment epithelium). The fact that these structures appear clinically to be on the same plane is especially important because it is the presence of pigmented structures deeper to the plane of the retinal pigment epithelium/Bruch’s Membrane complex that underlies the clinical recognition of an externally oblique Border Tissue.
These findings are contrary to previous reports in human eyes.4-7 Hogan et al defined the traditional view that the ophthalmoscopically visible optic nerve begins at the termination of the retinal pigment epithelium.6 Where the edge of the choroid and the edge of the retinal pigment epithelium align, an extension of densely packed collagenous tissue arising from the sclera is usually interposed between the choroid and the ONH.5 This structure, defined as the ‘Border Tissue of Elschnig’, is visible ophthalmoscopically as a white halo bounding the disc, terminating as a ‘scleral lip’.
The clinical appearance of the scleral ring has been referred to as the ‘scleral ring of Elschnig’ and has been the traditional clinical3 and stereophotographic13-18 definition of the disc margin. Although Bruch’s Membrane has been observed to extend beyond the termination of the retinal pigment epithelium and to cover the Border Tissue, this has not been described as a phenomenon detectable by ophthalmoscopic examination.5
The results of our study suggest that in those normal monkey eyes in which the Border Tissue had an internally oblique configuration and Bruch’s Membrane extended beyond the Border Tissue termination (by far the most common orientation), it was the termination of Bruch’s Membrane (BMO) that was the clinically visible disc boundary. In these eyes, the white crescent observed at the disc margin was due to a rim of unpigmented Bruch’s Membrane rather than a manifestation of a ‘scleral’ ring which in fact was shielded from clinical view (Figure 5A). Figure 7 further illustrates this point. The ring of Elschnig (white glyphs in panels C and E) does not co-localize to the innermost white reflective disc margin structure (indeed it is at a considerable distance external to it). The termination of unpigmented Bruch’s Membrane (red glyphs in panels C and E) does in fact co-localize to the white crescent, indicating that in this region of the disc, BMO is the disc margin. We have previously demonstrated that the termination of ‘lightly’ pigmented Bruch’s Membrane co-localizes to the disc margin.8 In Figure 7, however, we clearly demonstrate that unpigmented Bruch’s Membrane, effectively a transparent structure, is an ophthalmoscopically visible structure which co-localizes to the disc margin.
Figure 7. The termination of unpigmented Bruch’s Membrane is clinically visible and aligns to the disc margin.
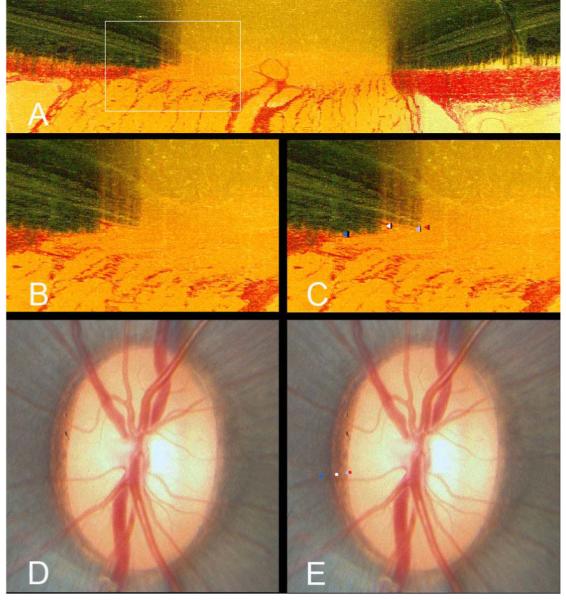
- A histomorphometric section taken at 67.5° from a left eye. A white box highlights the area of interest in the nasal region of the optic nerve head
- The area within the white box has been magnified to highlight the structures comprising the disc margin. In this section, there is a substantial overhang of Bruch’s Membrane (both pigmented and unpigmented) beyond the termination of the Border Tissue of Elschnig
- The structures comprising the disc margin have been delineated within Multiview; termination of unpigmented Bruch’s Membrane, in other words Bruch’s Membrane Opening (red glyph), termination of pigmented Bruch’s Membrane (light blue glyph), junction of Border Tissue of Elschnig with Bruch’s Membrane (scleral ring of Elschnig - white glyph) and the anterior scleral canal opening (dark blue glyph)
- The in vivo disc photograph of the eye from which the histomorphometric reconstruction was obtained
-
Following co-localization of the three-dimensional vessel reconstruction to the disc photograph, the termination of unpigmented Bruch’s Membrane (red glyph) coincides with the innermost white reflective halo at the disc margin. The termination of pigmented Bruch’s Membrane (light blue glyph) coincides with the inner edge of the pigment at the disc margin. The Border Tissue/Bruch’s Membrane junction (scleral ring - white glyph) coincides with a white reflective stripe within the mottled disc margin pigment; the anterior scleral canal opening (dark blue glyph) is external to this.It is apparent in this eye that the innermost white reflective structure at the disc margin is not the scleral ring of Elschnig but the edge of unpigmented Bruch’s Membrane. In this region of the disc, the scleral ring is considerably external to what the clinician perceives as the disc margin
Where the Border Tissue had an externally oblique configuration and the Bruch’s Membrane terminated before the edge of the Border Tissue, the clinical disc margin was indeed the edge of the Border Tissue. Unfortunately, in the one example where this was apparent, the histomorphometric presence of pigment in the underlying sclera combined with poorly focused stereophotographs did not allow for a true ‘scleral lip’ to be detected in the stereophotograph. In this circumstance, the pigment observed clinically was therefore composed of two zones. The first was a superficial external pigment rim which was within the plane of the retinal pigment epithelium and histomorphometrically co-localized to pigmented Bruch’s Membrane. The second was an internal pigment rim which was deep to the plane of the retinal pigment epithelium and histomorphometrically co-localized to pigment on the surface of the Border Tissue. This latter pigment may be seen stereoscopically to slope downwards and inwards, following the angle of incidence of the Border Tissue as it slopes down from Bruch’s Membrane to the sclera.
There have been two clinical reports of ‘gray’ optic disc crescents in human eyes.19, 20 A gray crescent is defined as ‘a crescent-shaped, slate gray pigmentation in the periphery of the neuroretinal rim that is completely inside of the scleral crescent’. In these reports, the authors used the existing ‘scleral lip’ definition of the disc margin to define the crescent and were uncertain of its histologic derivation. They did not specifically comment on whether the gray crescent appeared posterior to (and sloping away from) the Bruch’s Membrane/ retinal pigment epithelium plane. We now propose that the gray crescent is likely to be pigment on the surface of an externally oblique Border Tissue of Elschnig. Clinical awareness of the plane in which the crescent occurs, perhaps clarified by spectral domain OCT (SD-OCT) ONH imaging may provide further insight into this form of pigmentary crescent.
The co-localization method used in this study was prone to several inherent sources of error. The caliber and size of the photographed vessels and the 3-D reconstructed vessels may differ because of tissue shrinkage during processing, cardiac pulse and distention during the perfusion fixation process. Co-localization is unlikely to be 100% accurate because the process required the two-dimensional photographic plane to be aligned to a three-dimensional structure. It was therefore felt reasonable to explore co-localization error as a source of misalignment, and therefore to repeat the co-localization process in those eyes in which a poor alignment between the BMO and the clinical marks was observed. It should also be noted that ‘quantification’ of the accuracy of alignment would be rendered largely meaningless due to the difference in size between the in vivo imaged eye and the post perfusion-fixation histomorphometrically reconstructed eye. It is for these reasons that a qualitative approach to assessing alignment was adopted.
Our work in monkeys is pertinent to humans for two important reasons. Firstly, we believe that the anatomic relationships we describe will contribute to the histologic explanation of most forms of human disc margin anatomy. Secondly, by applying these concepts at the slit lamp and when interpreting SD-OCT ONH images, clinicians who have previously understood the disc margin to be the ring of Elschnig, will recognize the histologic origin of the disc margin to be the termination of Bruch’s Membrane in at the very least in the nasal region of most human optic discs. The principal explanation for this is that the nasal disc margin in both monkeys and humans is prone to an internally oblique Border Tissue configuration in order to enable the optic nerve to pass obliquely through the sclera to reach the more midline optic chiasm. These clinical implications of neural canal anatomy have been explained in detail, in our previous report.8
The results of this study have additional clinical implications. Recognition of the disc margin plays an important role in examining the neuroretinal rim (or indeed assigning a focal cup-to-disc ratio). The ability to recognize a, perhaps subtle, rim of unpigmented Bruch’s Membrane as the disc margin may yield a more accurate assessment of the neuroretinal rim. Likewise, ONH imaging modalities, such as scanning laser tomography, require the placement of a contour line around the disc margin. As disc margin anatomy is more clearly defined, this may be applied to imaging modalities enabling the reliability of contour line placement to be improved.
We have previously proposed BMO as a source structure for a reference plane for quantification of 3-D histomorphometric volumes.8 An expert consensus group has also previously proposed this concept.3 More recently we have confirmed that BMO is an anatomically continuous, relatively planar structure both in 3-D histomorphometric volumes and in 3-D SD-OCT volumes.1 The results of the current study further support the adoption of the BMO as a reference plane in clinical ONH imaging. The fact that the BMO is usually the innermost disc margin structure in the monkey eye suggests that it will be easily and reliably delineated by automated segmentation. It is necessary, however, to first confirm that the SD-OCT defined neural canal opening aligns with the clinical disc margin.2 This work will be the subject of a future report from our group.
In summary, the innermost edge of Bruch’s Membrane, the Bruch’s Membrane Opening, is a clinically visible structure in the monkey eye. It is usually the innermost disc margin structure and so constitutes the disc margin boundary in the majority of monkey eyes. The Border Tissue may be clinically visible in regions where it demonstrates an externally oblique configuration and the Border Tissue/scleral junction is internal to the termination of Bruch’s Membrane. We are beginning to study the relationship between Bruch’s Membrane Opening, internal versus external oblique Border Tissue configuration and the disc margin in monkey and human eyes using post-mortem histomorphometric and clinical SD-OCT ONH reconstructions.
ACKNOWLEDGEMENTS
The authors wish to gratefully acknowledge G Williams, E Dyrud, W Wang (all for delineation of histomorphometric reconstructions), J Grimm (for software support), J Reynaud (for software and hardware support) and J Couchman (for assistance with Figures).
Grant Information:
R01-EY11610, Legacy Good Samaritan Foundation, Heidelberg Engineering and Sears Medical Trust
Footnotes
Commercial Disclosure:
NGS is funded by an unrestricted educational grant from Heidelberg Engineering and by a Royal College of Ophthalmologists/Pfizer Travel Fellowship; CFB receives instrument and unrestricted research support but no honoraria from Heidelberg Engineering.
REFERENCES
- 1.Strouthidis NG, Yang H, Fortune B, Downs JC, Burgoyne CF. Detection of the Optic Nerve Head Neural Canal Opening within Three-Dimensional Histomorphometric and Spectral Domain Optical Coherence Tomography Data Sets. Invest Ophthalmol Vis Sci. doi: 10.1167/iovs.08-2302. First published on Aug 8,2008 as doi:10.1167/iovs.08-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manassakorn A, Ishikawa H, Kim JS, Wollstein G, Bilonick RA, Kagemann L, Gabriele ML, Sung KR, Mumcuoglu T, Duker JS, Fujimoto JG, Schuman JS. Comparison of optic disc margin identified by color disc photography and high-speed ultrahigh-resolution optical coherence tomography. Arch Ophthalmol. 2008;126:58–64. doi: 10.1001/archophthalmol.2007.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jonas JB, Airaksinen PJ, Robert Y. Definitionsentwurf der intra- und parapapillären Parameter für die Biomorphometrie des Nervus opticus. Klin Monatsbl Augenheilkd. 1988;192:621. [Google Scholar]
- 4.Anderson DR. Ultrastructure of human and monkey lamina cribrosa and optic nerve head. Arch Ophthalmol. 1969;82:800–814. doi: 10.1001/archopht.1969.00990020792015. [DOI] [PubMed] [Google Scholar]
- 5.Fantes FE, Anderson DR. Clinical histologic correlation of human peripapillary anatomy. Ophthalmology. 1989;96:20–25. doi: 10.1016/s0161-6420(89)32929-0. [DOI] [PubMed] [Google Scholar]
- 6.Hogan MJ, Alvarado JA, Esperson Weddell J. Histology of the human eye: an atlas and textbook. W.B. Saunders Company; Philadelphia: 1971. [Google Scholar]
- 7.Nevarez J, Rockwood EJ, Anderson DR. The configuration of peripapillary tissue in unilateral glaucoma. Arch Ophthalmol. 1988;106:901–903. doi: 10.1001/archopht.1988.01060140047021. [DOI] [PubMed] [Google Scholar]
- 8.Downs JC, Yang H, Girkin C, Sakata L, Bellezza AJ, Thompson H, Burgoyne CF. Three Dimensional Histomorphometry of the Normal and Early Glaucomatous Monkey Optic Nerve Head: Neural Canal and Subarachnoid Space Architecture. Invest. Ophthalmol. Vis. Sci. 2007;48:3195–3208. doi: 10.1167/iovs.07-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgoyne CF, Downs JC, Bellezza AJ, Hart RT. Three-dimensional reconstruction of normal and early glaucoma monkey optic nerve head connective tissues. Invest. Ophthalmol. Vis. Sci. 2004;45:4388–4399. doi: 10.1167/iovs.04-0022. [DOI] [PubMed] [Google Scholar]
- 10.Yang H, Downs JC, Burgoyne CF. Physiologic Inter-eye Differences in Monkey Optic Nerve Head Architecture and Their Relation to Changes in Early Experimental Glaucoma. Invest Ophthalmol Vis Sci. doi: 10.1167/iovs.08-2464. First published on Sep 4,2008 as doi:10.1167/iovs.08-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang H, Downs JC, Bellezza AJ, Thompson H, Burgoyne CF. 3-D Histomorphometry of the Normal and Early Glaucomatous Monkey Optic Nerve Head: Prelaminar Neural Tissues and Cupping. Invest. Ophthalmol. Vis. Sci. 2007;48:5068–5084. doi: 10.1167/iovs.07-0790. [DOI] [PubMed] [Google Scholar]
- 12.Yang H, Downs JC, Girkin C, Sakata L, Bellezza A, Thompson H, Burgoyne CF. 3-D Histomorphometry of the Normal and Early Glaucomatous Monkey Optic Nerve Head: Lamina Cribrosa and Peripapillary Scleral Position and Thickness. Invest. Ophthalmol. Vis. Sci. 2007;48:4597–4607. doi: 10.1167/iovs.07-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sommer A, Pollack I, Maumenee AE. Optic disc parameters and onset of glaucomatous field loss. I. Methods and progressive changes in disc morphology. Arch Ophthalmol. 1979;97:1444–1448. doi: 10.1001/archopht.1979.01020020106002. [DOI] [PubMed] [Google Scholar]
- 14.Britton RJ, Drance SM, Schulzer M, Douglas GR, Mawson DK. The area of the neuroretinal rim of the optic nerve in normal eyes. Am J Ophthalmol. 1987;103:497–504. doi: 10.1016/s0002-9394(14)74271-0. [DOI] [PubMed] [Google Scholar]
- 15.Airaksinen PJ, Tuulonen A, Alanko HI. Rate and pattern of neuroretinal rim area decrease in ocular hypertension and glaucoma. Arch Ophthalmol. 1992;110:206–210. doi: 10.1001/archopht.1992.01080140062028. [DOI] [PubMed] [Google Scholar]
- 16.Garway-Heath DF, Wollstein G, Hitchings RA. Aging changes of the optic nerve head in relation to open angle glaucoma. Br J Ophthalmol. 1997;81:840–845. doi: 10.1136/bjo.81.10.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moya FJ, Brigatti L, Caprioli J. Effect of aging on optic nerve appearance: a longitudinal study. Br J Ophthalmol. 1999;83:567–572. doi: 10.1136/bjo.83.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmer R, Schroeder S, Martus P, Viestenz A, Mardin CY. Quantification of neuroretinal rim loss using digital planimetry in long-term follow-up of normals and patients with ocular hypertension. J Glaucoma. 2007;16:430–436. doi: 10.1097/IJG.0b013e31804a5e80. [DOI] [PubMed] [Google Scholar]
- 19.Shields MB. Gray crescent in the optic nerve head. Am J Ophthalmol. 1980;89:238–244. doi: 10.1016/0002-9394(80)90117-8. [DOI] [PubMed] [Google Scholar]
- 20.Higginbotham L, Shafranov G, Shields MB. Gray optic disc crescent: influence of ethnicity in a glaucoma population. J Glaucoma. 2007;16:572–576. doi: 10.1097/IJG.0b013e31805342cb. [DOI] [PubMed] [Google Scholar]