Abstract
Female songbirds display preferences for certain song characteristics, but the neural and hormonal mechanisms mediating these preferences are not fully clear. The present study sought to further explore the role of estradiol, as well as assess potential roles of dopaminergic systems, on behavioral responses to song. Adult female zebra finches were treated with estradiol and exposed to tutored or untutored song or silence. Behavior was quantified and neurochemistry of the nucleus accumbens and striatum was examined with high performance liquid chromatography. As a control, the responses of these two systems to treatment with raclopride, a specific D2 receptor antagonist, were also evaluated. This manipulation did not affect dopamine (DA), but did increase DOPAC and the DOPAC/DA ratio. Estradiol reduced the display of two behaviors, distance calls and visual scanning, but had no effect on dopaminergic responses. Auditory stimulus exposure affected other vocalizations, but song presentation did not modulate the levels of DA or its metabolite, DOPAC in the nucleus accumbens or striatum. Collectively, the results suggest that both estradiol and auditory stimuli can modify the behavioral responses of adult zebra finches, but they may not change DA concentration or turnover in striatal dopamine neurons.
Keywords: songbird, song preference, estrogen, nucleus accumbens, striatum
Introduction
Female songbirds display preferences for certain song characteristics, and perhaps as a consequence, vary their behavioral responses to them. However, the neural mechanisms involved in these processes are not completely clear. Increased immediate early gene expression in response to male songs of higher quality is regularly seen in the auditory perception regions in a variety of songbird species [e.g., 1-7]. Information on responses of other neural regions in songbirds is more limited and not as consistent. The mesolimbic dopamine (DA) system is activated in mammals during reproduction in both sexes [reviewed in 8-10], as well as in response to reproductive stimuli alone [8, 11], and is also implicated in mediating motivation and motivated behavior in mammals [12, 13] and birds [14-16]. As song is a critical element of courtship [17], and females likely display specific responses to quality song, the involvement of this system in song responses is plausible. Exposure to song does not induce immediate early gene expression in female zebra finches in the mesolimbic DA system, [7], but these results do not completely eliminate the possibility that the regions respond to song stimuli. Quantifying the concentrations of DA and its metabolites will provide more direct assessments of the responses of striatal dopamine neurons.
The anatomical and physiological characteristics of the dopaminergic systems are highly similar in birds and mammals [reviewed in 18, 19]. However, the precise location and characteristics of the nucleus accumbens in avian species is still being discussed [see 20]. In birds, dopaminergic neurons from the substantia nigra and ventral tegmental area project to the basal ganglia, including the medial striatum (nigrostriatal system) and the nucleus accumbens (mesolimbic system) [18, 21, 22]. In vitro, neurons from the ventral tegmental area and substantia nigra have similar electrophysiological characteristics to mammalian dopaminergic neurons, and D2 receptor agonists block their activity [23]. In addition, in chickens and zebra finches release of DA in striatal slices decreases with D2 receptor agonist treatment [24, 25]. Few avian studies, however, have simultaneously examined the responses of the nigrostriatal and mesolimbic dopaminergic systems, as in this study.
In addition to physiological characteristics, functional similarities exist in these systems between mammals and birds [see 26 for review]. In both groups, the regions are important to the control of motor movements [see 27-30], as well as the mediation of reward [see 31-34]. In particular, these two systems are activated when males sing sexually motivated song (measured with immediate early gene expression in mesolimbic reward regions [15, 35] and microdialysis in the striatum [16]). Additionally, tyrosine hydroxylase-(the rate limiting enzyme in DA synthesis)-positive cells in the ventral tegmental area are ZENK-positive in male zebra finches singing undirected song [36] or courting females [37].
The response of neurons in the mesolimbic DA system to song exposure has only been examined using immediate early genes, which are either expressed in these regions to a greater degree in response to song [38] in white throated sparrows or are unchanged [7] in zebra finches. In components of the social behavior network, Riters et al. [39] observed decreased phosphorylated tyrosine hydroxylase in response to male starling song. Also, DA release is increased in the auditory forebrain (caudomedial nidopalium) in female starlings exposed to song [40]. It remains to be seen if exposure to song stimuli results in increased dopaminergic actvity in the striatum and nucleus accumbens of female songbirds.
Although song is a critical component of reproduction, and estrogen is often used to prime females in song response studies [i.e., 3, 41-44], the role of steroid hormones in neural responses to song has only recently been evaluated. The effects are not completely consistent [see 7, 38, 39, 45]. Zebra finch females present more distance calls in response to complex song versus other song types with estrogen but not control treatment [46, 47]. In our previous study in zebra finches [7], estradiol specifically reduced expression of the immediate early gene ZENK in the ventromedial hypothalamus but did not affect neural responses to song, behavioral preference for tutored versus untutored male song, or general calling behavior in response to these auditory stimuli. In contrast, a selective increase in ZENK expression in response to male song was observed following estrogen treatment in several regions in the social behavior network in white-throated sparrows [38].
Estrogen may also affect the response of dopaminergic systems. The avian ventral tegmental area contains estrogen receptors [45]. In female starlings, breeding condition affects the activity of dopaminergic neurons in the ventral tegmental area, as well as regions in the social behavior network in response to male song [39]. These results suggest potential for dopaminergic systems to be modified by estradiol.
The present study had two primary goals. First, we examined the specific behavioral responses of female zebra finches to tutored versus untutored song, and in particular how they might be affected by an increase in estradiol. Second, we characterized the response of the nucleus accumbens and striatum of estrogen-treated and control females to song stimuli using high performance liquid chromatography (HPLC) to measure the levels of DA and its metabolite 3,4-dihydroxyphenylacetic acid (DOPAC). Prior to this main experiment, a separate group of adult females was administered raclopride to verify that predictable changes in DA metabolism could be detected with this procedure. Raclopride is a specific D2 receptor antagonist, which blocks the action of DA on autoreceptors on the presynaptic neuron and increases DA neuronal activity.
Methods
Animals
Adult female zebra finches were raised in mixed-sex aviaries in our breeding colony at Michigan State University. They were kept on a 12:12 light:dark cycle, and seed and water were provided ad libitum. Orange and spinach as well as a hard-boiled egg/bread mixture were given once per week. After reaching adulthood (at least 100 days after hatching), they were housed in a unisex aviary, in acoustic (but not visual) contact with adult males for at least three weeks prior to testing. It is unlikely that these females had formed bonds, but they were housed in a separate room from their mates in the time prior to testing. Bonds are not typically maintained in this species when individuals are visually and acoustically separated, and new bonds can be formed shortly after separation [48]. All procedures were approved by the Michigan State IACUC and adhered to the guidelines of the National Institutes of Health.
Raclopride treatment
Females were removed from the all-female aviary, taken to a separate room in individual cages, and given an intramuscular injection of either 1mg/kg raclopride (1 mg/kg; Sigma-Aldrich Corp., St. Louis, MO) or vehicle (1ml/kg). They remained in cages in the room for 70 min, after which they were rapidly decapitated and brain tissue was collected, flash-frozen, and stored at -80°C until processing.
Hormone treatment
Estradiol treatment was conducted as in Svec and Wade [7]. Briefly, females were implanted subcutaneously with 5 mm long Silastic capsules (i.d. 0.076 mm, o.d. 1.65 mm) under isoflurane anesthesia. Implants were made from Silastic tubing, 2mm of which was packed with 17β-estradiol and sealed with silicone. In a recent study from this lab, implants of this size used for this time period successfully increased the level of circulating estradiol, and resulted in a change in basal ZENK expression within the ventromedial hypothalamus [7]. These estradiol levels were slightly above the natural range in a number of other studies in female zebra finches [49, 50] and were comparable to those detected in estrogen-treated birds in other songbird studies [42, 45, 51]. Females were not gonadectomized, as ovariectomy in this species has been shown to result in an increased level of circulating estradiol [49]. Control females received empty implants. Females were housed in individual cages in a room with males and females in group aviaries for 5 days following the surgery.
Song stimuli
The same auditory stimuli were used as in Svec and Wade [7], recorded by Dr. Adkins-Regan at Cornell University [also used in 52]. Songs were recorded from males that had been tutored (n= 3, raised with adult males present) or untutored (n= 3, raised in the absence of other males after 18 days of age) during development. Twelve different 30-min song clips (six tutored and six untutored) were created in Adobe Audition (Adobe Systems Inc., San Jose, CA) by combining 30 sec song clips from three randomly chosen males (from a pool of six males in each category) that were each separated by 30 sec of silence and repeated for 30 min.
Auditory stimulus exposure
Five days after implant surgery, females were taken to a novel room for auditory stimulus exposure. The procedure was conducted as described in Svec and Wade [7], except that tissue was collected 10 min rather than 1 hour after the conclusion of the song presentation. This time point was chosen following a pilot study to determine appropriate time point for euthanasia. Brains were collected from females 0, 10, or 20 min after the song presentation. The range in this pilot was based on Sasaki et al. [16], which demonstrated that DA levels remain above baseline for approximately 20 minutes after males stop singing. The levels of DA and DOPAC did not differ substantially among the three time points, so the middle one was selected
For stimulus exposure, females were placed in a sound isolated box in their individual cages, allowed 30 min for acclimation, and then exposed to 30 min of one of the two types of song. A speaker with a taxidermic model of an adult male zebra finch on top broadcast the song at 60 dB three inches from the cage. As a control, some birds were not exposed to song and remained in silence during the test. The stimulus exposure was videotaped, and behaviors displayed were quantified by an observer blind to treatment condition (see Table 1 for descriptions of behaviors, largely generated from [17]). Brain tissue was collected following rapid decapitation, flash frozen, and stored at -80°C until processing.
Table 1.
Descriptions of measured behaviors
| Behavior | Description |
|---|---|
| Distance calls | Long, loud sounds emitted by the female, typically presented by the female after male song begins and shortly after it stops, often used for localizing other individuals |
| Other calls | Shorter calls (∼half the duration of distance calls), at a lower volume, including both tets and stacks, presented frequently throughout the test in many cases |
| Duration of movement |
The amount of time an individual bird was moving throughout the cage, excluding time in which the bird was sitting still either on the perch or on the ground for more than 10 sec |
| Flights/Jumps | Movement to or from the perch or up to the sides of the cage |
| Beak wipes | Rhythmic movement of the beak back and forth along the perch |
| Allopreening | Cleaning feathers with beak |
| Visual Scanning | Turning head to one side, including craning the neck and/or moving one of the eyes in the direction of the speaker and bird model |
| Beak open | Top and bottom beak are separated slightly for more than 2 sec |
| Tail quiver | Rapid movement of the tail, often associated with a bowed position on the perch |
Quantification of neurochemicals
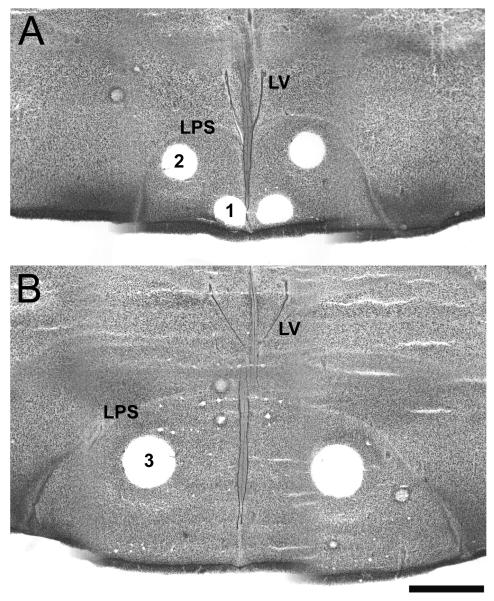
Tissue from both the preliminary study including raclopride-treated birds and those in the main experiment that were exposed to auditory stimuli was coronally sectioned frozen (500 μm thick). Samples of the nucleus accumbens, rostral medial striatum and caudal medial striatum were microdissected from these sections using the Palkovits method [53]. The lateral ventricles, as well as the lamino-pallio-subpallius (which defines the dorsal and lateral border of the striatum) were utilized to determine the location of the sampling regions (Figure 1). The more rostral portion of the nucleus accumbens was sampled in this study, where the division between the shell and the core is unknown in avian species [20]. A 21-gauge micropunch (i.d. 0.5 mm) was utilized for the nucleus accumbens and rostral medial striatum, and an 18-gauge punch (i.d. 0.75 mm) was used for the caudal medial striatum. Punches were placed in 0.1M phosphate-citric buffer in 20% methanol (pH 2.5; 50 μl for nucleus accumbens and rostral medial striatum, and 100 μl for caudal medial striatum) and stored at -20 °C. For visualization of the location of tissue samples, other brain tissue was sliced at 30 μm and stained with thionin.
Figure 1.
Example of microdissections taken from thionin-stained coronal sections of the zebra finch brain. Panel A depicts punches of (1) the nucleus accumbens and (2) rostral medial striatum (both 21-gauge). Panel B depicts punches from the caudal medial striatum (3; 18-gauge). LV= lateral ventricle, LPS= Lamina pallio-subpallialis. Scale bar = 1 mm.
Samples were thawed, sonicated with three 1-sec bursts (Sonicator Cell Disruptor, Heat Systems-Ultrasonic, Plainview, NY, USA) and centrifuged (12000 rpm) for 1 min. The supernatant was removed and stored at -20°C. HPLC coupled with electrochemical detection was conducted to determine the levels of DA and DOPAC as described in Lindley et al. [54] and Behrouz et al. [55]. Briefly, the supernatant was injected into a C-18 reverse phase analytical column and using a mobile phase of 1.0M phosphate citrate buffer with 0.1M EDTA, 0.35% sodium octylsulfate and 20% methanol. DA and DOPAC content were determined by comparing the heights of peaks generated by a Hewlett Packard Integrator (Model 3393A) with those generated the same day with standards. To determine the protein content, the pellet was dissolved in 1 N NaOH, sonicated, and assayed using the method developed by Lowry [56]. Briefly, samples were compared to a standard curve serially diluted from 12.5 μg to 50 μg of protein. Samples and the standard curve were reacted with a reagent of sodium carbonate, cupric sulfate, and KNA tartrate for 10 min and then reacted with Folin reagent (phenol in ddH20) for 30 min. Absorbance was measured with a microplate reader (MicroQuant, Biotek Instruments, Winooski, VT).
To control for variation in size of each sample, concentrations of DA and DOPAC were determined by dividing the absolute quantity of each neurochemical by the protein content within the sample. The DOPAC/DA ratio was also calculated as a measure of the ratio of metabolized to stored DA. No differences in the values were observed between the two portions of the striatum, so they were pooled to form a total striatum value.
Statistics
Two-way ANOVAs were conducted to examine effects of estrogen and auditory stimulus type on behaviors. These were followed by Tukey-Kramer post-hoc tests for pairwise comparisons when significant main effects were observed. The HPLC results were analyzed with mixed-model ANOVAs to compare DA and DOPAC concentrations and the DOPAC/DA ratio across treatments and song exposure (between animals) and brain regions (within animals) for both the raclopride and song exposure studies. All statistical analyses were conducted using StatView (SAS Institute; Carey, NC). Sample sizes for each group are indicated in the legends to Figures 2, 3, and 4 and Table 2.
Figure 2.
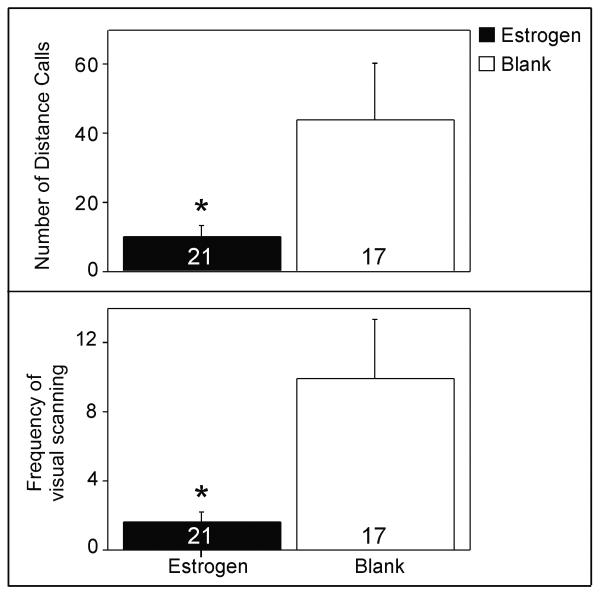
Effects of estradiol on distance calls (top) and visual scanning behavior (bottom). Data (mean±S.E.) were pooled across song exposure groups, as the effects of the auditory stimuli did not differ among them. Sample sizes: Estradiol-treated tutored song = 6, estradiol-treated untutored song = 8, estradiol-treated silence = 7, blank-treated tutored song = 5, blank-treated untutored song = 6, blank-treated silence = 6. * p< 0.031
Figure 3.
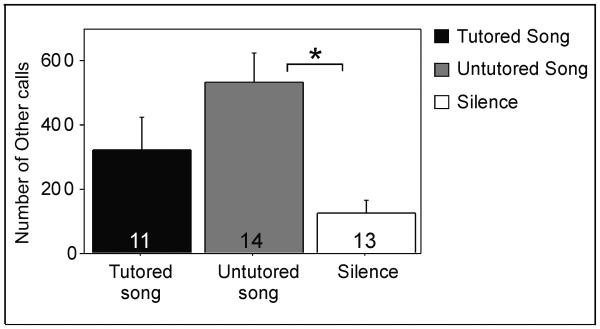
Effect of auditory stimulus on ‘other calls’, which included tets and stacks (mean±S.E.). Data were pooled across treatment groups, as estradiol did not affect the number of these vocalizations. Sample sizes: Estradiol-treated tutored song = 6, estradiol-treated untutored song = 8, estradiol-treated silence = 7, blank-treated tutored song = 5, blank-treated untutored song = 6, blank-treated silence = 6. * p= 0.006.
Figure 4.
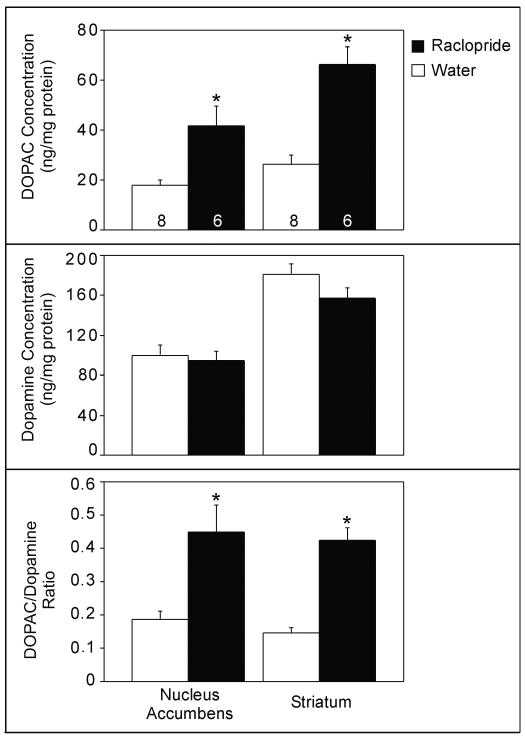
Effects of raclopride treatment (mean±S.E.) on the concentration of DOPAC (top) and DA (middle), and the DOPAC/DA ratio (bottom) in the nucleus accumbens and medial striatum. Sample sizes are indicated at the bottom of each bar; * p< 0.008.
Table 2.
Concentrations of DOPAC, Dopamine and the DOPAC/DA ratio in the nucleus accumbens and striatum following song presentation and estrogen treatment. No main effects or interactions were detected, all F< 1.92, p> 0.16.
| Brain Region |
Treatment | Song | DOPAC concentration (ng/mg)±SE |
Dopamine concentration (ng/mg)±SE |
DOPAC/DA ratio±SE |
|---|---|---|---|---|---|
| Nucleus Accumbens |
Estrogen | Tutored n= 7 |
11.95±1.85 | 20.63±3.15 | 0.6±0.10 |
| Untutored n= 7 |
12.26±2.37 | 25.83±6.25 | 0.51±0.06 | ||
| Silence n= 5 |
14.61±3.88 | 21.64±4.06 | 0.67±0.13 | ||
| Blank | Tutored n= 6 |
12.51±2.38 | 29.85±5.43 | 0.46±0.09 | |
| Untutored n= 8 |
9.70±1.96 | 17.12±1.91 | 0.55±0.80 | ||
| Silence n= 7 |
12.6±2.86 | 23.04±5.93 | 0.74±0.16 | ||
| Striatum * | Estrogen | Tutored | 22.88±4.51 | 85.07±12.37 | 0.29±0.06 |
| Untutored | 30.24±5.70 | 137.89±20.94 | 0.22±0.02 | ||
| Silence | 37.40±4.85 | 113.43±10.63 | 0.22±0.02 | ||
| Blank | Tutored | 29.49±4.92 | 125.58±18.83 | 0.26±0.05 | |
| Untutored | 32.98±5.34 | 110.15±8.88 | 0.29±0.04 | ||
| Silence | 38.81±9.52 | 131.95±16.58 | 0.24±0.05 | ||
Sample sizes same as in Nucleus Accumbens
Results
Estrogen and Song Exposure - Behavior
A main effect of treatment was observed, such that estradiol decreased the number of distance calls (F= 5.12, p= 0.031) and visual scanning (F= 6.26, p= 0.018), regardless of type of stimulus exposure (Figure 2; data pooled across the groups of auditory stimuli for ease of viewing in the figure since they did not differ). In addition, a main effect of auditory stimulus was observed in the number of ‘other calls’ (F= 5.93, p= 0.006); females produced more of them when exposed to untutored song than silence (Tukey Kramer, p< 0.05; Figure 3). No main effects of song or treatment or interactions between these variables, were observed for any of the other behaviors (all F< 3.99, p> 0.055).
Raclopride
A main effect of treatment was observed in the concentration of DOPAC (F= 23.35, p= 0.001) and the DOPAC/DA ratio (F= 25.95, p= 0.001); raclopride increased both compared to the control manipulation (Figure 4). An interaction between brain region and treatment indicated that the effect of raclopride on DOPAC concentration was greater in the striatum than in the nucleus accumbens (F= 10.14, p= 0.008). In addition, the concentrations of DOPAC and DA were higher in the striatum than in the nucleus accumbens (F= 39.70, p< 0.001; F= 40.48, p< 0.001, respectively). As expected, no effect of raclopride treatment on DA concentration was observed (F= 2.43, p= 0.145).
Estrogen and Song Exposure - Neurochemistry
A main effect of brain region was observed such that concentrations of DA (F= 250.314, p< 0.001) and DOPAC (F= 0.82.24, p<0.001) were greater in the striatum than in the nucleus accumbens. In addition, the DOPAC/DA ratio was higher in nucleus accumbens than in the striatum (F= 58.69, p< 0.001). A trend for an interaction among treatment and song type was detected in concentration of DA (F= 03.26, p= 0.051), but one-way ANOVAs between treatments or among song exposure groups revealed no significant differences (all F< 4.44, p> 0.059). No other effects of song or treatment on neurochemistry approached statistical significance (all F< 1.92, p> 0.16; Table 2).
Discussion
Summary
Estradiol decreased long-distance calls and visual scanning behavior, and across hormone manipulations, females presented a greater number of ‘other calls’ when hearing untutored song compared to silence. Through the raclopride study, we verified our measures of dopaminergic activity in the nucleus accumbens and striatum. However, neither estrogen treatment nor exposure to either tutored or untutored song affected these values in the two neural centers.
Behavioral response to estrogen treatment and song exposure
The present results provide a more detailed analysis of the effect of estradiol on behavior than our previous work on female zebra finches [7]. In the earlier study, we did not observe an effect of these manipulations on a general call measure. However, vocalizations were not separated into distance and ‘other calls’ in that study, and visual scanning behavior was not quantified. In the current study, the estrogen-induced decrease in distance calls and visual scanning behavior indicates that behavioral effects of the hormone do occur. These effects may be associated with the same function, involving a reduced need or desire for locating other individuals. Visual scanning behavior would be consistent with this idea, and distance calls are typically used for localization of other birds and often uttered when an individual is isolated [17]. It is interesting to note that this reduction in behavior parallels a general reduction of ZENK expression in the ventromedial hypothalamus following estrogen treatment in a previous study [7].
At least three potential explanations exist for the observed reductions in behaviors. First, it is possible that estrogen treatment results in decreased social interest. Perhaps the visual cues received from the model zebra finch were sufficient for estrogen-treated females and increased attempts to contact or find an individual were not required. Second, estradiol facilitates receptive behaviors in a variety of vertebrate species [see 57-59 for reviews]. Perhaps an increased focus on mating results in a decrease in the amount of time the female spends on other behaviors such as calling or scanning their environment. Third, it is possible that estrogen-treated females have decreased anxiety, as has been observed in mammals [reviewed in 60]. This reduction in anxiety may then result in a diminished response to the novelty of the testing environment, which is manifested in fewer distance calls and visual scanning. Although modulated by estradiol treatment, these behaviors were unaffected by auditory stimulus type. This result suggests that these behaviors may be displayed in response to some other aspect of the testing situation, perhaps isolation or one or more features of the novel environment.
Our results differ from those of Vyas et al. [46, 47] who showed that estrogen treatment resulted in increased selectivity of the behavioral response to song (i.e. increased number of distance calls in response to high versus lower quality song). However, several important differences exist between their studies and ours. First, Vyas et al. [46, 47] utilized three song stimuli: prototypical song (normal song), long bout song (which had additional motifs), and complex song (which included acoustically different song motifs and high numbers of unique syllables). Thus, female responses to normal song were compared to those of artificially higher quality. In our study, we compared behavior following exposure to normal versus lower quality song. It is possible that females employ distinct mechanisms to distinguish between the features, and estradiol mediates these mechanisms in different ways. Second, we exposed females to only one song type during the behavioral test, whereas in the other studies, females were exposed to all three song types during each test, and proportional responses to them were compared across the groups. Third, no silence group was utilized by Vyas et al. [46, 47].
In contrast, other types of calls (tets and stacks, collectively) were not affected by estradiol treatment in our study but were increased in response to untutored song compared to silence. This result is consistent with the idea that females are responding to the unfamiliarity of this song type, which they would not have previously heard. In parallel, exposure to novel stimuli can result in increased locomotion. Tets are used as contact calls and are often associated with movement, and stacks are frequently presented when taking off in flight [17]. We did not detect an effect of song type on movement, but that may be due to the small size of the cage in which the test was conducted. However, it is also possible that hearing this unusual and novel stimulus, untutored song, induces females to increase their attempts to contact other birds.
Dopaminergic response to raclopride treatment
The increased levels of DOPAC and DOPAC/DA ratio in both the striatum and nucleus accumbens of raclopride-treated birds indicates that activity of these neurons was increased by blocking D2 dopaminergic receptors. In mammals, D2 receptors serve as autoreceptors, and raclopride treatment results in an increase in DOPAC in both of the regions [61, 62]. Based on the results of several in vitro studies, it appears that this also holds true in birds. For example, DA release decreases when D2 receptor agonists are administered to chicken hyperstriatal slices [25], and the activity of striatal dopaminergic neurons is decreased with activation of the D2 receptor [63]. The results here confirm that this effect is also observed following in vivo treatment with raclopride, validating the use of DOPAC concentration and the DOPAC/DA ratio as reliable indicators of dopaminergic activity in birds, as in mammals.
Dopaminergic response to estrogen treatment
The lack of effects of estrogen treatment on DA, DOPAC, and the DOPAC/DA ratio was surprising, as estradiol increases DA release in both the striatum [64, 65] and the nucleus accumbens [66, 67] in mammals. Estradiol also increases labeling for tyrosine hydroxylase in white-throated sparrows in the ventral tegmental area, the location of dopaminergic cell bodies [45]. We cannot rule out the possibility that there may be a ceiling effect, such that more estradiol than baseline has no effect on these measures. It is also possible that the period of implantation may not have been long enough to affect dopamine responses in these regions, but the fact that behavior was affected suggests that some changes within the brain did occur. A more interesting potential explanation is the hypothesis that hormones play a different role in opportunistic breeders such as the zebra finch compared to seasonal breeders like sparrows. For example, it is possible that a physiological difference exists, such that various features might be inherently less sensitive to changes in hormone level. This idea would need to be tested in future experiments.
Parallel to the lack of DA response, we did not detect an effect of estrogen on ZENK expression in response to song in either the social behavior network or mesolimbic dopaminergic system in zebra finches [7]. Similarly, ZENK expression in catecholamine neurons (tyrosine hydroxylase positive neurons which may be dopaminergic or noradrenergic) following song exposure was unaffected by estrogen treatment in white-throated sparrows [45]. Perhaps in this system estradiol is involved with slower or more long-term modulation, but short-term neurochemical responses within these regions are less sensitive to the hormone.
Dopaminergic responses to song exposure
To our knowledge, the present study is the first to concurrently examine the effects of song perception on dopaminergic neurons in the striatum and nucleus accumbens in songbirds. Concentrations of DA, DOPAC and the DOPAC/DA ratio were not affected by song exposure in the nucleus accumbens or striatum. This result corresponds with the fact that presentation of the same auditory stimulus did not increase the density of ZENK immunoreactive nuclei in the nucleus accumbens or the ventral tegmental area in zebra finches [7]. Similarly, in female starlings, male song had no effect on immunoreactivity of phosphorylated tyrosine hydroxylase (an indicator of dopaminergic activity) in the ventral tegmental area [39]. In contrast, dopaminergic neurons in some brain regions do appear to be involved in song perception. For example, the level of DOPAC in the auditory perception regions increases following exposure to high quality song stimuli [40], and phosphorylated tyrosine hydroxylase increases after exposure to male song in the breeding season in the lateral septum and ventromedial hypothalamus of starlings [39].
Perception of zebra finch song may differ from what is observed in other songbirds in part because adult male zebra finch song is simple and highly stable in adulthood [17]. Therefore, it may not be as critical for female zebra finches to utilize additional neuronal processing (such as the activity of these dopaminergic systems) to assess the relative value of or distinguish among song types. Alternatively, females may utilize auditory perception systems to determine whether or not song is present and then process other cues such as visual courtship or tactile stimuli to determine the relative value of those signals via the dopaminergic system.
We also cannot completely rule out the possibility that the time point at which we collected the tissue may have affected our results. However, DA levels rapidly increase and remain elevated for 20-30 minutes in Area X (in the medial striatum) of male zebra finches after singing [16]. As tissue for the present experiment was collected 10 min after the conclusion of the song presentation, it seems likely that if dopaminergic activity had changed with song exposure, it would still be detectable.
Summary
The results from this study, along with other recently collected data from this lab, reveal that estrogen has specific effects on both behavior and neural activity in adult female zebra finches. Estrogen decreased the display of distance calls and visual scanning (present study) and inhibited the expression of ZENK in the ventromedial hypothalamus [7]. These effects, however, appear independent of auditory stimulus presentation, and therefore may result from some other aspect of the behavior testing environment. As estradiol in the ventromedial hypothalamus in other model systems affects both anxiety and sexual receptivity (see above), either of these factors could have influenced the present behavioral results. Other vocalizations were increased in response to untutored song (present study), and female zebra finches spend more time near tutored compared to untutored song [7]. Neither estradiol treatment nor song exposure appears to affect dopaminergic responses in the nucleus accumbens or the medial striatum. However, DA innervation is also present in cortical regions in songbirds [i.e., 68, 69], and it is possible that their activity plays a role in mediating responses to song. This idea should be investigated in future studies.
Acknowledgements
Research was supported through grants from the National Institutes of Health to Juli Wade (R01-MH55488 and K02-MH65907). We would like to thank Katie Licht for behavioral coding and Elizabeth Adkins-Regan for providing the tutored and untutored song stimuli.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Gentner TQ, Hulse SH, Duffy D, Ball GF. Response Biases in Auditory Forebrain Regions of Female Songbirds Following Exposure to Sexually Relevant Variation in Male Song. J Neurobiol. 2001;46:48–58. doi: 10.1002/1097-4695(200101)46:1<48::aid-neu5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [2].Bailey DJ, Rosebush JC, Wade J. The Hippocampus and Caudomedial Neostriatum Show Selective Responsiveness to Conspecific Song in the Female Zebra Finch. J Neurobiol. 2002;52:43–51. doi: 10.1002/neu.10070. [DOI] [PubMed] [Google Scholar]
- [3].Maney DL, MacDougall-Shackleton EA, MacDougall-Shackleton SA, Ball GF, Hahn TP. Immediate early gene response to hearing song correlates with receptive behavior and depends on dialect in a female songbird. J Comp Physiol A. 2003;189:667–674. doi: 10.1007/s00359-003-0441-z. [DOI] [PubMed] [Google Scholar]
- [4].Leitner S, Voigt C, Metzdorf R, Catchpole CK. Immediate early gene (ZENK, Arc) expression in the auditory forebrain of female canaries varies in response to male song quality. J Neurobiol. 2005;64(3):275–84. doi: 10.1002/neu.20135. [DOI] [PubMed] [Google Scholar]
- [5].Sockman KW, Gentner TQ, Ball GF. Complementary Neural Systems for the Experience-Dependent Integration of Mate-Choice Cues in European Starlings. J Neurobiol. 2005;62:72–81. doi: 10.1002/neu.20068. [DOI] [PubMed] [Google Scholar]
- [6].Tomaszycki ML, Sluzas EM, Sundberg KA, Newman SW, DeVoogd TJ. Immediate early gene (ZENK) responses to song in juvenile female and male zebra finches: effects of rearing environment. J Neurobiol. 2006;66(11):1175–82. doi: 10.1002/neu.20275. [DOI] [PubMed] [Google Scholar]
- [7].Svec LA, Wade J. Estradiol induces region-specific inhibition of ZENK but does not affect the behavioral preference for tutored song in adult female zebra finches. Behav Brain Res. 2009;199(2):298–306. doi: 10.1016/j.bbr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mitchell JB, Gratton A. Involvement of mesolimbic dopamine neurons in sexual behaviors: implications for the neurobiology of motivation. Rev Neurosci. 1994;5(4):317–29. doi: 10.1515/revneuro.1994.5.4.317. [DOI] [PubMed] [Google Scholar]
- [9].Melis MR, Argiolas A. Dopamine and sexual behavior. Neurosci Biobehav Rev. 1995;19(1):19–38. doi: 10.1016/0149-7634(94)00020-2. [DOI] [PubMed] [Google Scholar]
- [10].Paredes RG, Agmo A. Has dopamine a physiological role in the control of sexual behavior? A critical review of the evidence. Prog Neurobiol. 2004;73(3):179–226. doi: 10.1016/j.pneurobio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- [11].Fabre-Nys C, Ohkura S, Kendrick KM. Male faces and odours evoke differential patterns of neurochemical release in the mediobasal hypothalamus of the ewe during oestrus: an insight into sexual motivation? Eur J Neurosci. 1997;9(8):1666–77. doi: 10.1111/j.1460-9568.1997.tb01524.x. [DOI] [PubMed] [Google Scholar]
- [12].Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6(2):228–36. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- [13].Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137(12):3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- [14].Heimovics SA, Riters LV. Evidence that dopamine within motivation and song control brain regions regulates birdsong context-dependently. Physiol Behav. 2008;95(12):258–266. doi: 10.1016/j.physbeh.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Heimovics SA, Riters LV. Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J Neurobiol. 2005;65(3):207–24. doi: 10.1002/neu.20181. [DOI] [PubMed] [Google Scholar]
- [16].Sasaki A, Sotnikova TD, Gainetdinov RR, Jarvis ED. Social context-dependent singing-regulated dopamine. J Neurosci. 2006;26(35):9010–4. doi: 10.1523/JNEUROSCI.1335-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zann RA. In: The Zebra Finch: A Synthesis of Field and Laboratory Studies. Perrins CM, editor. Oxford University Press; New York: 1996. (Oxford Ornithology Series). [Google Scholar]
- [18].Durstewitz D, Kroner S, Gunturkun O. The dopaminergic innervation of the avian telencephalon. Prog Neurobiol. 1999;59(2):161–95. doi: 10.1016/s0301-0082(98)00100-2. [DOI] [PubMed] [Google Scholar]
- [19].Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Gunturkun O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473(3):377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Balint E, Csillag A. Nucleus accumbens subregions: hodological and immunohistochemical study in the domestic chick (Gallus domesticus) Cell Tissue Res. 2007;327(2):221–30. doi: 10.1007/s00441-006-0295-0. [DOI] [PubMed] [Google Scholar]
- [21].Szekely AD, Boxer MI, Stewart MG, Csillag A. Connectivity of the lobus parolfactorius of the domestic chicken (Gallus domesticus): an anterograde and retrograde pathway tracing study. J Comp Neurol. 1994;348(3):374–93. doi: 10.1002/cne.903480305. [DOI] [PubMed] [Google Scholar]
- [22].Metzger M, Jiang S, Wang J, Braun K. Organization of the dopaminergic innervation of forebrain areas relevant to learning: a combined immunohistochemical/retrograde tracing study in the domestic chick. J Comp Neurol. 1996;376(1):1–27. doi: 10.1002/(SICI)1096-9861(19961202)376:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- [23].Gale SD, Perkel DJ. Physiological properties of zebra finch ventral tegmental area and substantia nigra pars compacta neurons. J Neurophysiol. 2006;96(5):2295–306. doi: 10.1152/jn.01040.2005. [DOI] [PubMed] [Google Scholar]
- [24].Gale SD, Perkel DJ. Properties of dopamine release and uptake in the songbird basal ganglia. J Neurophysiol. 2005;93(4):1871–9. doi: 10.1152/jn.01053.2004. [DOI] [PubMed] [Google Scholar]
- [25].Jackson G, Hudson AL, Lalis M, Raj AB. Pharmacological characterisation of the electrically evoked release of monoamines from chicken brain in vitro. Br Poult Sci. 2007;48(1):76–83. doi: 10.1080/00071660601157485. [DOI] [PubMed] [Google Scholar]
- [26].Reiner A, Medina L, Veenman CL. Structural and functional evolution of the basal ganglia in vertebrates. Brain Res Brain Res Rev. 1998;28(3):235–85. doi: 10.1016/s0165-0173(98)00016-2. [DOI] [PubMed] [Google Scholar]
- [27].Rieke GK. Movement disorders and lesions of pigeon brain stem analogues of basal ganglia. Physiol Behav. 1981;26(3):379–84. doi: 10.1016/0031-9384(81)90162-1. [DOI] [PubMed] [Google Scholar]
- [28].Sanberg PR, Mark RF. The effect of striatal lesions in the chick on haloperidol-potentiated tonic immobility. Neuropharmacology. 1983;22(2):253–7. doi: 10.1016/0028-3908(83)90018-7. [DOI] [PubMed] [Google Scholar]
- [29].Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12(10):366–75. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- [30].Hauber W. Involvement of basal ganglia transmitter systems in movement initiation. Prog Neurobiol. 1998;56(5):507–40. doi: 10.1016/s0301-0082(98)00041-0. [DOI] [PubMed] [Google Scholar]
- [31].Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- [32].Esch T, Stefano GB. The neurobiology of pleasure, reward processes, addiction and their health implications. Neuro Endocrinol Lett. 2004;25(4):235–51. [PubMed] [Google Scholar]
- [33].Akins CK, Levens N, Prather R, Cooper B, Fritz T. Dose-dependent cocaine place conditioning and D1 dopamine antagonist effects in male Japanese quail. Physiol Behav. 2004;82(23):309–15. doi: 10.1016/j.physbeh.2004.03.035. [DOI] [PubMed] [Google Scholar]
- [34].Akins CK, Geary EH. Cocaine-induced behavioral sensitization and conditioning in male Japanese quail. Pharmacol Biochem Behav. 2008;88(4):432–7. doi: 10.1016/j.pbb.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Riters LV, Teague DP, Schroeder MB, Cummings SE. Vocal production in different social contexts relates to variation in immediate early gene immunoreactivity within and outside of the song control system. Behav Brain Res. 2004;155(2):307–18. doi: 10.1016/j.bbr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- [36].Lynch KS, Diekamp B, Ball GF. Catecholaminergic cell groups and vocal communication in male songbirds. Physiol Behav. 2008;93(45):870–6. doi: 10.1016/j.physbeh.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bharati IS, Goodson JL. Fos responses of dopamine neurons to sociosexual stimuli in male zebra finches. Neuroscience. 2006;143(3):661–70. doi: 10.1016/j.neuroscience.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL. Estradiol modulates neural responses to song in a seasonal songbird. J Comp Neurol. 2008;511(2):173–86. doi: 10.1002/cne.21830. [DOI] [PubMed] [Google Scholar]
- [39].Riters LV, Olesen KM, Auger CJ. Evidence that female endocrine state influences catecholamine responses to male courtship song in European starlings. Gen Comp Endocrinol. 2007;154(13):137–49. doi: 10.1016/j.ygcen.2007.05.029. [DOI] [PubMed] [Google Scholar]
- [40].Sockman KW, Salvante KG. The integration of song environment by catecholaminergic systems innervating the auditory telencephalon of adult female European starlings. Dev Neurobiol. 2008;68(5):656–68. doi: 10.1002/dneu.20611. [DOI] [PubMed] [Google Scholar]
- [41].Searcy WA, Marler P. A Test for Responsiveness to Song Structure and Programming in Female Sparrows. Science. 1981;213(4510):926–928. doi: 10.1126/science.213.4510.926. [DOI] [PubMed] [Google Scholar]
- [42].Clayton N, Prove E. Song Discrimination in Female Zebra Finches and Bengalese Finches. Anim Behav. 1989;38:352–362. [Google Scholar]
- [43].Searcy WA, Capp MS. Estradiol Dosage and the solicitation display assay in red-winged blackbirds. The Condor. 1997;99:826–828. [Google Scholar]
- [44].Ballentine B, Hyman J, Nowicki S. Vocal performance influences female response to male bird song: an experimental test. Behav Ecol. 2004;15(1):163–168. [Google Scholar]
- [45].LeBlanc MM, Goode CT, MacDougall-Shackleton EA, Maney DL. Estradiol modulates brainstem catecholaminergic cell groups and projections to the auditory forebrain in a female songbird. Brain Res. 2007;1171:93–103. doi: 10.1016/j.brainres.2007.06.086. [DOI] [PubMed] [Google Scholar]
- [46].Vyas A, Harding C, Borg L, Bogdan D. Acoustic characteristics, early experience, and endocrine status interact to modulate female zebra finches’ behavioral responses to songs. Horm Behav. 2009;55(1):50–9. doi: 10.1016/j.yhbeh.2008.08.005. [DOI] [PubMed] [Google Scholar]
- [47].Vyas A, Harding C, McGowan J, Snare R, Bogdan D. Noradrenergic neurotoxin, N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride (DSP-4), treatment eliminates estrogenic effects on song responsiveness in female zebra finches (Taeniopygia guttata) Behav Neurosci. 2008;122(5):1148–57. doi: 10.1037/0735-7044.122.5.1148. [DOI] [PubMed] [Google Scholar]
- [48].Silcox AP, Evans SM. Factors affecting the formation and maintenance of pair bonds in the zebra finch, Taeniopygia guttata. Anim Behav. 1982;30:1237–1243. [Google Scholar]
- [49].Adkins-Regan E, Abdelnabi M, Mobarak M, Ottinger MA. Sex Steroid Levels in Developing and Adult Male and Female Zebra Finches (Poephila guttata) Gen Comp Endocrinol. 1990;78:93–109. doi: 10.1016/0016-6480(90)90051-m. [DOI] [PubMed] [Google Scholar]
- [50].Hutchison JB, Wingfield JC, Hutchison RE. Sex differences in plasma concentrations of steroids during the sensitive period for brain differentiation in the zebra finch. J Endocrinol. 1984;103(3):363–9. doi: 10.1677/joe.0.1030363. [DOI] [PubMed] [Google Scholar]
- [51].Leboucher D, Kreutzer M, Dittami J. Copulation-solicitation Displays in Female Canaries (Serinus canaria): are Oestradiol Implants Necessary? Ethology. 1994;97:190–197. [Google Scholar]
- [52].Lauay C, Gerlach NM, Adkins-Regan E, DeVoogd TJ. Female zebra finches require early song exposure to prefer high-quality song as adults. Anim Behav. 2004;68:1249–1255. [Google Scholar]
- [53].Palkovits M. Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Res. 1973;59:449–50. doi: 10.1016/0006-8993(73)90290-4. [DOI] [PubMed] [Google Scholar]
- [54].Lindley SE, Gunnet JW, Lookingland KJ, Moore KE. 3,4-Dihydroxyphenylacetic acid concentrations in the intermediate lobe and neural lobe of the posterior pituitary gland as an index of tuberohypophysial dopaminergic neuronal activity. Brain Res. 1990;506(1):133–8. doi: 10.1016/0006-8993(90)91209-y. [DOI] [PubMed] [Google Scholar]
- [55].Behrouz B, Drolet RE, Sayed ZA, Lookingland KJ, Goudreau JL. Unique responses to mitochondrial complex I inhibition in tuberoinfundibular dopamine neurons may impart resistance to toxic insult. Neuroscience. 2007;147(3):592–8. doi: 10.1016/j.neuroscience.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- [57].Pfaff DW, Schwartz-Giblin S, McCarthy MM, Kow LM. Cellular and Molecular Mechanisms of Female Reproductive Behaviors. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven Press, Ltd.; New York: 1994. pp. 107–220. [Google Scholar]
- [58].Ball GF, Balthazart J. Non-Mammalian Hormone-Behavior Systems. Elsevier B.V.; San Diego: 2002. Neuroendocrine Mechanisms Regulating Reproductive Cycles and Reproductive Behavior in Birds; pp. 649–788. [Google Scholar]
- [59].Godwin J, Crews D. Hormones, Brain, and Behavior in Reptiles. In: Pfaff D, et al., editors. Non-Mammalian Hormone-Behavior Systems. Elsevier B.V.; San Diego: 2002. pp. 545–585. [Google Scholar]
- [60].Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31(6):1097–111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ogren SO, Hall H, Kohler C, Magnusson O, Sjostrand SE. The selective dopamine D2 receptor antagonist raclopride discriminates between dopamine-mediated motor functions. Psychopharmacology (Berl) 1986;90(3):287–94. doi: 10.1007/BF00179179. [DOI] [PubMed] [Google Scholar]
- [62].Eaton MJ, Tian Y, Lookingland KJ, Moore KE. Comparison of the effects of remoxipride and raclopride on nigrostriatal and mesolimbic dopaminergic neuronal activity and on the secretion of prolactin and alpha-melanocyte-stimulating hormone. Neuropsychopharmacology. 1992;7(3):205–11. [PubMed] [Google Scholar]
- [63].Ding L, Perkel DJ. Dopamine modulates excitability of spiny neurons in the avian basal ganglia. J Neurosci. 2002;22(12):5210–8. doi: 10.1523/JNEUROSCI.22-12-05210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Becker JB, Beer ME. The influence of estrogen on nigrostriatal dopamine activity: behavioral and neurochemical evidence for both pre- and postsynaptic components. Behav Brain Res. 1986;19(1):27–33. doi: 10.1016/0166-4328(86)90044-6. [DOI] [PubMed] [Google Scholar]
- [65].Becker JB, Cha JH. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res. 1989;35(2):117–25. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- [66].Thompson TL, Moss RL. Modulation of mesolimbic dopaminergic activity over the rat estrous cycle. Neurosci Lett. 1997;229(3):145–8. doi: 10.1016/s0304-3940(97)00450-3. [DOI] [PubMed] [Google Scholar]
- [67].Saigusa T, Takada K, Baker SC, Kumar R, Stephenson JD. Dopamine efflux in the rat nucleus accumbens evoked by dopamine receptor stimulation in the entorhinal cortex is modulated by oestradiol and progesterone. Synapse. 1997;25(1):37–43. doi: 10.1002/(SICI)1098-2396(199701)25:1<37::AID-SYN5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- [68].Bottjer SW. The distribution of tyrosine hydroxylase immunoreactivity in the brains of male and female zebra finches. J Neurobiol. 1993;24(1):51–69. doi: 10.1002/neu.480240105. [DOI] [PubMed] [Google Scholar]
- [69].Appeltants D, Ball GF, Balthazart J. The distribution of tyrosine hydroxylase in the canary brain: demonstration of a specific and sexually dimorphic catecholaminergic innervation of the telencephalic song control nulcei. Cell Tissue Res. 2001;304(2):237–59. doi: 10.1007/s004410100360. [DOI] [PubMed] [Google Scholar]